Anaplastic oligodendrogliomas (AO) are rare brain tumors, accounting for approximately 5% of adult gliomas and 0.5% of all primary tumors affecting the central nervous system. This article reports results of a study conducted within the frame of the French POLA network on the characteristics of survivors of AO, comparing populations with a disease-specific survival of less than 5 years with populations with a survival 5 years or more.
Keywords: anaplastic oligodendroglioma, age, seizure, Karnofsky Performance Status, proliferation, radiotherapy, chemotherapy, surgery
Abstract
Background
Anaplastic oligodendrogliomas IDH-mutant and 1p/19q codeleted (AO) occasionally have a poor outcome. Herein we aimed at analyzing their characteristics.
Methods
We retrospectively analyzed the characteristics of 44 AO patients with a cancer-specific survival <5 years (short-term survivors, STS) and compared them with those of 146 AO patients with a survival ≥5 years (classical survivors, CS) included in the POLA network.
Results
Compared to CS, STS were older (P = .0001), less frequently presented with isolated seizures (P < .0001), more frequently presented with cognitive dysfunction (P < .0001), had larger tumors (P = .= .003), a higher proliferative index (P = .= .0003), and a higher number of chromosomal arm abnormalities (P = .= .02). Regarding treatment, STS less frequently underwent a surgical resection than CS (P = .= .0001) and were more frequently treated with chemotherapy alone (P = .= .009) or with radiotherapy plus temozolomide (P = .= .05). Characteristics independently associated with STS in multivariate analysis were cognitive dysfunction, a number of mitosis > 8, and the absence of tumor resection. Based on cognitive dysfunction, type of surgery, and number of mitosis, patients could be classified into groups of standard (18%) and high (62%) risk of <5 year survival.
Conclusion
The present study suggests that although STS poor outcome appears to largely result from a more advanced disease at diagnosis, surgical resection may be particularly important in this population.
Implications for Practice.
Although anaplastic oligodendrogliomas are typically associated with a prolonged survival, approximately 20% of patients have a poor outcome and a survival inferior to 5 years. The present study demonstrates that these patients present aggressive baseline characteristics, highlights features that could enable their identification, and suggests an important role of surgical resection in these patients.
Introduction
Anaplastic oligodendrogliomas (AO) are rare brain tumors, accounting for approximately 5% of adult gliomas and 0.5% of all primary tumors affecting the central nervous system.1 They are defined by the 2016 World Health Organization (WHO) classification2 as IDH-mutant 1p/19q-codeleted diffuse gliomas with increased mitotic activity, microvascular proliferation, and/or necrosis.3 Among high-grade gliomas, AO have a better prognosis than high-grade IDH-mutant astrocytomas and a much better prognosis than IDH-wild-type glioblastomas.3 Standard treatment consists of maximal safe surgical resection followed by radiotherapy plus PCV chemotherapy (CT) regimen (procarbazine, CCNU [lomustine], and vincristine).4,5 Anaplastic oligodendrogliomas are typically associated with a prolonged survival with a median survival estimated to approximately 15 years.4,5 However, approximately 20% of patients have a poor outcome and survive less than 5 years.4-6 Poor prognostic factors have been identified in AO,7-9 yet the characteristics of AO short-term survivors (STS) remain to be described.10 The aim of the present study was to analyze the characteristics of STS, defined as patients with a disease-specific survival <5 years and to compare with those of AO patients with a survival ≥5 years (classical survivors, CS). It was conducted within the frame of the French POLA network dedicated to anaplastic oligodendroglial tumors.
Material and Methods
POLA Network and Patients
In 2008, the French Institut National du Cancer supported the creation of a national network named “Prise en charge des OLigodendrogliomes Anaplasiques” (POLA). This network prospectively collects samples, characteristics, and outcomes of patients diagnosed with high-grade oligodendroglial tumor in French academic centers. Among the 2189 patients included in the POLA network, patients with centrally reviewed confirmation of newly diagnosed AO were prospectively included in the present study. Formalin-fixed paraffin-embedded (FFPE) tumor tissue was available for pathological and immunohistochemical investigations for all cases. Patients provided written informed consent for clinical data collection and genetic analysis according to national and POLA network policies. We retrospectively analyzed data from all patients registered in the POLA network from 2008 to 2019. The following clinical data were collected: age at diagnosis, preoperative symptoms and Karnofsky Performance Status (KPS), surgical and postoperative treatments, tumor location and characteristics, survival status, and survival time. Extent of resection (EOR) was recorded as biopsy or resection. The initial postoperative treatment strategy was classified as radiotherapy (RT) alone, CT alone, RT + CT (sequential and/or concurrent), simple follow-up, and/or no treatment. CT regimen was defined as PCV, TMZ (temozolomide), or CCNU (lomustine). Tumor volumes could be measured for a subset of 64 patients and were calculated according to the 3 largest diameter technique using T2 or FLAIR-weighted magnetic resonance imaging.11
Pathological Review and Immunohistochemistry
All cases of supposed AO were centrally reviewed and included in the prospective POLA network if they met the pathological inclusion criteria of AO according to the WHO classification of brain tumors.2 The presence of mitoses (with mitotic index referring to the number of mitotic figures per 10 High Power Fields), marked atypia, areas of high cellularity, microvascular proliferation, and necrosis were assessed. In addition, automated immunohistochemistry was performed on 4-µm-thick FFPE sections with avidin-biotin-peroxydase complex on Benchmark XT (Ventana Medical System Inc., Tucson, AZ, USA) using the Ventana Kit including DAB reagent to search for expression of IDH1 R132H (clone H09; 1:75; Diavona), p53 (clone DO.7; 1:200; Dako), ATRX (polyclonal; Sigma), Ki67/MIB1 (clone Mib1;:100; Dako), EGFR (clone EGFR.25; 1:100; BNovocasta), and inactivating mutations in the transcriptional repression factor Capicua (CIC). EGFR positive expression was assessed using the Hirsch score as previously described.12 P53-positive expression was considered with a cutoff at 10%.
