Abstract
Background
The efficacy of irinotecan plus continuous trastuzumab beyond progression in patients with gastric cancer previously treated with trastuzumab plus standard first-line chemotherapy has not been reported.
Methods
Patients with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer who were previously treated with trastuzumab received trastuzumab every 3 weeks and irinotecan every 2 weeks. The primary endpoint was the overall response rate (ORR), and the secondary endpoints included progression-free survival (PFS), 6-month survival rates, safety, and subgroup analysis by HER2 status.
Results
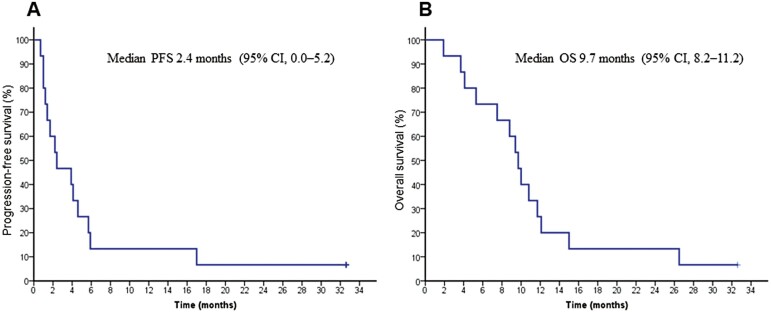
Sixteen patients were enrolled in a 3-year pre-planned registration period. This study was prematurely closed due to poor patient accrual. The ORR and disease control rate were 6.7% (95% CI, 0.2-32.0) and 53.3% (95% CI, 26.6-78.7). The median PFS and overall survival (OS) were 2.4 months (95% CI, 0.0-5.2) and 9.7 months (95% CI, 8.2-11.2), respectively. The most frequently reported grades 3-4 adverse events were neutropenia (40%), anemia (27%), anorexia (33%), and fatigue (33%).
Conclusion
With only 16 patients enrolled, the present study has very low power to detect any clinical benefit of trastuzumab plus irinotecan beyond disease progression in patients with HER2-positive advanced gastric cancer who previously received trastuzumab.
Trial Identifier: UMIN000007636.
Keywords: gastric cancer, HER2, trastuzumab, irinotecan, second-line treatment
This prospective study explored the significance of continuous trastuzumab plus irinotecan in the second-line treatment of HER2-positive gastric cancer that was refractory to initial trastuzumab combined with chemotherapy.
Lessons Learned.
Results showed that continuous trastuzumab plus irinotecan in the second-line treatment of HER2-positive gastric cancer refractory to an initial trastuzumab-containing regimen was not more effective than previous studies of irinotecan monotherapy indicated.
The study size may limit the strength of this conclusion.
Discussion
To our knowledge, this is the first prospective study to explore the significance of continuous trastuzumab plus irinotecan in the second-line treatment of HER2-positive gastric cancer that was refractory to initial trastuzumab combined with chemotherapy. Although limited by poor accrual, this study did not support that hypothesis. Continuous trastuzumab plus irinotecan treatment did not appear to be more effective than seen in previous reports of irinotecan monotherapy. The significance of continued anti-HER2 therapy is unknown. The present study, which closed early due to poor accrual, did not suggest the efficacy of trastuzumab past progression. This preliminary result was consistent with the results of the WJOG7112G study, a randomized phase II study comparing trastuzumab beyond disease progression in combination with weekly paclitaxel versus weekly paclitaxel alone after the failure of trastuzumab, fluoropyrimidine, and platinum-containing chemotherapy in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer that failed to observe a survival benefit for continued anti-HER2 therapy.1 Moreover, the efficacy of trastuzumab deruxtecan in previously treated HER2-positive gastric cancer was recently reported,2 and will likely become the standard of care.
Several reasons for poor accrual can be considered. The subset of HER2-positive gastric cancer is reported to comprise approximately 6.8-34.0% of all gastric cancers.3 Thus, it would have been necessary to screen 200-600 patients to accumulate 60 cases in this study. However, our clinical trial group lacked the ability to accumulate this number of cases. In addition, when disease progression was confirmed during primary treatment, some patients were not able to receive second-line treatment. Furthermore, the administration regimen for the combination of trastuzumab (every 3 weeks) and irinotecan (every 2 weeks) may have been considered too complicated by some.
