Abstract
Background
The degree of hospital‐level variation in the ratio of percutaneous coronary interventions to coronary artery bypass grafting procedures (PCI:CABG) and the association of the PCI:CABG ratio with clinical outcome are unknown.
Methods and Results
In a multicenter population‐based study conducted in Ontario, Canada, we identified 44 288 patients from 19 institutions who had nonemergent diagnostic angiograms indicating severe multivessel coronary artery disease (2013–2017) and underwent a coronary revascularization procedure within 90 days. Hospitals were divided into tertiles according to their adjusted PCI:CABG ratio into low (0.70–0.85, n=17 487), medium (1.01–1.17, n=15 275), and high (1.18–1.29, n=11 526) ratio institutions. Compared with low PCI:CABG ratio hospitals, hazard ratios (HRs) for major adverse cardiac and cerebrovascular events were higher at medium (HR, 1.19; 95% CI, 1.14–1.25) and high ratio (HR, 1.21; 95% CI, 1.15–1.27) hospitals during a median 3.3 (interquartile range 2.1–4.6) years follow‐up. When interventional cardiologists performed the diagnostic angiogram, the odds of the patient receiving PCI was higher (odds ratio, 1.37; 95% CI, 1.23–1.52) than when it was performed by noninterventional cardiologists, after accounting for patient characteristics. Having the diagnostic angiogram at an institution without cardiac surgical capabilities was independently associated with a higher risk of major adverse cardiac and cerebrovascular events (HR, 1.07; 95% CI, 1.02–1.11), death (HR, 1.09; 95% CI, 1.02–1.18), and myocardial infarction (HR, 1.10; 95% CI, 1.03–1.17).
Conclusions
Patients undergoing diagnostic angiography in hospitals with higher PCI:CABG ratio had higher rates of adverse outcomes, including major adverse cardiac and cerebrovascular events, myocardial infarction, and repeat revascularization. Presence of on‐site cardiac surgery was associated with better survival and lower major adverse cardiac and cerebrovascular events.
Keywords: coronary artery bypass surgery, coronary artery revascularization, percutaneous coronary intervention
Subject Categories: Cardiovascular Surgery, Health Services, Percutaneous Coronary Intervention, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- LM
left main
- MACCE
major adverse cardiac and cerebrovascular events
Clinical Perspective
What Is New?
In a population‐based study, we found a 2‐fold variation in adjusted percutaneous coronary interventions to coronary artery bypass grafting procedures ratio among institutions performing nonemergent coronary angiography for patients with severe multivessel coronary artery disease, with a high rate of ad hoc percutaneous coronary interventions (70%).
Patients undergoing diagnostic angiography in hospitals with higher percutaneous coronary interventions to coronary artery bypass grafting procedures ratio had higher rates of adverse outcomes, including major adverse cardiac and cerebrovascular events, myocardial infarction, and repeat revascularization, whereas the presence of on‐site cardiac surgery was associated with better survival and fewer major adverse cardiac and cerebrovascular events.
What Are the Clinical Implications?
Physicians should be cognizant that their treatment recommendations may be influenced by nonpatient‐related factors and institutional practices.
Surgical and percutaneous procedures are safe and effective methods of revascularization for patients with multivessel coronary artery disease (CAD). Since the 1990s, several studies have demonstrated an increase in percutaneous coronary interventions (PCI) and a decrease in coronary artery bypass grafting (CABG). 1 , 2 Annual volumes of both procedures have declined in recent years, possibly because of advances in medical therapy, data questioning the benefit of PCI in stable CAD, and increasing use and scrutiny of appropriateness criteria. 3 , 4 , 5 Furthermore, results of the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) and FREEDOM (Future Revascularization Evaluation in Patients with Diabetes mellitus: Optimal Management of Multivessel Disease) trials indicated that CABG may offer better outcomes compared with PCI in patients with multivessel CAD, particularly in patients with diabetes or complex CAD. 6 , 7 Current guidelines suggest that both strategies are options for stable multivessel CAD, unstable angina, or non–ST‐segment–elevation myocardial infarction (MI), with CABG receiving a stronger recommendation than PCI across most clinical scenarios. 8 , 9
The relative distribution of PCI and CABG differs markedly across regions, and the magnitude and reasons for these variations are not well understood. 10 , 11 Although patient characteristics (diabetes, comorbidities, life expectancy) are known to influence the choice of revascularization procedure, the impact of physician and institutional factors is poorly understood. 12 To minimize the influence of nonclinical factors in the choice of revascularization strategy, multispecialty guidelines recommend a heart team discussion for patients with multivessel CAD. 9 , 13
The objectives of this population‐based study were to determine (1) the presence and magnitude of variations in the rates of PCI and CABG across institutions in contemporary practice; (2) whether nonclinical factors influence the choice of revascularization; and (3) whether these nonclinical factors are associated with patient outcomes following revascularization.
Methods
Setting
The data set from this study is held securely in coded form at Institute for Clinical Evaluative Sciences (ICES). Although legal data sharing agreements between ICES and data providers (eg, health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). We performed a multicenter, population‐based retrospective analysis of all residents of Ontario, Canada, who were found to have severe multivessel CAD on coronary angiography from April 1, 2013 to December 31, 2017, and subsequently underwent revascularization with PCI or CABG within 90 days after the index angiogram. Clinical registries were linked to population‐based health databases using patient‐level encrypted health card numbers at ICES. ICES is an independent, nonprofit research institute whose legal status allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. These data sets were linked using unique encoded identifiers and analyzed at ICES.
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Data Sources and Definitions
The CorHealth Registry captures baseline demographics, coronary anatomy, and procedural details on all Ontario residents who undergo coronary angiography, PCI, or CABG. A significant coronary lesion was defined as ≥70% obstruction in a coronary artery, with the exception of the left main (LM) where ≥50% was considered significant. Severe multivessel CAD was defined as one of the following: 2‐vessel disease with left anterior descending (LAD) artery involvement AND either circumflex (or branches) OR right coronary artery (or branches) disease; 3‐vessel disease with LAD involvement AND circumflex AND right coronary artery disease; or LM disease. We excluded patients undergoing index angiography in the presence of acute ST‐segment–elevation MI, cardiogenic shock, during an emergency procedure and those with prior PCI or sternotomy. Patients with less severe CAD were also excluded, specifically those with single‐vessel disease or 2‐vessel disease without LAD involvement.
