Abstract
Background
In addition to primary neurodegenerative processes, vascular disorders, such as stroke, can lead to parkinsonism. However, some cardiovascular risk factors, such as smoking and elevated cholesterol levels, are associated with reduced risk of Parkinson disease. We examined the risk of Parkinson disease and secondary parkinsonism in 1‐year survivors of myocardial infarction (MI).
Methods and Results
We conducted a nationwide population‐based matched cohort study using Danish medical registries from 1995 to 2016. We identified all patients with a first‐time MI diagnosis and sampled a sex‐, age‐, and calendar year–matched general population comparison cohort without MI. Cox regression analysis was used to compute adjusted hazard ratios (aHRs) for Parkinson disease and secondary parkinsonism, controlled for matching factors and adjusted for relevant comorbidities and socioeconomic factors. We identified 181 994 patients with MI and 909 970 matched comparison cohort members (median age, 71 years; 62% men). After 21 years of follow‐up, the cumulative incidence was 0.9% for Parkinson disease and 0.1% for secondary parkinsonism in the MI cohort. Compared with the general population cohort, MI was associated with a decreased risk of Parkinson disease (aHR, 0.80; 95% CI, 0.73–0.87) and secondary parkinsonism (aHR, 0.72; 95% CI, 0.54–0.94).
Conclusions
MI was associated with a 20% decreased risk of Parkinson disease and 28% decreased risk of secondary parkinsonism. Reduced risk may reflect an inverse relationship between cardiovascular risk factors and Parkinson disease.
Keywords: epidemiology, myocardial infarction, Parkinson disease
Subject Categories: Myocardial Infarction, Epidemiology, Risk Factors
Nonstandard Abbreviations and Acronyms
- aHR
adjusted hazard ratio
- CCI
Charlson Comorbidity Index
- DNPR
Danish National Patient Registry
Clinical Perspective
What Is New?
In addition to primary neurodegenerative processes, cerebrovascular disorders can lead to parkinsonism.
Although the risk of cardiovascular disease is increased in patients with Parkinson disease, the risk of Parkinson disease in myocardial infarction survivors remains unknown.
In our study, myocardial infarction was associated with a 20% decreased risk of Parkinson disease and a 28% decreased risk of secondary parkinsonism compared with the general population.
What Are the Clinical Implications?
Our observations suggest that myocardial infarction is negatively associated with Parkinson disease.
This finding may reflect inverse associations between classic cardiovascular risk factors, such as smoking and elevated cholesterol, and Parkinson disease.
The risk of Parkinson disease need not be a focus area during follow‐up of patients with myocardial infarction.
The number of myocardial infarction (MI) survivors has increased worldwide 1 because of an ongoing demographic shift toward an elderly population combined with improved mortality rates following MI. 2 In this growing population, the risk of neurovascular complications, such as ischemic stroke 3 and vascular dementia, 4 is markedly increased. Although the risk of cardiovascular disease is increased in patients with Parkinson disease, 5 , 6 the risk of Parkinson disease in MI survivors remains unknown.
Parkinson disease is primarily a neurodegenerative disease, 7 whereas parkinsonism has several underlying causes besides primary neurodegenerative processes, including a variety of vascular mechanisms. 8 These may include lacunar infarcts and other vascular insults encompassing the substantia nigra, cerebral white matter, and other cerebral structures. 9 Complications after MI, such as atrial fibrillation and regional wall motion abnormalities, may increase the risk of such infarcts. Likewise, heart failure and hypotension following MI may facilitate formation of watershed infarcts in susceptible areas of the brain. 10 Cardiovascular drugs commonly initiated in the course of an MI (eg, verapamil and diltiazem) also are known to increase the risk of parkinsonism. 11 , 12
Shared risk factors between MI and Parkinson disease include age and male sex, whereas coffee consumption and physical activity each are associated with a lower risk of both diseases. 13 Conversely, several studies confirm that classic cardiovascular risk factors, such as smoking, hypercholesterolemia, increased blood pressure, and diabetes, are negatively associated with the risk of Parkinson disease. 14 , 15 , 16 However, it is not known whether this inverse relationship extends to manifest atherosclerotic disease. As the underlying cause of Parkinson disease is largely unknown, identification of potential risk factors would contribute to our understanding of the disease. We therefore examined the long‐term risk of Parkinson disease and secondary parkinsonism following first‐time MI and the impact of common MI treatments and complications.
Methods
Setting and Design
The data that support the findings of this study are available from the corresponding author on reasonable request.
We conducted this nationwide population‐based cohort study in Denmark, which had a cumulative population of 8 262 736 inhabitants during the study period (January 1, 1995, to December 31, 2016). 17 The Danish National Health Service provides tax‐supported health care, with free and equal access to general practitioners and hospitals for all Danish inhabitants. Accurate linkage of all registries at the individual level is possible in Denmark because of the unique central personal registry number assigned to each Danish citizen at birth and to residents on immigration. 18
Patients With MI
We used the Danish National Patient Registry 19 (DNPR) to identify all patients with a first‐time inpatient diagnosis of MI during the study period. The DNPR has recorded information on all admissions to Danish nonpsychiatric hospitals since 1977 and on emergency department and outpatient clinic visits since 1995. 19 Each hospital discharge or outpatient visit is recorded in the DNPR with one primary diagnosis and one or more secondary diagnoses classified according to the International Classification of Diseases, Eighth Revision (ICD‐8), through 1993 and International Classification of Diseases, Tenth Revision (ICD‐10), thereafter. 19 We identified patients with MI using both primary and secondary diagnoses.