DNA Extraction, Single Nucleotide Polymorphism Array, and Comparative Genomic Hybridization Array Procedures
Following the manufacturer’s recommendations, tumor DNA was extracted from frozen tissue, or FFPE samples using the iPrep ChargeSwith Forensic Kit. Qualification and quantification of tumor DNA were fulfilled using a NanoVue spectrophotometer and gel electrophoresis, respectively. When necessary, the genomic profile was assessed using single nucleotide polymorphism (SNP) or CGH arrays, as described previously.13TERT mutation were also assessed as previously described.14
Statistical Analysis
SNP and CGH array analysis were performed as previously described.15 For arrays, genomic imbalances were classified as loss, gain, homozygous deletion, or amplification. For correlation between chromosomal arm imbalances and histological variables, the Fisher’ exact test (for factors) or the Student’s t test (for quantitative variables, when they were scored as positive or negative) were used. Continuous variables were compared using Mann-Whitney U test. Overall survival (OS) was defined as the time from surgery to tumor-progression-related death (patients who died from other causes were excluded from the retrospective analyses). Progression-free survival (PFS) was defined as the time from surgery to first progression or last follow-up in case of unprogressive tumor. In order to identify clinical, radiological, pathological, and/or genomic factors related to OS, survival curves were obtained according to the Kaplan-Meier method and compared using the log-rank test for univariate comparisons. Cox proportional hazards models were used for multivariate analyses and for estimating hazard ratios in survival regression models. Because of the large number of potiential explanatory variables, multivariate analysis only included all the variables with a P-value of <.02 in univariate analyses. All variables obtained were searched for prognostic significance. The final model was fit using a backward method of selection. All statistical tests were 2-sided, and the final threshold for statistical significance was P-value = .05. Analysis was performed by the Clinical Investigation Center (Inserm CIC 1431) of Besançon and was conducted using SAS for windows version 9.4.
Results
Patient Selection
At the time of analysis, among the 519 AO patients included in the POLA network, 318 patients were alive and their follow-up was <5 years, 146 patients had a survival ≥ 5 years (and constituted the CS group) and 55 patients had a survival < 5 years. Among the latter patients, 44 patients (80%) died from tumor progression and 11 patients (20%) died from another cause (suicide n = 2, other cancer n = 2, post-operative cerebral hemorrhage n = 1, stroke n = 1, congestive heart failure n = 1, pulmonary embolism n = 1, aortic aneurysm rupture n = 1, sepsis n = 1, car crash n = 1), while their last evaluation indicated stable disease. We considered as AO STS the 44 patients with a disease-specific survival < 5 years. Patients who died from another cause than tumor progression were excluded from the analysis. The CONSORT flow diagram of patient selection for the study cohort is available in Supplementary Fig. 1. The median survival of STS patients was 2 years, the median survival of CS patients was not reached after a median follow-up of 7 years. The median PFS was 0.68 years for STS patients and 5 years in CS patients.
Clinical and Imaging Characteristics
The clinical and imaging characteristics of STS and CS are summarized in Table 1. Compared to CS, STS were older at diagnosis (median 57.4 vs 48.1 years; P = .= .0001), had a poorer pre-operative KPS (KPS < 80) (43.2% vs 67.1%; P = .= .005), more frequently presented with focal deficits (27.3% vs 11%; P = .= .02), intracranial hypertension (52.3% vs 29.5%; P = .= .0039), and cognitive dysfunction (59.1% vs 17.8%; P < .0001). They less frequently presented with seizures (36.4% vs 66.4% P = .= .0004), especially with isolated seizures (15.2% vs 58.6%, P < .0001).
Table 1.
Comparison of STS and CS patients: clinical and imaging characteristics.
| All patients, n (%) | STS, n (%) | CS, n (%) | P-valuesa | |
|---|---|---|---|---|
| N | 190 | 44 | 146 | |
| Age at diagnosis (median) | 50.2 | 57.4 | 48.1 | .0001 |
| <40 year | 41 (21.6%) | 4 (9.1%) | 37 (25.2%) | ref |
| [40-60] year | 106 (55.8%) | 23 (52.3%) | 83 (56.8%) | NS |
| >60 year | 43 (22.6%) | 17 (38.6%) | 26 (7.8%) | .0018 |
| ≤60 year vs > 60 year | .0047 | |||
| Sex | ||||
| Female | 77 (40.5%) | 15 (34%) | 62 (42%) | ref |
| Male | 113 (59.5%) | 29 (66%) | 84 (58%) | NS |
| Sex ratio M:F | 1.5 | 1.9 | 1.3 | |
| Preoperative KPS | ||||
| <80% | 73 (38.4%) | 25 (56.8%) | 48 (32.9%) | ref |
| ≥80% | 117 (61.6%) | 19 (43.2%) | 98 (67.1%) | .005 |
| Preoperative symptoms | ||||
| Seizures | 113 (59.5%) | 16 (36.4%) | 97 (66.4%) | .0004 |
| Isolated seizuresb | 73 (38.4%) | 5 (15.2%) | 68 (58.6%) | <.0001 |
| Intracranial hypertension | 66 (34.7%) | 23 (52.3%) | 43 (29.5%) | .0039 |
| Speech disorder | 11 (5.8%) | 4 (9.1%) | 7 (4.8%) | NS |
| Cognitive dysfunction | 52 (27.4%) | 26 (59.1%) | 26 (17.8%) | <.0001 |
| Focal deficits | 28 (14.7%) | 12 (27.3%) | 16 (11%) | .02 |
| Mnesic dysfunction | 27 (14.2%) | 10 (22.7%) | 17 (11.6%) | NS |
| Tumor location | ||||
| Frontal | 150 (78.9%) | 36 (81.82%) | 114 (78.1%) | NS |
| Temporal | 45 (23.7%) | 14 (31.8%) | 31 (21.2%) | NS |
| Parietal | 43 (22.6%) | 17 (38.6%) | 26 (17.8%) | .005 |
| Occipital | 19 (10%) | 8 (18.2%) | 11 (7.5%) | .04 |
| Insular | 30 (15.8%) | 11 (25%) | 19 (13.0% | NS |
| Corpus callosum | 52 (27.4%) | 18 (40.9%) | 34 (23.3%) | .023 |
| Extension | ||||
| Unilobar | 92 (48.4%) | 15 (34%) | 77 (52.7%) | ref |
| Multilobar | 98 (51.6%) | 29 (66%) | 69 (47.3%) | .03 |
| Hemipsherec | ||||
| Right | 90 (47.4%) | 20 (45.5%) | 70 (48%) | NS |
| Left | 71 (37.4%) | 12 (27.3%) | 59 (40.4%) | .04 |
| Midline cross | ||||
| No | 161 (84.7%) | 32 (72.7%) | 129 (88.4%) | ref |
| Yes | 29 (15.3%) | 12 (27.3%) | 17 (11.6%) | .01 |
| Tumor characteristics | ||||
| Contrast enhancement | 119 (62.6%) | 34 (77.3%) | 85 (58.2%) | .004 |
| Mass effect | 114 (60%) | 31 (70.5%) | 83 (59.9%) | NS |
| Edema | 76 (40%) | 24 (54.6%) | 52 (35.6%) | .03 |
| Intratumoral cyst | 40 (21.1%) | 11 (25%) | 29 (19.9%) | NS |
| Calcification | 57 (30%) | 20 (45.5%) | 37 (25.3%) | .01 |
| Necrosis | 32 (16.8%) | 8 (18.2%) | 24 (16.4%) | NS |
Univariate analysis: logistic regression without covariate adjustment.