Baseline demographics were obtained from the CIHI‐DAD (Canadian Institute for Health Information Discharge Abstract Database) and the CorHealth Registry. Statistics Canada’s census data were used to determine sociodemographic information based on median neighborhood income of individuals, to serve as a proxy for socioeconomic status. Presence of diabetes was determined using the Ontario Diabetes Database, a registry of all patients with the condition in the province. Physician characteristics were determined from the ICES Physicians Database.
Study Design
Nineteen Ontario hospitals performed diagnostic coronary angiography during the study period, with 2/19 (10.5%) not performing PCI or CABG, 6/19 (31.6%) performing only PCI, and 11/19 (57.9%) performing both PCI and CABG. Because the crude PCI:CABG ratio does not account for variation in case‐mix, we determined a risk‐adjusted ratio for each institution to indicate the likelihood of receiving PCI over CABG. The 19 institutions were divided into tertiles according to their risk‐adjusted PCI/CABG ratio: low, medium, or high. Baseline demographics, coronary anatomy, and procedural details were compared among the groups.
Patient characteristics were analyzed to determine which factors were associated with revascularization by PCI rather than CABG. Nonpatient‐related factors (the PCI:CABG ratio group of the institution and hospital CABG capabilities) were evaluated to determine their association with long‐term outcomes following revascularization after adjusting for all baseline characteristics.
Study End Points
The primary outcome of the study was major adverse cardiac and cerebrovascular events (MACCE), a composite of death, MI, stroke, and repeat revascularization from the index revascularization procedure. Secondary end points included MACCE‐1 (death, MI, and stroke), and the individual components of the primary outcome. Deaths were determined using the Registered Persons Database. Rates of MI, stroke, and repeat revascularization were identified using previously validated algorithms through the CIHI‐DAD. 14 Staged PCIs (identified by the operator physician as such in the CorHealth registry or by performance of repeat PCI of a different target‐vessel within 90 days from the index PCI) were not considered to be repeat revascularization procedures. Ad hoc PCI was defined as PCI performed in the same setting as the index diagnostic angiogram, without any previous angiogram in the 6 months before the procedure. The rate of ad hoc PCI was calculated using the total counts of PCIs in the denominator. Primary and secondary outcomes were defined a priori.
Statistical Analysis
A logistic regression model was fit to calculate the risk‐adjusted (PCI:CABG ratio) score of each institution and categorize them into tertiles. The model estimated the probability of a patient receiving PCI over CABG in the overall study population, accounting for all baseline characteristics in Table 1, including 19 variables from the following patient characteristics: demographic, socioeconomic status, cardiac risk factors, comorbidities, Canadian Cardiovascular Society angina classification (Table S1), estimated glomerular filtration rate, and coronary anatomy. The predicted probability of receiving PCI over CABG was calculated for each individual based on the coefficients of the logistic regression model. The probabilities of patients within the same institution were summed and divided by the number of patients in the institution to obtain the expected PCI rate. The observed PCI rate was also determined and each institution’s observed/expected PCI ratio was calculated, producing the hospital’s “PCI:CABG ratio.” If the score was >1, the institution was performing more PCI than expected based on clinical characteristics. Baseline demographics, coronary anatomy, and procedural details were compared among PCI:CABG ratio tertile groups using the Kruskal‐Wallis for continuous variables and χ2 test for categorical variables.
Table 1.
Baseline Characteristics, Coronary Anatomy, and Procedural Details of Patients With Multivessel Coronary Artery Disease Undergoing Nonemergent Angiography
| Variable | Hospital PCI/CABG ratio | |||
|---|---|---|---|---|
|
Low (0.70–0.85) n=17 487 |
Medium (1.01–1.17) n=15 275 n=15 275 |
High (1.18–1.29) n=11 526 |
P value | |
| Age, y, mean±SD | 67.2±10.6 | 66.8±11.0 | 66.9±11.3 | <0.01 |
| Male sex, n (%) | 13 331 (76.2%) | 11 588 (75.9%) | 8801 (76.4%) | 0.60 |
| Income quintile, n (%) | <0.01 | |||
| 1, lowest | 3458 (19.8%) | 3045 (19.9%) | 2702 (23.4%) | |
| 2 | 3517 (20.1%) | 3150 (20.6%) | 2643 (22.9%) | |
| 3 | 3584 (20.5%) | 3088 (20.2%) | 2597 (22.5%) | |
| 4 | 3609 (20.6%) | 3117 (20.4%) | 1692 (14.7%) | |
| 5, highest | 3283 (18.8%) | 2852 (18.7%) | 1858 (16.1%) | |
| Missing | 36 (0.2%) | 23 (0.2%) | 34 (0.3%) | |
| Rural, n (%) | 3351 (19.2%) | 2592 (17.0%) | 316 (2.7%) | <0.01 |
| Charlson index, mean±SD | 2.0±1.9 | 1.9±1.7 | 2.0±1.7 | 0.01 |
| Hypertension, n (%) | 12 375 (70.8%) | 10 623 (69.5%) | 8422 (73.1%) | <0.01 |
| Diabetes, n (%) | 5986 (34.2%) | 5469 (35.8%) | 4508 (39.1%) | <0.01 |
| Smoking status, n (%) | ||||
| Current | 3528 (20.2%) | 3675 (24.1%) | 1733 (15.0%) | <0.01 |
| Former | 6408 (36.6%) | 3723 (24.4%) | 2953 (25.6%) | |
| Never | 6399 (36.6%) | 7150 (46.8%) | 6210 (53.9%) | |
| Canadian Cardiovascular Society class, n (%)* | <0.01 | |||
| 0 | 1307 (7.5%) | 1823 (11.9%) | 1389 (12.1%) | |
| 1 | 1165 (6.7%) | 1199 (7.8%) | 1280 (11.1%) | |
| 2 | 2857 (16.3%) | 2863 (18.7%) | 2835 (24.6%) | |
| 3 | 2093 (12.0%) | 2033 (13.3%) | 1389 (12.1%) | |
| 4 | 473 (2.7%) | 554 (3.6%) | 312 (2.7%) | |
| ACS low risk | 3051 (17.4%) | 2224 (14.6%) | 737 (6.4%) | |
| ACS intermediate risk | 4676 (26.7%) | 2916 (19.1%) | 2865 (24.9%) | |
| ACS high risk | 1528 (8.7%) | 1599 (10.5%) | 690 (6.0%) | |