General Population Comparison Cohort
We created a general population comparison cohort using the Danish Civil Registration System, which has provided daily updates on vital statistics, including dates of birth, emigration, and death, since 1968. 18 For each patient in the MI cohort, 5 individuals from the general population without an MI diagnosis were randomly selected and matched on sex, age, and calendar year of MI diagnosis. We used matching with replacement (ie, individuals from the general population comparison cohort could be matched with more than one patient with MI). 20
The MI admission date defined the index date for patients with MI and their matched counterparts in the general population cohort. To ensure capture of only incident cases of Parkinson disease and secondary parkinsonism, we excluded patients with MI and people in the matched comparison cohort who had received a previous diagnosis of these diseases. If members of the general population cohort were diagnosed with MI after the index date, follow‐up in the comparison cohort was discontinued. They then were transferred to the MI cohort and matched with new members of the general population.
Parkinson Disease and Secondary Parkinsonism
Data on inpatient and outpatient Parkinson disease and secondary parkinsonism diagnoses were retrieved from the DNPR. 19 We also included secondary parkinsonism to consider patients with parkinsonism attributable to, for example, cerebrovascular insults following MI. In the DNPR, diagnoses are available for hospital admissions since 1977 and for outpatient clinic visits since 1995. 19 We identified Parkinson disease and secondary parkinsonism using both primary and secondary diagnoses. The validity of Parkinson disease in the DNPR is high at 91%. 21
Covariables
All patients’ medical histories were available in the DNPR since 1977 19 and the Danish Psychiatric Central Research Register since 1995. 22 We obtained information on comorbidities that may represent confounders or shared risk factors for MI and parkinsonism. These consisted of all hospital inpatient and outpatient diagnoses of heart failure, angina pectoris, atrial fibrillation/atrial flutter, heart valve disease, hypercholesterolemia, hypertension, stroke, intermittent claudication, obesity, diabetes, chronic pulmonary disease (as an indicator of chronic smoking), alcoholism‐related diseases, head trauma, osteoarthritis, anemia, chronic kidney disease, depression, and a modified Charlson Comorbidity Index (CCI) score (excluding congestive heart failure, MI, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, and diabetes from the index). We also obtained information on personal gross income, employment status during the year preceding the index date, and highest education achieved from the Integrated Database for Labour Market Research. 23 Finally, we included certain antipsychotics (specifically piperazine side chain neuroleptics) and calcium channel blockers from the nationwide prescription registry, 24 as they are known to cause extrapyramidal adverse effects mimicking Parkinson disease 11 , 25 and are commonly used in patients with cardiovascular disease. 26 , 27
Surgical Procedures
We obtained information on coronary artery bypass graft surgery, percutaneous coronary intervention, and pacemaker implantation from the DNPR, which has coded surgery according to the Danish Classification of Surgical Procedures and Therapies until January 1, 1996, and according to the NOMESCO Classification of Surgical Procedures thereafter. 19
Statistical Analysis
We characterized the MI and general population comparison cohorts according to sex, age groups (<60, 60–69, 70–79, and ≥80 years), index year calendar periods (1995–1999, 2000–2004, 2005–2009, and 2010–2016), comorbidities, and socioeconomic factors at baseline and at 1 year after MI. We followed up all patients with MI and members of the general population comparison cohort until the occurrence of any Parkinson disease or secondary parkinsonism diagnosis, emigration, death, or December 31, 2016, whichever came first. A priori, we disregarded the first year after MI and initiated follow‐up thereafter, because parkinsonism diagnosed shortly after admission for MI is unlikely to be a consequence of MI and is prone to diagnostic bias shortly after MI. The Figure provides a flowchart of exclusions within the first year of MI and the resulting final study population.
Figure 1. Study flowchart.
