Abstract
Limited data are available on antiretroviral drug concentrations in seminal plasma during a dosing interval. Further, since human ejaculate is composed of fluids originating from the testes, the seminal vesicles, and the prostate, all having different physiological characteristics, drug concentrations in total seminal plasma do not necessarily reflect concentrations in the separate compartments. Five human immunodeficiency virus type 1-infected patients on nevirapine (NVP; 200 mg twice a day [b.i.d.]) and/or indinavir (IDV; 800 mg b.i.d. with ritonavir, 100 mg b.i.d.) regimens used a split ejaculate technique to separate seminal plasma in two fractions, representing fluids from the testes and prostate (first fraction) and fluids from the seminal vesicles (second fraction). Split-ejaculate samples were provided at 0, 2, 5, and 8 h after drug ingestion, on separate days after 3 days of sexual abstinence. NVP and IDV showed time-dependent concentrations in seminal plasma, with peak concentrations in both fractions at 2 and 2 to 5 h, respectively, after drug ingestion. The NVP concentrations were not significantly different between the first and second fractions of the ejaculate at all time points measured and were in the therapeutic range, except for the predose concentration in two patients. The median (range) predose IDV concentrations in the first and second fractions of the ejaculate were 448 (353 to 1,015) ng/ml and 527 (240 to 849) ng/ml, respectively (P = 0.7). In conclusion, NVP and IDV concentrations in seminal plasma are dependent on the time after drug ingestion. Furthermore, our data suggest that NVP and IDV achieve therapeutic concentrations in both the testes and prostate and the seminal vesicles throughout the dosing interval.
Penetration of antiretroviral drugs into all body compartments is important in the treatment of human immunodeficiency virus (HIV) infection. The central nervous system and the male genital tract are considered anatomical reservoirs for HIV, as the blood-brain barrier and the blood-testis barrier may prevent antiretroviral drugs from entering these organs. Suboptimal antiretroviral drug concentrations in the male genital tract could allow continuing production of HIV-1 and the emergence of drug-resistant HIV type 1 (HIV-1) strains (4–6, 22).
Data available on drug concentrations in semen show that the penetration of the protease inhibitors nelfinavir, ritonavir (RTV), and saquinavir is poor (14; M. Reijers, R. van Heeswijk, H. Schuitemaker, P. Portegies, G. J. Weverling, J. Lange, and R. Hoetelmans, 7th Conf. Retrovir. Opportunistic Infect., abstr. 316, 2000). The nucleoside analogues zidovudine (ZDV), stavudine (d4T), and lamivudine (3TC), the nonnucleoside analogue nevirapine (NVP), and the protease inhibitors indinavir (IDV) and amprenavir penetrate well into the male genital tract (4, 10, 13, 15, 20). It is largely unknown whether drug concentrations in seminal plasma vary during the dosing interval (2, 10, 15).
Further, human ejaculate is composed of secretions from the testes (10% of ejaculate volume), the prostate (20 to 30% of ejaculate volume), and the seminal vesicles (50 to 70% of ejaculate volume), all of which have their own physiological characteristics (11). Therefore, the final concentration of a drug in the ejaculate does not necessarily reflect the concentration of the drug in the various compartments of the male genital tract. Drug concentrations in the various parts of the male genital tract can be studied using the so-called split ejaculate technique (8). The testicular and prostate fluids are discharged first during the ejaculatory process, whereas later during the ejaculatory process mainly fluids originating from the seminal vesicles are released.
We evaluated the pharmacokinetic profiles of NVP and IDV in seminal plasma during the dosing interval. By using the split ejaculate technique, we investigated the penetration of NVP and IDV in fluids originating from the testes and prostate and from the seminal vesicles.
MATERIALS AND METHODS
Patients and study design.
Five HIV-1-infected men who had used NVP and IDV as part of their antiretroviral regimen for at least 4 weeks participated in this study. Semen samples were obtained by masturbation. The patients were instructed to collect the ejaculate in such a way that the first fraction of the ejaculate was collected into a sterile container (representing the testes and prostate fraction of the ejaculate), and the remaining part of the ejaculate (second fraction) was collected into a second container (representing the seminal vesicles fraction). In both fractions, the concentrations of fructose and spermatozoa were measured. The testes and prostate fraction is characterized by a high spermatozoa concentration and a low amount of fructose, while the seminal vesicles fraction is characterized by a low spermatozoa concentration and a high amount of fructose (8). The patients ejaculated prior to drug ingestion (t = 0 h) and at 2, 5, and 8 h after drug ingestion, on separate days. Patients were instructed to have at least 3 days of sexual abstinence before each semen sample was obtained. Patients had no signs or symptoms of a genital infection.