When patients presented seizures as the only clinical manifestation.
When unilateral; CS, classical survivors; F, female; KPS, Karnofsky performance status; M, male; NS, not significant; STS, short-term survivors; y, years.
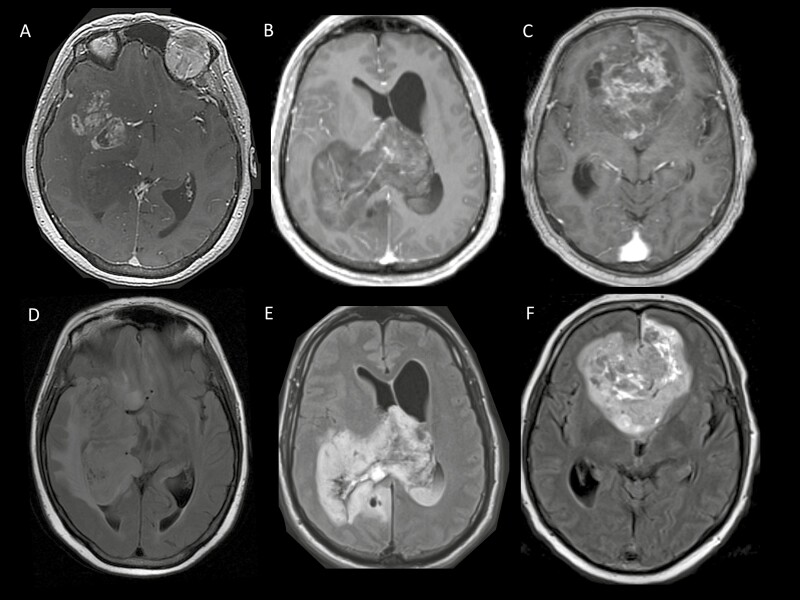
In terms of radiological characteristics, compared to CS, tumors in STS more frequently presented with contrast enhancement (77.3% vs 58.2%; P = .= .004), edema (54.6% vs 35.6%; P = .= .03), and calcifications (45.5% vs 25.3%; P = .= .01). They were more frequently located in the parietal lobe and occipital lobe respectively (38.6% vs 17.8%; P = .= .005 and 18.2% vs 7.5%; P = .= .04) and they more frequently affected the corpus callosum (40.9% vs 23.3%, P = .= .023). They also more frequently involved multiple lobes (66% vs 47.3%; P = .= .03) and crossed the midline (27.3% vs 11.6%; P = .= .01) (Fig. 1). Consistently, the mean tumor volume was higher for STS (186 cm3) than for CS (90 cm3; P < .001) in the subset of patients for whom it could be assessed (STS n = 16 and CS n = 48).
Figure 1.
Representative examples of magnetic resonance imaging (MRI) presentation in 3 STS patients. Top: post-gadolinium axial T1-weighted images in 3 different STS patients (A, B, C) Bottom: corresponding axial Fluid-attenuated inversion recovery (FLAIR) (D, E, F).
Histo-Molecular Characteristics
The histo-molecular of STS and CS is summarized in Table 2. Compared to CS, AO in STS were associated with a higher level of nuclear atypia (79.6% vs 48%; P = .= .0006), displayed a higher number of mitoses (10 vs 7; P = .= .02), a higher level of Ki67 expression (median 25% vs 15%; P = .= .0003), and a higher number of chromosome arm alterations (5.14 vs 3.76; P = .= .02). In addition, TP53 median expression was higher in STS (median 10.79% vs 3.92%; P = .= .01). Chromosome arm 9p loss and CDKN2A deletion were more frequent in STS than CS but the difference did not reach statistical significance.
Table 2.