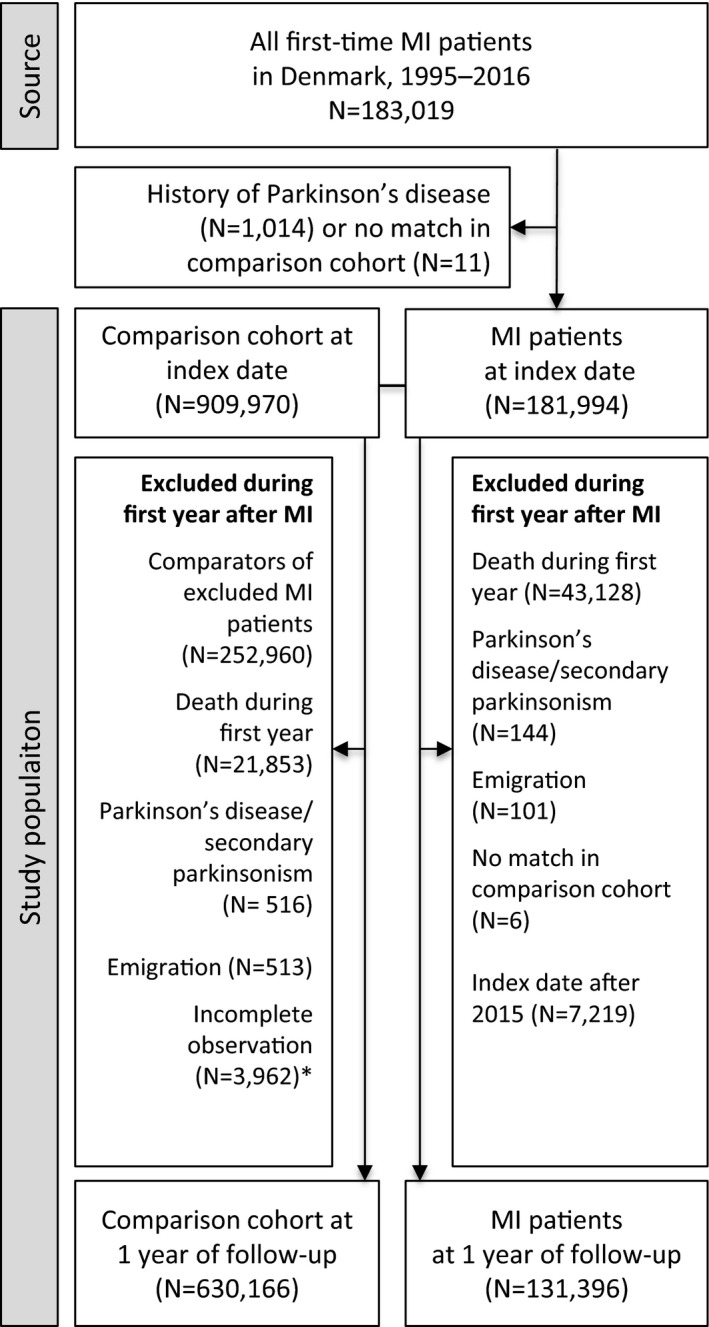
Population of first‐time myocardial infarction (MI) survivors and members of the matched general population comparison cohort. *Censored because they had an MI during the first year of follow‐up.
We used cumulative incidence functions with death as a competing risk to calculate risks of Parkinson disease or secondary parkinsonism during 1 to 22 years of follow‐up. This implies a maximum follow‐up of 21 years. Using multivariable stratified Cox proportional hazards regression, we computed adjusted hazard ratios (aHRs) with 95% CIs, comparing patients with MI with members of the general population comparison cohort. 28 The aHRs were controlled for sex, age, and calendar year by the matched study design and in multivariable analyses adjusted for preadmission diagnoses of heart failure, stable angina pectoris, atrial fibrillation or atrial flutter, valvular heart disease, hypercholesterolemia, hypertension, stroke, intermittent claudication, obesity, diabetes, chronic pulmonary disease, alcoholism‐related disease, head trauma, osteoarthritis, anemia, chronic kidney disease, depression, a modified CCI score, antipsychotics, calcium channel blockers, and socioeconomic factors (income and employment). The proportional‐hazards assumption was assessed graphically using log‐log plots, and there were indications of violation within the total follow‐up period. Therefore, we repeated all analyses using the Poisson regression approach, 29 which does not require proportionality of hazards. We found no difference in the estimates with these 2 approaches and therefore report results from the Cox regression analyses. All codes used in the study are provided in Tables S1 and S2.
Additional Analyses
To identify clinical pathways with a potential impact on the association between MI and Parkinson disease or secondary parkinsonism, we stratified by cardiac procedures performed during hospital admission for MI and by complications occurring between MI and start of follow‐up 1 year later.
The presence of potential interactions was examined in strata of sex, age groups, underlying preadmission comorbidity, different levels of comorbidity measured using modified CCI scores, post‐MI use of drugs associated with Parkinson disease, and subgroups of MI (ST‐segment–elevation MI and non–ST‐segment–elevation MI). We also investigated temporal differences in risk of Parkinson disease and secondary parkinsonism following MI by splitting up the index periods (1995–1999, 2000–2004, 2005–2009, and 2010–2016). In this analysis, we limited follow‐up to 5 years, to allow sufficient follow‐up time for patients in all time periods. We further stratified the analyses by type of MI diagnosis (primary or secondary), because the positive predictive value is lower (80%) for secondary diagnoses. 30 Socioeconomic status is associated with Parkinson disease risk. 31 Therefore, we also examined the impact of socioeconomic status as reflected in income, employment status, and educational attainment. In the analyses stratified by underlying preadmission comorbidity, modified CCI scores, and socioeconomic factors, the matching was dissolved and aHRs were additionally adjusted for matching variables. Dissolving the matching introduced a lack of independence among members of the comparison cohort because they were matched with replacement. 20 However, we did not use a robust variance estimator in the Cox regression analyses, because we did not detect any notable difference in doing so (the maximum difference was 0.01 in the CI) and because all estimates were tested with a Poisson regression model using a robust variance estimator, also with no difference compared with the original estimates.
Sensitivity Analyses
We performed several sensitivity analyses. First, given an assumed latency period for development of clinically overt parkinsonism following MI, we repeated the analyses sequentially excluding the initial 2, 3, and 5 years of follow‐up. Second, we additionally adjusted for education, which was not included in the main analysis because data were unavailable for ≈18% of participants and because there was a strong collinearity with the other socioeconomic factors (income and employment). Third, we divided follow‐up time into periods of 1 to 5 years, 6 to 10 years, 11 to 15 years, and 16 years to end of follow‐up to examine whether associations changed over time. Fourth, we continued follow‐up for members of the comparison cohort who experienced an MI during follow‐up. Fifth, in a sensitivity analysis, we also calculated E‐values 32 for main estimates and the corresponding upper limit of the 95% CI. This allowed us to assess how strongly a single unmeasured binary confounder would need to be associated with both the exposure (MI) and outcome (Parkinson disease/secondary parkinsonism) to fully explain away the observed exposure‐outcome association. 32
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The study was approved by the Danish Data Protection Agency (record number 1‐16‐02‐268‐14). According to Danish legislation, no approval from an ethics committee or informed consent from patients is required for registry‐based studies in Denmark.
