Abstract
To further elucidate the induction process of the carbapenem-hydrolyzing β-lactamase of Ambler class A, NmcA, ampD genes of the wild-type (WT) strain and of ceftazidime-resistant mutants of Enterobacter cloacae NOR-1 were cloned and tested in transcomplementation experiments. Ceftazidime-resistant E. cloacae NOR-1 mutants exhibited derepressed expression of the AmpC-type cephalosporinase and of the carbapenem-hydrolyzing β-lactamase NmcA. The ampD genes of Escherichia coli and E. cloacae WT NOR-1 transcomplemented the ceftazidime-resistant E. cloacae NOR-1 mutants to the WT level of β-lactamase expression, while the mutated ampD alleles of E. cloacae NOR-1 failed to do so. The deduced E. cloacae NOR-1 WT AmpD protein exhibited 95 and 91% amino acid identity with the E. cloacae O29 and E. cloacae 14 WT AmpD proteins, respectively. Of the 12 ceftazidime-resistant E. cloacae NOR-1 strains, 3 had AmpD proteins with amino acid changes, while the others had truncated AmpD proteins. Most of these mutations were located outside the conserved regions that link the AmpD proteins to the cell wall hydrolases. AmpD from E. cloacae NOR-1 is involved in the regulation of expression of both β-lactamases (NmcA and AmpC), suggesting that structurally unrelated genes may be under the control of an identical genetic system.
Several enterobacterial species express inducible, chromosomally encoded AmpC-type β-lactamases (cephalosporinase) (11, 37), which are class C enzymes according to Ambler's classification (2). The regulation of AmpC β-lactamase expression is intimately linked to cell wall recycling and involves at least three genes: ampR, which encodes a transcriptional regulator of the LysR family; ampG, which encodes a transmembrane permease; and ampD, which encodes a cytosolic N-acetyl-anhydromuramyl-l-alanine amidase hydrolyzing 1,6-anhydromuropeptides (11, 12, 15, 16, 19, 31). In the absence of β-lactam inducer, AmpR is repressed by the murein precursor UDP- MurNAc-pentapeptide (uridine-pyrophosphoryl-N-acetylmuramyl-l-alanyl-d-glutamyl-meso-diaminopimelic acid-d-alanyl-d-alanine) (14). Since β-lactams interfere with murein synthesis, their actions lead to an increased periplasmic accumulation of degradation products, such as 1,6-anhydromuropeptides, which are signal molecules for β-lactamase induction (8, 15). AmpG transports these products from periplasm to cytoplasm, where they are cleaved by AmpD, which acts as a negative regulator of AmpC β-lactamase expression (8, 15, 19, 31). In ampD mutants, the constitutive overproduction of AmpC β-lactamase is associated with an accumulation of an M tripeptide (monosaccharide tripeptide) (1,6-anhydro-N-acetylmuramyl-l-alanyl-d-glutamyl-meso-diaminopimelic acid) and an M pentapeptide (1,6-anhydro-N-acetylmuramyl-l-alanyl-d-glutamyl-meso-diaminopimelic acid-d-alanyl-d-alanine) in the cytoplasm (8, 15). Jacobs et al. suggested that the M tripeptide could be the AmpR-activating ligand, since this product can relieve the repressed state of AmpR in vitro, resulting in the activation of β-lactamase expression (14). However, potential interactions of the M pentapeptide with AmpR have not been investigated. Another gene, ampE, which encodes a transmembrane protein, forms an operon with ampD, but this gene is not involved in β-lactamase expression (11, 13, 32).
