Abstract
Background
This study aimed to evaluate the prognostic value of hyperemic coronary sinus flow (h‐CSF) and global coronary flow reserve (g‐CFR) obtained by phase‐contrast cine‐magnetic resonance imaging in patients with acute myocardial infarction (MI).
Methods and Results
This retrospective study analyzed patients with acute MI (n=523) who underwent primary (ST‐segment–elevation MI) or urgent (non–ST‐segment–elevation MI) percutaneous coronary intervention. Absolute coronary sinus blood flow (CSF) at rest and during vasodilator stress hyperemia was quantified at 30 days (24–36 days) after the index infarct‐related lesion percutaneous coronary intervention and revascularization of functionally significant non–infarct‐related lesions. We used Cox proportional hazards regression modeling to examine the association between h‐CSF, g‐CFR, and major adverse cardiac events defined as all‐cause death, nonfatal MI, hospitalization for congestive heart failure, and stroke. Finally, 325 patients with ST‐segment–elevation MI (62.1%) and 198 patients with non–ST‐segment–elevation MI (37.9%) were studied over a median follow‐up of 2.5 years. The rest CSF, h‐CSF, and g‐CFR were 0.94 (0.68–1.26) mL/min per g, 2.05 (1.42–2.73) mL/min per g, and 2.17 (1.54–3.03), respectively. Major adverse cardiac events occurred in 62 patients, and Cox proportional hazards analysis showed that h‐CSF and g‐CFR were independent predictors of major adverse cardiac events (h‐CSF: hazard ratio [HR], 0.64; 95% CI, 0.47–0.88; P=0.005; g‐CFR: HR, 0.62; 95% CI, 0.47–0.82; P=0.001). When stratified by h‐CSF and g‐CFR, cardiac event‐free survival was the worst in patients with concordantly impaired h‐CSF (<1.6 mL/min per g) and g‐CFR (<1.7) (P<0.001).
Conclusions
Global coronary sinus flow quantification using phase‐contrast cine‐magnetic resonance imaging provided significant prognostic information independent of infarction size and conventional risk factors in patients with acute MI undergoing revascularization.
Keywords: cardiac magnetic resonance imaging, coronary flow reserve, microvascular disease, myocardial blood flow, primary percutaneous coronary intervention
Subject Categories: Magnetic Resonance Imaging (MRI), Prognosis, Percutaneous Coronary Intervention, Acute Coronary Syndromes, Coronary Circulation
Nonstandard Abbreviations and Acronyms
- CS
coronary sinus
- CSF
coronary sinus blood flow
- FFR
fractional flow reserve
- g‐CFR
global coronary flow reserve
- h‐CSF
hyperemic coronary sinus flow
- LGE
late gadolinium enhancement
- MACE
major adverse cardiac event
- MBF
myocardial blood flow
- MVO
microvascular obstruction
- PC‐CMR
phase‐contrast cine‐magnetic resonance imaging
Clinical Perspective
What Is New?
In patients with acute myocardial infarction revascularized with primary or urgent percutaneous coronary intervention and staged percutaneous coronary intervention of non–infarct‐related functionally significant lesions, global coronary flow assessments using phase‐contrast cine‐magnetic resonance imaging provided significant prognostic information independent of myocardial injury caused by the index myocardial infarction and other confounding risk factors.
The integration of hyperemic coronary sinus flow and global coronary flow reserve obtained by phase‐contrast cine‐magnetic resonance imaging provided significantly increased predictive efficacy of major adverse cardiac events in comparison with the prediction model using each one of these 2 factors.
What Are the Clinical Implications?
The stratification of patients with acute myocardial infarction based on absolute global coronary flow assessments using phase‐contrast cine‐magnetic resonance imaging might help identify high‐risk patients for future adverse events after percutaneous coronary intervention, independent of established risk factors, such as ejection fraction, late gadolinium enhancement, and microvascular obstruction, and other confounding variables.
Future intensive therapeutic management monitored by global coronary flow reserve and hyperemic coronary sinus flow may potentially provide a novel therapeutic strategy for improving prognosis in patients with acute myocardial infarction early after revascularization.
Primary or emergent revascularization and optimal medical therapy in patients with acute coronary syndrome (ACS) provide considerable improvement of outcomes. 1 Nevertheless, ACS remains associated with high rates of subsequent major adverse cardiac events (MACEs), even after successful revascularization and intensive secondary prevention. 2 Therefore, risk stratification tools that enable personalized risk assessment and help guide therapeutic decision making for identifying patients with ACS at high risk for worse outcomes after percutaneous coronary intervention (PCI) are needed.
Myocardial perfusion quantification using positron emission tomography (PET) has been demonstrated to yield important prognostic value for predicting cardiovascular events in patients with known or suspected coronary artery disease (CAD), independent of the presence or absence of obstructive atherosclerotic coronary lesions. 3 , 4 Cardiac magnetic resonance imaging (CMR) is a useful modality for cardiac assessment of pathological and functional conditions, and can be used as an alternative tool to PET with respect to myocardial blood flow (MBF) quantification. Phase‐contrast cine‐magnetic resonance imaging (PC‐CMR) allows noninvasive MBF quantification and global coronary flow reserve (g‐CFR) by quantifying coronary sinus blood flow (CSF) without need for ionizing radiation, radioactive tracers, gadolinium, or intravascular catheterization. Recently, MBF and g‐CFR by PET or CMR have been reported to be important predictors of worse outcomes in patients with known or suspected CAD. 4 , 5 , 6 , 7 , 8 g‐CFR represents the ability of the coronary microvasculature to dilate in response to vasodilator stress and is also influenced by the flow‐limiting epicardial stenosis, indicating the potential of g‐CFR to integrate epicardial functional stenosis severity, diffuse disease, and microvascular function.
However, the prognostic information obtained by PC‐CMR–derived hyperemic coronary sinus flow (h‐CSF) and g‐CFR in patients with ACS who underwent primary or emergent PCI of the infarct‐related and non–infarct‐related functionally significant lesions remains undetermined. Furthermore, the incremental capability of the integration of h‐CSF and g‐CFR remains elusive. Therefore, in this study, we tested the hypothesis that post‐revascularization h‐CSF and g‐CFR using PC‐CMR provided prognostic values in patients with ACS. We further evaluated if the integration of h‐CSF and g‐CFR demonstrated the predictive efficacy of MACEs.
METHODS
The data underlying this article are available on reasonable request to the corresponding author.