| Unknown | 337 (1.9%) | 64 (0.4%) | 29 (0.3%) | |
| Congestive heart failure, n (%) | 1224 (7.0%) | 1141 (7.5%) | 770 (6.7%) | 0.04 |
| Previous myocardial infarction, n (%) | 4126 (23.6%) | 2800 (18.3%) | 2513 (21.8%) | <0.01 |
| Peripheral vascular disease, n (%) | 1287 (7.4%) | 1103 (7.2%) | 695 (6.0%) | <0.01 |
| Cerebrovascular disease, n (%) | 1376 (7.9%) | 982 (6.4%) | 743 (6.4%) | <0.01 |
| Chronic obstructive pulmonary disease, n (%) | 1181 (6.8%) | 1060 (6.9%) | 462 (4.0%) | <0.01 |
| Creatinine, mean±SD | 97.4±74.6 | 100.1±95.9 | 103.3±94.1 | <0.01 |
| Dialysis, n (%) | 287 (1.6%) | 242 (1.6%) | 296 (2.6%) | <0.01 |
| Estimated glomerular filtration rate, n (%) | <0.01 | |||
| <30 | 526 (3.0%) | 507 (3.3%) | 482 (4.2%) | |
| 30–59 | 2981 (17.0%) | 2294 (15.0%) | 1846 (16.0%) | |
| 60–89 | 7861 (45.0%) | 6206 (40.6%) | 5137 (44.6%) | |
| ≥90 | 5220 (29.9%) | 4433 (29.0%) | 3596 (31.2%) | |
| Missing | 899 (5.1%) | 1835 (12.0%) | 465 (4.0%) | |
| Left main disease, n (%) | 4075 (23.3%) | 3295 (21.6%) | 2023 (17.6%) | <0.001 |
| 3‐VD with proximal LAD, n (%) | 3036 (17.4%) | 2168 (14.2%) | 2147 (18.6%) | <0.01 |
| 3‐VD without proximal LAD, n (%) | 3440 (19.7%) | 3439 (22.5%) | 2418 (21.0%) | <0.01 |
| 2‐VD with LAD, n (%) | 6936 (39.7%) | 6373 (41.7%) | 4938 (42.8%) | <0.01 |
| PCI, n (%) | 6774 (38.7%) | 8055 (52.7%) | 6989 (60.6%) | <0.01 |
| Physician performing index angiogram, n (%) | <0.01 | |||
| Diagnostic cardiologist | 2430 (13.9%) | 2558 (16.7%) | 3338 (29.0%) | |
| Interventional cardiologist | 15 057 (86.1%) | 12 717 (83.3%) | 8188 (71.0%) | |
| Hospital type, n (%) | <0.01 | |||
| Community | 8969 (51.3%) | 8010 (52.4%) | 5169 (44.8%) | |
| Teaching | 8518 (48.7%) | 7265 (47.6%) | 6357 (55.2%) | |
| Hospital capability, n (%) | <0.01 | |||
| Angiography only | 0 (0.0%) | 0 (0.0%) | 1647 (14.3%) | |
| Angiography and PCI only | 2620 (15.0%) | 4677 (30.6%) | 3522 (30.6%) | |
| Angiography, PCI and CABG | 14 867 (85.0%) | 10 598 (69.4%) | 6357 (55.2%) | |
| Primary reason for referral, n (%) | <0.01 | |||
| E: elective, stable coronary Disease | 4180 (23.9%) | 4103 (26.9%) | 4855 (42.1%) | |
| N: non–ST‐segment–elevation myocardial infarction | 6998 (40.0%) | 5633 (36.9%) | 3274 (28.4%) | |
| R: rule out coronary artery disease | 2091 (12.0%) | 2241 (14.7%) | 1152 (10.0%) | |
| U: unstable angina | 3362 (19.2%) | 2517 (16.5%) | 1907 (16.5%) | |
| O: other | 856 (4.9%) | 781 (5.1%) | 338 (2.9%) | |
ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; LAD, left anterior descending; PCI, percutaneous coronary intervention; and VD, vessel disease.
A second logistic regression model was fit to determine patient, physician, and hospital characteristics associated with revascularization by PCI rather than CABG. A 3‐level hierarchical model was fit that included hospital‐specific and physician‐specific random effects, with the procedural physician nested within the procedural hospital. We examined whether the index diagnostic coronary angiogram was performed by an interventional cardiologist, who is able to perform PCI procedures, or by a catheterization cardiologist, who performs diagnostic angiography but not coronary interventions. Hospital characteristics included teaching status and capability to perform PCIs and/or CABG on site in addition to diagnostic coronary angiography. Odds ratios (OR) and 95% CIs were calculated for each risk factor.
Time‐to‐event analyses were performed using Cox proportional hazards models to calculate predictors of long‐term primary and secondary outcomes. Frailty (or random effects) terms were included for each hospital. Hazard ratios (HR) were determined up to 5 years after the revascularization procedure. Proportional hazards were assessed for each fitted model.
We conducted several sensitivity analyses. First, we conducted sensitivity analyses by examining patient subgroups: (1) excluding all patients presenting with a non–ST‐segment–elevation MI using the CIHI‐DAD, and (2) including those who had diabetes. Second, we conducted institution‐ and provider‐based sensitivity analyses by adjusting for (3) annualized volume of total revascularization procedures, (4) physician age, or (5) number of years since medical school graduation. For these sensitivity analyses, the PCI:CABG ratios were recalculated, hospitals were divided into tertiles, and baseline demographics, coronary anatomy, procedural details, and long‐term outcomes were compared. All tests were 2 sided and P values <0.05 were considered significant. All analyses were conducted with SAS (version 9.4; SAS Institute, Inc, Cary, NC).
Results
Population Characteristics
A total of 44 288 patients underwent nonemergent diagnostic coronary angiograms demonstrating severe multivessel CAD and had a coronary revascularization procedure performed within 90 days (Figure 1). This population was used to create a risk‐adjusted PCI/CABG ratio (observed to expected PCI:CABG) for each participating hospital. The adjusted hospital‐specific PCI:CABG ratios ranged from 0.70 (fewer PCIs than expected compared with CABG) to 1.29 (more PCIs than expected compared with CABG). Hospitals were divided into tertiles according to their PCI:CABG ratio: 7 low (0.70–0.85), 6 medium (1.01–1.17), and 6 high (1.18–1.29) PCI:CABG ratio institutions (Figure 2). Two (33%) of the institutions in the high PCI:CABG ratio group performed only diagnostic angiograms. Box and whisker plots of cardiologist‐specific PCI:CABG ratios indicated that, overall, the distribution of physician‐specific PCI:CABG ratios tracked with the subdivided institutional categories (Figure S1). The median (25th, 75th percentile) crude revascularization rates of eligible study patients across the 3 categories were 76.6% (68.7%, 77.7%) for low, 79.9% (78.6%, 81.8%) for medium, and 79.6% (76.9%, 84.4%) for high PCI:CABG ratio groups.