Results
The study included 181 994 patients in the MI cohort and 909 970 individuals in the matched general population comparison cohort. Median age at MI diagnosis was 71 (interquartile range, 60–80) years, and 62% of the study population were men. Median follow‐up time was 4.1 (25th–75th percentile, 0.7–9.6) years for patients with MI and 6.6 (25th–75th percentile, 2.9–11.6) years for members of the comparison cohort. The difference in follow‐up time arose mainly from the competing risk of death after MI. The MI cohort had an expected higher prevalence of cardiovascular conditions and CCI levels. Patients in the MI cohort had slightly lower income and educational levels, and a larger proportion were unemployed compared with the general population comparison cohort (Table 1). Findings were similar in MI survivors and members of the comparison cohort at 1 year after MI (Table S3).
Table 1.
Characteristics of Patients at Hospital Admission for First‐Time MI and Members of the General Population Comparison Cohort, Denmark, 1995 to 2016
| Characteristics | MI cohort (n=181 994) |
Comparison cohort (n=909 970) |
|---|---|---|
| Men | 112 362 (61.7) | 561 795 (61.7) |
| Age, y | ||
| <60 | 43 829 (24.1) | 219 145 (24.1) |
| 60–69 | 41 579 (22.8) | 207 895 (22.8) |
| 70–79 | 48 668 (26.7) | 243 340 (26.7) |
| ≥80 | 47 918 (26.3) | 239 590 (26.3) |
| Median (25th–75th percentile) | 71.0 (60–80) | 71.0 (60–80) |
| Decade of diagnosis/index year | ||
| 1995−1999 | 41 201 (22.6) | 206 005 (22.6) |
| 2000−2004 | 46 685 (25.7) | 233 425 (25.7) |
| 2005−2009 | 40 965 (22.5) | 204 825 (22.5) |
| 2010–2016 | 53 143 (29.2) | 265 715 (29.2) |
| Comorbidity | ||
| Heart failure | 14 059 (7.7) | 31 218 (3.4) |
| Angina pectoris | 29 771 (16.4) | 59 753 (6.6) |
| Atrial fibrillation or flutter | 14 558 (8.0) | 53 802 (5.9) |
| Valvular heart disease | 7265 (4.0) | 17 522 (1.9) |
| Hypercholesterolemia | 9888 (5.4) | 24 847 (2.7) |
| Hypertension | 63 119 (34.7) | 216 747 (23.8) |
| Stroke | 14 062 (7.7) | 45 301 (5.0) |
| Intermittent claudication | 5426 (3.0) | 9445 (1.0) |
| Obesity | 7213 (4.0) | 18 799 (2.1) |
| Diabetes | 25 853 (14.2) | 69 485 (7.6) |
| Chronic pulmonary disease | 18 988 (10.4) | 58 456 (6.4) |
| Alcoholism‐related diseases | 5975 (3.3) | 22 344 (2.5) |
| Head trauma | 12 291 (6.8) | 55 411 (6.1) |
| Osteoarthritis | 23 633 (13.0) | 104 967 (11.5) |
| Anemia | 9588 (5.3) | 29 929 (3.3) |
| Chronic kidney disease | 6650 (3.7) | 12 044 (1.3) |
| Depression | 6244 (3.4) | 24 152 (2.7) |
| Drugs frequently associated with parkinsonism | ||
| Typical antipsychotics (piperazine side chain neuroleptics) | 3437 (1.9) | 14 288 (1.6) |
| Calcium channel blockers | 56 081 (30.8) | 184 974 (20.3) |
| Modified CCI score* | ||
| Normal | 132 575 (72.8) | 724 597 (79.6) |
| Moderate | 25 091 (13.8) | 81 607 (9.0) |
| Severe | 17 008 (9.3) | 78 669 (8.6) |
| Very severe | 7320 (4.0) | 25 097 (2.8) |
| Income | ||
| Low | 55 289 (30.4) | 242 797 (26.7) |
| Intermediate | 53 763 (29.5) | 250 765 (27.6) |
| High | 40 612 (22.3) | 210 864 (23.2) |
| Very high | 32 148 (17.7) | 204 862 (22.5) |
| Missing | 182 (0.1) | 682 (0.1) |
| Employment | ||
| Employed | 50 493 (27.7) | 288 209 (31.7) |
| Early retirement | 23 474 (12.9) | 95 216 (10.5) |
| Unemployed | 4988 (2.7) | 19 668 (2.2) |
| State pensioner | 102 535 (56.3) | 503 890 (55.4) |
| Missing | 504 (0.3) | 2987 (0.3) |
| Education | ||
| Basic education or primary school | 72 566 (39.9) | 314 529 (34.6) |
| Youth education, high school, or similar education | 55 330 (30.4) | 284 647 (31.3) |
| Higher education | 20 385 (11.2) | 146 426 (16.1) |
| Unknown | 33 713 (18.5) | 164 368 (18.1) |
Values are given as number (percentage), unless otherwise indicated. CCI indicates Charlson Comorbidity Index; and MI, myocardial infarction.