In all patients, several random heparinized blood samples were obtained, allowing for comparison with plasma population pharmacokinetics. Plasma was isolated by centrifugation for 10 min at 1,200 × g, and samples were stored at −70°C until analysis. The study was approved by the local Medical Ethics Committee and informed consent was obtained from all patients.
Fructose and spermatozoa concentrations in ejaculate fractions.
All semen samples were processed within 1 h after ejaculation. After liquefaction, concentration and motility of spermatozoa were assessed in both fractions using a Makler counting chamber (Sefi-Medical Instruments, Haifa, Israel) and computer-aided sperm analysis equipment (Hobson Tracking Systems, Sheffield, United Kingdom). Subsequently, the two fractions were centrifuged at 1,200 × g for 10 min to isolate seminal plasma and 100 μl was used for fructose analysis. Fructose concentration was determined using an automated spectrophotometer (Hoffman-La Roche, Basel, Switzerland) at a wavelength of 340 nm. Seminal plasma was stored at −20°C until analysis.
Bioanalysis of NVP and IDV in blood plasma and seminal plasma.
The NVP and IDV concentrations in blood plasma and seminal plasma were measured using a high-performance liquid chromatographic procedure (16, 17). NVP and IDV concentrations in seminal plasma were assessed after 1:1 dilution with blank human heparinized plasma. The within- and between-day precision of the NVP assay was less than 4.5% and that of the IDV assay was less than 6.2%.
HIV-1 RNA in blood plasma and seminal plasma.
In patients 027 and 028, the HIV-1 RNA concentration in EDTA plasma was measured using the quantiplex bDNA assay (Bayer Corporation, Emeryville, Calif.) with a lower limit of quantification (LLQ) of 50 copies/ml. In patients 010, 014, and 021, the HIV-1 RNA concentration in EDTA plasma was measured using the NucliSens HIV-1 QT assay (Organon Teknika, Boxtel, The Netherlands). When HIV-1 RNA concentrations decreased to below 50 copies/ml (23), an initial input volume in the assay of 2 ml of plasma was used with the ultrasensitive protocol adaptation, resulting in an LLQ of 5 copies/ml.
HIV-1 RNA concentrations in seminal plasma were measured using the NucliSens HIV-1 QT assay. An input volume of 0.2 ml was used with the ultrasensitive protocol adaptation (23), resulting in an LLQ of 50 copies/ml.
Pharmacokinetic analysis.
The seminal plasma concentration (C) versus time (t) data were analyzed by noncompartmental methods (7). The highest observed concentration in seminal plasma was defined as the peak concentration (Cmax), with the corresponding sampling time defined as tmax. The concentration in seminal plasma measured prior to drug ingestion was defined as the trough concentration (Cmin).
Statistical analysis.
Differences in concentrations of NVP and IDV between the first and second ejaculate fractions were tested with the Wilcoxon signed ranks test. Statistical significance was defined as P ≤ 0.05. Data were analyzed using SPSS software (version 9.0.0; SPSS Inc., Chicago, Ill.).
RESULTS
The five patients had a median CD4+ T-cell count of 490 cells/mm3 (range, 360 to 1,010 cells/mm3), and HIV-1 RNA levels in blood were below the LLQ at the time of semen sample collection. HIV-1 RNA levels in seminal plasma were below the LLQ in four patients, whereas the seminal plasma of one patient yielded an invalid test result despite repeated testing. Patient and treatment characteristics are described in Table 1.
TABLE 1.