Al-Shamsi et al suggested that the continuation of trastuzumab beyond disease progression in patients with HER2-positive metastatic gastric cancer was feasible and safe in their retrospective analysis.4 These findings coincided with the absence of cardiac events and other major adverse events (AEs) in our study population.
Due to incomplete accrual, the present study has very low power to detect any clinical benefit of trastuzumab and irinotecan beyond disease progression in patients with HER2-positive metastatic or advanced gastric cancer who previously received trastuzumab due to the limited sample size.
Trial Information
| Disease | Gastric cancer |
| Stage of disease/treatment | Metastatic/advanced |
| Prior therapy | 1 prior regimen |
| Type of study | Phase II, single-arm |
| Primary endpoint | Overall response rate (ORR) |
| Secondary endpoints | Progression-free survival, safety |
| Investigator’s Analysis | The present study has a very low power to detect any clinical benefit due to the limited sample size. |
Additional Details of Endpoints or Study Design
6-month survival rates
Subgroup analysis by HER2 status (IHC 3+ group, IHC 2+/FISH-positive group).
Subgroup analysis by retesting of HER2 status (retest or no retest).
Subgroup analysis by HER2 status in the HER2-retested population (changed from positive to negative or not changed).
The Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) was used to evaluate the response rate. Progression-free survival was defined as the time from registration until the date of first documented disease progression or death of any cause. Overall survival (OS) was defined as the time from registration until the date of death of any cause. Common Terminology Criteria for Adverse Events, version 4.0, was used to evaluate AEs.
The primary endpoint was the overall response rate (ORR). The secondary endpoints were PFS, 6-month survival rates, safety, subgroup analysis by HER2 status (immunohistochemistry (IHC) 3+ group, IHC 2+/ fluorescence in situ hybridization (FISH)-positive group), subgroup analysis by retesting of HER2 status (retest or no retest), and subgroup analysis by HER2 status in the HER2-retested population (changed from positive to negative or not changed). ORR was calculated according to the RECIST, version 1.1, criteria. At the time this study was drafted, it had been reported that irinotecan monotherapy for second-line treatment resulted in a response rate of 0.0%-20.0%, and the median OS was approximately 5 months.5-8 With a null hypothesis threshold ORR of 10%, alternative hypothesis expected ORR of 20%, one-sided α of 0.10, and 80% power, a total of 60 evaluable patients were required according to the SWOG’s 2-stage design. Due to the low recruitment of this study, we modified the statistical plan as a descriptive analysis. The overall response rate (ORR) was estimated with 95% exact confidence intervals. The Kaplan-Meier method was used to determine the summary estimates of PFS and OS. In the subgroup analysis by HER2 status, the comparison between groups was examined using the log-rank test, and a 2-sided P-value of <0.05 was considered significant. These were calculated by IBM SPSS Statics, version 20.0 (IBM, Armonk, NY).
The study included patients aged 20 years or older who had histologically confirmed inoperable locally advanced, recurrent, or metastatic adenocarcinoma of the stomach with HER2 status scored as 3+ on immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH)-positive. Further inclusion criteria were: prior trastuzumab therapy and radiologically confirmed disease progression within 28 days after the last dose of first-line therapy including trastuzumab; no prior irinotecan treatment; Eastern Cooperative Oncology Group performance status 0-2, adequate organ function within 14 days of enrollment. Patients were excluded if they had cardiac effusion, pleural, and/or peritoneal effusion requiring treatment or drainage. Further exclusion criteria were: serious and/or uncontrolled complications (infection, pulmonary fibrosis, bowel paralysis, bowel obstruction, uncontrolled diabetes, hepatic cirrhosis, uncontrolled hypertension, current history of myocardial infarction, and/or unstable angina); active gastrointestinal hemorrhage.