Figure 1. Cohort flow diagram.
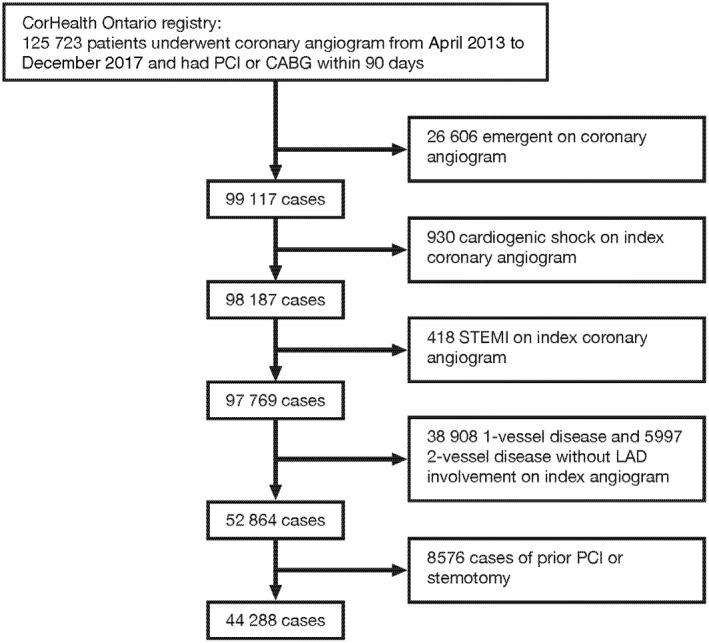
CABG indicates coronary artery bypass graft; LAD, left anterior descending; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation myocardial infarction.
Figure 2. Variation in PCI:CABG ratio by institution.
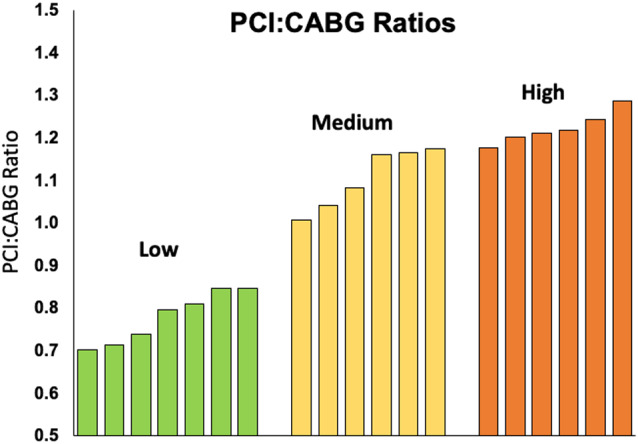
CABG indicates coronary artery bypass grafting; and PCI, percutaneous coronary intervention.
Table 1 presents the baseline characteristics, coronary anatomy, and procedural details from the 3 PCI:CABG ratio groups. High PCI:CABG ratio hospitals had more patients with diabetes (P<0.01) and fewer acute coronary syndrome (non–ST‐segment–elevation MI or unstable angina) cases (P<0.01). High PCI/CABG ratio hospitals had a lower incidence of LM disease (high: 17.6%, medium: 21.6, low: 23.3, P<0.01) but more patients with 3‐vessel disease with proximal LAD involvement (high: 18.6%, low: 17.4, medium: 14.2 P<0.01). Nearly half of the patients from the high PCI:CABG ratio group (44.8%) had their diagnostic angiogram in institutions without cardiac surgery capabilities. In contrast, fewer patients from the medium (30.6%) and low (15%) PCI:CABG ratio groups had their diagnostic angiograms in hospitals without CABG capabilities (P<0.01).
PCI was the procedure of choice for 38.7%, 52.7%, and 60.6% of cases in the low, medium, or high PCI:CABG groups, respectively (P<0.01). Figure 3a depicts the frequency of PCI as the revascularization method of choice for each group, stratified by coronary anatomy. Hospitals from the high PCI:CABG group performed more PCIs than medium and low ratio institutions among all anatomical subgroups (LM, 3‐vessel disease with or without proximal LAD involvement, or 2‐vessel disease with LAD involvement). The proportion of patients with diabetes in low, medium, and high PCI:CABG hospitals is shown in Figure 3b, stratified by anatomy. There was a higher rate of diabetes prevalence in hospitals with higher PCI:CABG ratios overall.
Figure 3. Diabetes and revascularization of choice by coronary anatomy.
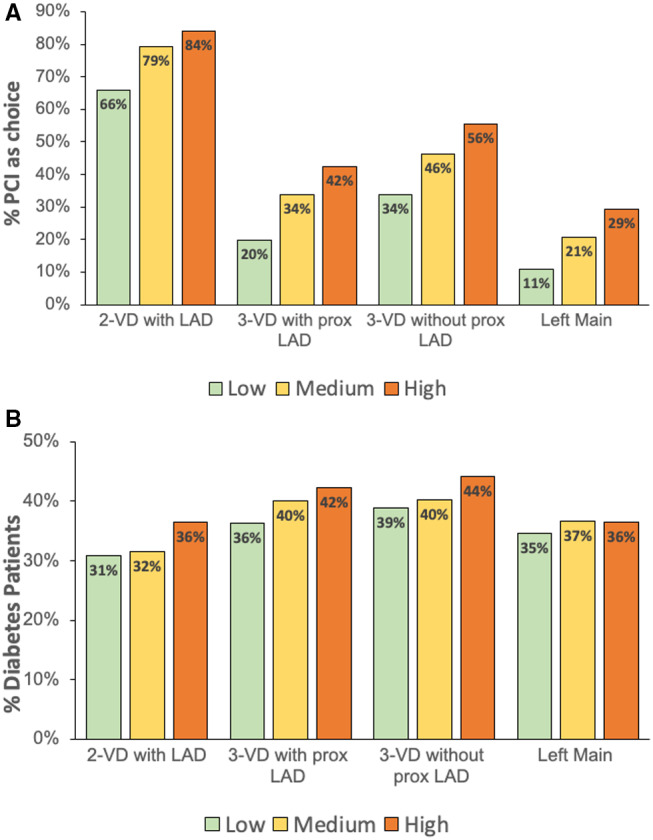
A, Frequency of PCI as the revascularization of choice, among all revascularized patients for each category of coronary anatomy. B, Proportion of patients with diabetes for each category of coronary anatomy, among all PCI procedures. LAD indicates left anterior descending; PCI, percutaneous coronary intervention; and VD, vessel disease.
