Abstract
Background
Anthracycline use in metastatic breast cancer (MBC) is hindered by cumulative exposure limits and risk of cardiotoxicity. Pixantrone, a novel aza-anthracenedione with structural similarities to mitoxantrone and anthracyclines, is theorized to exhibit less cardiotoxicity, mainly due to lack of iron binding. We conducted a randomized phase II study to evaluate the efficacy and safety of 2 dosing schedules of pixantrone in patients with refractory HER2-negative MBC.
Methods
Intravenous pixantrone was administered at 180 mg/m2 every 3 weeks (group A) versus 85 mg/m2 on days 1, 8, and 15 of a 28-day cycle (group B). Primary endpoint was objective response rate (ORR) and secondary endpoints included progression-free survival (PFS), median 6-month PFS, overall survival (OS), safety, quality of life, and serial assessment of circulating tumor cells. A 20% ORR was targeted as sufficient for further testing of pixantrone in this patient population.
Results
Forty-five patients were evaluable, with 2 confirmed partial responses in group A and 1 in group B. The trial was terminated due to insufficient activity. Overall median PFS and OS were 2.8 (95% confidence interval [CI]: 2.0-4.1) and 16.8 (95% CI: 8.9-21.6) months, respectively. Notable overall grade 3-4 adverse events were the following: neutrophil count decrease (62%), fatigue (16%), and decrease in ejection fraction (EF) (4%).
Conclusion
Pixantrone has insufficient activity in the second- and third-line MBC setting. It appears, however, to have limited cardiotoxicity. (ClinicalTrials.gov ID: NCT01086605).
Keywords: pixantrone, breast cancer, anthracycline, randomized phase II, cardiotoxicity
This randomized phase II study evaluated the efficacy and safety of two dosing schedules of pixantrone in patients with refractory HER2-negative metastatic breast cancer.
Lessons Learned.
Pixantrone has limited single agent antitumor activity in the second- and third-line settings in patients with metastatic breast cancer.
Pixantrone appears safe with little cardiotoxicity in anthracycline pretreated patients.
Discussion
Anthracyclines are active in MBC but limited by cumulative cardiotoxicity. Epirubicin, liposomal-doxorubicin and mitoxantrone were developed, in part, to improve on cardiac safety. However, all compounds continue to be toxic.1,2 Pixantrone, a novel aza-anthracenedione, does not bind iron and forms no secondary alcohol metabolites, thus an improved cardiotoxicity profile is postulated.3 Mouse models4 and clinical trials have demonstrated the cardiac safety of pixantrone, with congestive heart failure (CHF) observed in 2%-3% of patients previously exposed to doxorubicin.5 Pixantrone is approved in Europe as a salvage treatment for relapsed/refractory non-Hodgkin’s lymphoma.6
The NCCTG N1031 randomized phase-II study assessed the efficacy and safety of 2 dosing schedules of pixantrone as second- to fourth-line chemotherapy in HER2-negative MBC, after progression on taxanes. Primary endpoint was ORR. Forty-seven patients enrolled between July 2010 and July 2011. One patient was ineligible and one never started treatment leaving 45 evaluable patients (24 in group A and 21 in group B). Baseline characteristics were balanced. Twenty-two percent had triple-negative breast cancer. Most patients (80%) received prior doxorubicin and 71% received between 2 and 3 prior lines of chemotherapy. A median of 3 cycles was given (range 1-12). All 45 patients discontinued treatment, primarily due to progression (62.2%) or adverse events (17.8%). One patient completed the maximum 12 cycles.
Overall 3 patients (7%; 95%CI: 1%-18%) achieved a confirmed PR; 2 in group A (8%; 95%CI: 1%-27%), and 1 in group B (5%; 95%CI: 0%-24%). Only 1 patient among the first 16 patients, enrolled in each group, achieved a PR. The early stopping rule required at least 2 confirmed responses among the first 16 evaluable patients for each dose level to continue accrual. Thus, the study was terminated due to insufficient activity.