Drug Information
| Trastuzumab | |
|---|---|
| Generic/working name | Trastuzumab |
| Drug type | Antibody |
| Drug class | Her-2/Neu |
| Dose | 8 mg/kg initial dose, followed by 6 mg/kg every 3 week mg/kg |
| Route | IV |
| Schedule of administration | Trastuzumab was administered at an initial dose of 8 mg/kg, followed by a tri-weekly maintenance infusion at a dose of 6 mg/kg. |
| Irinotecan | |
|---|---|
| Generic/working name | Irinotecan |
| Drug type | Irinotecan hydrochloride hydrate |
| Drug class | Topoisomerase I |
| Dose | 150 mg per squared meter (m2) |
| Route | IV |
| Schedule of administration | Irinotecan was administered biweekly at a dose of 150 mg/m2. Doses could be modified to manage treatment-related toxic effects. The protocol treatment was continued until disease progression was confirmed or treatment could not continue by adverse events. |
Patient Characteristics
| Number of patients, male | 9 |
| Number of patients, female | 6 |
| Age | Median (range): 65 (42-78) years |
| Number of prior systemic therapies | Median: 1 |
| Performance status: Eastern Cooperative Oncology Group (ECOG) | 0-8 1-7 2-0 3-0 Unknown-0 |
| Other | Histology: adenocarcinoma, 15 Extent of disease: metastatic, 13; recurrent, 2 The number of metastatic sites per patient: median 2 (range 1-3) Metastatic site: lymph node, 11; liver, 10; lung, 3; peritoneum, 2 Previous gastrectomy: 3 Previous adjuvant therapy 2 HER2 status: IHC 2+/FISH positive, 5; IHC 3+, 10 Prior therapy: trastuzumab with capecitabine plus cisplatin, 12; trastuzumab with S-1 plus cisplatin, 3 |
| Cancer types or histologic subtypes | Intestinal, 9; diffuse, 6 |
Primary Assessment Method
| Title | Overall response rate |
|---|---|
| Number of patients screened | 16 |
| Number of patients enrolled | 16 |
| Number of patients evaluable for toxicity | 15 |
| Number of patients evaluated for efficacy | 15 |
| Evaluation method | RECIST 1.1 |
| Response assessment CR | n = 1 (6.7%) |
| Response assessment PR | n = 0 (0%) |
| Response assessment SD | n = 7 (46.7%) |
| Response assessment PD | n = 7 (46.7%) |
| Response assessment OTHER | n = 0 (0%) |
| (Median) duration assessments PFS | 2.4 months, CI: 0.0-5.2 |
| (Median) duration assessments OS | 9.7 months, CI: 8.2-11.2 |
| (Median) duration assessments duration of treatment | 1.9 months |
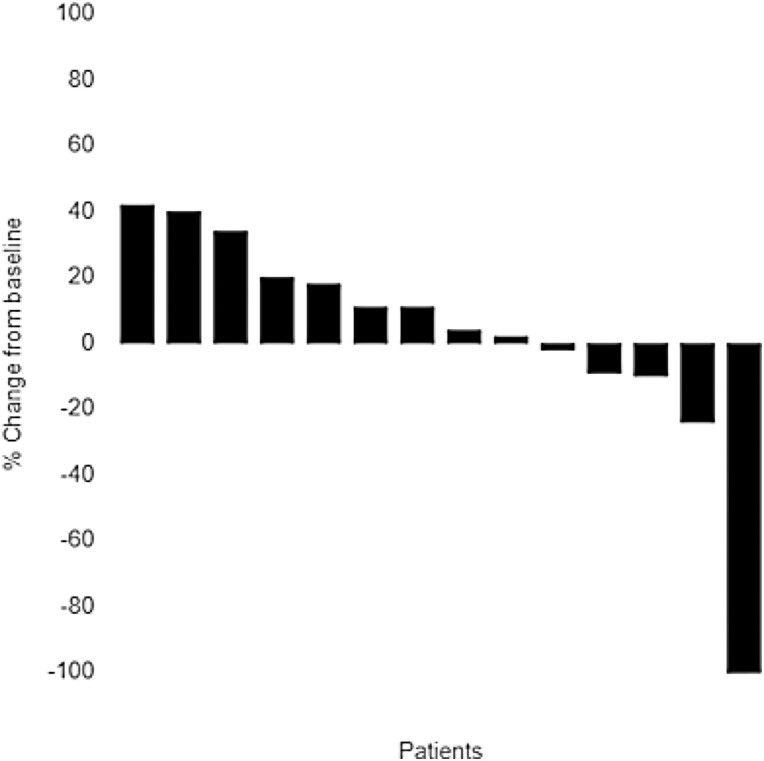
% Best response in 15 patients is plotted. Best response of patients 1, 2, 3, 4, 5, 6, and 9 was progressive disease. Patient 5, 6, and 9 had progression of non-target lesion. Best response of patients 7, 8, 10, 11, 12, 13, and 14 was stable disease, and that of patient 15 was complete response.