Categories of comorbidity were based on modified CCI scores: 0 (normal), 1 (moderate), 2 (severe), and ≥3 (very severe).
Risk of Parkinson Disease and Secondary Parkinsonism
During follow‐up, 668 patients in the MI cohort were diagnosed with Parkinson disease and 71 with secondary parkinsonism. The cumulative incidence in the MI cohort after 21 years of follow‐up was 0.9% for Parkinson disease and 0.1% for secondary parkinsonism. As a result of the competing risk of death, the cumulative incidence of Parkinson disease and secondary parkinsonism was higher in the general population comparison cohort than in the MI cohort (Table 2). MI was associated with a moderately decreased risk of both Parkinson disease (aHR, 0.80; 95% CI, 0.73–0.87) and secondary parkinsonism (aHR, 0.72; 95% CI, 0.54–0.94).
Table 2.
Risk of Parkinson Disease and Secondary Parkinsonism in Patients With MI and Members of the General Population Comparison Cohort
| Disease | Comparison cohort | Patients with MI | ||||
|---|---|---|---|---|---|---|
| Events/No. at risk | Cumulative incidence,% (95% CI) | Events/No. at risk | Cumulative incidence,% (95% CI) | Hazard ratio controlled for matching factors (95% CI)* | Adjusted hazard ratio (95% CI) † | |
| Parkinson disease | 4352/630 166 | 1.25 (1.20–1.29) | 668/131 396 | 0.86 (0.79–0.94) | 0.82 (0.75–0.89) | 0.80 (0.73–0.87) |
| Secondary parkinsonism | 474/630 166 | 0.14 (0.12–0.16) | 71/131 396 | 0.09 (0.07–0.12) | 0.77 (0.59–1.00) | 0.72 (0.54–0.94) |
MI indicates myocardial infarction.
Age, sex, and calendar year.
Controlled for matching factors by study design and adjusted for heart failure, stable angina pectoris, atrial fibrillation/atrial flutter, valvular heart disease, hypercholesterolemia, hypertension, stroke, intermittent claudication, obesity, diabetes, chronic pulmonary disease, alcoholism‐related disease, head trauma, osteoarthritis, anemia, chronic kidney disease, depression, a modified Charlson Comorbidity Index score, antipsychotics, calcium channel blockers, income, and employment.
Additional Analyses
In analyses stratified by procedures and complications (including stroke) after MI, we observed no impact on the risk of Parkinson disease or secondary parkinsonism (Table 3). In age‐stratified analyses, the decreased risk of Parkinson disease was more pronounced for older age groups, whereas estimates were too imprecise to draw firm conclusion for secondary parkinsonism. We observed no sex differences (Table S4). Apart from a null association for patients with atrial fibrillation or flutter, alcoholism‐related disease, and chronic kidney disease, the results remained largely unchanged in subgroup analyses of cardiac and noncardiac comorbidity, CCI levels, and use of drugs associated with extrapyramidal adverse effects mimicking Parkinson disease (Table S5). We observed no temporal difference in the association observed during early versus late time periods, apart from a weakened association from 2005 to 2009 for Parkinson disease (Table S6). In analyses stratified by primary versus secondary diagnoses of MI, the results also remained unchanged (Table S6). Across levels of income, employment status, and education, the results agreed with those of the main analysis, apart from a near null association for employed patients with MI (Table S7). Despite no apparent statistical significance at the 95% level of confidence in these additional analyses, we cannot entirely rule out type 1 errors attributable to limited sample sizes in these subgroup analyses.
Table 3.
Risk of Parkinson Disease and Secondary Parkinsonism in Patients With MI and Members of the General Population Cohort, by Procedures and Complications After MI
| Variable | Parkinson disease | Secondary parkinsonism | ||
|---|---|---|---|---|
| Cumulative risk, % (95% CI) | Adjusted hazard ratio (95% CI)* | Cumulative risk, % (95% CI) | Adjusted hazard ratio (95% CI)* | |
| Procedures and complications during admission for MI | ||||
| Coronary artery bypass grafting | ||||
| Yes | 1.10 (0.69–1.69) | 1.12 (0.61–2.05) | … | … |
| No | 0.85 (0.78–0.94) | 0.79 (0.73–0.87) | 0.09 (0.07–0.12) | 0.74 (0.56–0.97) |
| Percutaneous coronary intervention | ||||
| Yes | 0.98 (0.80–1.20) | 0.83 (0.71–0.98) | 0.04 (0.02–0.08) | 0.51 (0.26–0.99) |
| No | 0.80 (0.73–0.89) | 0.78 (0.71–0.87) | 0.10 (0.08–0.13) | 0.78 (0.57–1.06) |
| Pacemaker | ||||
| Yes | 0.51 (0.21–1.06) | 0.55 (0.23–1.34) | … | … |
| No | 0.86 (0.79–0.95) | 0.80 (0.74–0.88) | 0.09 (0.07–0.12) | 0.74 (0.56–0.98) |
| Complications during first year after MI | ||||
| Cardiogenic shock or pulmonary edema | ||||
| Yes | 0.87 (0.62–1.20) | 0.87 (0.60–1.27) | 0.07 (0.03–0.15) | 0.27 (0.07–0.96) |
| No | 0.86 (0.79–0.95) | 0.79 (0.72–0.87) | 0.09 (0.07–0.12) | 0.77 (0.58–1.03) |
| Stroke (ischemic or hemorrhagic) | ||||
| Yes | 0.10 (0.01–0.56) | 0.21 (0.02–2.49) | 0.11 (0.01–0.60) | … |
| No | 0.87 (0.79–0.95) | 0.80 (0.74–0.88) | 0.09 (0.07–0.12) | 0.71 (0.54–0.94) |
| Heart failure | ||||
| Yes | 0.73 (0.59–0.90) | 0.88 (0.70–1.11) | 0.09 (0.05–0.15) | 0.72 (0.31–1.71) |
| No | 0.88 (0.80–0.97) | 0.78 (0.71–0.86) | 0.09 (0.07–0.12) | 0.69 (0.51–0.93) |
| Hypertension | ||||
| Yes | 0.88 (0.76–1.01) | 0.76 (0.68–0.86) | 0.09 (0.06–0.12) | 0.72 (0.49–1.06) |
| No | 0.85 (0.75–0.96) | 0.85 (0.75–0.96) | 0.09 (0.07–0.14) | 0.72 (0.48–1.09) |
| Atrial fibrillation or flutter | ||||
| Yes | 0.91 (0.70–1.16) | 1.01 (0.75–1.36) | 0.19 (0.08–0.42) | 0.78 (0.32–1.88) |
| No | 0.86 (0.78–0.94) | 0.79 (0.72–0.86) | 0.08 (0.06–0.11) | 0.69 (0.51–0.93) |
Ellipses (…) indicate insufficient data to compute a meaningful estimate. MI, myocardial infarction.