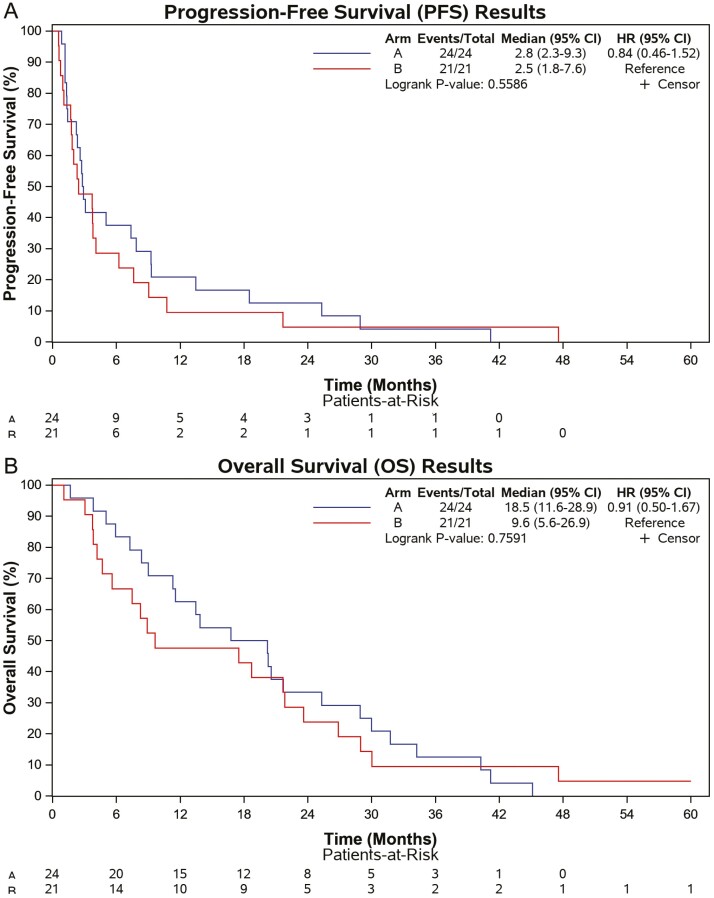
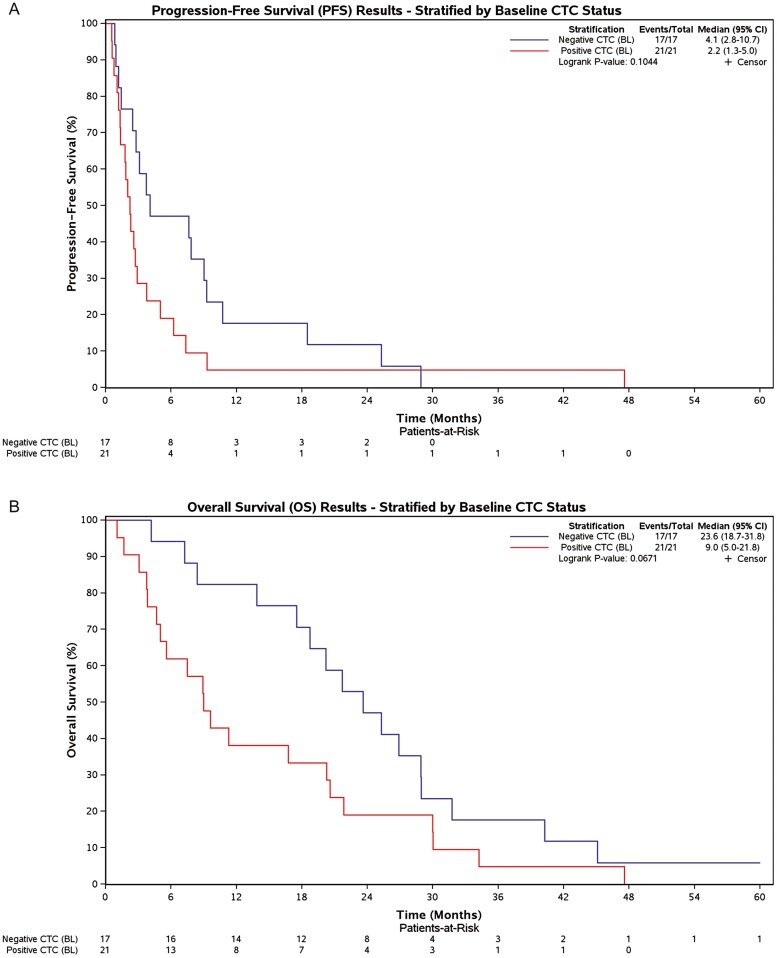
All patients were followed until death with median OS for groups A and B 18.5 months (95%CI: 11.6-28.9) and 9.6 months (95%CI: 5.6-26.9), respectively (Fig. 1A), and median PFS 2.8 months (95%CI: 2.3-9.3) and 2.5 months (95%CI: 1.8-7.6), respectively (Fig. 1B). Overall median PFS and OS were 2.8 (95% CI: 2.0-4.1) and 16.8 (95% CI: 8.9-21.6) months, respectively. No significant QOL differences in change over time were observed (data not presented). Patients with <5 CTCs at baseline (n = 17) had a trend toward improved OS compared to patients with ≥5 CTCs (n = 21), with median OS 23.6 months (95%CI: 18.7-31.8) versus 9.0 months (95%CI: 5.0-21.8), respectively (P = .0671).