Patient and treatment characteristics at the time of semen sample collection
| Patient no. | Therapya | Time on regimen (wk) | CD4+ T cells (cells/mm3) | Plasma HIV-1 RNA (copies/ml) |
|---|---|---|---|---|
| 010 | ZDV, 3TC, IDV, RTV | 160 | 410 | <5c |
| 014 | ZDV, 3TC, ABC, NVP, IDVb RTV | 144 | 360 | <5 |
| 021 | d4T, 3TC, ABC, NVP, IDV, RTV | 64 | 800 | <5 |
| 027 | d4T, 3TC, NVP, IDV, RTV | 4 | 1,010 | <50d |
| 028 | d4T, 3TC, NVP, IDV, RTV | 10 | 490 | <50 |
ZDV, zidovudine at 300 mg b.i.d.; 3TC, lamivudine at 150 mg b.i.d.; d4T, stavudine at 40 mg b.i.d.; ABC, abacavir at 300 mg b.i.d.; NVP, nevirapine at 200 mg b.i.d.; IDV, indinavir at 800 mg b.i.d.; RTV, ritonavir at 100 mg b.i.d.
This patient received IDV at 1,000 mg b.i.d. and RTV at 100 mg b.i.d.
Plasma HIV-1 RNA was measured using the NucliSens HIV-1 QT assay (Organon Teknika).
Plasma HIV-1 RNA was measured using the quantiplex bDNA assay (Bayer Corporation).
Split ejaculates.
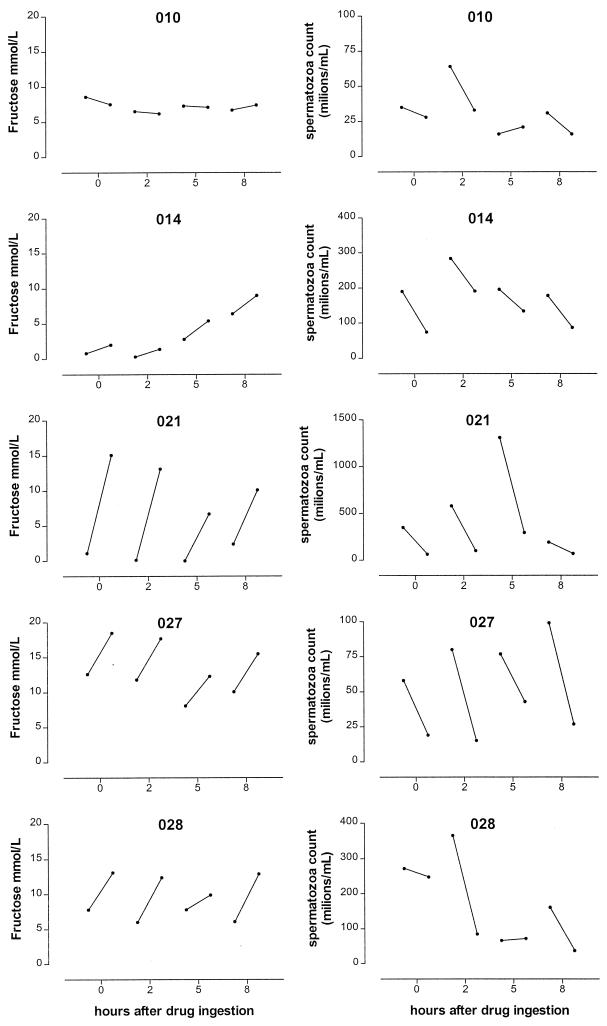
The patients were able to provide split ejaculate samples at all required time points. The median (range) volume of the first and second fractions was 1.2 (0.3 to 3.0) ml and 1.6 (0.5 to 3.1) ml, respectively. The concentrations of fructose and spermatozoa in the first and second ejaculate fractions are shown in Fig. 1. According to the concentrations of fructose and/or spermatozoa, all patients were able to split the ejaculate into a first fraction derived from the testes and prostate and a second fraction derived from the seminal vesicles. The split ejaculate samples of patient 010 showed no increase in fructose concentration, but the spermatozoa concentration decreased significantly between the first and second ejaculate fraction for this patient, indicating a reliable split ejaculate technique.
FIG. 1.
Fructose and spermatozoa concentrations in split ejaculate samples at 0, 2, 5, or 8 h after drug ingestion. The left circle of each line represents the fructose or spermatozoa concentration in the first ejaculate fraction; the right circle represents the fructose or spermatozoa concentration in the second ejaculate fraction.