Several chromosomally mediated Ambler class A β-lactamases are regulated in a manner similar to AmpC. In Proteus vulgaris, the chromosomal class A β-lactamase CumA is under the control of CumR, a LysR-type regulator, and is dependent on the presence of CumD (an AmpD analog) and CumG (an AmpG analog) for its induction (7). Similar observations have been made for Citrobacter koseri (formerly Citrobacter diversus) where CdiA, a class A β-lactamase, is under the control of CdiR (17) and for Burkholderia cepacia, which expresses a class A β-lactamase, PenA, that is under the control of a transcriptional regulator, PenR (41). Serratia fonticola expresses an inducible oxyimino cephalosporin-hydrolyzing class A β-lactamase named SFO-1 (33). This enzyme was recently found to be plasmid mediated in Enterobacter cloacae 8009 and regulated by an AmpR-type regulator (24). An inducible β-lactamase from Proteus penneri that is closely related to class A β-lactamases from a biochemical point of view has been described (25).
In E. cloacae NOR-1, in addition to the inducible AmpC β-lactamase that is under the control of the transcriptional regulator AmpR (29, 30), a second β-lactamase, NmcA, had been identified (30). NmcA, unlike most carbapenem-hydrolyzing β-lactamases that are involved in acquired carbapenem resistance, belongs to Ambler class A (26). NmcA expression is also regulated by the presence of a LysR-type regulator, NmcR, which is necessary for NmcA expression and induction (26, 30). To further elucidate the NmcA induction process, ampD genes of the wild-type (WT) NOR-1 strain and of ceftazidime-resistant E. cloacae NOR-1 (NOR-1D) mutants were cloned and tested in transcomplementation experiments. Furthermore, the inducibility of NmcA expression was tested when nmcA and nmcR were located on a plasmid at different copy numbers.
MATERIALS AND METHODS
Bacterial strains, antimicrobial agents, and MIC determinations.
The strains and plasmids used in this study are described in Table 1. Electrocompetent Escherichia coli DH10B (Life Technologies, Eragny, France) was used as host for construction and propagation of recombinant plasmids. Electrocompetent E. coli MC4100, E. coli JRG582, E. cloacae MHN1, E. cloacae MHN2, and E. cloacae NOR-1 variants were prepared as described previously (34). Bacterial cells were grown in Trypticase Soy (TS) broth or on TS agar plates (Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France). When required, kanamycin (50 μg/ml), chloramphenicol (30 μg/ml), and spectinomycin (50 μg/ml) were added.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Source or reference(s) |
|---|---|---|
| Strains | ||
| E. coli DH10B | F′ mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 araΔ139 Δ(ara leu)7697 galU galK λ− rpsL endA1 nupG | Gibco-BRL-Life Technologies, Eragny, France |
| E. cloacae NOR-1 | Inducible AmpC and NmcA β-lactamases | 26, 30 |
| E. cloacae NOR-1D1→12b | Ceftazidime-resistant mutants of E. cloacae NOR-1 | This work |
| E. cloacae MHN1 | Inducible AmpC β-lactamase | 29 |
| E. cloacae MHN2 | ΔampDE derivative of E. cloacae MHN1 | 29 |
| E. coli MC4100 | ΔampC | 13 |
| E. coli JRG582 | ΔampC ΔampDE | 13 |
| Plasmids | ||
| pK19 | Neor/Kanr | 36 |
| pACYC184 | Cmr/Tetr | 5 |
| pGB2 | Strr/Spcr | 6 |
| pNH5 | ampD of E. coli | 13 |
| pPCRScriptCm | Cmr | Stratagene |
| pPTN-1 | 5.2-kb Sau3AI fragment from E. cloacae NOR-1 containing nmcA and nmcR into pACYC184 BamHI | 26, 30 |
| pPTN-3 | NaeI fragment from pPTN-1 containing nmcA and nmcR into pK19 SmaI | This report |
| pPTN-7 | Blunt-ended NaeI fragment from pPTN-1 containing nmcA and nmcR into blunt-ended pACYC184 BamHI | This report |
| pPTN-9 | NaeI fragment from pPTN-1 containing nmcA and nmcR into pGB2 | This report |
| pMS1–12 | E. cloacae NOR-1 ampD1–12 mutant genes cloned into pPCRScript | This report |
| pMS13 | E. cloacae NOR-1 WT ampD gene cloned into pPCRScript | This report |
Antibiotic resistance phenotypes for plasmids: Neor/Kanr, neomycin or kanamycin resistance; Cmr/Tetr, chloramphenicol or tetracycline resistance; Strr/Spcr, streptomycin or spectinomycin resistance.
