Abstract
Stroke is one of the most important acute diseases that endanger human health and result in death, including acute cerebral hemorrhage and acute cerebral ischemia. Acute onset is its most prominent feature. Carbon monoxide (CO) is a colorless and odorless gas existing at room temperature. It is not only a common air pollutant, but also has been found to be closely related to stroke. A large amount of exogenous CO has an important impact on the incidence and prognosis of stroke, while endogenous CO as a gas signal also has an important impact on neuroprotection after stroke. Both low-dose CO inhalation and CO-releasing molecule-3 (a molecule that emits CO) treatment have shown the benefits of stroke, and perhaps the role of CO in stroke is one of the key areas for future research.
Keywords: air pollution, carbon monoxide, cerebral hemorrhage, cerebral ischemia, CORM-3, HO-1, neuroinflammation, neuroprotection, oxidative stress, stroke
INTRODUCTION
Stroke is one of the most important acute diseases endangering human health in the world today, and it is also an important factor in the death and disability of the global population.1 Stroke is divided into acute ischemic stroke and acute hemorrhagic stroke according to the type of incidence. Ischemic stroke is divided into arteriosclerotic thrombotic cerebral infarction, cardiogenic cerebral embolism and lacunar infarction; hemorrhagic stroke is divided into cerebral hemorrhage and subarachnoid hemorrhage.2,3 Regarding cerebral ischemic stroke, there is no operation or medicine that can achieve a consistent and satisfactory effect on cerebral infarction that has occurred.4 Thrombolytic therapy is currently the only treatment with definite curative effect for acute ischemic stroke, while other medications are used to prevent recurrence and systemic protective treatment.4 The incidence of hemorrhagic stroke is lower than that of ischemic stroke, and the main treatment is surgical treatment of intracranial lesions and postoperative medication to control blood pressure and prevent vasospasm.5 Because of the great harm of stroke, unsatisfactory treatment effect, and poor prognosis, the prevention and treatment of stroke is very urgent and important.
Epidemiological studies on risk factors of stroke have not been stopped, some risk factors have been recognized, and some have not been confirmed. The unchangeable risk factors of stroke include age, sex, race, and so on, while the variable risk factors of stroke have a wider range, including internal and external environmental factors, such as hypertension, smoking, unreasonable diet, lack of exercise, etc.6,7 Most of the risk factors of stroke are related to lifestyle, which can be prevented through healthy lifestyle change.7 However, previous stroke studies also show that aging, air pollution and smoking accelerate the prevalence of stroke and other diseases.8,9 A large number of related literatures show that carbon monoxide (CO) in air pollution has become one of the new risk factors for stroke, leading us to summarize the research on the relationship between stroke and CO.10,11,12
CO in the atmosphere is a common colorless and odorless gas pollutant, or a large number and widely distributed pollutant gas produced by incomplete combustion of carbon-containing energy sources such as coal and petroleum.13,14 In addition, a small amount of CO is also produced in the chemical reaction of the upper atmosphere, the slight dissociation of carbon dioxide and the metabolism of animals.13 Generally, the concentration of CO in fresh air is harmless to humans, but with the continuous development of industrialization, the use of coal and petroleum has increased CO emissions and has an impact on health, such as stroke.11,12
In the body, endogenous CO is the second gas signal molecule found after nitric oxide (NO).15 The CO in cells is mainly produced by the decomposition of heme under the catalysis of heme oxygenase (HO).16 Endogenous CO can not only freely pass through the membrane structure and mediate cell function changes; it can also interact with enzymes and ion channels containing transition metals, such as soluble guanylyl cyclase, sodium ion channels, NO synthase, and cytochrome c oxidase, etc., combine to play a related physiological role.16,17,18 Exogenous CO enters the blood through the respiratory system, and forms a reversible combination with hemoglobin (Hb) in the blood and some other iron-containing proteins outside the blood (such as myoglobin, cytochromes of Fe2+, etc.).19,20 More than 90% of CO is combined with Hb to form carboxyhemoglobin (COHb), about 7% of CO is combined with myoglobin, and only a small amount is combined with cytochrome.19 Especially in the nervous system, CO can also bind to the oxygen-carrying protein neuroglobin in brain tissue.21 With the gradual increase of COHb content, the dissociation of oxygen in oxygenated Hb and the output of CO2 in the tissue are hindered, which eventually leads to tissue hypoxia and carbon dioxide retention, resulting in symptoms of poisoning.19,21 Although the formation of COHb causes tissue hypoxia, it is difficult to explain many phenomena after poisoning, such as common arrhythmia and brain damage during poisoning.21 Therefore, we would like to review the specific role of carbon monoxide in the body, especially in stroke, which has a great impact on us. We searched PubMed for relevant articles published by the end of 2020 and summarized them.