Study Population
This retrospective analysis of the institutional ACS registry enrolled all patients with ACS who were admitted in Tsuchiura Kyodo General Hospital. From this registry, we included consecutive patients aged ≥20 years, admitted between October 2013 and May 2018, who underwent post‐PCI vasodilator stress PC‐CMR, provided written informed consent, and had >1‐year follow‐up data available. A total of 1317 patients were diagnosed with ACS, and 912 patients underwent primary (ST‐segment–elevation myocardial infarction [STEMI]) or urgent PCI (non–ST‐segment–elevation myocardial infarction [NSTEMI]). We excluded those with prior coronary bypass grafting, clinical diagnosis of cardiomyopathy, ongoing dialysis, renal insufficiency with a baseline serum creatinine level >1.5 mg/dL, significant valvular disease, contraindication to CMR (eg, pacemaker, internal defibrillator or other incompatible intracorporeal foreign bodies, pregnancy, and claustrophobia), and failed PCI, leaving a final cohort of 565 patients who underwent CMR examination at 1 month from the index PCI. The patients with unidentified infarct‐related lesions were excluded from the current study. We included patients who met the electrocardiographic criteria of STEMI and underwent primary PCI within 12 hours from symptom onset, or who were admitted to the intensive care unit with a diagnosis of NSTEMI within 48 hours of the last appearance of symptoms suggestive of myocardial ischemia and/or ST‐segment change in at least 2 leads and elevated cardiac marker (cardiac troponin I) on admission, and underwent PCI with an early invasive strategy <48 hours after admission. 9 Patients with multivessel CAD, defined as the presence of additional angiographic stenosis >50% in at least one coronary artery other than the infarct‐related vessel, were eligible for inclusion. When the non–infarct‐related coronary arteries were considered significant by symptomatic or objective ischemia according to stress tests, including exercise test and fractional flow reserve (FFR) ≤0.80 or a visually assessed diameter stenosis ≥90%, and these significant non–infarct‐related artery stenoses were considered as candidates for revascularization, ad hoc procedure at the time of the index primary/urgent PCI or a planned staged procedure during the index hospitalization was performed. Unsuitable lesions for PCI, including heavily calcified lesions, diffuse lesions, and those with small subtended myocardial mass, were left untreated on the consensus of the institutional heart team. The staged procedure was performed between 3 and 9 days after the index infarct‐related lesion PCI. CMR imaging was performed after non–infarct‐related lesion revascularization in all patients in this study. Prompt optimal medical therapy was initiated in all patients after enrollment. The present study protocol was in accordance with the Declaration of Helsinki and approved by the institutional ethics committee. All patients provided written informed consent before the institutional ACS registry enrollment for future investigations.
Cardiac Catheterization
Invasive coronary angiography and revascularization of the infarct‐related artery were performed by ad hoc PCI via the routine use of drug‐eluting stents with a 6F system. Before the PCI procedure, all patients received a loading dose of 200 mg aspirin and 300 mg clopidogrel or 20 mg prasugrel. Coronary angiograms were analyzed quantitatively using QAngio XA system (Medis Medical Imaging Systems, Leiden, the Netherlands). The infarct‐related lesion was identified by the combination of electrocardiography, echocardiography, and coronary angiographic findings. The stent type and procedure strategy selected were at the operator’s discretion. After reperfusion therapy, standard dual‐antiplatelet therapy was continued according to current guidelines. Physiological measurements (FFR) were performed for all lesions showing intermediate stenosis (visual estimation between 30% and 90% diameter stenosis) in the patients with stable hemodynamics after the infarct‐related lesion PCI. All patients were instructed to strictly refrain from ingesting caffeinated beverages after admission. FFR was determined using Radi Analyzer Xpress instrument with a Certus coronary pressure wire (Abbott Vascular, St. Paul, MN). FFR was calculated as the ratio of the mean distal coronary pressure/the mean aortic pressure during stable hyperemia induced by intravenous adenosine (140 μg/kg per minute through a central vein).
CMR Image Acquisition
Images were acquired on a 1.5‐T scanner (Philips Achieva; Philips Medical Systems, Best, the Netherlands) with 32‐channel cardiac coils after 30 days (24–36 days) of PCI for the infarct‐related and non–infarct‐related significant lesions. Cardiac gating and heart rate recording were achieved using the vector‐cardiogram device. Blood pressure and heart rate were monitored throughout the protocol. Cine‐CMR was performed using a retrospectively gated steady‐state free precession sequence. Twelve short‐axis slices of the left ventricle (LV) were acquired from the apex to the base. The cine‐CMR parameters were as follows: repetition time/echo time, 4.1/1.4 ms; slice thickness, 6 mm; flip angle, 55°; field of view, 350×350 mm2; matrix size, 128×128; and number of phases per cardiac cycle, 20. LV mass and volumes were calculated according to the Simpson rule using CMR data. 10 After the acquisition of PC‐CMR images, gadolinium contrast was infused intravenously at a total dose of 0.10 mmol/kg. Fifteen minutes after this injection, late gadolinium enhancement (LGE) images were acquired in the same planes as cine images, and imaging parameters were as follows: repetition time/echo time, 3.8/1.28 ms; flip angle, 15°; field of view, 350×350 mm2; acquisition matrix, 200×175; number of phases per cardiac cycle, 20; and slice thickness, 8 mm. The infarcted myocardium was quantified on the LGE images as myocardium with a signal intensity exceeding the mean signal intensity of the remote myocardium by >5 SDs and using a semiautomatic algorithm. Microvascular obstruction (MVO) was defined as the hypoenhanced region within and included in the infarcted myocardium. LV mass was normalized to body surface area as LV mass index.
Coronary Sinus Flow and g‐CFR Measurement by CMR
The coronary sinus (CS) was identified in the atrioventricular groove using basal slices of the short‐axis stack. The plane for flow measurement by PC‐CMR was positioned perpendicular to CS at 1 to 2 cm from the ostium. 10 Velocity‐encoded images were acquired using retrospective electrocardiographic gating during 15‐second breath holds, and the imaging parameters were as follows: repetition time/echo time, 7.3/4.4 ms; flip angle, 10°; field of view, 250×250 mm2; acquisition matrix, 128×128; 20 phases per cardiac cycle; encoding, 200 cm/s; and slice thickness, 6 mm. Maximal stable hyperemia was induced by intravenous adenosine (140 μg/kg per minute through a central vein). The duration between the end of hyperemia and the resting image acquisition was 10 minutes.