NVP.
Twelve blood plasma samples and 32 split ejaculate fractions from four patients were available for NVP measurement. The NVP concentrations measured in random blood plasma samples of these patients were comparable to concentrations normally observed in a reference population of HIV-1-infected patients using NVP at 200 mg twice a day (b.i.d.) (Fig. 2A) (19).
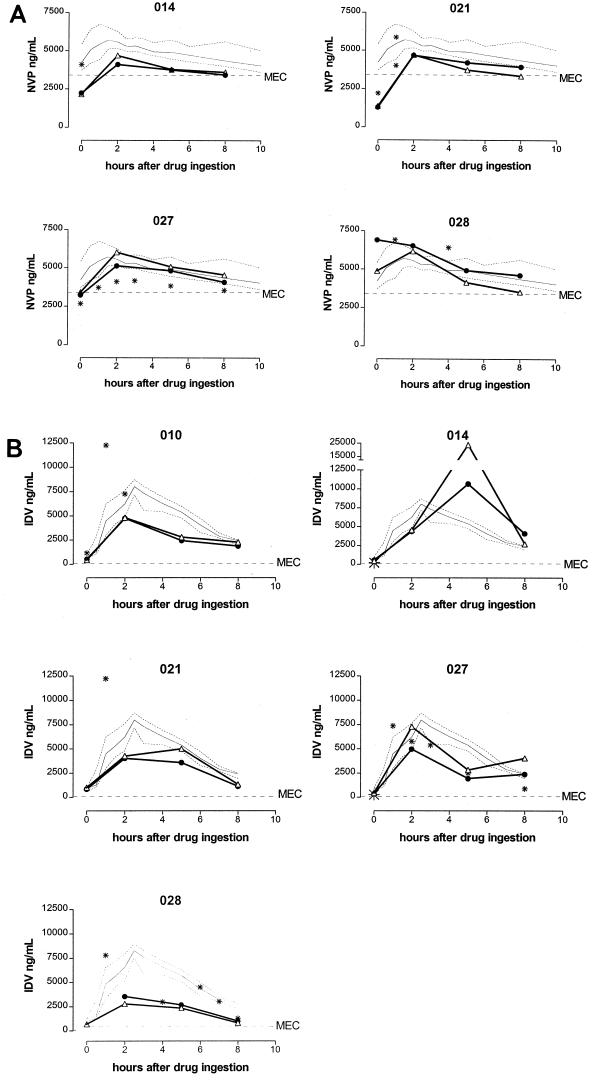
FIG. 2.
(A) NVP concentrations in blood plasma and seminal plasma during the dosing interval. All patients used NVP at 200 mg b.i.d. Open triangles, NVP concentration in the first ejaculate fraction; black circles, NVP concentration in the second ejaculate fraction; asterisks, NVP concentration in a random blood plasma sample; solid thin line, median NVP concentrations measured in a reference population of HIV-1-infected patients using NVP at 200 mg b.i.d., with interquartile ranges represented as dotted lines (19); MEC, minimal effective concentration of NVP in blood plasma (3.4 μg/ml) (9, 21). The lower limits of quantification of NVP in blood plasma and seminal plasma were 50 and 100 ng/ml, respectively (17). (B) IDV concentrations in blood plasma and seminal plasma during the dosing interval. All patients used IDV (800 mg b.i.d.) and RTV (100 mg b.i.d.), except for patient 014, who used IDV (1,000 mg b.i.d.) and RTV (100 mg b.i.d.). Open triangles, IDV concentration in the first ejaculate fraction; black circles, IDV concentration in the second ejaculate fraction; asterisks, IDV concentration in a random blood plasma sample; solid thin line, median IDV concentrations measured in a reference population of HIV-1-infected patients using IDV (800 mg b.i.d.) plus RTV (100 mg b.i.d.), with the interquartile ranges represented as dotted lines (18); MEC, minimal effective concentration of IDV in blood plasma (100 ng/ml) (3). The lower limits of quantification of IDV in blood plasma and seminal plasma were 25 and 50 ng/ml, respectively (16).
In both ejaculate fractions, we observed NVP peak concentrations in samples collected 2 h after drug ingestion (Fig. 2A). At all time points, concentrations in the first and second fractions were not significantly different (P > 0.1 in all cases).