Ad hoc PCI was common in all groups but occurred slightly more often in the low PCI:CABG group (75.2%), compared with the medium (72.2%) and high (70.0%) PCI:CABG groups (P<0.01). The 2 institutions that only performed diagnostic angiograms and did not offer any type of revascularization including ad hoc PCI were in the high PCI:CABG group, and their PCIs were not included in the ad hoc rate calculation. The rate of ad hoc PCI was similar for centers performing PCI with or without CABG capabilities (72.2% versus 73.4%, P=0.11). The highest rate of ad hoc PCI occurred in patients with 2‐vessel disease with LAD involvement (77.7%), whereas the lowest rate of ad hoc PCI occurred in patients with LM disease (53.8%) (Figure 4).
Figure 4. Frequency of ad hoc PCI for each category of coronary anatomy.
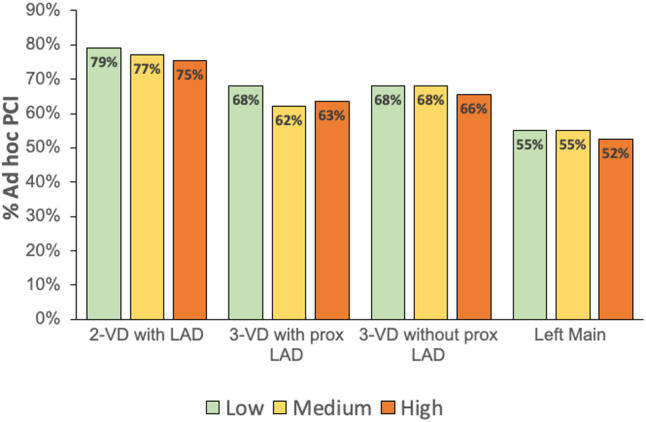
LAD indicates left anterior descending; PCI, percutaneous coronary intervention; and VD, vessel disease.
Predictors of Receiving PCI Preferentially Over CABG
Table 2 presents the patient, physician, and hospital factors that were independently associated with undergoing PCI rather than CABG, based on a 3‐level hierarchical model. Older patients (OR, 1.23 per 10 years), those with more comorbidities, and those with 2‐vessel disease (OR, 8.74) or 3‐vessel disease without proximal LAD involvement (OR, 1.97) were more likely to undergo PCI rather than CABG. In contrast, male patients (OR, 0.69), those with diabetes (OR, 0.68), and those with LM disease (OR, 0.47) were more likely to undergo CABG. Compared with angiograms performed by a noninterventional cardiologist, if the index cardiac catheterization procedure was performed by an interventional cardiologist, the odds of receiving PCI were 37% higher (OR, 1.37; 95% CI, 1.23–1.52). After adjusting for patient and physician characteristics, the institutional capabilities (angiography only, angiography and PCI, or on‐site cardiac surgery) were not independent predictors of receiving PCI or CABG.
Table 2.
Patient, Physician, and Hospital Factors Associated With Receiving Percutaneous Coronary Intervention Rather Than Coronary Artery Bypass Graft
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Canadian Cardiovascular Society class | ||
| 0 | Referent | |
| 1 | 0.99 (0.89–1.10) | 0.82 |
| 2 | 0.98 (0.89–1.07) | 0.63 |
| 3 | 0.97 (0.88–1.07) | 0.55 |
| 4 | 1.51 (1.29–1.75) | <0.01 |
| ACS low risk | 1.47 (1.33–1.63) | <0.01 |
| ACS intermediate risk | 1.65 (1.51–1.80) | <0.01 |
| ACS high risk | 2.22 (1.99–2.48) | <0.01 |
| Age, per 10 years | 1.23 (1.20–1.26) | <0.01 |
| Male sex | 0.69 (0.66–0.73) | <0.01 |
| Congestive heart failure history | 1.34 (1.22–1.47) | <0.01 |
| Smoking status | ||
| Nonsmoker | Referent | |
| Current | 1.03 (0.97–1.10) | 0.33 |
| Former | 0.94 (0.89–0.99) | 0.03 |
| Chronic obstructive pulmonary disease | 1.26 (1.15–1.40) | <0.01 |
| Cerebrovascular disease | 1.11 (1.02–1.22) | 0.02 |
| Diabetes | 0.68 (0.65–0.72) | <0.01 |
| Hypertension | 0.88 (0.83–0.93) | <0.01 |
| Estimated glomerular filtration rate | ||
| <30 | 1.32 (1.15–1.51) | <0.01 |
| 30–59 | 1.18 (1.10–1.28) | <0.01 |
| 60–89 | 0.94 (0.89–0.99) | 0.03 |
| ≥90 | Referent | |
| Previous myocardial infarction | 1.22 (1.15–1.29) | <0.01 |
| Coronary anatomy | ||
| Left main disease | 0.47 (0.43–0.50) | <0.01 |
| 3‐VD with proximal LAD | Referent | |
| 3‐VD without proximal LAD | 1.97 (1.83–2.11) | <0.01 |
| 2‐VD with LAD | 8.74 (8.18–9.34) | <0.01 |
| Physician performing index angiogram | ||
| Diagnostic cardiologist | Referent | |
| Interventional cardiologist | 1.37 (1.23–1.52) | <0.01 |
| Hospital capabilities | ||
| Angiography only | 2.31 (0.96–5.56) | 0.08 |
| Angiography and PCI | 1.16 (0.66–2.03) | 0.62 |
| Angiography, PCI, and coronary artery bypass graft | Referent | |
ACS indicates acute coronary syndrome; LAD, left anterior descending; PCI, percutaneous coronary intervention; and VD, vessel disease.
Predictors of Long‐Term Outcomes
Physician and hospital factors independently associated with our primary and secondary outcomes of interest, adjusting for all patients’ baseline characteristics, are presented in Table 3. A total of 144 145 person‐years of follow‐up were examined. The median follow‐up was 3.3 (interquartile range 2.1–4.6) years and the date of last follow‐up was December 2019. The physician subspecialty (interventional versus noninterventional cardiologist) performing the index angiogram was not associated with MACCE, MACCE‐1, death, MI, stroke, or repeat revascularization.
Table 3.