Figure 1.
Kaplan-Meier plots for progression-free and overall survival by dosing schedule. Group A received pixantrone at 180 mg/m2 every 3 weeks and group B received pixantrone 85 mg/m2 on days 1, 8, and 15 of 4-week cycles.
Safety signals were similar to previously reported toxicities. All-grade adverse events included neutropenia (91%), fatigue (89%), anemia (78%), alopecia (76%), and nausea (71%). Six patients experienced an EF drop (4 grade 2, 2 grade 3) with a 2% mean drop.
Pixantrone has insufficient single agent activity in anthracycline and/or taxane pretreated patients with MBC. Adverse effects are in keeping with prior experience. Cardiotoxicity was minimal.
Trial Information
| Disease | Breast cancer |
| Stage of disease/treatment | Metastatic/Advanced |
| Prior therapy | 2 prior regimens |
| Type of study | Phase II, Randomized |
| Primary endpoint | Overall response rate |
| Secondary endpoints | Progression-free survival, Overall survival, Safety, Correlative endpoint |
| Investigator’s analysis | Inactive because results did not meet primary endpoint |
Additional Details of Endpoints or Study Design
Patient Eligibility
Eligible patients included adult men or women with (1) histologically or cytologically confirmed metastatic breast adenocarcinoma, (2) measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST v1.0), (3) use of taxanes in the metastatic setting (patients had up to 2-3 prior lines of chemotherapy in the metastatic setting if no prior (neo)-adjuvant chemotherapy was given, while only up to 1 or 2 prior treatments were allowed if prior (neo)-adjuvant chemotherapy was received, (4) unlimited prior hormonal therapies were allowed, (5) left ventricular ejection fraction (LVEF) ≥50% and EKG within institutional normal limits prior to registration. Key exclusion criteria included (1) Prior cumulative anthracycline dose exceeding 400 mg/m2 doxorubicin equivalent dose, (2) HER2-positive breast cancer, (3) leptomeningeal disease or active brain metastases, (4) uncontrolled hypertension or clinically significant heart disease. Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Study Design and Treatment
NCCTG N1031 was a 2-stage randomized (1:1) phase II study design testing 2 dose schedules of pixantrone (weekly vs 3-weekly). Group A patients received 180 mg/m2 IV every 3 weeks, and group B patients 85 mg/m2 IV on days 1, 8, 15 every 4 weeks. Patients were treated until disease progression, unacceptable toxicity, or voluntary withdrawal from the study. Up to 2 dose modifications were allowed per patient for toxicity. Due to lack of long-term cardiac safety data no more than 12 cycles were allowed. The study complies with the published CONSORT guidelines (http://www.consort-statement.org/). NCCTG is now part of the Alliance for Clinical Trials in Oncology.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on January 23, 2020.
Evaluation of Response and Toxicity
Evaluation of response was performed every 2 cycles of treatment. Criteria for response and progression were based on RECIST v1.0. A confirmed tumor response was defined to be either a complete response (CR) or partial response (PR) noted as the objective status on 2 consecutive evaluations at least 6-8 weeks apart. Evaluation for toxicity was based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE v4.0). Toxicities were evaluated with every visit. Cardiac monitoring was performed by either echocardiogram (ECHO) or MUGA scan (based on institutional preference) after cycles 4, 8, 10, and 12, and 6-8 months after end of treatment if possible.
QOL
QOL was measured as a secondary outcome using the Linear Analogue Self-Assessment (LASA-6).7,8,9
Circulating tumor cells
Optional circulating tumor cell (CTC) collections were conducted at baseline, prior to cycle 3 and at the end of treatment. A total of 10 mL of whole blood was collected using CellSearch Kits (provided by Cell Therapeutics, currently named CTI BioPharma) per the manufacturer’s instructions (Menarini Silicon Biosystems, San Diego, CA). A cutoff of ≥ 5 CTCs/7.5 mL blood was used for a positive result.