Controlled for matching factors by study design and adjusted for heart failure, stable angina pectoris, atrial fibrillation/atrial flutter, valvular heart disease, hypercholesterolemia, hypertension, stroke, intermittent claudication, obesity, diabetes, chronic pulmonary disease, alcoholism‐related disease, head trauma, osteoarthritis, anemia, chronic kidney disease, depression, a modified Charlson Comorbidity Index score, antipsychotics, calcium channel blockers, income, and employment.
Sensitivity Analyses
Results of the sensitivity analyses are presented in Table S8. The results were essentially unchanged when we sequentially excluded the initial 2, 3, and 5 years of follow‐up. The results also remained robust when the model was extended to adjust for education, and when the 4 follow‐up periods (1–5 years, 6–10 years, 11–15 years, and 16 years–end of follow‐up) were considered separately. In addition, type of MI (ST‐segment–elevation MI/non–ST‐segment–elevation MI) did not substantially affect the results, although results were less precise in these MI subgroups. Finally, results were consistent when we continued follow‐up for members of the population comparison cohort who experienced MI during follow‐up (data not shown). The derived E‐values were relatively large (E‐values of 1.56 for Parkinson disease and 1.32 for secondary parkinsonism) in comparison with their main estimate counterparts (aHRs of 0.80 for Parkinson disease and 0.72 for secondary parkinsonism) (Figure S1). These results indicate that an unmeasured confounder would need to be strongly associated with both MI and Parkinson disease to fully explain our findings but still leave the potential for a single confounder with a moderate or large effect (eg, smoking) to explain away the observed association.
Discussion
In this nationwide matched population‐based cohort study with virtually complete follow‐up of 181 994 MI survivors for 21 years, we found a moderately lower risk of both Parkinson disease and secondary parkinsonism compared with the general population. Apart from a null association in patients with MI who were employed or had specific comorbidity (atrial fibrillation or flutter, alcoholism‐related disease, and chronic kidney disease), our results for Parkinson disease remained unchanged across subgroup and sensitivity analyses. For secondary parkinsonism, subgroups analyses were hampered by imprecise estimates attributable to smaller sample sizes, and should be interpreted with caution. Our study is the first to examine the MI–Parkinson disease association, and from a clinical perspective our findings do not suggest the need for increased attention to development of Parkinson disease among MI survivors. Reduced risk of Parkinson disease and secondary parkinsonism after MI might be related to an inverse association with cardiovascular risk factors, such as smoking and blood cholesterol.
Although studies of the relation between classic cardiovascular risk factors and Parkinson disease have been inconsistent, most studies point to smoking as negatively associated with Parkinson disease. 14 , 15 , 33 , 34 , 35 Our finding of a lower risk of Parkinson disease and secondary parkinsonism in MI survivors may partially be driven by a higher prevalence of smoking in MI survivors compared with the general population, although we adjusted for chronic pulmonary disease as a proxy for chronic smoking exposure. High blood levels of cholesterol also have been negatively associated with risk of Parkinson disease. A prospective study from the Netherlands reported high serum cholesterol levels to be associated with a decreased risk of Parkinson disease after adjusting for other cardiovascular risk factors (HR, 0.77; 95% CI, 0.64–0.94). 36 This finding has been confirmed in a case‐control study 14 and a population‐based cohort study. 16 Although we adjusted for hypercholesterolemia diagnoses, the prevalence in the MI cohort is only 5.4% (Table 1). Hence, residual confounding of hypercholesterolemia may also partly drive our results of lower risk of Parkinson disease and secondary parkinsonism. High body mass index has been described as a risk factor for Parkinson disease in a Finnish observational study reporting a 2‐fold increased risk for body mass index ≥30 kg/m2, 37 whereas a recent Swedish population‐based cohort study reported no association with Parkinson disease. 38 This null association has further been supported in a case‐control study 39 and a population‐based cohort study. 15 High blood pressure has been both positively 40 and negatively 14 , 15 associated with the risk of Parkinson disease. It is interesting that stroke among MI survivors did not increase Parkinson disease risk in our study.