The CSF quantitative analyses were performed in a blinded manner by 2 expert investigators (T.M. and Y.K.), using a proprietary software (Philips View Forum, Best, the Netherlands). The CS contour was traced on the magnified images throughout the cardiac cycle. The CSF was quantified by integrating the flow rates from each cardiac cycle and multiplying them by the mean heart rate during the acquisition period (Figure 1). The resting CSF value was corrected using rate pressure products as follows 10 , 11 : rate pressure product = systolic blood pressure (mm Hg) × heart rate; corrected CSF = (CSF/rate pressure product) ×10 000; and corrected CSF (mL/min per g) = corrected CSF/left ventricular mass (g). G‐CFR was evaluated by CSF reserve, which was calculated as CSF during maximal hyperemia divided by resting CSF.
Figure 1. Phase‐contrast cine‐magnetic resonance images of the coronary sinus flow measurement.
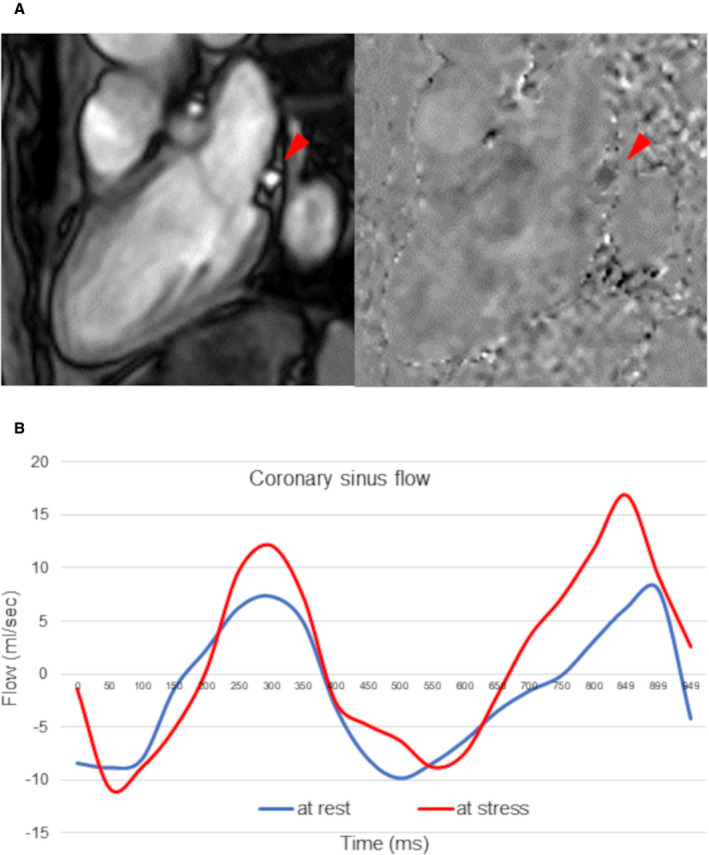
A, The proximal coronary sinus was detected in cross‐section on the magnitude and phase‐contrast images. (Red arrows show coronary sinus.) B, The coronary sinus blood flow curves (blue line, resting flow; red line, hyperemic flow) were generated.
Assessment of Outcomes
Patients were followed up for the primary outcome of MACEs: all‐cause death, nonfatal myocardial infarction (MI), hospitalization for congestive heart failure, and stroke. Clinical end points were determined by the blinded assessment of hospital records or via telephone interviews. Time to event was calculated as the period between the CMR study and the first occurrence of MACEs. Patients without MACEs were censored at the time of last follow‐up.
Statistical Analysis
The patients were divided into 2 groups, with and without MACEs. Clinical characteristics and CMR‐derived variables were compared between these 2 groups. Statistical analyses were performed using SPSS version 25.0 (IBM Corporation, Armonk, NY) and R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria) software. Categorical data were expressed as numbers and percentages and compared by the χ2 or Fisher exact tests. Continuous data were expressed as median (interquartile range) and analyzed using Mann‐Whitney U test. The ANOVA was used for variables with nonnormal and normal distributions to evaluate the difference between the groups with and without MACEs, respectively. Receiver operating characteristic curves were analyzed to assess the best cutoff values of h‐CSF and g‐CFR for predicting MACEs. The optimal cutoff value was calculated using the Youden index. Survival curves were estimated using Kaplan‐Meier estimates and were compared using log‐rank tests. A Cox proportional hazards regression model was used to identify independent predictors of MACEs. The covariates with P<0.10 in the univariate analysis were included in the multivariate analysis. A collinearity index was used for checking linear combinations among covariates, and Akaike information criterion was used for avoiding overfitting. Integration of h‐CSF and g‐GFR was achieved by creating 4 groups based on concordant or discordant impairment of these indexes. Intraobserver and interobserver variability of g‐CFR was analyzed using intraclass correlation coefficients. Reproducibility was also evaluated via Brand‐Altman analysis in the first 100 cases. A 2‐sided P<0.05 was considered statistically significant.
RESULTS
Patient Characteristics
From initially studied 565 patients, 22 were excluded because of unsatisfactory CMR image acquisition. Eight patients could not complete the CMR examination because of atrioventricular block and bradycardia resulting from adenosine infusion. Among the 535 eligible patients for the analysis, 12 (2.2%) were lost to follow‐up. Thus, the final analysis was performed on 523 patients (Figure 2), of whom, 268 (51.2%) patients showed multivessel disease (angiographic diameter stenosis >50%) (summarized clinical characteristics and CMR findings of patients are shown in Table 1, and the detailed data set is shown in Table S1). In these patients, non–infarct‐related vessel stenoses were subsequently treated with PCI at the time of the index primary PCI according to the results of physiological examination or angiographically severe diameter stenosis >90% in 21 patients. A stress test, including exercise test and FFR, was positive in 173 patients; and staged PCI for non–infarct‐related vessel lesion was performed in 140 patients of the 247 remaining multivessel disease. In 33 patients with non–infarct‐related vessel showing functionally significant stenosis, revascularization was not performed on the basis of the consensus of the institutional heart team. There was no significant difference in the prevalence of untreated lesions between the 2 groups with or without MACEs (P=0.482). Optical medical therapy and revascularization were achieved according to the guidelines. 12 Patients who experienced MACEs were older, more often had previous MI and revascularization, and were more likely to have multivessel disease, worse Killip class, and high NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels at admission. Moreover, they had a lower estimated glomerular filtration rate than those without MACEs (all P<0.05). These results were similar when 33 patients with untreated functionally significant lesions were excluded from the analysis (Table S2).
Figure 2. Study flowchart.