EFFECTS OF CARBON MONOXIDE ON THE NERVOUS SYSTEM
CO is like every coin has two sides to the nervous system. When the intracellular CO content exceeds a certain physiological concentration, it can produce neuroprotective effects by synergistically acting with NO or increasing the production of intracellular cyclic guanosine phosphate (cGMP).21,22 When the dose continues to increase, including the concentration and exposure time, it may cause poisoning, but the dose boundary from the protective effect to the poisoning effect is not clear.22 When excessive CO in cells causes poisoning, it not only forms excessive COHb and causes hypoxia, but also can promote the production of reactive oxygen species and oxidative stress injury by damaging mitochondria, increasing Hb level, binding platelet Hb, promoting NO production and other functions.21,22,23,24 When CO is poisoned, excessive CO binds to the iron atom of the heme group in soluble guanylyl cyclase to change its configuration and activate the enzyme, increasing the production of cGMP in the cell.23,25 Excessive cGMP can make the CO signal regulation system malfunction.25 In addition to the above, when CO is poisoned, CO can act on ion channels containing transition metals to change their configuration and affect ion passage, such as inhibiting neuronal K+ channels and activating a voltage-gated Na+ channel to excite cells sexual increase.23,26 In addition to the aforementioned low-dose CO nerve cell protection and a large number of CO nerve damage functions, CO also affects the circadian rhythm.27 In the nervous system, CO can regulate the circadian rhythm through neuronal PAS domain protein 2 (NPAS-2, another transcription factor involved in transcription, which can be combined with the response element in the nucleus through the heterodimer formed by clock and brain and muscle aryl-hydrocarbon-receptor-nuclear-translocator-like 1 after adjusting the circadian rhythm).27
CO and stroke incidence
With the development of technology and industry, global air pollution has become increasingly serious, and CO has become one of the most important gases in air pollution.28 A large amount of data shows that CO exposure in the air is closely related to stroke admission, and reducing environmental health issues such as CO and other air pollution can reduce the occurrence of stroke.10,11 Stroke caused by CO is not only related to air pollution such as CO, but also to seasons.12 Increased CO exposure in polluted air during warm seasons increased the risk of stroke, while CO concentration in cold seasons was not associated with stroke incidence.12 This effect may be due to seasonal differences in CO exposure or CO air pollution.29 Air pollution such as CO is also related to the race to increase the risk of stroke hospitalization and death, especially Asians and ischemic stroke.30 In China, studies on the relationship between stroke cases and environmental pollution in many cities have found that exposure to major air pollution gases such as sulfur dioxide and CO is positively correlated with the hospitalization rate of ischemic stroke, while hemorrhagic stroke is only associated with nitrogen dioxide.31 In Hong Kong, China, the maximum daily concentration of CO in the air is negatively correlated with the number of people admitted to the emergency department due to stroke. This may be due to the protective effect of CO exposure on stroke.32 This is different from the research results in China's mainland. This may be because the air quality is different. The air in Hong Kong, China has a neuroprotective effect because it contains low doses of CO, while the CO air pollution in northern China is heavier due to industrial development. In general, however, these all suggest that CO and stroke are inextricably linked.
CO poisoning is also one of the suspected risk factors for stroke, more and more stroke cases after CO poisoning are reported.33,34 In South Korea, 3.36% of patients were diagnosed with stroke after CO poisoning, and about 50% of stroke occurred within 1 year after CO poisoning.35 In Taiwan, China, the incidence of ischemic stroke after CO poisoning is 2.5 times higher than that of the normal population. In particular, compared with patients who did not have CO exposure, the risk of CO poisoning is greater in young people aged 20–34 years, and the incidence of stroke is also greater.34 Not only does the concentration of CO exposure in the air and CO poisoning affect the stroke of patients, the CO gas we exhale is also related to stroke, which sounds more interesting. The CO in the body is excreted through our lungs. American researchers measured the concentration of exhaled CO and followed up for 13 years, and found that higher exhaled CO is negatively related to cerebrovascular disease, which is related to stroke/transient ischemic attack Increased risk related.36 Higher exhale CO levels (over 3 ppm) are associated with an increased risk of ischemic stroke, not with sex, although there seems to be an independent association with male smokers.37 These results may be due to the fact that endogenous CO at high concentrations reduces the antithrombotic activity of the endothelial layer by inhibiting endothelial NO and enhances the migration of inflammatory cells by stimulating cGMP.36 In addition, endogenous CO can also increase blood sugar, high blood pressure, oxidative stress and free radicals, thereby increasing the risk of stroke.36,38
CO and cerebral ischemia