Outcome Notes
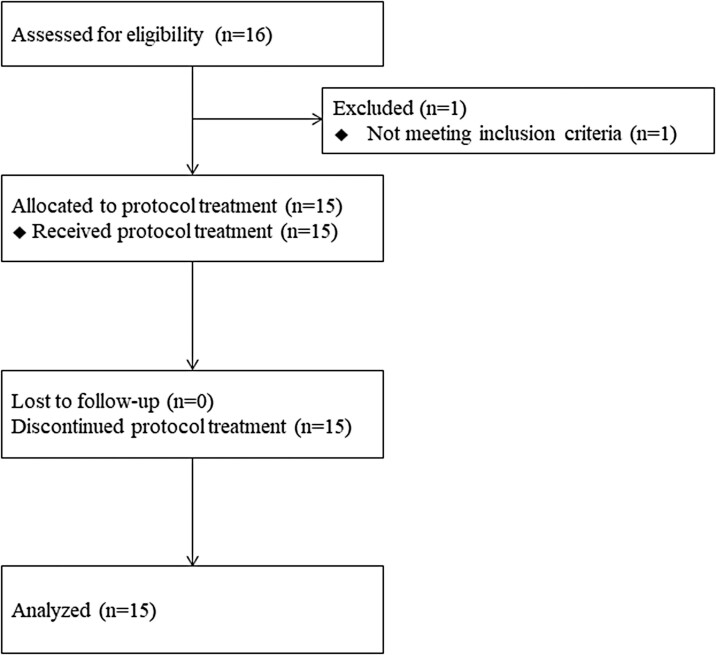
Between April 2012 and March 2015, 16 patients were registered during the planned registration period from 9 institutions. This study was prematurely closed because of poor patient accrual. Among 16 total patients, one patient was excluded because of inadequate liver function and 15 patients were analyzed (Fig. 1).
Figure 1.
Progression-free survival (PFS) and overall survival (OS) in HGCSG 1201. (A) The median PFS was 2.4 months (95% CI, 0.0-5.2), and the 6-month PFS rate was 13%. (B) The median OS was 9.7 months (95% CI, 8.2-11.2), and the 6- and 12-month OS rates were 73 and 27%, respectively.
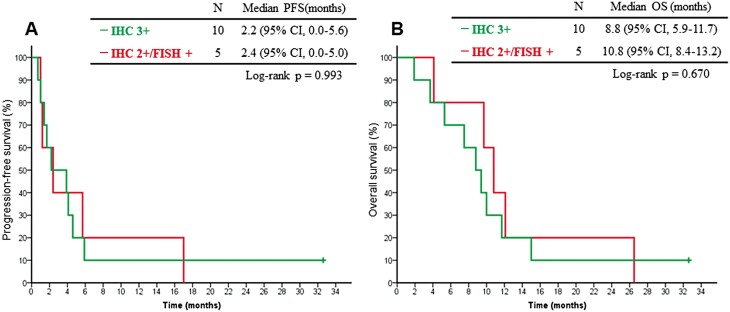
The ORR was 6.7% (95% CI, 0.2-32.0), and the disease control rate was 53% (95% CI, 26.6-78.7). The median PFS was 2.4 months (95% CI, 0.0-5.2), and the 6-month PFS rate was 13.3% (95% CI, 3.4-40.5). The median OS was 9.7 months (95% CI, 8.2-11.2), and the 6- and 12-month OS rates were 73.3% (95% CI, 46.7-89.6) and 26.7% (95% CI, 10.4-53.3), respectively (Fig. 2). These results revealed that continuous trastuzumab plus irinotecan treatment was not more effective than past irinotecan monotherapy treatment. In the subgroup analysis by HER2 status, the median PFS for the IHC 3+ and IHC 2+/FISH-positive subgroups were 2.2 months (95% CI, 0.0-5.6) and 2.4 months (95% CI, 0.0-5.0), respectively (P = .993). The median OS for these subgroups was 8.8 months (95% CI, 5.9-11.7) and 10.8 months (95% CI, 8.4-13.2), respectively (P = .670) (Fig. 3).