Statistical Design and Analysis
This trial was designed to assess the efficacy of 2 doses of pixantrone in patients with metastatic breast cancer using a 2-stage phase II study design. With 25 evaluable patients in each dose cohort and assuming a 10% significance level, there was a greater than 90% chance of detecting a tumor response rate of at least 30% in this patient population; where a response rate of 10% or less would lead to the conclusion that this regimen lacks sufficient anti-tumor activity to recommend it for further testing in this patient population, while a response rate of 20% (5 out of 25 patients) would indicate that further testing would be recommended. The primary endpoint for the study was overall response rate, defined as the proportion of confirmed tumor responses at each dose level of pixantrone.
If at most 1 of the first 16 evaluable patients, enrolled at a given dose level, achieved a confirmed tumor response then enrollment would be terminated and the given dose level would be considered inactive in this patient population. If at least 5 of the first 25 evaluable patients enrolled into a given dose level achieved a confirmed tumor response, consideration would be given to recommending this dose level for further testing in this patient population. Accrual continued while the first 16 evaluable patients were being evaluated for the early stopping rule.
Survival time is defined as the time from registration to death due to any cause. The distribution of survival time was estimated using the method of Kaplan-Meier at each dose level. Time to disease progression is defined as the time from registration to the earliest date of documentation of disease progression. If a patient dies without a documentation of disease progression the patient will be considered to have had disease progression at the time of their death. The distribution of time to progression will be estimated using the method of Kaplan-Meier at each dose level.
Overall QOL, QOL subdomains items were assessed at each time point by each dose level using the mean and median, each with an appropriate confidence interval. Exploratory comparisons of overall QOL and QOL subdomains were made between the 2 different dose levels using Wilcoxon rank sum tests at single data time points.
Drug Information: Group A, Pixantrone
| Generic/Working name | Pixantrone |
| Trade name | Pixurvi |
| Company name | Servier |
| Drug type | Chemotherapy |
| Drug class | Anthracenedione |
| Dose | 180 milligrams (mg) per squared meter (m²) |
| Route | IV |
| Schedule of administration | Every 3 weeks |
Drug Information: Group B, Pixantrone
| Generic/Working name: | Pixantrone |
| Trade name: | Pixurvi |
| Company name: | Servier |
| Drug type: | Chemotherapy |
| Drug class: | Anthracenedione |
| Dose: | 85 milligrams (mg) per squared meter (m²) |
| Route: | IV |
| Schedule of administration: | days 1, 8, 15, of 4 week cycle |
Dose Escalation Table
| Dose Level | Dose of Drug: Pixantrone | Number Enrolled | Number Evaluable for Toxicity |
|---|---|---|---|
| Group A | 180 mg/m2 q3w | 24 | 24 |
| Group B | 85 mg/m2 days 1, 8, and 15 of a 28-day cycle | 21 | 21 |
Patient Characteristics: Group A
| Number of patients, male | 1 |
| Number of patients, female | 23 |
| Stage | IV |
| Age | Median (range): 57 (42-79) years |
| Number of prior systemic therapies | Median (range): 2(1-3) |
| Performance Status: ECOG | 0 — 11 1 — 12 2 — 1 3 — 0 Unknown — 0 |
Patient Characteristic: Group B
| Number of patients, male | 0 |
| Number of patients, female | 21 |
| Stage | IV |
| Age | Median (range): 55 (38-75) years |
| Number of prior systemic therapies | Median (range): 2(1-3) |
| Performance Status: ECOG | 0 — 6 1 — 12 2 — 3 3 — 0 Unknown — 0 |
Primary Assessment Method: Group A
| Title | Overall Response Rate |
|---|---|
| Number of patients screened | 24 |
| Number of patients enrolled | 24 |
| Number of patients evaluable for toxicity | 24 |
| Number of patients evaluated for efficacy | 24 |
| Evaluation Method | RECIST 1.0 |
| Response assessment CR | n = 0 (0%) |
| Response assessment PR | n = 2 (8%) |
| Response assessment SD | n = 14 (58%) |
| Response assessment PD | n = 7 (29%) |