After cerebral ischemia, the distribution and concentration of CO in brain tissue will change. The appropriate concentration of internal and exogenous low-dose CO has neuroprotective effects during ischemia-reperfusion. In a mouse model of transient middle cerebral artery occlusion, when 125 or 250 ppm CO was inhaled immediately at the beginning of ischemia/reperfusion, the infarct volume and cerebral edema were reduced, and the neurological function after 48 hours of ischemia was improved neurological defect score.39 These findings indicate that low CO levels can protect the brain from transient focal ischemic injury and reperfusion, and its potential application in the treatment of cerebral ischemic stroke. Ischemic stroke involves multiple mechanisms after vascular occlusion and reperfusion. One of the mechanism components comes from the formation of oxygen free radicals in hypoxic mitochondria.40 HO is considered to be an effective antioxidant in the nervous system and it can degrade heme from heme protein to produce CO, iron and biliverdin.16 HO has two subtypes, HO-1 and HO-2. HO-1 is an inducible enzyme, mainly concentrated in the liver and spleen, but in the brain it can be induced by hypoxia and inflammation to induce HO-1 protein synthesis.41,42 HO-2 is a constitutively expressed enzyme, mainly concentrated in the brain and testis, and accounts for most of the HO activity in the brain.42,43 In the rat cerebral ischemia model, HO-1 is induced to increase, and it is highly concentrated in the border of the infarcted tissue and glial cells.44 In the in vitro ischemic stroke model, the induced increase of HO-1 in astrocytes also occurs, which leads to the increase of intracellular CO in neurons, and the increase of CO may reduce the activity of caspase-3 by mediating intracellular cGMP to protect the hypoxic-mediated apoptosis and death of neurons.45 HO-1 can also reduce lipid peroxidation and protein nitrification in mice with cerebral ischemic stroke, thereby reducing infarct volume and reducing neurological deficits.46 In addition, HO-1 can slightly reduce the ischemic brain NO concentration, inhibit the brain expression of inducible NO synthase protein expression, and maintain NO bioavailability by increasing the level of endothelial NO synthase phosphorylation.46 In the animal model of cerebral ischemia lacking HO-2, nerve damage is aggravated, because HO-2 usually exerts a neuroprotective effect in the brain, and the CO produced can also affect the vasodilation function through cGMP.44
Under oxidative stress conditions after cerebral ischemia, the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf-2) dissociates from Kelch-like ECH-associated protein 1, an important regulator of oxidative stress, and then passes through the nucleus, and binds to antioxidant response elements to induce expression HO-1.47 After middle cerebral artery occlusion in mice, the infarct area of mice exposed to CO was smaller than that not treated with CO.48 This was because after CO exposure, Kelch-like ECH-associated protein 1 bound Nrf-2 in mice was significantly reduced, and the binding activity of Nrf-2 and HO-1 antioxidant reaction elements was increased.47,48 CO treatment cannot save the mice with Nrf-2 gene deletion, which shows that low concentration of CO is mediated by the activation of Nrf-2 pathway in the treatment of ischemic stroke.48
CO releasing molecule 3 (CORM-3) produces low-dose CO and has neuroprotective functions.49 The use of CORM-3 to treat mice with transient middle cerebral artery occlusion can reduce cerebral infarction, cerebral edema, neuroinflammation, blood-brain barrier destruction and improve the prognosis after brain injury.50 This is because CORM-3 treatment inhibits the activation of microglia, down-regulates the expression of ionized calcium-binding adaptor molecule-1, tumor necrosis factor-α and interleukin-1β, and reduces matrix metalloproteinase-9. The protein level increases the expression of platelet-derived growth factor receptor-β, tight junction protein zonula occluden-1 and matrix protein laminin.50
Restoration of spontaneous circulation after cardiac arrest, it can also cause cerebral ischemia/reperfusion injury.51 After low dose CO treatment of restoration of spontaneous circulation, the expression level of mitochondrial biological factors (peroxisome proliferator-activated receptor-γ coactivator-1α, nuclear respiratory factor-1, nuclear respiratory factor-2 and mitochondrial transcription factor A) in hippocampus and cerebral cortex increased, which indicated that CO could improve cerebral ischemia by increasing mitochondrial activity after brain injury.52
In children with hypoxic-ischemic encephalopathy, levels of CO and NO are positively correlated with the severity of hypoxic-ischemic encephalopath.53 CO treatment can reduce the apoptosis of hippocampal cells induced by hypoxia and ischemia in neonatal mice, limit the release of cytochrome c from mitochondria, reduce the activation of caspase-3, and increase the expression of Bcl-2 in neurons, thus resulting in neuroprotective effect of cerebral ischemia.54
CO and cerebral hemorrhage
After hemorrhagic stroke, inflammation and oxidative stress can cause brain damage and increase the expression of HO-1.55,56 Heme can inhibit the antioxidant activity of peroxidase I, so the co-expression of HO-1 and peroxidase I is the mechanism to maintain its antioxidant function.57 In astrocytes and microglia in the rat brain hemorrhage area, HO-1 and peroxidase I co-expression produced antioxidant effects.55 When CORM-3 was used to treat intracerebral hemorrhage induced by collagenase in rats, pretreatment and 3-day treatment of intracerebral hemorrhage could reduce inflammation and plasma tumor necrosis factor-α.58
Subarachnoid hemorrhage (SAH) is also a common form of hemorrhagic stroke.59 The common vasospasm after the onset may be caused by Hb and Hb released by hemolytic red blood cells.60 After SAH, the stress of HO-1 in the whole brain glial of rats increases.56 In particular, the HO-1 response of microglia can not only increase heme clearance and iron chelation, but also increase the amount of antioxidant bilirubin to reduce lipid peroxidation and vasospasm.56 After SAH, the mice were treated with low-dose CO for 1 hour, which improved neurological deficits and vasospasm, which may indicate that CO has vasodilation, neurotransmission, inhibition of platelet aggregation, and anti-smooth muscle proliferation.61,62 The amount of hematoma in SAH patients has also been shown to be related to the expression of HO-1, because the sudden accumulation of blood in the subarachnoid space will stimulate the increase of HO-1 expression in the cerebrospinal fluid of the patient.63 Therefore, the production of HO-1 and CO is also very important for reducing neuronal cell death and clearing cerebral hematoma after SAH.