Comparison of STS and CS patients: histo-molecular characteristics.
| All patients, n (%) | STS, n (%) | CS, n (%) | P-valuesa | |
|---|---|---|---|---|
| N | 190 | 44 | 146 | .0006 |
| Morphology | ||||
| Nuclear atypia | 105 (55.3%) | 35 (79.6%) | 70 (48%) | .02 |
| Number of mitoses (median) | 7 | 10 | 7 | .004 |
| Mitoses >8 | 61 (32.1%) | 23 (57.5%) | 38 (31.4%) | NS |
| Necrosis | 41 (21.6%) | 11 (25%) | 30 (20.6%) | NS |
| MVP | 147 (77.4%) | 37 (84.1%) | 110 (75.3%) | .0003 |
| Immunohistochemistry | ||||
| KI67 expression (median) | 20 | 25 | 15 | .0001 |
| Ki67<25 vs ≥25 | .01 | |||
| TP53 expression (median) | 5.54 | 10.79 | 3.92 | NS |
| TP53-positive expression | 36 (18.9%) | 10 (22.7%) | 26 (17.8%) | NS |
| EGFR-positive expression | 31 (16.3%) | 5 (11.4%) | 26 (17.8%) | NS |
| CIC loss | 67 (35.3%) | 18 (41%) | 49 (33.6%) | NS |
| Genomic alterations | ||||
| Chr 9p loss | 71 (37.4%) | 19 (43.2%) | 52 (35.6%) | NS |
| Chr 9q loss | 30 (15.8%) | 8 (18.2%) | 22 (15.1%) | NS |
| Chr 7 gain | 21 (11.1%) | 5 (11.4%) | 16 (11%) | NS |
| Chr 10q loss | 22 (11.6%) | 8 (18.2%) | 14 (9.6%) | |
| CDKN2A deletion | 14 (7.4%) | 6 (13.6%) | 8 (5.5%) | NS |
| Mean number of chr arm alteration (total) | 4.08 | 5.14 | 3.76 | .02 |
| TERT promoter mutationsb | ||||
| C228T | 116 (72%) | 30 (78%) | 86 (69.9%) | NS |
| C250T | 38 (23%) | 6 (15.7%) | 32 (26%) | NS |
| None | 8 (4.9%) | 2 (5.2%) | 6 (4.8%) | NS |
Univariate analysis: logistic regression without covariate adjustment.
TERT promoter mutation was not available in 29 tumors and 1 tumor presented both mutations.
Chr, chromosome; CS, classical survivors; MVP, microvascular proliferation; NS, not significant; STS, short-term survivors.
Treatment
Treatment characteristics of STS and CS are summarized in Table 3. Short-term survivors less frequently underwent tumor resection than CS (61.9% vs 88.8%; P = .= .0001) and more frequently needed postoperative steroids (66% vs 42%; P = .= .009). Median time from surgery to postoperative treatment onset tended to be shorter in STS than in CS (47 vs 65 days, P = .08). After surgery, STS less frequently received the treatment that was considered as the standard treatment at the time of diagnosis than CS. Before 2012, they were less frequently treated with RT alone, and after 2012, less frequently treated with RT plus PCV (40.9% vs 63%, P = .= .01). Compared to CS, STS were more frequently treated with CT alone (22.7% vs 6.8%, P = .= .009) or radiotherapy plus TMZ (29.6% vs 15.7%, P = .= .05), and less frequently with RT alone (18.2% vs 49.3%, P = .= .0009).
Table 3.
Comparison of STS and CS patients: treatments.
| All patients, n (%) | STS, n (%) | CS, n (%) | P- valuesa | |
|---|---|---|---|---|
| N | 190 | 44 | 146 | .0001 |
| Extent of resectionb | ||||
| Biopsy | 32 (16.8%) | 16 (38.1%) | 16 (11.2%) | |
| Surgery | 153 (80.5%) | 26 (61.9%) | 127 (88.8%) | |
| Postoperative corticotherapy | 91 (47.9%) | 29 (65.9%) | 62 (42.5%) | .009 |
| Postoperative treatment | .0009 | |||
| Radiotherapy alone | 80 (42.1%) | 8 (18.2%) | 72 (49.3%) | |
| Chemotherapy alone | 20 (10.5%) | 10 (22.7%) | 10 (6.8%) | |
| PCV | 7 (3.7%) | 5 (11.4%) | 2 (1.4%) | |
| TMZ | 13 (6.8%) | 5 (11.4%) | 8 (5.5%) | |
| Radiochemotherapy | 75 (39.5%) | 24 (54.6%) | 51 (35%) | |
| PCV | 36 (18.9%) | 10 (22.7%) | 26 (17.8%) | |
| TMZ | 36 (18.9%) | 13 (29.6%) | 23 (15.7%) | |
| CCNU | 1 (0.5%) | 1 (2.3%) | 0 (0.0%) | |
| TMZ plus BCNU | 2 (1%) | 0 (0%) | 2 (1.4%) | |
| No treatment/surveillance | 15 (7.9%) | 2 (4.5%) | 13 (8.9%) | |
| Standard treatment | 110 (57.9%) | 18 (40.9%) | 92 (63%) | .01 |
| RT alone <2012 | 8/17 (47%)c | 68/110 (62%)c | ||
| RT+PCV≥2012 | 10/27 (37%)d | 24/36 (66%)d |
Univariate analysis: logistic regression without covariate adjustment.
Extent of surgery was unavailable in 2 of the 44 STS and 3 of the 146 CS patients for technical reasons.
% calculated based on the number of STS (n = 17) and CS (n = 110) patients treated before 2012.
% calculated based on the number of STS (n = 27) and CS (n = 36) patients treated after 2012.
CS, classical survivors; STS, short-term survivors; NS, not significant; TMZ, temozolomide; PCV, lomustine + procarbazine + vincristine CCNU, lomustine; RT, radiotherapy.