Figure 2.
Flow chart of HGCSG 1201. Sixteen patients were registered during the planned registration period from 9 institutions. One patient was excluded because of inadequate liver function, and 15 patients were analyzed.
Figure 3.
Kaplan-Meier curves of progression-free survival (PFS) (A) and overall survival (B) by HER2 status. (A) The median PFS for the IHC 3+ and IHC 2+/FISH-positive subgroups were 2.2 (95% CI, 0.0-5.6) and 2.4 (95% CI, 0.0-5.0) months, respectively (P = .993). (B) The median OS for these subgroups were 8.8 (95% CI, 5.9-11.7) and 10.8 (95% CI, 8.4-13.2) months, respectively (P = .670). IHC, immunocytochemistry; FISH, fluorescence in situ hybridization.
Subgroup analysis according to retesting of HER2 status could not be conducted because retesting was only performed in 4 patients. Regarding these patients, 3 patients had HER2 IHC scores of 3+ and one patient had a score of 0. The best response among patients with IHC 3+ was stable disease in 2 patients and progressive disease in one patient, and progressive disease was noted in the patient with an IHC score of 0.
Adverse Events, All Cycles
| Leukocytes (total WBC) | 20 | 7 | 53 | 20 | 0 | 0 | 80 |
| Neutrophils/granulocytes (ANC/AGC) | 33 | 7 | 20 | 33 | 7 | 0 | 67 |
| Hemoglobin | 33 | 0 | 40 | 27 | 0 | 0 | 67 |
| Platelets | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Albumin, serum-low (hypoalbuminemia) | 40 | 27 | 33 | 0 | 0 | 0 | 60 |
| Aspartate aminotransferase increased | 73 | 20 | 7 | 0 | 0 | 0 | 27 |
| Alanine aminotransferase increased | 67 | 20 | 7 | 7 | 0 | 0 | 33 |
| Alkaline phosphatase increased | 60 | 40 | 0 | 0 | 0 | 0 | 40 |
| Diarrhea | 53 | 27 | 13 | 7 | 0 | 0 | 47 |
| Nausea | 33 | 33 | 20 | 13 | 0 | 0 | 67 |
| Vomiting | 73 | 20 | 7 | 0 | 0 | 0 | 27 |
| Anorexia | 33 | 20 | 13 | 33 | 0 | 0 | 67 |
| Fatigue | 33 | 20 | 13 | 33 | 0 | 0 | 67 |
| Alopecia | 67 | 13 | 20 | 0 | 0 | 0 | 33 |
The most frequently reported grades 3-4 AEs were neutropenia (40%), anemia (27%), anorexia (33%), and fatigue (33%). The median left ventricular ejection fractions before and after treatment were 66% and 62%, respectively.
Regarding the causes of treatment discontinuation, 12 patients had disease progression, and 3 patients withdrew consent in response to AEs. Specifically, 2 patients withdrew because of grade 3 anorexia and fatigue, and 1 patient withdrew because of grade 2 anorexia and grade 1 alopecia.
Assessment, Analysis, and Discussion
| Completion | Did not fully accrue |
| Investigator’s Assessment | The present study had very low power to detect any clinical benefit due to the limited sample size |
Gastric cancer was the fourth leading cause of cancer death worldwide in 2020.9 Approximately 60% of patients with gastric cancer worldwide are diagnosed in East Asian countries, including Japan, Korea, and China.10
Based on several clinical trials investigating primary chemotherapy for unresectable advanced or recurrent gastric- and gastro-esophageal junction cancer, standard chemotherapy includes fluoropyrimidine plus platinum for human epidermal growth factor receptor 2 (HER2)-negative gastric cancer and fluoropyrimidine plus platinum in combination with trastuzumab for HER2-positive gastric cancer.