| Response assessment OTHER | n = 1 (5%) |
| (Median) duration assessments PFS | 2.8 Days, CI: 2.3-9.3 |
| (Median) duration assessments OS | 18.5 Days, CI: 11.6-28.9 |
Outcome Notes
Hazard ratio for PFS was 0.84 (95% CI: 0.46-1.52)
Hazard ratio for OS was 0.91 (95% CI: 0.50-1.67)
Primary Assessment Method: Group B
| Title | Overall Response Rate |
|---|---|
| Number of patients screened | 23 |
| Number of patients enrolled | 21 |
| Number of patients evaluable for toxicity | 21 |
| Number of patients evaluated for efficacy | 21 |
| Evaluation Method | RECIST 1.0 |
| Response assessment CR | n = 0 (0%) |
| Response assessment PR | n = 1 (5%) |
| Response assessment SD | n = 10 (48%) |
| Response assessment PD | n = 10 (48%) |
| Response assessment OTHER | n = 0 (0%) |
| (Median) duration assessments PFS | 2.5 months, CI: 1.8-7.6 |
| (Median) duration assessments OS | 9.6 months, CI: 5.6-26.9 |
Adverse Events: Group A (all cycles, regardless of attribution)
| Name | *NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
|---|---|---|---|---|---|---|---|
| Neutrophil count decreased | 17% | 13% | 13% | 46% | 13% | 0% | 83% |
| White blood cell decreased | 67% | 4% | 13% | 17% | 0% | 0% | 33% |
| Lymphocyte count decreased | 92% | 0% | 4% | 4% | 0% | 0% | 8% |
| Fatigue | 13% | 54% | 17% | 17% | 0% | 0% | 88% |
| Anemia | 25% | 54% | 17% | 4% | 0% | 0% | 75% |
| Left ventricular systolic dysfunction | 83% | 0% | 13% | 4% | 0% | 0% | 17% |
| Pneumonitis | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Pleural effusion | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Dyspnea | 92% | 0% | 8% | 0% | 0% | 0% | 8% |
| ALT, SGPT (serum glutamic pyruvic transaminase) | 83% | 8% | 0% | 4% | 4% | 0% | 17% |
| AST, SGOT(serum glutamic oxaloacetic transaminase) | 79% | 13% | 0% | 4% | 4% | 0% | 21% |
| Blood bilirubin increased | 92% | 4% | 0% | 4% | 0% | 0% | 8% |
| Hyponatremia | 88% | 4% | 0% | 8% | 0% | 0% | 13% |
| Potassium, serum-low (hypokalemia) | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypercalcemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypoalbuminemia | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hepatobiliary disorders - Other, specify | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Hypertension | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Blood and lymphatic system disorders - Other, specify | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Nausea | 33% | 38% | 25% | 4% | 0% | 0% | 67% |
| Vomiting | 63% | 29% | 4% | 4% | 0% | 0% | 38% |
| Peripheral sensory neuropathy | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Alopecia | 25% | 42% | 33% | 0% | 0% | 0% | 75% |
| Skin and subcutaneous tissue disorders - Other, specify | 50% | 50% | 0% | 0% | 0% | 0% | 50% |
| Diarrhea | 58% | 42% | 0% | 0% | 0% | 0% | 42% |
| Platelet count decreased | 79% | 17% | 4% | 0% | 0% | 0% | 21% |
| Mucositis oral | 67% | 21% | 13% | 0% | 0% | 0% | 33% |
| Activated partial thromboplastin time prolonged | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Lung infection | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Syncope | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Pain in extremity | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Chest wall pain | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Back pain | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Buttock pain | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Neck pain | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Voice changes/dysarthria (e.g., hoarseness, loss or alteration invoice,laryngitis) | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
Adverse Events: Group B (all cycles, regardless of attribution)
| Name | *NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
|---|---|---|---|---|---|---|---|
| Neutrophil count decreased | 0% | 10% | 24% | 62% | 5% | 0% | 100% |
| White blood cell decreased | 57% | 5% | 10% | 19% | 10% | 0% | 43% |
| Lymphocyte count decreased | 76% | 0% | 14% | 5% | 5% | 0% | 24% |
| Fatigue | 10% | 38% | 38% | 14% | 0% | 0% | 90% |
| Anemia | 19% | 48% | 29% | 5% | 0% | 0% | 81% |
| Left ventricular systolic dysfunction | 90% | 0% | 5% | 5% | 0% | 0% | 10% |