The disturbance of the biological clock after subarachnoid hemorrhage prompted researchers to study the effects of HO-1 and CO on the restoration of circadian rhythm. The results show that the increased expression of clock genes Per-1 (period 1), Per-2 (period 2) and NPAS-2 (neuronal PAS domain protein 2) after SAH is related to the basic expression of HO-1, and can reduce cerebral vasospasm, neuronal apoptosis and microglia activation significantly reduced.64 After SAH in HO-1 knockout mice, low-dose CO treatment can restore the low expression of Per-1, Per-2 and NPAS-2, and reduce neuronal cell apoptosis.63
CONCLUSION
CO can be considered as a combination of devil and angel. He is a devil because people still know him about the effects of hypoxia/reoxygenation on the nerve center after CO poisoning, such as dizziness, nausea and memory loss. Moreover, a large number of retrospective studies have shown that a large number of CO exposure or poisoning can increase the risk of stroke (Table 1). The concentration of CO in pure air is about 1 ppm, and that in general urban air is about 10 ppm. Systematic reviews and meta-analyses have shown that stroke hospitalization rate and stroke mortality are closely related to the increase of CO concentration in normal air (relative risk 1.015/1 ppm).11
Table 1.
High levels of CO or CO poisoning increase the risk of stroke
| Study | Year | Region | Design | Results |
|---|---|---|---|---|
| Hong et al.10 | 2002 | Korea | Case-crossover study | Air pollutants are significantly associated with ischemic stroke mortality, which suggests an acute pathogenetic process in the cerebrovascular system induced by air pollution. |
| Kettunen et al.12 | 2007 | Finland | Case-crossover study | PM2.5, ultrafine particles and carbon monoxide, are associated with increased risk of fatal stroke, but only during the warm season. |
| Yang et al.30 | 2014 | Global | Case-crossover study | Air pollution may transiently increase the risk of stroke hospitalizations and stroke mortality. |
| Shah et al.11 | 2015 | Global | Systematic review and Meta-analysis | Gaseous and particulate air pollutants have a marked and close temporal association with admissions to hospital for stroke or mortality from stroke. |
| Lin et al.34 | 2016 | Taiwan, China | Retrospective cohort analysis | CO poisoning is associated with a long-term risk of increased incident ischemic stroke. |
| Nayor et al.36 | 2016 | USA | Case-crossover study | Higher exhaled CO was associated with a greater burden of subclinical cerebrovascular disease cross-sectionally and with increased risk of stroke/transient ischemic attack prospectively. |
| Liu et al.31 | 2017 | China | Case-crossover study | PM10, NO2, SO2, CO, and O3 exposures were positively associated with ischemic stroke. |
| Kim et al.35 | 2020 | Korea | Case-crossover study | CO poisoning is a high-risk factor for the development of stroke, evidenced by the high incidences of stroke after CO poisoning. |
Note: CO: Carbon monoxide; NO2: nitrogen dioxide; O3: ozone; PM10: particulate matter less than 10 μm; PM2.5: particulate matter less than 2.5 μm; SO2: sulfur dioxide.
By reviewing the impact of CO on stroke, he can also become a beautiful angel, among which HO/CO is an important neuroprotective pathway (Table 2). The elevated HO-1 of stress after stroke can reduce oxidative stress damage and inflammation through several important ways, while maintaining the circadian rhythm after stroke (Figure 1). Both low-dose CO inhalation and CORM-3 treatment have shown benefits in addition to stroke. Exposure to 125 ppm and 250 ppm CO after MACO showed neuroprotective effects, especially when inhalation 18 hours of 250 ppm CO after ischemia/reperfusion provided the best neuroprotective.39,48 Exposure to 250 ppm CO at 2 hours after SAH for 1 hour also demonstrated neuroprotective effects.61 Similarly, immediate intravenous injection of CORM-3 (4 mg/kg) after intracerebral hemorrhage or cerebral ischemia can also improve the neuroprotective effect.50,58
Table 2.