IDV.
Seventeen blood plasma samples and 39 split ejaculate fractions of five patients were available for IDV measurement (one split ejaculate fraction contained insufficient material for analysis). The IDV concentrations measured in random blood plasma samples of these patients were comparable to concentrations normally observed in a reference population of HIV-1-infected patients using IDV (800 mg b.i.d.) and RTV (100 mg b.i.d.) (Fig. 2B) (18).
In both ejaculate fractions, we observed IDV peak concentrations in the samples collected at 2 or 5 h after drug ingestion (Fig. 2B). The median (range) IDV trough concentration in the first and second fractions of the ejaculate were 448 (353 to 1015) ng/ml and 527 (240 to 849) ng/ml, respectively (Wilcoxon P = 0.7) (Fig. 2B). At 2, 5, and 8 h, concentrations in the first and second fractions were also not significantly different (in all cases, P > 0.3).
DISCUSSION
The results of this study demonstrate that concentrations of NVP and IDV in seminal plasma are dependent on the time after drug ingestion, with peak concentrations 2 and 2 to 5 h after drug ingestion, respectively. This is in contrast with previously described relatively stable ZDV concentrations in semen (2, 10). These pharmacokinetic profiles of NVP and IDV indicate that in contrast to cerebrospinal fluid, where IDV concentrations are more stable (12), the concentrations of NVP and IDV in a semen sample cannot be used as a measure of exposure without taking into account the time of drug ingestion. We did not provide seminal plasma/blood plasma ratios, as only the absolute concentrations of NVP and IDV in seminal plasma are clinically relevant.
This study confirms previous data describing the good penetration of NVP into semen (15). A potential in vivo threshold concentration of NVP is in the range of 3.4 μg/ml (9, 21). Given the lower protein concentration in seminal plasma (35 to 55 g/liter) (8), and assuming the same percentage of protein binding in blood plasma and seminal plasma (i.e., 60% bound), the free drug available for antiretroviral activity exceeded the therapeutic level at all time points except for the predose concentration in two patients.
The minimal effective trough concentration (MEC) of IDV in serum is at least 100 ng/ml (3). The MEC in seminal plasma, assuming the same percentage of protein binding in both fluids (i.e., 60% [1]), is therefore also estimated to be at least 100 ng/ml. The median IDV concentration in both ejaculate fractions exceeded this MEC at least four- to fivefold during the whole dosing interval. We showed earlier that the addition of RTV to an IDV-containing regimen is important, as it significantly increases IDV concentrations in seminal plasma from IDV concentrations just above the MEC to IDV concentrations exceeding the MEC by several fold (20).
Both drugs penetrated equally well in testes and prostate and in seminal vesicles, as indicated by similar drug concentrations in the two ejaculate fractions. The split ejaculate technique does not distinguish between fluids derived from the testes (10% of total ejaculate volume) and fluids from the prostate (20 to 30% of total ejaculate volume) (11). Theoretically, all NVP or IDV measured in the first fraction could originate from either the testes or from the prostate. If all NVP or IDV originated from the prostate, this would mean that the actual drug concentration in the prostate, before dilution with fluids from the testes containing no drugs, must be higher than the drug concentrations in the seminal vesicles. This might be possible, if the weak bases NVP or IDV accumulate in acidic prostate fluid (pH 6.5). However, the value of the dissociation constants of NVP (pKa 2.8) and IDV (pKa 6.2) provide a strong argument against trapping of NVP or IDV in prostate fluid (11). Also, the fact that NVP and IDV concentrations are dependent on the time after drug intake suggests that accumulation of these drugs in the prostate is unlikely. On the other hand, preferential accumulation of these drugs in the fluid from the testes is also not very likely, considering the presence of the blood-testis barrier and the continuous efflux of testicular fluids. Therefore, we hypothesize that NVP and IDV concentrations measured in the first ejaculate fraction represent actual drug concentrations in both testicular and prostate fluid.
In conclusion, NVP and IDV concentrations in seminal plasma are dependent on the time after drug ingestion. It is therefore not justified to use drug concentrations of NVP and IDV in a random semen sample as a measure of exposure without taking into account the time of drug ingestion. Furthermore, our data suggest that NVP and IDV achieve therapeutic concentrations in both the testes and prostate and in the seminal vesicles.