Nonpatient‐Related (Institutional) Predictors of Long‐Term Adverse Outcomes
| Outcome | Risk factor | HR (95% CI) | P value |
|---|---|---|---|
| MACCE | Low PCI:CABG ratio | Referent | |
| Medium | 1.19 (1.14–1.25) | <0.01 | |
| High | 1.21 (1.15–1.27) | <0.01 | |
| With CABG capabilities | Referent | ||
| Without CABG | 1.07 (1.02–1.11) | <0.01 | |
| MACCE‐1 | Low PCI:CABG ratio | Referent | |
| Medium | 1.13 (1.07–1.19) | <0.01 | |
| High | 1.03 (0.97–1.09) | 0.30 | |
| With CABG capabilities | Referent | ||
| Without CABG | 1.07 (1.02–1.13) | <0.01 | |
| Death | Low PCI:CABG ratio | Referent | |
| Medium | 1.05 (0.97–1.13) | 0.24 | |
| High | 0.99 (0.91–1.07) | 0.76 | |
| With CABG capabilities | Referent | ||
| Without CABG | 1.09 (1.02–1.18) | 0.02 | |
| Myocardial infarction | Low PCI:CABG ratio | Referent | |
| Medium | 1.25 (1.17–1.34) | <0.01 | |
| High | 1.11 (1.03–1.20) | <0.01 | |
| With CABG capabilities | Referent | ||
| Without CABG | 1.10 (1.03–1.17) | <0.01 | |
| Repeat revascularization | Low PCI:CABG ratio | Referent | |
| Medium | 1.30 (1.17–1.45) | <0.01 | |
| High | 1.57 (1.41–1.76) | <0.01 | |
| With CABG capabilities | Referent | ||
| Without CABG | 1.08 (0.98–1.19) | 0.13 |
CABG indicates coronary artery bypass surgery; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events – composite of death, stroke, myocardial infarction, and repeat revascularization; MACCE‐1, composite of death, stroke, and myocardial infarction; and PCI, percutaneous coronary intervention.
Compared with those undergoing coronary angiography at low PCI:CABG ratio hospitals, those undergoing the procedure at medium (HR, 1.19; 95% CI, 1.14–1.25) or high (HR, 1.21; 95% CI, 1.15–1.27) PCI:CABG ratio hospitals demonstrated higher rates of MACCE, after adjusting for baseline demographics. When the individual components of the composite outcome were examined, rate of late MI was independently increased in patients treated at medium (HR, 1.25; 95% CI, 1.17–1.34) and high (HR, 1.11; 95% CI, 1.03–1.20) PCI:CABG hospitals. The rate of repeat revascularization up to 5 years was also independently increased in patients undergoing diagnostic angiography at medium (HR, 1.30; 95% CI, 1.17–1.45) and high (HR, 1.57; 95% CI, 1.41–1.76) PCI:CABG hospitals. The rate of death was not increased in patients from medium (HR, 1.05) or high (HR, 0.99) PCI:CABG ratio hospitals.
Patients undergoing the index angiogram at an institution without CABG capabilities had worse long‐term outcomes including MACCE (HR, 1.07; 95% CI, 1.02–1.11) and MACCE‐1 (death, MI, stroke) (HR, 1.07; 95% CI, 1.02–1.13), after accounting for baseline characteristics. Furthermore, having an index angiogram performed at a hospital without CABG capabilities was independently associated with a higher rate of death (HR, 1.09; 95% CI, 1.02–1.18) and MI (HR, 1.10; 95% CI, 1.03–1.17) over the follow‐up period. Hospital factors were not associated with rates of stroke or repeat revascularization.
Statistical Analysis
Sensitivity Analyses at Patient Level
After excluding patients who presented with a non–ST‐segment–elevation MI, the 28 383 remaining patients were divided into low, medium, and high PCI:CABG ratio groups. Baseline demographics, coronary anatomy, and procedural details are presented in Table S2. Similar to the overall analysis, high PCI:CABG group hospitals performed more PCI than CABG across all subgroups of coronary anatomy (Figure S2). The rate of ad hoc PCI ranged from 45% for LM disease to 68% for 2‐vessel disease with LAD involvement (Figure S3). The association of hospital factors with long‐term outcomes is presented in Table S3. After adjusting for all baseline characteristics, medium and high PCI:CABG ratio hospitals were associated with an increased rate of MACCE (HR, 1.10 and HR, 1.22, respectively) and repeat revascularization (HR, 1.24 and HR, 1.59, respectively) compared with low PCI:CABG ratio groups. Having the index angiogram performed in hospitals without CABG capabilities was independently associated with a higher rate of MACCE (HR, 1.08), MACCE‐1 (HR, 1.09), death (HR, 1.10), and MI (HR, 1.15), compared with having the index angiogram performed at hospitals without CABG capability. In another sensitivity analysis, we examined only those with diabetes, and the results were similar to the overall findings (Table S4).
Sensitivity Analyses at Institution/Provider Level
Furthermore, we adjusted for annualized total volume of coronary revascularization procedures. As shown in Table S5, the annualized volume of coronary revascularization procedures was not significantly associated with receiving PCI versus CABG surgery (P=0.74). After forcing revascularization procedure volume into the model, the association of PCI:CABG surgery remained robust relative to the primary and secondary outcomes (Table S6). Of the cohort, information on years since medical school graduation and age of the cardiologist performing the coronary angiogram was available for 42 899 (96.9%) and 42 681 (96.4%) patients, respectively. More years since graduation of the physician performing the coronary angiogram (Table S7) was associated with a lower likelihood of receiving PCI (OR, 0.96 per 5 years; 95% CI, 0.93–0.99, P=0.01), but the associations persisted even after inclusion of this variable (Table S8). Similarly, older age of the cardiologist performing the angiogram (Table S9) was associated with a lower probability of PCI (OR, 0.97 per 5 years; 95% CI, 0.94–0.99; P=0.02). However, the association of PCI:CABG with outcomes was unchanged after including age of the interventional cardiologist (Table S10). When the analysis incorporated hospital‐specific random effects, we found that the results for death and repeat revascularizations were similar to the main analyses reported in Table 3 (see Table S11).
Discussion
In this large, multicenter, population‐based analysis of variations in coronary revascularization practices between hospitals, we observed a 2‐fold variation in adjusted PCI/CABG ratio among institutions performing nonemergent coronary angiography for patients with severe multivessel CAD. Importantly, undergoing coronary angiography at institutions with higher PCI/CABG ratios was associated with an increased risk of MACCE, MI, and repeat revascularization. Furthermore, having the index angiogram performed at institutions with CABG capabilities was associated with lower rates of MACCE, MACCE‐1, death, and MI.