| Pneumonitis | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
| Pleural effusion | 90% | 0% | 5% | 5% | 0% | 0% | 10% |
| Dyspnea | 90% | 0% | 5% | 5% | 0% | 0% | 10% |
| ALT, SGPT (serum glutamic pyruvic transaminase) | 71% | 24% | 0% | 5% | 0% | 0% | 29% |
| AST, SGOT(serum glutamic oxaloacetic transaminase) | 57% | 19% | 10% | 14% | 0% | 0% | 43% |
| Blood bilirubin increased | 76% | 10% | 5% | 5% | 5% | 0% | 24% |
| Hyponatremia | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
| Hypokalemia | 90% | 5% | 0% | 5% | 0% | 0% | 10% |
| Hypercalcemia | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
| Hypoalbuminemia | 81% | 10% | 5% | 5% | 0% | 0% | 19% |
| Hepatobiliary disorders - Other, specify | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Hypertension | 95% | 0% | 5% | 0% | 0% | 0% | 5% |
| Blood and lymphatic system disorders - Other, specify | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
| Nausea | 24% | 62% | 14% | 0% | 0% | 0% | 76% |
| Vomiting | 57% | 38% | 5% | 0% | 0% | 0% | 43% |
| Peripheral sensory neuropathy | 71% | 19% | 5% | 5% | 0% | 0% | 29% |
| Alopecia | 24% | 24% | 52% | 0% | 0% | 0% | 76% |
| Skin and subcutaneous tissue disorders - Other, specify | 62% | 33% | 5% | 0% | 0% | 0% | 38% |
| Diarrhea | 71% | 19% | 10% | 0% | 0% | 0% | 29% |
| Platelet count decreased | 67% | 19% | 14% | 0% | 0% | 0% | 33% |
| Mucositis oral | 95% | 5% | 0% | 0% | 0% | 0% | 5% |
| Lung infection | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Activated partial thromboplastin time prolonged | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Syncope | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| Pain in extremity | 90% | 0% | 0% | 10% | 0% | 0% | 10% |
| Chest wall pain | 90% | 5% | 0% | 5% | 0% | 0% | 10% |
| Back pain | 90% | 0% | 5% | 5% | 0% | 0% | 10% |
| Buttock pain | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
| Neck pain | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
| Voice changes/dysarthria (eg, hoarseness, loss or alteration invoice, laryngitis) | 95% | 0% | 0% | 5% | 0% | 0% | 5% |
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator’s Assessment | Inactive Because Results Did Not Meet Primary Endpoint |
Pixantrone has insufficient single-agent activity in the MBC setting after multiple lines of therapy. The primary endpoint of the study was the proportion of confirmed tumor responses at each dose level of pixantrone. Standard chemotherapeutic options in the second and third line setting have historically shown a response rate between 10% and 35%.10-15 Pixantrone was to move forward with further testing in the Her-2 negative, metastatic breast cancer setting if the null hypothesis of a 10% response rate was rejected in either group. Since at interim analysis, only 1 patient per group had a confirmed tumor response rate (6%) the study was halted to further enrollment.
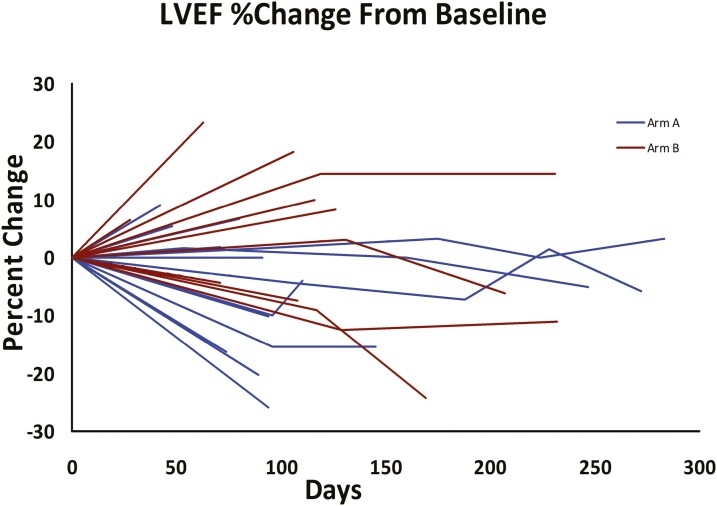
Pixantrone exhibited minimal cardiotoxicity in the studied patients, many of whom had prior anthracycline use. Six patients experienced an EF drop (4 grade 2 and 2 grade 3). Follow-up echocardiogram was available for 27 patients (those with at least 4 cycles of treatment) and mean EF drop was 2.0% (Fig. 2). Pixantrone was specifically designed to reduce anthracycline-induced cardiotoxicity.16 By not binding iron, pixantrone generates less reactive oxygen species.3,17 At the same time pixantrone has no secondary alcohol metabolites, the accumulation of which is thought to result in chronic cardiac toxicity.3 Animal studies have supported both the relative safety of pixantrone in this setting4 and the comparable or superior effectiveness of pixantrone compared to mitoxantrone or doxorubicin.18 Prior early-phase studies showed that, as a single agent, pixantrone was associated with a 2% chance of reduced LVEF. In our study, 1 patient per group (4%-5%) had a grade 3 reduction in LVEF, both in patients with prior doxorubicin use (Fig. 2). This is in keeping with the 3% LVEF reduction seen in patients in a study of refractory, aggressive non-Hodgkin’s lymphoma with prior exposure to anthracyclines.5
Figure 2.