Several study strengths and limitations should be considered when interpreting our results. An important strength is the size of the study, allowing for examination of several possible interactions and mediators of the association between MI and parkinsonism, while retaining precision. The population‐based design, within the setting of a tax‐supported universal health care system with complete follow‐up of all patients, largely eliminated selection biases. 18 Registration of the MI diagnosis in the DNPR is accurate, with validation studies consistently reporting positive predictive values of >90% throughout the study period. 19 , 30 , 41 , 42 The accuracy of Parkinson disease in the DNPR also is high at 91%. 21 A concern is the unknown sensitivity of the diagnosis of Parkinson disease and secondary parkinsonism. The sensitivity may be higher in the MI cohort because of surveillance bias. However, this would lead to increased risk among patients with MI compared with the general population, which we did not observe. Despite extensive confounder adjustment for sex, age, comorbidity, and socioeconomic factors, our study is limited by its observational design. Thus, residual and unmeasured confounding cannot be ruled out and may partly underlie our results. More important, we lacked information on smoking and incompletely captured hypercholesterolemia, both of which are positively associated with MI 43 and negatively associated with Parkinson disease. 34 , 36
For the growing population of MI survivors and their managing physicians, the primary implication of our results is that the risk of Parkinson disease and parkinsonism is not increased compared with the general population. Conversely, our results point to a moderately decreased risk, which may reflect inverse associations between cardiovascular risk factors, such as smoking and elevated cholesterol, and Parkinson disease.
In conclusion, MI was associated with a moderately decreased risk of Parkinson disease and secondary parkinsonism, with robust results across subgroups and in sensitivity analyses.
Sources of Funding
This work was supported by a grant from Lundbeckfonden (grant R248‐2017‐521). Neither funding source had a role in the design, conduct, analysis, or reporting of the study. Dr Henderson’s effort was supported by a grant from National Institutes of Health (grant P30AG066515).
Disclosures
None.
Supporting information
Tables S1–S8
Figure S1
Acknowledgments
Dr Sørensen is the guarantor of this work, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Supplemental material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022768
For Sources of Funding and Disclosures, see page 8.
References
- 1. Koch MB, Davidsen M, Andersen LV, Juel K, Jensen GB. Increasing prevalence despite decreasing incidence of ischaemic heart disease and myocardial infarction: a national register based perspective in Denmark, 1980‐2009. Eur J Prev Cardiol. 2015;22:189–195. doi: 10.1177/2047487313509495 [DOI] [PubMed] [Google Scholar]
- 2. Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT. 25 Year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. doi: 10.1136/bmj.e356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sundbøll J, Horváth‐Puhó E, Schmidt M, Pedersen L, Henderson VW, Bøtker HE, Sørensen HT. Long‐term risk of stroke in myocardial infarction survivors: thirty‐year population‐based cohort study. Stroke. 2016;47:1727–1733. doi: 10.1161/STROKEAHA.116.013321 [DOI] [PubMed] [Google Scholar]
- 4. Sundbøll J, Horváth‐Puhó E, Adelborg K, Schmidt M, Pedersen L, Bøtker HE, Henderson VW, Sørenson HT. Higher risk of vascular dementia in myocardial infarction survivors. Circulation. 2018;137:567–577. doi: 10.1161/CIRCULATIONAHA.117.029127 [DOI] [PubMed] [Google Scholar]
- 5. Hong CT, Hu HH, Chan L, Bai CH. Prevalent cerebrovascular and cardiovascular disease in people with Parkinson's disease: a meta‐analysis. Clin Epidemiol. 2018;10:1147–1154. doi: 10.2147/CLEP.S163493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JH, Kim DH, Park YG, Kwon DY, Choi M, Jung JH, Han K. Association of Parkinson disease with risk of cardiovascular disease and all‐cause mortality: a nationwide population‐based cohort study. Circulation. 2020;141:1205–1207. doi: 10.1161/CIRCULATIONAHA.119.044948 [DOI] [PubMed] [Google Scholar]
- 7. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 8. Korczyn AD. Vascular parkinsonism–characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61 [DOI] [PubMed] [Google Scholar]
- 9. de Laat KF, van Norden AGW, Gons RAR, van Uden IWM, Zwiers MP, Bloem BR, van Dijk EJ, de Leeuw F‐E. Cerebral white matter lesions and lacunar infarcts contribute to the presence of mild parkinsonian signs. Stroke. 2012;43:2574–2579. doi: 10.1161/STROKEAHA.112.657130 [DOI] [PubMed] [Google Scholar]
- 10. Abete P, Della‐Morte D, Gargiulo G, Basile C, Langellotto A, Galizia G, Testa G, Canonico V, Bonaduce D, Cacciatore F. Cognitive impairment and cardiovascular diseases in the elderly: a heart‐brain continuum hypothesis. Ageing Res Rev. 2014;18:41–52. doi: 10.1016/j.arr.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 11. Alvarez MVG, Evidente VGH. Understanding drug‐induced parkinsonism: separating pearls from oy‐sters. Neurology. 2008;70:e32–e34. doi: 10.1212/01.wnl.0000302255.49113.51 [DOI] [PubMed] [Google Scholar]