Multivariate Analysis
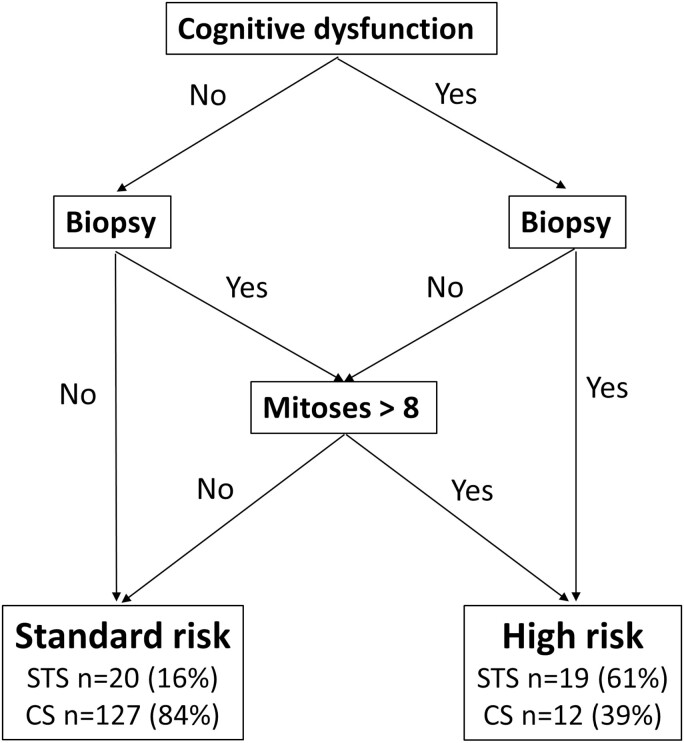
The following characteristics were associated with the STS profile in multivariate analysis: the presence of cognitive dysfunction at diagnosis (odds ratio [OR] = 4.94; 95% confidence interval [CI] [2.02; 12.08]; P = .= .0005), the presence of a number of mitosis > 8 (OR = 0.25; 95% CI [0.10; 0.60]; P = .= .0022), and the absence of tumor resection (OR = 5.24; 95% CI [1.89; 14.51]; P = .= .0014; Table 4). Based on these 3 characteristics, patients could be classified into groups of standard (16%) and high (61%) risk of < 5 year survival (Fig. 2).
Table 4.
Multivariate analyses of factors associated with STS.
| All patients, n (%) | STS, n (%) | CS, n (%) | OR [95% CI] | P-valuesa | |
|---|---|---|---|---|---|
| N | 190 | 44 | 146 | ||
| Cognitive dysfunction | 52 (27.4%) | 26 (59.1%) | 26 (17.8%) | 4.49 [2.02-12.08] | .0005 |
| Extent of resection | |||||
| Surgery | 153 (80.5%) | 26 (61.9%) | 127 (88.8%) | 5.24 [1.89-14.51] | .0014 |
| Biopsy | 32 (16.8%) | 16 (38.1%) | 16 (11.2%) | ref | ref |
| Number of mitoses | |||||
| >8 | 61 (37.8%) | 23 (57.5%) | 38 (31.4%) | 0.25 [0.10-0.60] | .0022 |
| ≤8 | 100 (62.2%) | 17 (42.5%) | 83 (68.6%) | ref | ref |
Multivariate analysis: logistic regression without covariate adjustment.
CS, classical survivors; STS, short-term survivors; OR, odds ratio; CI, confidence interval.
Figure 2.
Diagram of patient classification based on the presence of cognitive dysfunction, extent of surgery, and number of mitoses.
Discussion
Although AO are frequently associated with prolonged survival, approximately 20% of patients die within 5 years after diagnosis.4-6 The reason why some patients have a poor prognosis remains to be fully understood. Several studies have analyzed prognostic factors in AO but many of these studies included both 1p/19q codeleted and non-codeleted tumors as well as low-grade and high-grade oligodendrogliomas (Table 5). To our knowledge, our study is the first one to analyze the characteristics of STS. The present study showed that although the poor prognosis of STS appears to largely result from more aggressive baseline characteristics and a more advanced disease at diagnosis, surgical resection may be a particularly important determinant of survival in these patients.
Table 5.
Summary of studies reporting prognostic factors in anaplastic oligodendrogliomas within the 10 last years.
| Author, year | Study population | Number of patients | 1p/19q codeletion (n = %) | Analysis restricted to 1p/19q codeleted AO | Characteristics associated with worse prognosis | (Refs.) | ||
|---|---|---|---|---|---|---|---|---|
| Clinico-radiological | Histo-molecular | Treatment related | ||||||
| Roux, 2020 | O, AO | 108 | 108 (100) | No | Growth rate ≥8 mm/year | 16 | ||
| Garton, 2020 | O, AO | 2514 | 1067 (42) | No | Older age, worse comorbidity index, infratentorial location | Grade III | No debulking, RT | 17 |
| Shin, 2020 | AO | 95 | 31 (32) | No | Ki67 (>20%), no IDH mutation | 18 | ||
| Lin, 2020 | O, AO | 186 | 186 (100) | No | Older age, lower KPS | 19 | ||
| Pouget, 2020 | AO | 220 | 220 (100) | Yes | Older age | MCM6 ≥ 50% or Ki67 ≥ 15% |
20 | |
| Appay, 2019 | AO, AA, GBM IDHm | 911 | 483 (53) | Yes | Older age | CDKN2A deletion | 21 | |
| Kinslow, 2019 | O, AO | 3135 | unknown | No | Year of diagnosis, older age, sex (male), single status, infratentorial location | Grade III | No debulking, RT | 22 |
| Chen, 2019 | O, AO | 412 | 333 (80) | No | Older age, grade III | No 1p19q codeletion, 1p19q codeletion with polysomy |
No GTR | 23 |
| Liu, 2019 | AO | 1899 | Unknown | No | Older age, single status, multiple primary malignancies | No surgery | 9 | |
| Yeboa, 2018 | O, AO | 1618 | 1618 (100) | No | Older age, worse comorbidity index, lower income | Astrocytoma/mixed glioma, grade III | 24 | |
| Halani, 2018 | O, AO | 169 | 169 (100) | No | Older age | Grade III, PI3K mutations | 25 | |
| Appay, 2018 | AO | 227 | 227 (100) | Yes | Older age | Necrosis, higher proliferative index, absence of SSTR2A expression | 26 | |
| Rosenberg, 2018 | AO | 197 | 197 (100) | Yes | Older age | Necrosis, severe nuclear atypia, deletion of peak 9p21.3; amplification of peaks 14q13.1, 11q14.2, 20q13.33, and 1q21.3; CN‐LOH peak 17p11.2; gain of 11p | 27 | |
| Aoki, 2018 | O, AO, A, AA | 414 | 164 (39) | No | Age (≥60) | Notch1 mutation | No GTR | 28 |
| Zetterling, 2017 | O, AO | 214 | 64 (30) | No | Older age, non-frontal location, neurological deficit or personality change | No IDH-mt and no 1p/19q codel | 8 | |
| Figarella-Brnager, 2016 | AO | 157 | 157 (100) | Yes | Older age | MVP, necrosis, mitosis, KI67 LI | 29 | |
| Hu, 2017 | O, AO | 374 | 374 (100) | No | Older age | Grade III, gene expression profile | 30 | |
| Kamoun, 2016 | AO, AA, GBM IDHm | 156 | 80 (51) | No | Older age | Grade III, gene expression profile | 31 | |
| Kang, 2015 | AO | 376 | 95 (25) | Yes | Age (>45), KPS (<70), non-frontal lobe location | No complete removal, no RT | 32 | |
| Preusser, 2012 | AO | 281 | 76 (27) | No | Higher proliferative index | 33 | ||
| Lassman, 2011 | AO | 1013 | 301 (29) | No | Age (≥50), KPS (<70), non-frontal lobe location, bilateral hemispheric involvement | No 1p/19q codeletion | Biopsy, RT alone | 7 |
AO, anaplastic oligodendroglioma; A, astrocytoma; AA, anaplastic astrocytoma; CN-LOH, copy-neutral loss of heterozygosity; GBM, glioblastoma; GTR, gross total resection; KPS, Karnofsky performance status; LI, label index; MVP, microvascular proliferation; O, oligodendroglioma; RT, radiotherapy.