ACKNOWLEDGMENTS
We thank Glaxo-Wellcome for providing abacavir and Boehringer-Ingelheim for providing nevirapine. We also thank S. Jansen and M. Nievaard for excellent patient care, S. Jurriaans (Department of Human Retrovirology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands) for HIV-1 RNA measurements, M. T. L. Roos (Department of Clinical Viro-Immunology, CLB and Laboratory for Experimental and Clinical Immunology, Academic Medical Center Amsterdam, The Netherlands) for CD4+ T-cell measurements, and R. P. G. van Heeswijk (Department of Pharmacy and Pharmacology, Slotervaart Hospital, Amsterdam, The Netherlands) for critically reading the manuscript. Most of all, we thank the patients who volunteered to participate in this study.
The study was financially supported by a private foundation which does not wish to be named.
REFERENCES
- 1.Anderson P L, Brundage R C, Bushman L, Kakuda T N, Remmel R P, Fletcher C V. Indinavir plasma protein binding in HIV-1-infected adults. AIDS. 2000;14:2293–2297. doi: 10.1097/00002030-200010200-00010. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P L, Noormohamed S E, Henry K, Brundage R C, Balfour H H, Fletcher C V. Semen and serum pharmacokinetics of zidovudine and zidovudine-glucuronide in men with HIV-1 infection. Pharmacotherapy. 2000;20:917–922. doi: 10.1592/phco.20.11.917.35263. [DOI] [PubMed] [Google Scholar]
- 3.Burger D M, Hoetelmans R M W, Hugen P W H, Mulder J W, Meenhorst P L, Koopmans P P, Brinkman K, Keuter M, Dolmans W, Hekster Y A. Low plasma concentrations of indinavir are related to virological treatment failure in HIV-1 infected patients on indinavir-containing triple therapy. Antivir Ther. 1998;3:215–220. [PubMed] [Google Scholar]
- 4.Eron J J, Smeaton L M, Fiscus S A, Gulick R M, Currier J S, Lennox J L, D'Aquila R T, Rogers M D, Tung R, Murphy R L. The effects of protease inhibitor therapy on human immunodeficiency virus type 1 levels in semen (AIDS clinical trials group protocol 850) J Infect Dis. 2000;181:1622–1628. doi: 10.1086/315447. [DOI] [PubMed] [Google Scholar]
- 5.Eron J J, Vernazza P L, Johnston D M, Seillier-Moiseiwitsch F, Alcorn T M, Fiscus S A, Cohen M S. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS. 1998;12:181–189. doi: 10.1097/00002030-199815000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Eyre R C, Zheng G, Kiessling A A. Multiple drug resistance mutations in human immunodeficiency virus in semen but not in blood of a man on antiretroviral therapy. Urology. 2000;55:591X–VII-591X. doi: 10.1016/s0090-4295(99)00592-0. X. [DOI] [PubMed] [Google Scholar]
- 7.Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. Philadelphia, Pa: Lea & Febiger; 1991. [Google Scholar]
- 8.Hafez E S E, editor. Human semen and fertility regulation in men. St. Louis, Mo: Mosby Co.; 1976. [PubMed] [Google Scholar]
- 9.Havlir D, Cheeseman S H, McLaughlin M, Murphy R, Erice A, Spector S A, Greenough T C, Sullivan J L, Hall D, Myers M, Lamson M, Richman D D. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis. 1995;171:537–545. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 10.Henry K, Chinnock B J, Quinn R P, Fletcher C V, de Miranda P, Balfour H H., Jr Concurrent zidovudine levels in semen and serum determined by radioimmunoassay in patients with AIDS or AIDS-related complex. JAMA. 1988;259:3023–3026. [PubMed] [Google Scholar]
- 11.Kashuba A D M, Dyer J R, Kramer L M, Raasch R H, Eron J J, Cohen M S. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43:1817–1826. doi: 10.1128/aac.43.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin C, Sonnerborg A, Svensson J O, Stahle L. Indinavir-based treatment of HIV-1 infected patients: efficacy in the central nervous system. AIDS. 1999;13:1227–1232. doi: 10.1097/00002030-199907090-00012. [DOI] [PubMed] [Google Scholar]