Change in ejection fraction at the end of treatment as compared to baseline.
The adverse event profile of pixantrone was in keeping with prior experience. Neutropenia is known to be the most frequent grade 3 toxicity of pixantrone. In our study we observed 62% grade 3-4 neutropenia in the every 3 week schedule (group A) and 58% grade 3-4 neutropenia in the weekly schedule (group B). In the setting of refractory non-Hodgkin lymphoma a rate of 41% grade 3-4 neutropenia has been shown.5 It is not clear why a higher rate of neutropenia was seen in our study, given that the patients in the refractory Non-Hodgkin lymphoma study were also heavily pretreated. On the other hand, grade 3-4 thrombocytopenia was lower in our study (0%) in comparison to the non-Hodgkin lymphoma study 12%).
Correlative studies included assessment of circulating tumors cells (CTCs), which have been confirmed to have potential relevance and importance in assessing prognosis and clinical response to systemic therapy.19,20 The presence and persistence of ≥5 CTCs/7.5 mL blood in MBC patients, before and after treatment, is prognostic for poor clinical outcome and treatment failure.21,22 CTCs were collected at baseline (n = 38), before cycle 3 (n = 25), and end of treatment (n = 30). When using ≥5 CTCs/7.5 mL for cutoff value of a positive test, patients with <5 CTCs at baseline had a trend toward improved overall survival and progression-free survival (PFS) (Fig. 3). The failure to conclusively demonstrate this, in our study, likely relates to the small size of our cohort. In addition, it is of interest that <5 CTCs/7.5 mL, both at cycle 3 and at end of treatment, are associated with improved overall survival and PFS in our study. However, given the optional nature of the CTC study collection, as well as the significant missing data at these time points, bias cannot be excluded and we thus do not present the data.
Figure 3.
(A) Kaplan-Meier plots for progression-free survival (PFS) and overall survival by CTC cut-off (negative <5 CTCs, positive ≥5 CTCs). (B) Kaplan-Meier plots for overall survival (OS) and overall survival by CTC cut-off (negative <5 CTCs, positive ≥5 CTCs).
In conclusion, pixantrone exhibited insufficient activity in a cohort of chemotherapy pretreated patients with metastatic breast cancer to warrant further investigation as a single agent in this setting. Cardiotoxicity, however, was minimal despite prior use of anthracyclines in the majority of patients.
Click here to access other published clinical trials.
Acknowledgments
This study was sponsored by CTIBIOPHARMA. This study was conducted as a collaborative trial of North Cancer Center Treatment Group-Alliance for Clinical Trials in Oncology, the Nacional Cancer Institute and the Mayo Clinic and was supported in part by Public Health Service grants U10CA180821 and U10CA025224 from the National Cancer Institute Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. ClinicalTrials.gov Identifier: NCT01086605.
Additional participating institutions: Cancer Center of Kansas, Wichita, KS; Essentia Health Duluth Clinic, Duluth, MN; Carle Cancer Center COOP, Urbana, IL; Oncology Associates at Mercy Medical Center, Cedar Rapids, IA; Mcfarland Clinic, Ames, IA; Illinois Cancer Care, IL; Sanford Cancer Center, Sioux Falls, SD; Metro-Minnesota COOP, MN; Riverside Methodist Hospital Cancer Care, Columbus, OH; Grant Medical Center Cancer Care, Columbus, OH; Upstate Carolina COOP, Spartanburg, SC; Providence Portland Medical Center, Portland, OR; University of New Mexico Cancer Center, NM; Saint Joseph Mercy Medical Center, Ann Arbor, MI; Billings Clinic, Billings, MT; St. Vincent Hospital Cancer Center, Green Bay, WI; Baptist Medical Center, Missouri Baptist Medical Center, MO
Conflict of Interest
The authors indicated no financial relationships.