The neuroprotective effect of CO on stroke
| Study | Year | Animal/cells | Model | Results |
|---|---|---|---|---|
| Matz et al.56 | 1996 | Rat | SAH | Heme oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. |
| Doré et al.44 | 1999 | Mouse | MCAO | Heme oxygenase-2 is neuroprotective in cerebral ischemia. |
| Shi et al.53 | 2000 | Human | MCAO | Role of CO and nitric oxide in newborn infants with postasphyxial hypoxic-ischemic encephalopathy pediatrics. |
| Nakaso et al.55 | 2000 | Rat | ICH | CO-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. |
| Imuta et al.45 | 2007 | Rat | MCAO | Hypoxia-mediated induction of heme oxygenase-1 and CO release from astrocytes protects nearby cerebral neurons from hypoxia-mediated apoptosis. |
| Zeynalov and Doré39 | 2009 | Mouse | MCAO | Low doses of CO protect against experimental focal brain ischemia. |
| Wang et al.48 | 2011 | Mouse | MCAO | CO–activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. |
| Queiroga et al.54 | 2012 | Mouse | MCAO | Preconditioning triggered by CO provides neuronal protection following perinatal hypoxia-ischemia. |
| Yabluchanskiy et al.59 | 2012 | Rat | ICH | CORM-3, a CO-releasing molecule, alters the inflammatory response and reduces brain damage in a rat model of hemorrhagic stroke. |
| Chao et al.46 | 2013 | Mouse | MCAO | Up-regulation of heme oxygenase-1 attenuates brain damage after cerebral ischemia via simultaneous inhibition of superoxide production and preservation of NO bioavailability. |
| Schallner et al.63 | 2015 | Mouse | SAH | Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. |
| Wang et al.52 | 2016 | Rat | MCAO | CO improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. |
| Schallner et al.64 | 2017 | Mouse | SAH | CO preserves circadian rhythm to reduce the severity of subarachnoid hemorrhage in mice. |
| Wang et al.50 | 2018 | Mouse | MCAO | CORM-3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood-brain barrier disruption. |
| Kamat et al.61 | 2019 | Mouse | SAH | CO attenuates vasospasm and improves neurobehavioral function after subarachnoid hemorrhage. |
Note: CO: Carbon monoxide; CORM-3: carbon monoxide releasing molecule 3; ICH: intracerebral hemorrhage; MCAO: middle cerebral artery occlusion; NO: nitric oxide; SAH: subarachnoid hemorrhage.
Figure 1.
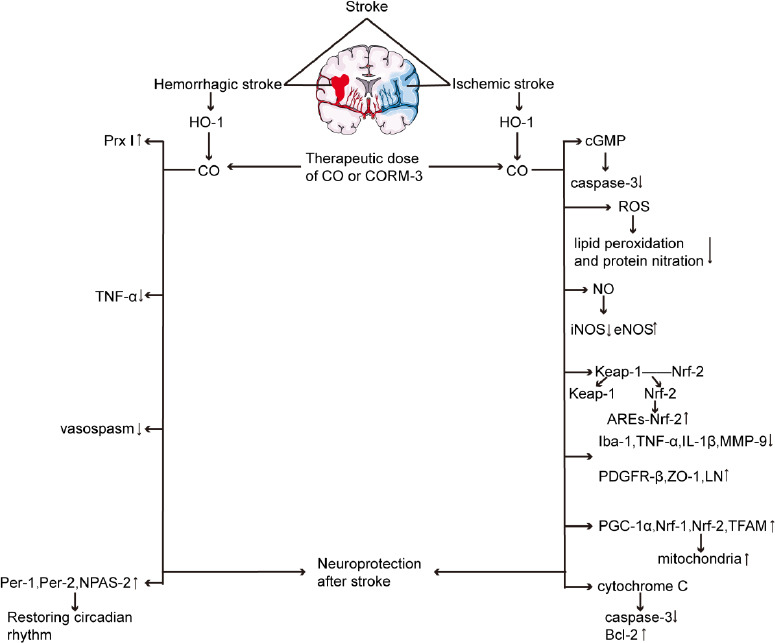
The neuroprotective effect of CO on ischemic stroke and hemorrhagic stroke.