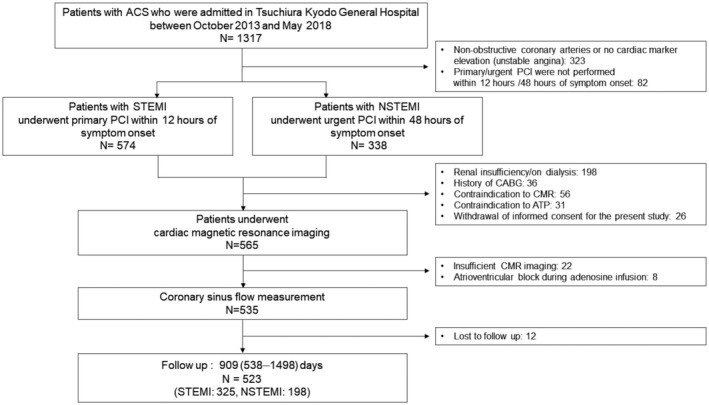
The screening and enrollment process with 523 patients in the final analysis. ACS indicates acute coronary syndrome; CMR, cardiac magnetic resonance imaging; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Table 1.
Clinical Characteristics and CMR Findings of Patients With and Without MACEs
| Variable | Total (N=523) | MACEs (+) (N=62) | MACEs (−) (N=461) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean±SD, y | 65±12 | 68±9 | 65±2 | 0.013 |
| Men, n (%) | 425 (81.3) | 49 (79.0) | 376 (81.6) | 0.630 |
| Medical history, n (%) | ||||
| History of MI | 58 (11.1) | 12 (19.4) | 46 (10.0) | 0.028 |
| Hypertension | 327 (62.5) | 41 (66.1) | 286 (62.0) | 0.532 |
| Hyperlipidemia | 253 (48.4) | 29 (46.8) | 224 (48.6) | 0.790 |
| Diabetes | 184 (35.2) | 24 (38.7) | 160 (34.7) | 0.536 |
| Current smoker | 211 (40.3) | 28 (45.2) | 183 (39.7) | 0.493 |
| Family history | 54 (10.3) | 5 (8.1) | 49 (10.6) | 0.533 |
| ACS presentation, n (%) | ||||
| STEMI/NSTEMI | 325 (62.1)/198 (37.9) | 40 (64.5)/22 (35.5) | 285 (61.8)/176 (38.2) | 0.681 |
| Killip class | <0.001 | |||
| I | 433 (82.8) | 46 (74.2) | 387 (83.9) | |
| II | 42 (8.0) | 2 (3.2) | 40 (8.7) | |
| III | 29 (5.5) | 9 (14.5) | 20 (4.3) | |
| IV | 19 (3.6) | 5 (8.1) | 14 (3.0) | |
| Coronary angiography, n (%) | ||||
| Infarct‐related lesion location: RCA/LAD/LCx | 173 (33.1)/269 (51.4)/81 (15.5) | 24 (38.7)/26 (43.6)/11 (17.7) | 149 (32.3)/242 (52.5)/70 (15.2) | 0.423 |
| TIMI flow grade at baseline | 0.133 | |||
| 0 | 228 (43.6) | 26 (41.9) | 202 (43.8) | |
| 1 | 47 (9.0) | 1 (1.6) | 46 (10.0) | |
| 2 | 115 (22.0) | 17 (27.4) | 98 (21.3) | |
| 3 | 133 (25.4) | 18 (29.0) | 115 (24.9) | |
| TIMI flow grade at final | 0.743 | |||
| 0 | 2 (0.4) | 0 (0) | 2 (0.4) | |
| 1 | 9 (1.7) | 2 (3.2) | 7 (1.5) | |
| 2 | 55 (10.5) | 6 (9.7) | 49 (10.6) | |
| 3 | 457 (87.4) | 54 (87.1) | 403 (87.4) | |
| Multivessel disease | 268 (51.2) | 41 (66.1) | 227 (49.2) | 0.012 |
| Ad hoc PCI of the non–infarct‐related artery during index procedure | 21 (4.0) | 3 (4.8) | 18 (3.9) | 0.725 |
| Staged PCI of the non–infarct‐related artery during index hospitalization | 140 (26.8) | 20 (32.3) | 120 (26.0) | 0.298 |
| Laboratory data | ||||
| LDL‐chol, mg/dL | 111 (88–135) | 104 (87–132) | 112 (88–136) | 0.179 |
| eGFR, mL/min per 1.73 m2 | 68.7 (57.7–80.9) | 64.5 (52.9–75.4) | 69.6 (58.8–81.3) | 0.021 |
| HbA1c, % | 6.0 (5.6–6.8) | 6.2 (5.6–7.0) | 6.0 (5.6–6.8) | 0.468 |
| NT‐proBNP, ng/L | 409 (134–1141) | 1016 (324–2678) | 364 (125–957) | <0.001 |
| Peak CK, IU/L | 1248 (281–2815) | 1562 (225–3225) | 1210 (289–2793) | 0.875 |
| Peak CK‐MB, IU/L | 115 (28–281) | 129 (23–369) | 111 (29–274) | 0.817 |
| hs‐CRP, mg/dL | 0.220 (0.090–0.750) | 0.390 (0.120–0.950) | 0.210 (0.090–0.712) | 0.056 |
| CMR indexes | ||||
| EDV, mL | 117.9 (97.3–140.2) | 127.7 (103.2–158.2) | 117.0 (96.3–139.4) | 0.021 |
| ESV, mL | 51.0 (38.7–73.1) | 65.9 (42.2–104.3) | 50.6 (38.5–69.6) | 0.007 |
| LVMI, g/m2 | 83.1 (69.9–95.6) | 92.6 (78.6–107.7) | 82.0 (69.3–92.9) | <0.001 |
| EF, % | 55.4 (45.7–63.2) | 49.6 (37.3–61.8) | 56.0 (47.8–63.2) | 0.012 |
| CSF at rest, mL/min per g | 0.79 (0.55–1.05) | 0.85 (0.63–1.03) | 0.76 (0.55–1.04) | 0.197 |
| Corrected CSF at rest, mL/min per g | 0.94 (0.68–1.26) | 0.94 (0.77–1.28) | 0.93 (0.67–1.24) | 0.508 |
| CSF at hyperemia, mL/min per g | 2.05 (1.42–2.73) | 1.46 (1.16–2.21) | 2.11 (1.49–2.75) | <0.001 |
| g‐CFR | 2.54 (1.82–3.70) | 1.86 (1.36–2.63) | 2.69 (1.91–3.81) | <0.001 |
| Corrected g‐CFR | 2.17 (1.54–3.03) | 1.60 (1.13–2.20) | 2.25 (1.62–3.10) | <0.001 |
| LGE volume, cm3 | 9.3 (3.4–16.0) | 12.3 (4.9–20.4) | 9.1 (2.7–15.6) | 0.005 |
| MVO presence, n (%) | 142 (27.2) | 24 (38.7) | 118 (25.6) | 0.043 |
ACS indicates acute coronary syndrome; CK, creatine kinase; CK‐MB, CK–myocardial band; CMR, cine‐magnetic resonance imaging; CSF, coronary sinus flow; EDV, end‐diastolic volume; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESV, end‐systolic volume; g‐CFR, global coronary flow reserve; HbA1c, glycated hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LDL‐chol, low‐density lipoprotein cholesterol; LGE, late gadolinium enhancement; LVMI, left ventricular mass index; MACE, major adverse cardiac event; MI, myocardial infarction; MVO, microvascular obstruction; NSTEMI, non–ST‐segment–elevation MI; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST‐segment–elevation MI; and TIMI, Thrombolysis in Myocardial Infarction.