The wide variation in the mode of revascularization in contemporary practice in Ontario hospitals, after adjusting for a large set of baseline characteristics, coronary anatomy, and socioeconomic factors are potentially concerning because some patients may not be receiving the optimal form of revascularization, which was dependent on the hospital where they present for care. Randomized controlled trials of multivessel stenting versus CABG surgery in similar groups of patients, such as the SYNTAX trial, found that MACCE was significantly lower with surgery at 1‐year follow‐up. 15 The FREEDOM trial reported that death, MI, and stroke were significantly lower with CABG surgery, with curves separating at 2.5 years. In FREEDOM, the occurrence of MACCE events was significantly lower with CABG surgery 1 year after randomization. 16
Some earlier studies investigating PCI:CABG ratio variations among institutions have observed more pronounced variations than our study; however, these studies did not adjust for differences in patient characteristics. 10 , 17 Chan and colleagues also demonstrated significant institutional variations of rates of nonacute PCI throughout the United States, with 12% of patients considered inappropriate. 18 Among 24 458 patients undergoing PCI in the State of New York, 14% were deemed inappropriate for the procedure, whereas only 1% of CABGs were considered to be inappropriate. 19 Other studies have also reported variations between regions and institutions; however, there was no statistical adjustment for patient preoperative characteristics and these studies were performed before the Grade I recommendations of a heart team approach in patients with multivessel CAD. 20 , 21 , 22
We observed that by having the index angiogram performed by an interventional cardiologist, the odds of receiving a PCI rather than CABG were 32% higher, consistent with previous reports. 10 , 23 In New York State, Hannan and colleagues reported that treatment recommendations for patients with severe CAD were determined by the cardiologist in the catheterization laboratory in 64% of cases. Among this group, 16.7% had indications for either CABG or PCI, and of these, 93% underwent PCI. 23 Furthermore, there was a weak inverse association between the number of years in practice and age with the PCI:CABG ratio; however, the magnitude of effect was small, and the underlying reasons underpinning this link could not be delineated in our study.
Some of the PCIs observed in the high PCI:CABG ratio group may be due to the lack of a heart team discussion. Almost half of the patients from the high PCI:CABG ratio group underwent their diagnostic angiogram at institutions without CABG capabilities and therefore without imminent availability of a cardiac surgeon. Despite adjustment for patient characteristics, patients treated in high PCI:CABG ratio centers experienced worse outcomes. This could be related to patients with high coronary disease burden with multiple lesions receiving PCI or having more incomplete revascularization compared with CABG.
The observational nature of this study may not account for the increased risk of PCI in patients deemed inoperable. Surgical ineligibility has been previously associated with poor outcomes following PCI. 24 Nevertheless, in our study, there was an unexpectedly high rate of ad hoc PCI in all coronary subgroups, which may indicate that a surgeon was not involved in the revascularization strategy selection. Furthermore, a recent study of patients with multivessel CAD and diabetes reported that among patients undergoing multivessel PCI, only 8.3% had pre‐PCI cardiac surgical consultation. 25 In fact, in our study, the proportion of patients with diabetes was higher as the PCI:CABG ratio increased irrespective of coronary anatomy. Therefore, for the reasons discussed previously, we believe that the unadjusted confounder of higher PCI risk in inoperable patients was not a major driver of our results.
The rate of ad hoc PCI for nonemergent severe multivessel CAD was high (70%), and similar for hospitals with or without on‐site cardiac surgery. The role of ad hoc PCI has been demonstrated in patients with ST‐segment–elevation MI and whenever there is no equipoise in the revascularization strategy (single‐vessel disease or double‐vessel disease without LAD involvement, frail patients who are not CABG candidates). 26 Nonetheless, our study excluded most of these groups, in which one treatment strategy would be preferred over another. A potential advantage of ad hoc is the cost saved by avoiding multiple angiographies. However, given the higher rate of adverse events that we observed in hospitals performing higher than expected rates of PCI (most of which were ad hoc), performing PCI at the time of the diagnostic angiogram may not be cost saving in patients with multivessel stable CAD. Finally, patient preference towards a less invasive technique (PCI) could not be accounted for and may have partially explained the rate of ad hoc procedures for stable complex CAD.
Our study demonstrated that long‐term outcomes (MACCE, MACCE‐1, MI, death, and repeat revascularization) are influenced by the institutional proclivity toward PCI when surgical revascularization might be a more appropriate option. Our statistical analysis accounted for baseline patient characteristics, coronary anatomy, and socioeconomic status before investigating the association of the PCI:CABG ratio with our primary and secondary outcomes. Physicians should be cognizant that their treatment recommendations may be influenced by nonpatient‐related factors and institutional practices. Closer adherence to guidelines recommending multidisciplinary discussions for treatment of severe multivessel CAD and instituting quality improvement initiatives toward a heart team discussion may lead to better decisions and improved outcomes. Other potential approaches to building systems of care that encourage evidence‐based care could also be beneficial.
Strengths and Limitations
This study has important limitations including its retrospective nature and inability to account for unmeasured confounders. We were unable to determine whether or not the chosen revascularization strategy was appropriate or why a patient underwent ad hoc PCI. We did not have information about coronary complexity (ie, SYNTAX score) nor about the patient’s surgical risk (ie, Society of Thoracic Surgeons or EuroScore). It is unclear whether or not a discussion occurred with members of the heart team or if a patient was previously deemed to be of prohibitive risk for CABG. Our study was also unable to identify any barriers to using a heart team approach, and this may require future study. Our analyses started upon revascularization; however, we excluded those with conditions that could increase the risk of patients before planned revascularization procedures (eg, cardiogenic shock, ST‐segment–elevation MI, emergent procedures) to mitigate potential for survival bias. Finally, we were not able to account for referral patterns among institutions and unmeasured patient characteristics.
Conclusions
There exists a large degree of variation in PCI:CABG ratios among patients with multivessel coronary disease undergoing nonemergent coronary angiography. The choice of revascularization strategy was influenced by the physician performing the diagnostic angiogram, with a substantial proportion of ad hoc PCIs being performed. Having a diagnostic angiogram in hospitals with nonelevated PCI:CABG ratios or at centers with on‐site cardiac surgery capability was associated with improved outcomes. Institutions providing coronary revascularization procedures should consider monitoring the relative proportion of PCIs and CABG surgeries in their pursuit of the delivery of optimal cardiac care.