Note: ARE: Antioxidant response element; cGMP: cyclic guanosine phosphate; CO: carbon monoxide; CORM-3: carbon monoxide releasing molecule 3; eNOS: endothelial nitric oxide synthase; HO-1: heme oxygenase-1; Iba-1: ionized calcium-binding adaptor molecule-1; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; Keap-1: Kelch-like ECH-associated protein 1; LN: laminin; MMP-9: matrix metalloproteinase-9; NO: nitric oxide; NPAS-2:neuronal PAS domain protein 2; Nrf: nuclear factor erythroid 2-related factor; PDGFR-β: platelet-derived growth factor receptor-β; Per: period; PGC-1α: peroxisome proliferator-activated receptor-γ coactivator-1α; Prx I: peroxidase I; ROS: reactive oxygen species; TFAM: mitochondrial transcription factor A; TNF-α: tumor necrosis factor-α; ZO-1: zonula occluden-1.
Therefore, CO may be very important in the physiological function of stroke, and we should strengthen cognition and utilization. Especially in today's severe CO air pollution, prevent the destruction caused by the devil on the one hand, and use the beneficial side of the angels at the same time. Perhaps the role of CO in stroke is one of the key areas of future research.
Footnotes
Conflicts of interest.
The authors declare that they have no competing interests.
Editor note: GC is an Editorial Board member of Neural Regeneration Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer review handled independently of this Editorial Board member and his research group.
REFERENCES
- 1.Caracciolo L, Marosi M, Mazzitelli J, et al. CREB controls cortical circuit plasticity and functional recovery after stroke. Nat Commun. 2018;9:2250. doi: 10.1038/s41467-018-04445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammann EM, Jones MP, Link BK, et al. Intravenous immune globulin and thromboembolic adverse events in patients with hematologic malignancy. Blood. 2016;127:200–207. doi: 10.1182/blood-2015-05-647552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awada Z, Abboud R, Nasr S. Risk of serious bleeding with antiplatelet therapy for secondary prevention post ischemic stroke in middle east population. Cureus. 2019;11:e4942. doi: 10.7759/cureus.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin H, Liang W, Mang J, et al. Relationship of gelatinases-tight junction proteins and blood-brain barrier permeability in the early stage of cerebral ischemia and reperfusion. Neural Regen Res. 2012;7:2405–2412. doi: 10.3969/j.issn.1673-5374.2012.31.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Noori H, Burkovskiy I, Lafreniere JD, Kelly MEM, Lehmann C. Modulation of the endocannabinoid system following central nervous system injury. Int J Mol Sci. 2019;20:388. doi: 10.3390/ijms20020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adebayo OD, Culpan G. Diagnostic accuracy of computed tomography perfusion in the prediction of haemorrhagic transformation and patient outcome in acute ischaemic stroke: a systematic review and meta-analysis. Eur Stroke J. 2020;5:4–16. doi: 10.1177/2396987319883461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26:871–895. doi: 10.1016/j.ncl.2008.07.003. vii. [DOI] [PubMed] [Google Scholar]
- 8.Maheswaran R, Pearson T, Beevers SD, Campbell MJ, Wolfe CD. Outdoor air pollution, subtypes and severity of ischemic stroke--a small-area level ecological study. Int J Health Geogr. 2014;13:23. doi: 10.1186/1476-072X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Babadjouni R, Radwanski R, et al. Stroke damage is exacerbated by nano-size particulate matter in a mouse model. PLoS One. 2016;11:e0153376. doi: 10.1371/journal.pone.0153376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong YC, Lee JT, Kim H, Kwon HJ. Air pollution: a new risk factor in ischemic stroke mortality. Stroke. 2002;33:2165–2169. doi: 10.1161/01.str.0000026865.52610.5b. [DOI] [PubMed] [Google Scholar]
- 11.Shah AS, Lee KK, McAllister DA, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. doi: 10.1136/bmj.h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kettunen J, Lanki T, Tiittanen P, et al. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke. 2007;38:918–922. doi: 10.1161/01.STR.0000257999.49706.3b. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock B, Niki H. Carbon monoxide balance in nature. Science. 1972;176:290–292. doi: 10.1126/science.176.4032.290. [DOI] [PubMed] [Google Scholar]
- 14.Barbulescu A, Barbes L. Modeling the carbon monoxide dissipation in Timisoara, Romania. J Environ Manage. 2017;204:831–838. doi: 10.1016/j.jenvman.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234-235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magierowska K, Brzozowski T, Magierowski M. Emerging role of carbon monoxide in regulation of cellular pathways and in the maintenance of gastric mucosal integrity. Pharmacol Res. 2018;129:56–64. doi: 10.1016/j.phrs.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Arngrim N, Schytz HW, Hauge MK, Ashina M, Olesen J. Carbon monoxide may be an important molecule in migraine and other headaches. Cephalalgia. 2014;34:1169–1180. doi: 10.1177/0333102414534085. [DOI] [PubMed] [Google Scholar]