CMR Findings
In patients with MACEs, end‐diastolic left ventricular volume, end‐systolic left ventricular volume, LV mass index, CSF at rest, LGE volume, and MVO presence were significantly higher, and h‐CSF and g‐CFR were significantly lower, than in those without MACEs (all P<0.05) (Table 1).
Primary Outcome
Of 523 patients, 62 (11.9%) experienced MACEs during the follow‐up of 2.5 (1.5–4.1) years, including 21 deaths (4.0%), 17 nonfatal MIs (3.3%), 13 heart failure admissions (2.5%), and 11 strokes (2.1%) (Table 2).
Table 2.
Primary Outcomes
| MACEs, n (%) | Total (N=523) |
|---|---|
| All‐cause death | 21 (4.0) |
| Cardiovascular death | 12 (2.3) |
| Nonfatal MI | 17 (3.3) |
| Nontarget vessel related | 11 (2.1) |
| Hospitalization attributable to HF | 13 (2.5) |
| Stroke | 11 (2.1) |
| Total | 62 (11.9) |
HF indicates heart failure; MACE, major adverse cardiac event; and MI, myocardial infarction.
Predictors of MACEs
The Cox proportional hazards regression analysis demonstrated that each of h‐CSF and g‐CFR remained significant as independent predictors of MACEs in the total cohort (h‐CSF: hazard ratio [HR], 0.64; 95% CI, 0.47–0.88; P=0.005; g‐CFR: HR, 0.62; 95% CI, 0.47–0.82; P=0.001) after adjustment for potential confounders. (The multivariable models for the coronary flow variables and MACEs are presented in Table 3, and the detailed results of the univariable and multivariable analysis are shown in Table S3.) g‐CFR was also a significant predictor in each of STEMI and NSTEMI subgroups (Tables S4 and S5). The optimal cutoff values of h‐CSF and g‐CFR obtained by receiver operating characteristic curve analyses for predicting MACEs were 1.6 (area under the curve, 0.66; sensitivity, 62.9%; specificity, 72.0%) and 1.7 (area under the curve, 0.67; sensitivity, 59.7%; specificity, 70.9%), respectively. On the basis of these thresholds, g‐CFR was impaired in 166 of 523 patients (31.7%), whereas h‐CSF was impaired in 173 of 523 patients (33.1%). Kaplan‐Meier analysis showed a significantly increased risk of MACEs in patients with impaired h‐CSF compared with those with preserved h‐CSF, and the patients with impaired g‐CFR compared with those with preserved g‐CFR (P<0.001 and P<0.001, respectively) (Figure 3). When stratified by 4 groups with concordant or discordant impairment of h‐CSF and g‐CFR, these were concordantly impaired or preserved in 100 of 523 (19.1%) and 284 of 523 patients (54.3%), respectively, and the discordance between h‐CSF and g‐CFR occurred in 139 of 523 patients (26.6%); 73 (14.0%) were with impaired h‐CSF and preserved g‐CFR, 66 (12.6%) were impaired with preserved h‐CSF and impaired g‐CFR (Figure 4A and 4B). The patients with concordantly impaired h‐CSF and g‐CFR showed significantly higher frequency of MACEs (P<0.001; Figure 4B), and the integration of h‐CSF and g‐CFR led to significant identification of high‐risk patients of MACEs (P<0.001; Figure 4C).
Table 3.
Cox Proportional‐Hazard Regression Analysis of MACEs
| Variable | Multivariable analysis 1 | Multivariable analysis 2 | Multivariable analysis 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| CSF at hyperemia, mL/min per g | 0.64 | 0.47–0.88 | 0.005 | Not selected | Not selected | ||||
| Corrected g‐CFR | Not selected | 0.62 | 0.47–0.82 | 0.001 | Not selected | ||||
| Concordantly impaired h‐CSF and g‐CFR | Not selected | Not selected | 2.80 | 1.68–4.65 | <0.001 | ||||
Adjusted for age, Killip class 3 or 4, log (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]), left ventricular mass index, and late gadolinium enhancement. CSF indicates coronary sinus flow; g‐CFR, global coronary flow reserve; h‐CSF, hyperemic CSF; HR, hazard ratio; and MACE, major adverse cardiac event.
Figure 3. Kaplan‐Meier curve for event‐free survival stratified by hyperemic coronary sinus flow (h‐CSF) (A) and global coronary flow reserve (g‐CFR) (B).