Sources of Funding
This study was supported by a Foundation Grant from the Canadian Institutes of Health Research (grant # FDN 148446). Dr. Austin is supported by a Mid‐Career Investigator Award from the Heart and Stroke Foundation. Dr. Lee is the Ted Rogers Chair in Heart Function Outcomes at University Health Network, University of Toronto. Dr. Ouzounian is the Antonio and Helga De Gasperis Chair in Clinical Trials and Outcomes at University Health Network, University of Toronto. This study was also supported by ICES, which is funded by an annual grant from the Ministry of Health and Long‐Term Care. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long‐Term Care is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s) and not necessarily those of Canadian Institute for Health Information. The authors acknowledge that the clinical registry data used in this analysis are from participating hospitals through CorHealth Ontario, which serves as an advisory body to the Ministry of Health, is funded by the Ministry of Health, and is dedicated to improving the quality, efficiency, access, and equity in the delivery of the continuum of adult cardiac, vascular, and stroke care in Ontario, Canada.
Disclosures
None.
Supporting information
Tables S1–S11
Figures S1–S3
Acknowledgments
We are thankful to Jiming Fang at the ICES, Toronto, Canada, for the assistance in the data analysis.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022770
For Sources of Funding and Disclosures, see page 11.
References
- 1. Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–1776.– 10.1001/jama.2011.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frutkin AD, Lindsey JB, Mehta SK, House JA, Spertus JA, Cohen DJ, Rumsfeld JS, Marso SP. Drug‐eluting stents and the use of percutaneous coronary intervention among patients with class I indications for coronary artery bypass surgery undergoing index revascularization: analysis from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2009;2:614–621. doi: 10.1016/j.jcin.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 3. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 4. Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes. 2011;4:193–197. doi: 10.1161/CIRCOUTCOMES.110.958744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai NR, Bradley SM, Parzynski CS, Nallamothu BK, Chan PS, Spertus JA, Patel MR, Ader J, Soufer A, Krumholz HM, et al. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA. 2015;314:2045–2053. doi: 10.1001/jama.2015.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serruys PW, Morice M‐C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 7. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 8. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 9. Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, DiSesa VJ, Hiratzka LF, Hutter AM, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e652–735. doi: 10.1161/CIR.0b013e31823c074e [DOI] [PubMed] [Google Scholar]
- 10. Tu JV, Ko DT, Guo H, Richards JA, Walton N, Natarajan MK, Wijeysundera HC, So D, Latter DA, Feindel CM, et al. Determinants of variations in coronary revascularization practices. CMAJ. 2012;184:179–186. doi: 10.1503/cmaj.111072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko W, Tranbaugh R, Marmur JD, Supino PG, Borer JS. Myocardial Revascularization in New York State: variations in the PCI‐to‐CABG Ratio and Their Implications. J Am Heart Assoc. 2012;1:e001446. doi: 10.1161/JAHA.112.001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ouzounian M, Ghali W, Yip AM, Buth KJ, Humphries K, Stukel TA, Norris CM, Southern DA, Galbraith PD, Thompson CR, et al. Determinants of percutaneous coronary intervention vs coronary artery bypass grafting: an interprovincial comparison. Can J Cardiol. 2013;29:1454–1461. doi: 10.1016/j.cjca.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 13. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 14. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. doi: 10.1161/CIRCOUTCOMES.114.001416 [DOI] [PubMed] [Google Scholar]
- 15. Serruys PW, Morice M‐C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. The New England Journal of Medicine. 2009;360:961–972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 16. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for multivessel revascularization in patients with diabetes. The New England Journal of Medicine. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 17. Matlock DD, Groeneveld PW, Sidney S, Shetterly S, Goodrich G, Glenn K, Xu S, Yang L, Farmer SA, Reynolds K, et al. Geographic variation in cardiovascular procedure use among Medicare fee‐for‐service vs Medicare Advantage beneficiaries. JAMA. 2013;310:155–162. doi: 10.1001/jama.2013.7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS, et al. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53–61. doi: 10.1001/jama.2011.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hannan EL, Cozzens K, Samadashvili Z, Walford G, Jacobs AK, Holmes DR, Stamato NJ, Sharma S, Venditti FJ, Fergus I, et al. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J Am Coll Cardiol. 2012;59:1870–1876. doi: 10.1016/j.jacc.2012.01.050 [DOI] [PubMed] [Google Scholar]
- 20. Ko DT, Tu JV, Samadashvili Z, Guo H, Alter DA, Cantor WJ, Hannan EL. Temporal trends in the use of percutaneous coronary intervention and coronary artery bypass surgery in New York State and Ontario. Circulation. 2010;121:2635–2644. doi: 10.1161/CIRCULATIONAHA.109.926881 [DOI] [PubMed] [Google Scholar]
- 21. Kim AM, Park JH, Cho S, Kang S, Yoon TH, Kim Y. Factors associated with the rates of coronary artery bypass graft and percutaneous coronary intervention. BMC Cardiovasc Disord. 2019;19:275. doi: 10.1186/s12872-019-1264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan A, Newman A, Ko DT, Rinfret S, Hirsch G, Ghali WA, Tu JV. Increasing rates of angioplasty versus bypass surgery in Canada, 1994–2005. Am Heart J. 2010;160:958–965. doi: 10.1016/j.ahj.2010.06.052 [DOI] [PubMed] [Google Scholar]
- 23. Hannan EL, Racz MJ, Gold J, Cozzens K, Stamato NJ, Powell T, Hibberd M, Walford G. Adherence of catheterization laboratory cardiologists to American College of Cardiology/American Heart Association guidelines for percutaneous coronary interventions and coronary artery bypass graft surgery: what happens in actual practice? Circulation. 2010;121:267–275. doi: 10.1161/CIRCULATIONAHA.109.887539 [DOI] [PubMed] [Google Scholar]
- 24. Waldo SW, Secemsky EA, O'Brien C, Kennedy KF, Pomerantsev E, Sundt TM 3rd, McNulty EJ, Scirica BM, Yeh RW. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2014;130:2295–2301. doi: 10.1161/CIRCULATIONAHA.114.011541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tam DY, Dharma C, Rocha R, Farkouh ME, Abdel‐Qadir H, Sun LY, Wijeysundera HC, Austin PC, Udell JA, Gaudino M, et al. Long‐term survival after surgical or percutaneous revascularization in patients with diabetes and multivessel coronary disease. J Am Coll Cardiol. 2020;76:1153–1164. doi: 10.1016/j.jacc.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blankenship JC, Gigliotti OS, Feldman DN, Mixon TA, Patel RA, Sorajja P, Yakubov SJ, Chambers CE. Society for Cardiovascular A and Interventions. Ad hoc percutaneous coronary intervention: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;81:748–758. doi: 10.1002/ccd.24701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S11
Figures S1–S3