- 19.Kim HH, Choi S. Therapeutic aspects of carbon monoxide in cardiovascular disease. Int J Mol Sci. 2018;19:2381. doi: 10.3390/ijms19082381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reboul C, Thireau J, Meyer G, et al. Carbon monoxide exposure in the urban environment: an insidious foe for the heart? Respir Physiol Neurobiol. 2012;184:204–212. doi: 10.1016/j.resp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Raub JA, Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev. 2002;26:925–940. doi: 10.1016/s0149-7634(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 22.Mannaioni PF, Vannacci A, Masini E. Carbon monoxide: the bad and the good side of the coin, from neuronal death to anti-inflammatory activity. Inflamm Res. 2006;55:261–273. doi: 10.1007/s00011-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 23.Hanafy KA, Oh J, Otterbein LE. Carbon Monoxide and the brain: time to rethink the dogma. Curr Pharm Des. 2013;19:2771–2775. doi: 10.2174/1381612811319150013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durante W, Schafer AI. Carbon monoxide and vascular cell function (review) Int J Mol Med. 1998;2:255–262. doi: 10.3892/ijmm.2.3.255. [DOI] [PubMed] [Google Scholar]
- 25.Abramochkin DV, Konovalova OP, Kamkin A, Sitdikova GF. Carbon monoxide modulates electrical activity of murine myocardium via cGMP-dependent mechanisms. J Physiol Biochem. 2015;71:107–119. doi: 10.1007/s13105-015-0387-y. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson WJ, Kemp PJ. Carbon monoxide: an emerging regulator of ion channels. J Physiol. 2011;589:3055–3062. doi: 10.1113/jphysiol.2011.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilun P, Stefanczyk-Krzymowska S, Romerowicz-Misielak M, Tabecka-Lonczynska A, Przekop F, Koziorowski M. Carbon monoxide-mediated humoral pathway for the transmission of light signal to the hypothalamus. J Physiol Pharmacol. 2013;64:761–772. [PubMed] [Google Scholar]
- 28.Maga M, Janik MK, Wachsmann A, et al. Influence of air pollution on exhaled carbon monoxide levels in smokers and non-smokers. A prospective cross-sectional study. Environ Res. 2017;152:496–502. doi: 10.1016/j.envres.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Hajat S, Haines A, Goubet SA, Atkinson RW, Anderson HR. Association of air pollution with daily GP consultations for asthma and other lower respiratory conditions in London. Thorax. 1999;54:597–605. doi: 10.1136/thx.54.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang WS, Wang X, Deng Q, Fan WY, Wang WY. An evidence-based appraisal of global association between air pollution and risk of stroke. Int J Cardiol. 2014;175:307–313. doi: 10.1016/j.ijcard.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Tian Y, Xu Y, et al. Association between ambient air pollution and hospitalization for ischemic and hemorrhagic stroke in China: A multicity case-crossover study. Environ Pollut. 2017;230:234–241. doi: 10.1016/j.envpol.2017.06.057. [DOI] [PubMed] [Google Scholar]
- 32.Tian L, Qiu H, Pun VC, Ho KF, Chan CS, Yu IT. Carbon monoxide and stroke: a time series study of ambient air pollution and emergency hospitalizations. Int J Cardiol. 2015;201:4–9. doi: 10.1016/j.ijcard.2015.07.099. [DOI] [PubMed] [Google Scholar]
- 33.Bayramoglu A, Kocak AO, Kadioglu E. Ischemic stroke due to carbon monoxide intoxication: Two case reports. World J Emerg Med. 2018;9:73–75. doi: 10.5847/wjem.j.1920-8642.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CW, Chen WK, Hung DZ, et al. Association between ischemic stroke and carbon monoxide poisoning: A population-based retrospective cohort analysis. Eur J Intern Med. 2016;29:65–70. doi: 10.1016/j.ejim.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Kim HH, Choi S, Jung YS, Min YG, Yoon D, Lee SE. Stroke Incidence in Survivors of Carbon Monoxide Poisoning in South Korea: A Population-Based Longitudinal Study. Med Sci Monit. 2020;26:e926116. doi: 10.12659/MSM.926116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nayor M, Enserro DM, Beiser AS, et al. Association of exhaled carbon monoxide with stroke incidence and subclinical vascular brain injury: Framingham heart study. Stroke. 2016;47:383–389. doi: 10.1161/STROKEAHA.115.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu G, Yu K, Yu C, et al. Association of exhaled carbon monoxide with risk of cardio-cerebral-vascular disease in the China Kadoorie Biobank cohort study. Sci Rep. 2020;10:19507. doi: 10.1038/s41598-020-76353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med. 2010;52:258–262. doi: 10.1097/JOM.0b013e3181ceff7a. [DOI] [PubMed] [Google Scholar]