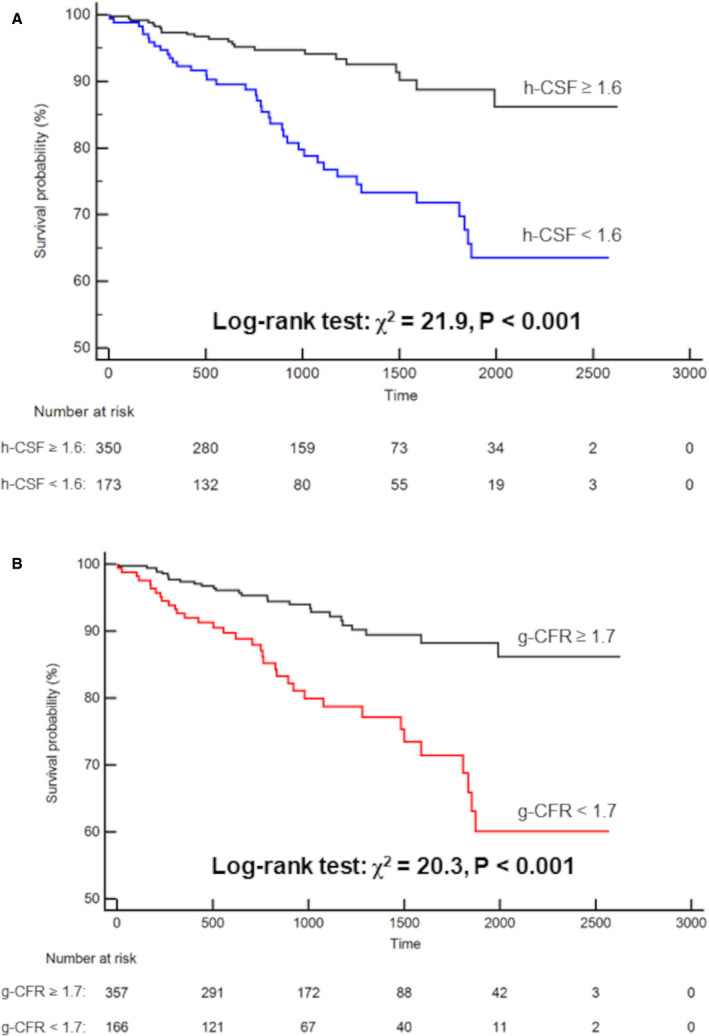
Event‐free survival was significantly worse in patients with impaired h‐CSF (<1.6) and g‐CFR (<1.7).
Figure 4. Four groups stratified by impairment of hyperemic coronary sinus flow (h‐CSF) and global coronary flow reserve (g‐CFR).
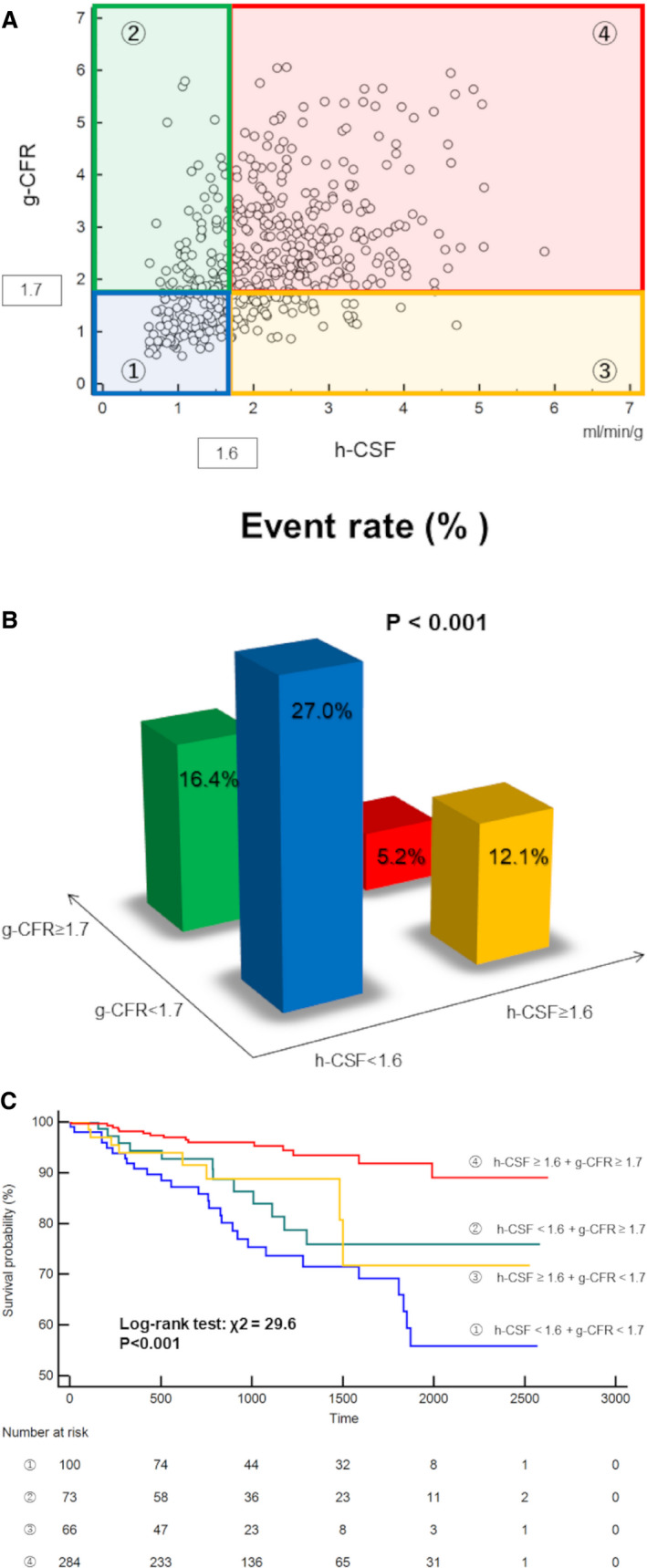
Group ① is 100 of 523 (19.1%) patients with concordantly impaired h‐CSF and g‐CFR; blue. Group ② is 73 of 523 (14.0%) patients with impaired h‐CSF and preserved g‐CFR; green. Group ③ is 66 of 523 (12.6%) patients with preserved h‐CSF and impaired g‐CFR; yellow. Group ④ is 284 of 523 (54.3%) patients with concordantly preserved h‐CSF and g‐CFR; red. A, The distribution of 523 patients with acute myocardial infarction stratified by 4 groups with concordant or discordant impairment of h‐CSF (<1.6) and g‐CFR (<1.7). B, Frequency of major adverse cardiac events (MACEs) stratified by 4 groups with concordant or discordant impairment of h‐CSF (<1.6) and g‐CFR (<1.7). The patients with concordantly impaired h‐CSF and g‐CFR showed significantly higher frequency of MACEs. C, Kaplan‐Meier curve for event‐free survival stratified by 4 groups with concordant or discordant impairment of h‐CSF (<1.6) and g‐CFR (<1.7). Event‐free survival was significantly worse in patients with concordantly impaired h‐CSF and g‐CFR.
CS Flow Reproducibility
The reproducibility of g‐CFR measurements was satisfactory for interobserver (intraclass correlation coefficient, 0.91) and intraobserver (intraclass correlation coefficient, 0.89) reproducibility analysis. Bland‐Altman analysis of interobserver reproducibility of g‐CFR for the first 100 cases showed a bias of 0.012; 95% limits of agreement were −0.294 to 0.279.