- 12. Morgan JC, Sethi KD. Drug‐induced tremors. Lancet Neurol. 2005;4:866–876. doi: 10.1016/S1474-4422(05)70250-7 [DOI] [PubMed] [Google Scholar]
- 13. Potashkin J, Huang X, Becker C, Chen H, Foltynie T, Marras C. Understanding the links between cardiovascular disease and Parkinson's disease. Mov Disord. 2020;35:55–74. doi: 10.1002/mds.27836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scigliano G, Musicco M, Soliveri P, Piccolo I, Ronchetti G, Girotti F. Reduced risk factors for vascular disorders in Parkinson disease patients: a case‐control study. Stroke. 2006;37:1184–1188. doi: 10.1161/01.STR.0000217384.03237.9c [DOI] [PubMed] [Google Scholar]
- 15. Vikdahl M, Bäckman L, Johansson I, Forsgren L, Håglin L. Cardiovascular risk factors and the risk of Parkinson's disease. Eur J Clin Nutr. 2015;69:729–733. doi: 10.1038/ejcn.2014.259 [DOI] [PubMed] [Google Scholar]
- 16. Rozani V, Gurevich T, Giladi N, El‐Ad B, Tsamir J, Hemo B, Peretz C. Higher serum cholesterol and decreased Parkinson's disease risk: a statin‐free cohort study. Mov Disord. 2018;33:1298–1305. doi: 10.1002/mds.27413 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, Sørensen HT. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 19. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heide‐Jørgensen U, Adelborg K, Kahlert J, Sørensen HT, Pedersen L. Sampling strategies for selecting general population comparison cohorts. Clin Epidemiol. 2018;10:1325–1337. doi: 10.2147/CLEP.S164456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case‐control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39:54–57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 23. Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39:95–98. doi: 10.1177/1403494811408483 [DOI] [PubMed] [Google Scholar]
- 24. Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44:445–448. [PubMed] [Google Scholar]
- 25. Shin HW, Chung SJ. Drug‐induced parkinsonism. J Clin Neurol. 2012;8:15–21. doi: 10.3988/jcn.2012.8.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundbøll J, Schmidt M, Adelborg K, Pedersen L, Bøtker HE, Videbech P, Sørensen HT. Impact of pre‐admission depression on mortality following myocardial infarction. Br J Psychiatry. 2017;210:356–361. doi: 10.1192/bjp.bp.116.194605 [DOI] [PubMed] [Google Scholar]
- 27. Adelborg K, Schmidt M, Sundbøll J, Pedersen L, Videbech P, Bøtker HE, Egstrup K, Sørensen HT, Egstrup K, Sorensen HT. Mortality risk among heart failure patients with depression: a nationwide population‐based cohort study. J Am Heart Assoc. 2016;5:e004137. doi: 10.1161/JAHA.116.004137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therneau T. Modeling Survival Data: Extending the Cox Model. Springer Science & Business Media; 2000:44–48. [Google Scholar]
- 29. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 30. Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang F, Johansson ALV, Pedersen NL, Fang F, Gatz M, Wirdefeldt K. Socioeconomic status in relation to Parkinson's disease risk and mortality: a population‐based prospective study. Medicine (Baltimore). 2016;95:e4337. doi: 10.1097/MD.0000000000004337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 33. Linder J, Stenlund H, Forsgren L. Incidence of Parkinson's disease and parkinsonism in northern Sweden: a population‐based study. Mov Disord. 2010;25:341–348. doi: 10.1002/mds.22987 [DOI] [PubMed] [Google Scholar]
- 34. Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990 [DOI] [PubMed] [Google Scholar]
- 35. Hernán MA, Zhang SM, Rueda‐deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson's disease in two prospective studies. Ann Neurol. 2001;50:780–786. doi: 10.1002/ana.10028 [DOI] [PubMed] [Google Scholar]
- 36. de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson's disease. Am J Epidemiol. 2006;164:998–1002. doi: 10.1093/aje/kwj283 [DOI] [PubMed] [Google Scholar]
- 37. Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67:1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5 [DOI] [PubMed] [Google Scholar]
- 38. Roos E, Grotta A, Yang F, Bellocco R, Ye W, Adami HO, Wirdefeldt K, Trolle LY. Body mass index, sitting time, and risk of Parkinson disease. Neurology. 2018;90:e1413–e1417. doi: 10.1212/WNL.0000000000005328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ragonese P, D'Amelio M, Callari G, Di Benedetto N, Palmeri B, Mazzola MA, Terruso V, Salemi G, Savettieri G, Aridon P. Body mass index does not change before Parkinson's disease onset. Eur J Neurol. 2008;15:965–968. doi: 10.1111/j.1468-1331.2008.02236.x [DOI] [PubMed] [Google Scholar]
- 40. Qiu C, Hu G, Kivipelto M, Laatikainen T, Antikainen R, Fratiglioni L, Jousilahti P, Tuomilehto J. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension. 2011;57:1094–1100. doi: 10.1161/HYPERTENSIONAHA.111.171249 [DOI] [PubMed] [Google Scholar]
- 41. Coloma PM, Valkhoff VE, Mazzaglia G, Nielsson MS, Pedersen L, Molokhia M, Mosseveld M, Morabito P, Schuemie MJ, van der Lei J, et al. Identification of acute myocardial infarction from electronic healthcare records using different disease coding systems: a validation study in three European countries. BMJ Open. 2013;3:e002862. doi: 10.1136/bmjopen-2013-002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316:1043–1047. doi: 10.1136/bmj.316.7137.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figure S1