Baseline Characteristics of STS
Older age and poorer KPS have been identified as poor prognostic factors in AO across multiple studies and characterized STS in the present study.7-9,17-32 Being aged > 60 years was associated with STS, which is consistent with a large retrospective study reporting an approximately 2 times higher median survival in 1p/19q codeleted AO patients aged < 60 years compared to those aged > 60 years.34 In addition, we found that STS had a more aggressive clinical presentation than CS, with less frequent seizures, more frequent neurological and cognitive deficits, the latter feature was the only clinical feature independently associated with STS in multivariate analysis. Except in one study (which however included both low- and high-grade oligodendrogliomas and 1p/19q codeleted and non-codeleted tumors), the clinical presentation has not been related to prognosis in AO.8 In contrast, neurological deficits and the absence of seizures are well-described poor prognostic factors in low-grade gliomas.35,36 Most IDH-mutant glioma patients display seizures and it has been suggested that 2-hydroxyglutarate, the oncometabolite resulting from the IDH mutation, could explain their epileptogenicity.37 The reason why some IDH-mutant glioma patients do not display seizures remains to be determined but these patients could have a poorer outcome due to a longer time to diagnosis.
Regarding radiological characteristics, STS also had a more aggressive presentation than CS. Tumors in STS were larger and more frequently presented with contrast-enhancement. Consistently, contrast-enhancement has been associated with more aggressive molecular features in AO.38 Initial tumor volume has not been reported as a prognostic factor in AO; however, this finding is consistent with the reported poor prognostic value of bilateral hemispheric involvement, which was in the present study one of the radiological feature associated with STS.7
At the histo-molecular level, STS were characterized by a higher proliferative index and a number of mitosis > 8 was independently associated with STS in multivariate analysis. A higher proliferative index has been shown to be associated with a poorer outcome in AO20,21,29,18,33 and in a recent study a radiological growth rate > 8 mm/year has been found as an independent factor of poorer PFS in oligodendrogliomas.35 Higher proliferation index and reduced epileptogenicity could explain why STS presented larger and more symptomatic tumors in older patients. In addition, STS were characterized by a higher level of chromosomal instability compared to CS. In IDH-mutant astrocytomas, chromosomal instability is an established poor prognostic factor.39 Its prognostic value in AO remains to be fully established, yet it has been associated with more frequent contrast-enhancement, larger tumor volume, and a poorer prognosis.23,38 Chromosome 9p loss and CDKN2A deletion have been shown to be important poor prognostic factors in AO.21 Herein, there was a trend toward more frequent 9p loss in STS compared to CS, but this trend was not statistically significant, possibly because of the small sample size. Other molecular alterations that have been associated with poorer outcome in AO include NOTCH1 and PI3KCA mutations, as well as specific gene expression profiles.24,28 Yet these alterations were not assessed in the present study. Future comprehensive molecular analyses will be important to determine whether STS are characterized by specific alterations that could facilitate their identification and constitute therapeutic targets.
Treatment Characteristics of STS
Although we observed important differences regarding the treatment of STS and CS, the only treatment-related characteristic independently associated with STS in multivariate analysis was the absence of surgical resection. Surgical resection has been associated with better prognosis in several studies7,9,17,22,23,28,32 and the present study suggests that it may be particularly important in AO patients presenting aggressive baseline characteristics. The possibility of a surgical resection should therefore be reconsidered in AO patients who have only undergone a biopsy, possibly because a diagnosis of AO was not suspected pre-operatively. Indeed, approximately 20% of AO patients have a “glioblastoma-like” presentation that may lead some teams to perform a biopsy rather than a surgical resection, especially in older patients with cognitive dysfunction.36 After surgery, we observed that compared to CS, STS were more frequently treated with CT alone or with radiotherapy plus temozolomide. Older age and larger tumor volume could explain why STS were more frequently treated with CT alone than CS, while one can hypothesize that a more aggressive “glioblastoma-like” presentation could explain partly why STS were more frequently treated with radiotherapy plus temozolomide. Although the optimal treatment of patients at risk for poor outcome remains to be determined, CT alone, especially with temozolomide may not be the optimal treatment AO.31,40 Whether these patients benefit from the addition of CT to radiotherapy is also unclear. Indeed, in both the RTOG and EORTC trials, survival curves of patients treated with RT plus PCV or RT only started to diverge after 5 years, as if the addition of PCV to RT had no clear impact on the outcome of AO patients at risk for poor survival.4,5 Analysis of STS characteristics in ongoing clinical trials dedicated to AO (NCT00887146, NCT02444000) will be important to determine the impact of post-operative treatment in these patients.