DISCUSSION
This is the first study demonstrating the prognostic significance of quantification of h‐CSF and g‐CFR using PC‐CMR specifically in patients with acute myocardial infarction (AMI) revascularized with primary or urgent PCI. The major findings of this study are as follows: (1) impaired h‐CSF and g‐CFR at 1 month after PCI of infarct‐related and non–infarct‐related significant lesions were both robust predictors of MACEs; and (2) the integration of h‐CSF and g‐CFR could provide the significantly increased predictive efficacy of MACEs.
Quantification of MBF on PET imaging has been established to provide powerful prognostic information in numerous studies. 3 , 13 These prognostic implications have been validated in patients with stable CAD. 3 , 13 , 14 , 15 The clinical utilities of absolute myocardial or coronary flow quantifications in patients with AMI are of interest and remain elusive. This study demonstrated the independent prognostic efficacy of h‐CSF and g‐CFR obtained by PC‐CMR, and the significant prognostic information of MACEs by integrating these 2 metrics in patients with AMI, including both STEMI and NSTEMI early after PCI. Although studies have shown that noninvasive assessment of global CFR with PET showed significant prognostic information in patients with stable CAD, 3 , 13 , 14 , 15 the requirement of ionizing radiation and less modality accessibility limited the widespread uptake in clinical practice. Quantification by CMR can provide both regional and global myocardial flow assessment. Knott et al showed that an artificial intelligence–based approach using perfusion CMR mapping could be an effective way to allow both regional and global myocardial flow quantification without time‐consuming postprocessing requiring manual editing. 8 Although artificial intelligence methods might be an effective way to improve the patient care and management, these new methods still need to be validated as prognostic tools. In contrast, PC‐CMR of CS has been validated as an alternative way to assess global myocardial flow and can be performed without ionizing radiation and a meticulous and complex postprocessing using a proprietary but widely accessible software. 10 , 16 , 17 We demonstrated the prognostic significance of h‐CSF and g‐CFR in patients with AMI revascularized of infarct‐related and non–infarct‐related significant lesions by a relatively simple PC‐CMR method, which might change the current clinically underused situation of MBF quantification.
Potential Mechanisms Linking g‐CFR and h‐CSF to MACEs
Recently, microvascular dysfunction has been reported to play a pivotal role in the pathophysiological mechanisms of cardiovascular disease in the setting of both obstructive epicardial and nonobstructive diseases. 18 , 19 The mechanisms of impaired myocardial perfusion linking to worse outcomes are likely multifactorial and are undetermined. In this cohort, injured myocardium, residual diffuse disease, and microvascular function can all be contributing factors of MACEs. Even without functionally significant epicardial lesions, impaired vasodilatory ability may cause ischemia. 20 , 21 Furthermore, our results indicated that h‐CSF and g‐CFR were not significantly associated with LGE volumes, ejection fraction, peak creatine kinase–myocardial band, or the presence of MVO in both overall cohort and each of STEMI and NSTEMI subgroups (Tables S4 and S5), indicating that h‐CSF and g‐CFR might be used as prognostic metrics in combination with other established prognostic factors, including LGE, MVO, ejection fraction, and the injured mass by the index MI. Recent reports also showed a significant association between microvascular dysfunction and cardiovascular morbidity and mortality, including atherosclerotic progression and heart failure. 6 , 22 , 23 Our results agree with these studies, and extend the clinical significance of quantitative global myocardial perfusion to post‐MI patients. In these patients, coexisting microvascular functional impairment of diffuse disease extending to the whole heart beyond the damage of the index AMI and infarct‐related vessel atherosclerotic burden might affect global myocardial perfusion, resulting in worse outcomes linking with impaired h‐CSF and g‐CFR. Nevertheless, this hypothesis, based on our study, is merely a speculative explanation of the mechanism linking quantitative myocardial perfusion and MACEs, and should be tested in further large prospective studies.
Prognostic Value of the Integration of g‐CFR and h‐CSF
In this study, the patients with concordantly impaired g‐CFR and h‐CSF showed the highest frequency of MACEs (27/100 [27.0%]) (Figure 4B). Johnson and Gould reported on the prognostic value of the combined assessment of hyperemic MBF and flow reserve derived from the quantitative analysis of regional PET perfusion imaging, termed as coronary flow capacity, and showed impaired coronary flow capacity defined by concordant severe impairment of hyperemic MBF and CFR was an independent predictor of all‐cause death. 24 Our results are in accordance with the concept of coronary flow capacity (Figure 4), although our results are presented by 2×2 classification not using coronary flow capacity maps but g‐CFR and h‐CSF cutoff values. In post‐MI patients after revascularization, the severity of diffuse disease and microvascular dysfunction may lead to worse outcomes. This study strongly suggests that the integration of g‐CFR and h‐CSF is useful for comprehensive understanding and classification of impaired myocardial tissue perfusion in post‐MI patients treated by revascularization.
Clinical Implications
Our findings are clinically relevant and help identify high‐risk patients of worse outcomes after revascularization of the infarct‐related and residual functionally significant non–infarct‐related epicardial lesions because CS flow quantification is feasible and easy to perform in a short period of time. Patients with impaired h‐CSF and g‐CFR may need continued close follow‐up with aggressive risk factor modification and optimal medical therapy because of the existence of diffuse and microvascular disease. In the present study, LGE and MVO, which indicate myocardial viability and fibrosis and have indicated the prognostic value of adverse outcomes in patients with AMI, were not significant predictors of MACEs on multivariable analyses. It might be explained by relatively small myocardial damage and small difference in the prevalence of MVO between the 2 groups with or without MACEs in our study compared with previous studies. In addition, the statistical analyses are underpowered because of the small number of events and study population. Meanwhile, our results suggested the clinical significance of the stratification of patients with AMI based on CSF quantification by PC‐CMR, which may help identify high‐risk patients with AMI for worse outcomes independent of myocardial injury, established risk factors, such as LGE and MVO, and other confounding variables. Future intensive therapeutic management monitored by g‐CFR and h‐CSF may potentially provide a novel management option for improving prognosis in patients with AMI early after revascularization.
Study Limitations
The results of this study should be interpreted with consideration of several limitations. First, this was a retrospective single‐center study and may have resulted in a selection bias. Furthermore, because of a small number of MACEs, unadjusted confounding factors, mixed inclusion criteria, and stroke, as included in MACEs, our results that h‐CSF and g‐CFR might be superior to ejection fraction or LGE in predicting worse outcomes should not be taken as a decisive finding. Finally, the assessment of ischemia induction by CMR perfusion imaging was not performed, although all CMR studies were conducted after revascularization of the infarct‐related and clinically indicated non–infarct‐related significant lesions. In patients with stable CAD, g‐CFR has been reported as a significant predictor of MACEs, independent of ischemia extent measured by abnormal stress perfusion, 7 and the effect of ischemia extent on g‐CFR in patients with AMI remains elusive. Further studies evaluating the relationship between the quantification of global myocardial perfusion and CMR‐derived regional perfusion data would provide further important insights of coronary physiology.