Trastuzumab is a HER2-targeted humanized monoclonal antibody. The addition of trastuzumab to chemotherapy improved survival in patients with chemotherapy-naïve advanced gastric- or gastroesophageal junction cancer compared with the efficacy of chemotherapy alone in the ToGA study.11 In that study, the median OS for the combination of trastuzumab and chemotherapy (capecitabine plus cisplatin or fluorouracil plus cisplatin) for 13.8 months in patients with HER2-positive gastric cancer, versus 11.1 months (hazard ratio [HR] 0.74 [95% CI, 0.60-0.91]; P = .0046) for chemotherapy alone.
For gastric cancer that failed to respond to initial treatment, the efficacy of paclitaxel plus ramucirumab was reported in the RAINBOW study.12 The median OS was significantly longer in the ramucirumab plus paclitaxel group than in the placebo plus paclitaxel group (9.6 months [95% CI, 8.5-10.8] versus 7.4 months [95% CI, 6.3-8.4], HR = 0.807 [95% CI, 0.678-0.962]; P = .017). Later lines of treatment have shown the efficacy of immune checkpoint inhibitors and trifluridine-tipiracil.13-14
Regarding the efficacy of irinotecan, a phase III clinical trial from the German AIO Group compared best supportive care with irinotecan.5 The study had a planned registration number of 120 patients; however, it was closed prematurely because of poor patient accrual with 40 patients registered. The primary endpoint, namely median OS, was 4.0 months for irinotecan, compared with 2.4 months for best supportive care (HR = 0.48 [95% CI, 0.25-0.92]; P = .012, one-sided log-rank test, corresponding to a 2-sided P = .023). Thus, irinotecan could be an option for second-line treatment. In addition, irinotecan was compared with paclitaxel as a second-line treatment in the WJOG 4007 study. The median OS times for the irinotecan and paclitaxel arms were 8.4 and 9.5 months, respectively, and the median PFS times were 2.3 and 3.6 months, respectively.15
When disease progression is confirmed during treatment, chemotherapy is commonly changed. However, it is unknown whether molecular targeted therapy such as trastuzumab should be continued. In a pre-clinical examination reported for breast cancer, trastuzumab continued to inhibit cell proliferation, and sudden tumor growth was observed after stopping trastuzumab administration.16-17 In addition, it has been reported that patients with metastatic breast cancer who continued treatment with trastuzumab beyond disease progression experienced more favorable clinical outcomes than those who stopped trastuzumab at progression.18 Meanwhile, retrospective analyses reported that continued trastuzumab after disease progression was not inferior to starting trastuzumab in second-line treatment; however, an unknown factor explaining the results associated with physician bias regarding stopping or continuing trastuzumab cannot be excluded.19-20
In patients with HER2-positive breast cancer that progressed during treatment with trastuzumab, von Minckwitz et al reported no significant difference in the median OS in a randomized phase III study comparing capecitabine alone with capecitabine combined with continued trastuzumab (20.4 months versus 25.5 months; P = .257).21 However, the HR for time-to-progression, the primary endpoint of the study, was 0.69% (95% CI, 0.48-0.97; P = .0338), and the response rates for the 2 groups were 27.0% and 48.1%, respectively (P = .0115).