- 39.Zeynalov E, Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res. 2009;15:133–137. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–275. doi: 10.1016/j.redox.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parfenova H, Tcheranova D, Basuroy S, Fedinec AL, Liu J, Leffler CW. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am J Physiol Heart Circ Physiol. 2012;302:H2257–2266. doi: 10.1152/ajpheart.01011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 43.Doré S, Goto S, Sampei K, et al. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience. 2000;99:587–592. doi: 10.1016/s0306-4522(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 44.Doré S, Sampei K, Goto S, et al. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 45.Imuta N, Hori O, Kitao Y, et al. Hypoxia-mediated induction of heme oxygenase type I and carbon monoxide release from astrocytes protects nearby cerebral neurons from hypoxia-mediated apoptosis. Antioxid Redox Signal. 2007;9:543–552. doi: 10.1089/ars.2006.1519. [DOI] [PubMed] [Google Scholar]
- 46.Chao XD, Ma YH, Luo P, et al. Up-regulation of heme oxygenase-1 attenuates brain damage after cerebral ischemia via simultaneous inhibition of superoxide production and preservation of NO bioavailability. Exp Neurol. 2013;239:163–169. doi: 10.1016/j.expneurol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang B, Cao W, Biswal S, Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen VG, Garza JI. Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul Fibrinolysis. 2014;25:801–805. doi: 10.1097/MBC.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Zhang D, Fu X, et al. Carbon monoxide-releasing molecule-3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood-brain barrier disruption. J Neuroinflammation. 2018;15:188. doi: 10.1186/s12974-018-1226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parnia S, Yang J, Nguyen R, et al. Cerebral oximetry during cardiac arrest: a multicenter study of neurologic outcomes and survival. Crit Care Med. 2016;44:1663–1674. doi: 10.1097/CCM.0000000000001723. [DOI] [PubMed] [Google Scholar]
- 52.Wang P, Yao L, Zhou LL, et al. Carbon monoxide improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. Int J Biol Sci. 2016;12:1000–1009. doi: 10.7150/ijbs.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Pan F, Li H, Pan J, Qin S, Shen C. Role of carbon monoxide and nitric oxide in newborn infants with postasphyxial hypoxic-ischemic encephalopathy. Pediatrics. 2000;106:1447–1451. doi: 10.1542/peds.106.6.1447. [DOI] [PubMed] [Google Scholar]
- 54.Queiroga CS, Tomasi S, Widerøe M, Alves PM, Vercelli A, Vieira HL. Preconditioning triggered by carbon monoxide (CO) provides neuronal protection following perinatal hypoxia-ischemia. PLoS One. 2012;7:e42632. doi: 10.1371/journal.pone.0042632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakaso K, Kitayama M, Mizuta E, et al. Co-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. Neurosci Lett. 2000;293:49–52. doi: 10.1016/s0304-3940(00)01491-9. [DOI] [PubMed] [Google Scholar]
- 56.Matz P, Turner C, Weinstein PR, Massa SM, Panter SS, Sharp FR. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 1996;713:211–222. doi: 10.1016/0006-8993(95)01511-6. [DOI] [PubMed] [Google Scholar]
- 57.Duan X, Wen Z, Shen H, Shen M, Chen G. Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid Med Cell Longev 2016. 2016 doi: 10.1155/2016/1203285. 1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yabluchanskiy A, Sawle P, Homer-Vanniasinkam S, Green CJ, Foresti R, Motterlini R. CORM-3, a carbon monoxide-releasing molecule, alters the inflammatory response and reduces brain damage in a rat model of hemorrhagic stroke. Crit Care Med. 2012;40:544–552. doi: 10.1097/CCM.0b013e31822f0d64. [DOI] [PubMed] [Google Scholar]
- 59.Kremer B, Coburn M, Weinandy A, et al. Argon treatment after experimental subarachnoid hemorrhage: evaluation of microglial activation and neuronal survival as a subanalysis of a randomized controlled animal trial. Med Gas Res. 2020;10:103–109. doi: 10.4103/2045-9912.296039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamat PK, Ahmad AS, Doré S. Carbon monoxide attenuates vasospasm and improves neurobehavioral function after subarachnoid hemorrhage. Arch Biochem Biophys. 2019;676:108117. doi: 10.1016/j.abb.2019.108117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahan VL. Effects of lactate and carbon monoxide interactions on neuroprotection and neuropreservation. Med Gas Res. 2021;11:158–173. doi: 10.4103/2045-9912.318862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schallner N, Pandit R, LeBlanc R, 3rd, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schallner N, Lieberum JL, Gallo D, et al. Carbon monoxide preserves circadian rhythm to reduce the severity of subarachnoid hemorrhage in mice. Stroke. 2017;48:2565–2573. doi: 10.1161/STROKEAHA.116.016165. [DOI] [PMC free article] [PubMed] [Google Scholar]