CONCLUSIONS
This study demonstrated that h‐CSF and g‐CFR, obtained by PC‐CMR, provided prognostic value for MACEs in patients with AMI who underwent primary or urgent PCI, independent of myocardial injury caused by the index MI and other confounding risk factors. Concordant impairments of h‐CSF and g‐CFR demonstrated significant incremental predictive efficacy of MACEs. Because accurate risk stratification and therapeutic strategy are essential in patients with AMI, our results extend the potential benefit of myocardial perfusion quantification from stable patients with CAD to patients with AMI treated by primary or urgent PCI and provide an additional potential insight for the risk stratification of patients with AMI.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S5
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023519
For Sources of Funding and Disclosures, see page 12.
References
- 1. Puymirat E, Simon T, Steg PG, Schiele F, Gueret P, Blanchard D, Khalife K, Goldstein P, Cattan S, Vaur L, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST‐elevation myocardial infarction. JAMA. 2012;308:998–1006. doi: 10.1001/2012.jama.11348 [DOI] [PubMed] [Google Scholar]
- 2. Hanssen M, Cottin Y, Khalife K, Hammer L, Goldstein P, Puymirat E, Mulak G, Drouet E, Pace B, Schultz E, et al. Myocardial infarction 2010: FAST‐MI 2010. Heart. 2012;98:699–705. doi: 10.1136/heartjnl-2012-301700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–2336. doi: 10.1161/CIRCULATIONAHA.117.029992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. doi: 10.1093/eurheartj/ehx721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Indorkar R, Kwong RY, Romano S, White BE, Chia RC, Trybula M, Evans K, Shenoy C, Farzaneh‐Far A. Global coronary flow reserve measured during stress cardiac magnetic resonance imaging is an independent predictor of adverse cardiovascular events. JACC Cardiovasc Imaging. 2019;12:1686–1695. doi: 10.1016/j.jcmg.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 8. Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, Aung N, Petersen SE, Cooper JA, Manisty C, Bhuva AN, et al. The prognostic significance of quantitative myocardial perfusion: an artificial intelligence‐based approach using perfusion mapping. Circulation. 2020;141:1282–1291. doi: 10.1161/CIRCULATIONAHA.119.044666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puymirat E, Taldir G, Aissaoui N, Lemesle G, Lorgis L, Cuisset T, Bourlard P, Maillier B, Ducrocq G, Ferrieres J, et al. Use of invasive strategy in non‐ST‐segment elevation myocardial infarction is a major determinant of improved long‐term survival: FAST‐MI (French Registry of Acute Coronary Syndrome). JACC Cardiovasc Interv. 2012;5:893–902. doi: 10.1016/j.jcin.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 10. Schwitter J, DeMarco T, Kneifel S, von Schulthess GK, Jörg MC, Arheden H, Rühm S, Stumpe K, Buck A, Parmley WW, et al. Magnetic resonance‐based assessment of global coronary flow and flow reserve and its relation to left ventricular functional parameters: a comparison with positron emission tomography. Circulation. 2000;101:2696–2702. doi: 10.1161/01.cir.101.23.2696 [DOI] [PubMed] [Google Scholar]
- 11. Kato S, Saito N, Nakachi T, Fukui K, Iwasawa T, Taguri M, Kosuge M, Kimura K. Stress perfusion coronary flow reserve versus cardiac magnetic resonance for known or suspected CAD. J Am Coll Cardiol. 2017;70:869–879. doi: 10.1016/j.jacc.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 12. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133:1135–1147. doi: 10.1161/CIR.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 13. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long‐term prognostic value of 13N‐ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069 [DOI] [PubMed] [Google Scholar]
- 14. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135:566–577. doi: 10.1161/CIRCULATIONAHA.116.023266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bom MJ, van Diemen PA, Driessen RS, Everaars H, Schumacher SP, Wijmenga J‐T, Raijmakers PG, van de Ven PM, Lammertsma AA, van Rossum AC, et al. Prognostic value of [15O]H2O positron emission tomography‐derived global and regional myocardial perfusion. Eur Heart J Cardiovasc Imaging. 2020;21:777–786. doi: 10.1093/ehjci/jez258 [DOI] [PubMed] [Google Scholar]
- 16. Koskenvuo JW, Hartiala JJ, Knuuti J, Sakuma H, Toikka JO, Komu M, Saraste M, Niemi P. Assessing coronary sinus blood flow in patients with coronary artery disease: a comparison of phase‐contrast MR imaging with positron emission tomography. AJR Am J Roentgenol. 2001;177:1161–1166. doi: 10.2214/ajr.177.5.1771161 [DOI] [PubMed] [Google Scholar]
- 17. Koskenvuo JW, Sakuma H, Niemi P, Toikka JO, Knuuti J, Laine H, Komu M, Kormano M, Saraste M, Hartiala JJ. Global myocardial blood flow and global flow reserve measurements by MRI and PET are comparable. J Magn Reson Imaging. 2001;13:361–366. doi: 10.1002/jmri.1051 [DOI] [PubMed] [Google Scholar]
- 18. Elgendy IY, Pepine CJ. Heart failure with preserved ejection fraction: is ischemia due to coronary microvascular dysfunction a mechanistic factor? Am J Med. 2019;132:692–697. doi: 10.1016/j.amjmed.2018.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mejia‐Renteria H, van der Hoeven N, van de Hoef TP, Heemelaar J, Ryan N, Lerman A, van Royen N, Escaned J. Targeting the dominant mechanism of coronary microvascular dysfunction with intracoronary physiology tests. Int J Cardiovasc Imaging. 2017;33:1041–1059. doi: 10.1007/s10554-017-1136-9 [DOI] [PubMed] [Google Scholar]
- 20. De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, Gould KL, Wijns W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but "normal" coronary angiography. Circulation. 2001;104:2401–2406. doi: 10.1161/hc4501.099316 [DOI] [PubMed] [Google Scholar]
- 21. Kotecha T, Martinez‐Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, Patel D, Coghlan G, Moon J, Plein S, et al. Automated pixel‐wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12:1958–1969. doi: 10.1016/j.jcmg.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889 [DOI] [PubMed] [Google Scholar]
- 23. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook‐Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of abnormal coronary reactivity on long‐term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5:430–440. doi: 10.1016/j.jcmg.2011.12.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