Identification of STS
Identification of patients at risk for poor survival is crucial to test more effective treatment strategies in this population. Herein, combining 3 characteristics independently associated with STS (cognitive dysfunction, mitosis count, and type of surgery) enabled to distinguish 2 groups of patients with different risk of short-term survival. However, this finding needs to be validated in an independent series and future studies should try to determine baseline features that are easier to assess for the identification of STS and explore classifications. Indeed, the identification of cognitive dysfunction may depend on testing method, mitosis count may lack reproducibility, and the type of surgery performed may depend on neurosurgeons’ experience.
Study Limits
Our study is limited by the absence of volumetric analysis for all patients, the absence of in-depth molecular analyses, and the heterogeneity of post-operative treatments. Because of its retrospective design, it is also difficult to determine to what extent differences regarding treatment resulted from differences in baseline characteristics and to what extent they influenced the outcome. Despite these limits, our study provides the first description of STS characteristics, highlights features that could help identifying these patients, and suggests that surgical resection may be particularly important in this population. However, these findings require validation in independent series. In addition, although cancer-specific survival may be difficult to assess in retrospective studies, our study strongly suggests that future studies on this population should carefully analyze the cause of death in poor prognosis AO patients, since here approximately 20% of the patients who died < 5 years after diagnosis very likely died from an AO-unrelated cause. In a large series from the Surveillance, Epidemiology, and End Result (SEER) database, the rate of non-cancer death was 11.7% in adult oligodendrogliomas20 but this rate may be higher in the first years after AO diagnosis.
Conclusions
The present study suggested that STS poor survival largely results from more aggressive baseline characteristics and a more advanced disease at diagnosis. In these patients, reduced epileptogenicity and a higher proliferation index could lead to the diagnosis of large and symptomatic tumors in older patients. Future studies will have to determine how to optimally identify and treat AO patients at risk for poor outcome, yet surgical resection may be particularly important in this population.
Supplementary Material
Acknowledgments
We thank Mrs. Hélène Boyer for English reviewing of the manuscript. We also thank the Hospices Civils de Lyon Biological Resource Center—Tissus Tumorothèque Est & Neurobiotec for their precious help in collecting data. Cases from Marseille were retrieved from the AP-HM tumor bank (authorization number: AC2018-31053; CRB BB-0033-00097). Members of the POLA network include the following: Amiens (C. Desenclos, H. Sevestre), Angers (A. Rousseau), Annecy (T. Cruel, S. Lopez), Besançon (M.-I. Mihai, A. Petit), Bicêtre (C. Adam, F. Parker), Brest (R. Seizeur, I. Quintin-Roué), Bordeaux (S. Eimer, H. Loiseau), Caen (F. Chapon), Clamart (D. Ricard), Clermont-Ferrand (C. Godfraind, T. Khallil), Clichy (D. Cazals-Hatem, T. Faillot), Colmar (C. Gaultier, M.C. Tortel), Cornebarrieu (I. Carpiuc, P. Richard) Créteil (W. Lahiani) Dijon (M.H. Aubriot-Lorton, F. Ghiringhelli), Lille (C.A. Maurage), Limoges (E.M. Gueye, F. Labrousse), Lyon (D. Meyronet), Montpellier (L. Bauchet, V. Rigau), Nancy (G. Gauchotte), Nantes (M. Campone, D. Loussouarn), Nice (D. Fontaine, F. Vandenbos-Burel), Orléans (C. Blechet, M. Fesneau), Paris (F. Bielle, A. Carpentier, M. Polivka), Poitiers (S. Milin, M Wager), Reims (M.D. Diebold), Rennes (D. Chiforeanu, E. Vauleon), Rouen (A. Laquerriere), Saint-Etienne (F. Forest, M.J. Motso Fotso), Saint-Pierre de la Réunion (M. Andraud, G. Runavot), Strasbourg (B. Lhermitte, G. Noel), Suresnes (A.L. Di Stéfano, C. Villa), Tours (C. Rousselot-Denis, I. Zemmoura), Toulon (N. Desse), Toulouse (E. Uro-Coste).
Conflict of Interest
Elisabeth Cohen-Jonathan Moyal: Novocure (ET); Novocure, Incyte, Bayer, Astra Zeneca (RF); Elsa Curtit: Novartis, Pfizer, ExactSciences (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: L.G., F.D. Provision of study material/patients: L.G., O.C., E.C.-J. M., A.D., C.B., L.B., L.T., J.-S.F., O.L., P.C., P.M., F.D., C.C., E.C., D.F.-B., C.D., F.D.. Collection and/or assembly of data: L.G. Data analysis and interpretation: L.G., C.V., A.G., F.D. Manuscript writing: L.G., F.D. Final approval of manuscript: All authors.