The aforementioned results were all obtained in patients with breast cancer, and it has been unclear whether similar findings would be observed for gastric cancer. However, because HER2 is the growth driver in HER-positive gastric cancer, continued trastuzumab might suppress tumor progression after previous trastuzumab therapy. Only 8 of 132 (6%) patients treated with lapatinib plus PTX received continuous anti-HER2 treatment in the TyTAN study.22 In that study, the treatment outcomes of patients who received continuous anti-HER2 therapy were not reported. In the GATSBY study, 174 of 228 (76%) patients received continuous anti-HER2 treatment consisting of trastuzumab emtansine,23 which did not provide an overall treatment benefit. Therefore, the significance of continued anti-HER2 therapy is unknown. Our goal was to evaluate the efficacy and safety of trastuzumab plus irinotecan for patients with unresectable advanced or recurrent gastric cancer with HER2 overexpression that was refractory to initial chemotherapy including trastuzumab. However, the number of patients who could be enrolled was small, and clinical benefits could not be verified. Among the limited sample size, no clinically meaningful efficacy was found for continuation of trastuzumab in combination with irinotecan. WJOG7112G study showed no efficacy of continued trastuzumab in combination with PTX, and trastuzumab deruxtecan emerged in this population.1-2
Acknowledgments
We express our thanks to the patients and their families, as well as to the investigators, study coordinators, and nurses at each center, and to Hokkaido University Hospital Clinical Research and Medical Innovation Center (Data Center). We thank Enago (www.enago.jp) for the English language review.
Conflict of Interest
Y. Kawamoto reports personal fees from Eli Lilly Japan, Taiho, Yakult, and Merck. Y. Komatsu reports personal fees from Eli Lilly Japan, Taiho, Yakult, Bristol-Myers, Merck, Chugai, Takeda, Novartis, Pfizer, Bayer, and Daiichi-Sankyo. The other authors indicated no financial relationships.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Makiyama A, Sukawa Y, Kashiwada T, et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin Oncol. 2020;38(17):1919-1927. [DOI] [PubMed] [Google Scholar]
- 2. Shitara K, Bang YJ, Iwasa S, et al. ; DESTINY-Gastric01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419-2430. [DOI] [PubMed] [Google Scholar]
- 3. Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797-805. [DOI] [PubMed] [Google Scholar]
- 4. Al-Shamsi HO, Fahmawi Y, Dahbour I, et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience. J Gastrointest Oncol. 2016;7(4):499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer—a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47(15):2306-2314. [DOI] [PubMed] [Google Scholar]
- 6. Futatsuki K, Wakui A, Nakao I, et al. [Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. CPT-11 Gastrointestinal Cancer Study Group]. Gan To Kagaku Ryoho. 1994;21(7):1033-1038. [PubMed] [Google Scholar]
- 7. Chun JH, Kim HK, Lee JS, et al. Weekly irinotecan in patients with metastatic gastric cancer failing cisplatin-based chemotherapy. Jpn J Clin Oncol. 2004;34(1):8-13. [DOI] [PubMed] [Google Scholar]
- 8. Kanat O, Evrensel T, Manavoglu O, et al. Single-agent irinotecan as second-line treatment for advanced gastric cancer. Tumori. 2003;89(4):405-407. [DOI] [PubMed] [Google Scholar]
- 9. The Global Cancer Observatory. Available at https://gco.iarc.fr. Accessed June 11, 2021.
- 10. Ferlay J, Parkin DM, Steliarova-Foucher E.. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765-781. [DOI] [PubMed] [Google Scholar]
- 11. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. [DOI] [PubMed] [Google Scholar]
- 12. Wilke H, Muro K, Van Cutsem E, et al. ; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224-1235. [DOI] [PubMed] [Google Scholar]
- 13. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471. [DOI] [PubMed] [Google Scholar]
- 14. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437-1448. [DOI] [PubMed] [Google Scholar]
- 15. Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438-4444. [DOI] [PubMed] [Google Scholar]
- 16. Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ.. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96(10):739-749. [DOI] [PubMed] [Google Scholar]
- 17. Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ.. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17(17):2235-2249. [DOI] [PubMed] [Google Scholar]
- 18. Bullock K, Blackwell K.. Clinical efficacy of taxane-trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist. 2008;13(5):515-525. [DOI] [PubMed] [Google Scholar]
- 19. Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25(25):3853-3858. [DOI] [PubMed] [Google Scholar]
- 20. Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22(6):1063-1070. [DOI] [PubMed] [Google Scholar]
- 21. von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27(12):1999-2006. [DOI] [PubMed] [Google Scholar]
- 22. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32(19):2039-2049. [DOI] [PubMed] [Google Scholar]
- 23. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640-653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.