Data Availability
De-identified participant data and the associated data dictionary to replicate all analysis in the publication will be made available at https://nctn-data-archive.nci.nih.gov/ beginning 6 months after earliest publication date.
References
- 1. Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryberg M, Nielsen D, Cortese G, Nielsen G, Skovsgaard T, Andersen PK.. New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst. 2008;100(15):1058-1067. [DOI] [PubMed] [Google Scholar]
- 3. Salvatorelli E, Menna P, Paz OG, et al. The novel anthracenedione, pixantrone, lacks redox activity and inhibits doxorubicinol formation in human myocardium: insight to explain the cardiac safety of pixantrone in doxorubicin-treated patients. J Pharmacol Exp Ther. 2013;344(2):467-478. [DOI] [PubMed] [Google Scholar]
- 4. Cavalletti E, Crippa L, Mainardi P, et al. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: comparative studies against doxorubicin and mitoxantrone. Invest New Drugs. 2007;25(3):187-195. [DOI] [PubMed] [Google Scholar]
- 5. Pettengell R, Coiffier B, Narayanan G, et al. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2012;13(7):696-706. [DOI] [PubMed] [Google Scholar]
- 6. Péan E, Flores B, Hudson I, et al. The European Medicines Agency review of pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin’s B-cell lymphomas: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2013;18(5):625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchanan DR, O’Mara AM, Kelaghan JW, Minasian LM.. Quality-of-life assessment in the symptom management trials of the National Cancer Institute-supported Community Clinical Oncology Program. J Clin Oncol. 2005;23(3):591-598. [DOI] [PubMed] [Google Scholar]
- 8. Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C; Clinical Significance Consensus Meeting Group. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002;77(5):479-487. [PubMed] [Google Scholar]
- 9. Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249-4255. [DOI] [PubMed] [Google Scholar]
- 10. Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer. 2001;92(7):1759-1768. [DOI] [PubMed] [Google Scholar]
- 11. Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40(4):536-542. [DOI] [PubMed] [Google Scholar]
- 12. Gasparini G, Caffo O, Barni S, et al. Vinorelbine is an active antiproliferative agent in pretreated advanced breast cancer patients: a phase II study. J Clin Oncol. 1994;12(10):2094-2101. [DOI] [PubMed] [Google Scholar]
- 13. Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25(23):3407-3414. [DOI] [PubMed] [Google Scholar]
- 14. Reichardt P, Von Minckwitz G, Thuss-Patience PC, et al. Multicenter phase II study of oral capecitabine (Xeloda(“)) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003;14(8):1227-1233. [DOI] [PubMed] [Google Scholar]
- 15. Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25(33):5210-5217. [DOI] [PubMed] [Google Scholar]
- 16. Menna P, Salvatorelli E, Minotti G.. Rethinking drugs from chemistry to therapeutic opportunities: pixantrone beyond anthracyclines. Chem Res Toxicol. 2016;29(8):1270-1278. [DOI] [PubMed] [Google Scholar]
- 17. Hasinoff BB, Wu X, Patel D, Kanagasabai R, Karmahapatra S, Yalowich JC.. Mechanisms of action and reduced cardiotoxicity of pixantrone; a topoisomerase ii targeting agent with cellular selectivity for the topoisomerase IIα isoform. J Pharmacol Exp Ther. 2016;356(2):397-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beggiolin G, Crippa L, Menta E, et al. Bbr 2778, an AZA-anthracenedione endowed with preclinical anticancer activity and lack of delayed cardiotoxicity. Tumori. 2001;87(6):407-416. [DOI] [PubMed] [Google Scholar]
- 19. Ignatiadis M, Perraki M, Apostolaki S, et al. Molecular detection and prognostic value of circulating cytokeratin-19 messenger RNA-positive and HER2 messenger RNA-positive cells in the peripheral blood of women with early-stage breast cancer. Clin Breast Cancer. 2007;7(11):883-889. [DOI] [PubMed] [Google Scholar]
- 20. Reinholz MM, Nibbe A, Jonart LM, et al. Evaluation of a panel of tumor markers for molecular detection of circulating cancer cells in women with suspected breast cancer. Clin Cancer Res. 2005;11(10):3722-3732. [DOI] [PubMed] [Google Scholar]
- 21. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781-791. [DOI] [PubMed] [Google Scholar]
- 22. Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920-928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified participant data and the associated data dictionary to replicate all analysis in the publication will be made available at https://nctn-data-archive.nci.nih.gov/ beginning 6 months after earliest publication date.