Ethics Statement
All patients provided written informed consent for clinical data collection and genetic analysis according to national and POLA network policies.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ostrom QT, Gittleman H, Liao Pet al.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncol 2017;19:v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al.. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 3. Tabouret E, Nguyen AT, Dehais C, et al. ; For POLA Network . Prognostic impact of the 2016 WHO classification of diffuse gliomas in the French POLA cohort. Acta Neuropathol. 2016;132(4):625-634. [DOI] [PubMed] [Google Scholar]
- 4. van den Bent MJ, Brandes AA, Taphoorn MJ, et al.. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344-350. [DOI] [PubMed] [Google Scholar]
- 5. Cairncross G, Wang M, Shaw E, et al.. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wick W, Roth P, Hartmann C, et al. ; Neurooncology Working Group (NOA) of the German Cancer Society . Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lassman AB, Iwamoto FM, Cloughesy TF, et al.. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zetterling M, Berhane L, Alafuzoff I, Jakola AS, Smits A.. Prognostic markers for survival in patients with oligodendroglial tumors; a single-institution review of 214 cases. PLoS One. 2017;12(11):e0188419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu S, Liu X, Xiao Y, Chen S, Zhuang W.. Prognostic factors associated with survival in patients with anaplastic oligodendroglioma. PLoS One. 2019;14(1):e0211513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holdhoff M, Cairncross GJ, Kollmeyer TM, et al.. Genetic landscape of extreme responders with anaplastic oligodendroglioma. Oncotarget. 2017;8(22):35523-35531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mandonnet E, Delattre JY, Tanguy ML, et al.. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524-528. [DOI] [PubMed] [Google Scholar]
- 12. Coulibaly B, Nanni I, Quilichini B, et al.. Epidermal growth factor receptor in glioblastomas: correlation between gene copy number and protein expression. Hum Pathol. 2010;41(6):815-823. [DOI] [PubMed] [Google Scholar]
- 13. Figarella-Branger D, Mokhtari K, Dehais C, et al. ; POLA Network . Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro Oncol. 2014;16(9):1244-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meyronet D, Esteban-Mader M, Bonnet C, et al.. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19(8):1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Idbaih A, Ducray F, Dehais C, et al. ; POLA Network . SNP array analysis reveals novel genomic abnormalities including copy neutral loss of heterozygosity in anaplastic oligodendrogliomas. PLoS One. 2012;7(10):e45950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roux A, Tauziede-Espariat A, Zanello M, et al.. Imaging growth as a predictor of grade of malignancy and aggressiveness of IDH-mutant and 1p/19q-codeleted oligodendrogliomas in adults. Neuro Oncol. 2020;22(7):993-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garton ALA, Kinslow CJ, Wang TJC.. Erratum. Extent of resection, molecular signature, and survival in 1p19q-codeleted gliomas. J Neurosurg. 2020;134(5):1675. [DOI] [PubMed] [Google Scholar]
- 18. Shin DW, Lee S, Song SW, et al.. Survival outcome and prognostic factors in anaplastic oligodendroglioma: a single-institution study of 95 cases. Sci Rep. 2020;10(1):20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin AJ, Kane LT, Molitoris JK, et al.. A multi-institutional analysis of clinical outcomes and patterns of care of 1p/19q codeleted oligodendrogliomas treated with adjuvant or salvage radiation therapy. J Neurooncol. 2020;146(1):121-130. [DOI] [PubMed] [Google Scholar]
- 20. Pouget C, Hergalant S, Lardenois E, et al.. Ki-67 and MCM6 labeling indices are correlated with overall survival in anaplastic oligodendroglioma, IDH1-mutant and 1p/19q-codeleted: a multicenter study from the French POLA network. Brain Pathol. 2020;30(3):465-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Appay R, Dehais C, Maurage CA, et al. ; POLA Network . CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019;21(12):1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kinslow CJ, Garton ALA, Rae AI, et al.. Extent of resection and survival for oligodendroglioma: a U.S. population-based study. J Neurooncol. 2019;144(3):591-601. [DOI] [PubMed] [Google Scholar]
- 23. Chen H, Thomas C, Munoz FA, et al.. Polysomy is associated with poor outcome in 1p/19q codeleted oligodendroglial tumors. Neuro Oncol. 2019;21(9):1164-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yeboa DN, Yu JB, Liao E, et al.. Differences in patterns of care and outcomes between grade II and grade III molecularly defined 1p19q co-deleted gliomas. Clin Transl Radiat Oncol. 2019;15:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halani SH, Yousefi S, Velazquez Vega J, et al.. Multi-faceted computational assessment of risk and progression in oligodendroglioma implicates NOTCH and PI3K pathways. NPJ Precis Oncol. 2018;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Appay R, Tabouret E, Touat M, et al. ; POLA network . Somatostatin receptor 2A protein expression characterizes anaplastic oligodendrogliomas with favorable outcome. Acta Neuropathol Commun. 2018;6(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenberg S, Ducray F, Alentorn A, et al. ; POLA Network . Machine learning for better prognostic stratification and driver gene identification using somatic copy number variations in anaplastic oligodendroglioma. Oncologist. 2018;23(12):1500-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aoki K, Nakamura H, Suzuki H, et al.. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20(1):66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Figarella-Branger D, Mokhtari K, Dehais C, et al. ; POLA Network . Mitotic index, microvascular proliferation, and necrosis define 3 pathological subgroups of prognostic relevance among 1p/19q co-deleted anaplastic oligodendrogliomas. Neuro Oncol. 2016;18(6):888-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu X, Martinez-Ledesma E, Zheng S, et al.. Multigene signature for predicting prognosis of patients with 1p19q co-deletion diffuse glioma. Neuro Oncol. 2017;19(6):786-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamoun A, Idbaih A, Dehais C, et al. ; POLA network . Integrated multi-omics analysis of oligodendroglial tumours identifies three subgroups of 1p/19q co-deleted gliomas. Nat Commun. 2016;7:11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang HC, Yu T, Lim DH, et al.. A multicenter study of anaplastic oligodendroglioma: the korean radiation oncology group study 13-12. J Neurooncol. 2015;125(1):207-215. [DOI] [PubMed] [Google Scholar]
- 33. Preusser M, Hoeftberger R, Woehrer A, et al.. Prognostic value of Ki67 index in anaplastic oligodendroglial tumours–a translational study of the european organization for research and treatment of cancer brain tumor group. Histopathology. 2012;60(6):885-894. [DOI] [PubMed] [Google Scholar]
- 34. Panageas KS, Reiner AS, Iwamoto FM, et al.. Recursive partitioning analysis of prognostic variables in newly diagnosed anaplastic oligodendroglial tumors. Neuro Oncol. 2014;16(11):1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pallud J, Audureau E, Blonski M, et al.. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(Pt 2):449-462. [DOI] [PubMed] [Google Scholar]
- 36. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group . Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076-2084. [DOI] [PubMed] [Google Scholar]
- 37. Chen H, Judkins J, Thomas C, et al.. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reyes-Botero G, Dehais C, Idbaih A, et al. ; POLA Network . Contrast enhancement in 1p/19q-codeleted anaplastic oligodendrogliomas is associated with 9p loss, genomic instability, and angiogenic gene expression. Neuro Oncol. 2014;16(5):662-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shirahata M, Ono T, Stichel D, et al.. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153-166. [DOI] [PubMed] [Google Scholar]
- 40. Jaeckle KA, Ballman KV, van den Bent M, et al.. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro-Oncol 2021;23:457-4–67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.