- 13.Pereira A S, Kashuba A D M, Fiscus A A, Hall J E, Tidwell R R, Troiani L, Dunn J A, Eron J J, Cohen M S. Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis. 1999;180:2039–2043. doi: 10.1086/315149. [DOI] [PubMed] [Google Scholar]
- 14.Taylor S, Back D J, Workman J, Drake S M, White D J, Choudhury B, Cane P A, Beards G M, Halifax K. Poor penetration of the male genital tract by HIV-1 protease inhibitors. AIDS. 1999;13:859–860. doi: 10.1097/00002030-199905070-00017. [DOI] [PubMed] [Google Scholar]
- 15.Taylor S, van Heeswijk R P G, Hoetelmans R M W, Workman J, Drake S M, White D J, Pillay D. Concentrations of nevirapine, lamivudine and stavudine in semen of HIV-1 infected men. AIDS. 2000;14:1979–1984. doi: 10.1097/00002030-200009080-00014. [DOI] [PubMed] [Google Scholar]
- 16.van Heeswijk R P G, Hoetelmans R M W, Harms R, Meenhorst P L, Mulder J W, Lange J M A, Beijnen J H. Simultaneous quantitative determination of the HIV protease inhibitors amprenavir, indinavir, nelfinavir, ritonavir, and saquinavir in human plasma by ion-pair high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1998;719:159–168. doi: 10.1016/s0378-4347(98)00392-2. [DOI] [PubMed] [Google Scholar]
- 17.van Heeswijk R P G, Hoetelmans R M W, Meenhorst P L, Mulder J W, Beijnen J H. Rapid determination of nevirapine in human plasma by ion-pair reversed phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1998;713:395–399. doi: 10.1016/s0378-4347(98)00217-5. [DOI] [PubMed] [Google Scholar]
- 18.van Heeswijk R P G, Veldkamp A I, Hoetelmans R M W, Mulder J W, Schreij G, Hsu A, Lange J M A, Beijnen J H, Meenhorst P L. The steady-state plasma pharmacokinetics of indinavir alone and in combination with a low dose of ritonavir in twice daily dosing regimens in HIV-1-infected individuals. AIDS. 1999;13:F95–F99. doi: 10.1097/00002030-199910010-00001. [DOI] [PubMed] [Google Scholar]
- 19.van Heeswijk R P G, Veldkamp A I, Mulder J W, Meenhorst P L, Wit F W N M, Lange J M A, Danner S A, Foudraine N A, Kwakkelstein M O, Reiss P, Beijnen J H, Hoetelmans R M W. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1 infected individuals. AIDS. 2000;14:F77–F82. doi: 10.1097/00002030-200005260-00001. [DOI] [PubMed] [Google Scholar]
- 20.van Praag R M E, Weverling G J, Portegies P, Jurriaans S, Zhou X J, Turner-Foisy M D, Sommadossi J-P, Burger D M, Lange J M A, Hoetelmans R M W, Prins J M. Enhanced penetration of indinavir in cerebrospinal fluid and semen after the addition of low-dose ritonavir. AIDS. 2000;14:1187–1194. doi: 10.1097/00002030-200006160-00016. [DOI] [PubMed] [Google Scholar]
- 21.Veldkamp A I, Weverling G J, Lange J M, Montaner J S, Reiss P, Cooper D A, Vella S, Hall D, Beijnen J H, Hoetelmans R M. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS. 2001;15:1089–1095. doi: 10.1097/00002030-200106150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Vernazza P L, Troiani L, Flepp M J, Cone R W, Schock J, Roth F, Boggian K, Cohen M S, Fiscus S A, Eron J J the Swiss Cohort Study. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding. AIDS. 2000;14:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 23.Weverling G J, Lange J M A, Jurriaans S, Prins J M, Lukashov V V, Notermans D W, Roos M, Schuitemaker H, Hoetelmans R M W, Danner S A, Goudsmit J, de Wolf F. Alternative multidrug regimen provides improved suppression of HIV-1 replication over triple therapy. AIDS. 1998;12:F117–F122. doi: 10.1097/00002030-199811000-00003. [DOI] [PubMed] [Google Scholar]