The subscript 1→12 indicates the ceftazidime-resistant E. cloacae NOR-1D mutants.
Routine antibiograms were determined by the disk diffusion method on Mueller-Hinton (MH) agar plates (Sanofi Diagnostics Pasteur). The antimicrobial agents and their sources have been described elsewhere (27, 30). MICs of selected β-lactams were determined by an agar dilution technique on MH agar plates with a Steers multiple inoculator and an inoculum of 104 CFU per spot (27, 30, 34). All plates were incubated at 37°C for 18 h. MICs of β-lactams were determined alone or with a fixed concentration of either clavulanic acid (2 μg/ml) or tazobactam (4 μg/ml). MIC results were interpreted according to the National Committee for Clinical Laboratory Standards guidelines (28).
In vitro selection of extended-spectrum cephalosporin-resistant mutants.
Frequencies of in vitro selection of antibiotic-resistant mutants were determined by counting the number of colonies that arose by plating a large inoculum (109 CFU) of E. cloacae NOR-1 on MH agar plates containing ceftazidime (32 μg/ml).
Kinetic measurements.
β-Lactamase extracts were obtained as described previously (34). The specific β-lactamase activity of the extracts was measured by UV spectrophotometry (ULTROSPEC 2000 spectrophotometer; Amersham Pharmacia Biotech, Orsay, France) as described previously (34). Basal and induced β-lactamase levels were determined as previously described (34). β-Lactamase activity was induced with imipenem (10 μg/ml) and cefoxitin (2 μg/ml for E. cloacae WT NOR-1 and 50 μg/ml for ceftazidime-resistant E. cloacae NOR-1D mutants). The specific β-lactamase activities were obtained as previously described with cephalothin and imipenem as substrates (30, 34). One unit of enzyme activity was defined as the activity which hydrolyzes 1 μmol of cephalothin or imipenem per min. The total protein content was measured with the Bio-Rad DC protein assay kit (Bio-Rad, Ivry/Seine, France).
Hydridization, PCR analyses, and sequencing.
Standard PCR experiments were performed as described previously (36). All the PCR amplifications were performed using the following amplification program: 10 min at 94°C; 35 cycles, with 1 cycle consisting of 1 min at 94°C, 1 min at 55°C, and 3 min at 72°C; followed by a final extension step of 10 min at 72°C. In order to PCR amplify the ampD genes of E. cloacae WT NOR-1 and mutant strains, two primers derived from the E. cloacae O-14 ampD sequence (18, 23) were synthesized. The sequences of the primers were as follows: AmpDF, 5′-ATGTTGTTAGAAAACGGATG-3′; and AmpDB, 5′-TCATGTTATCTCCTTATCTG-3′. A 564-bp DNA fragment encompassing the entire ampD gene was amplified by PCR with the Taq DNA polymerase (PE Biosystems, Les Ulis, France) and whole-cell DNAs of E. cloacae WT NOR-1 and E. cloacae NOR-1D mutants.
The amplicons were purified using the Qiaquick PCR purification kit (Qiagen). The nucleotide and amino acid sequences were analyzed using the software available at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov). Multiple-sequence alignment of deduced peptide sequences was performed online at the University of Cambridge website using the ClustalW program (http://www.ebi.uk/clustallW).
In order to screen for the presence of the ampD gene in E. cloacae NOR-1D mutants that were negative by PCR analysis, 2-μg samples of heat-denatured whole-cell DNAs were spotted onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech) and were subsequently UV cross-linked for 2 min (UV cross-linker; Stratagene, Amsterdam, The Netherlands). Hybridizations were performed as described by the manufacturer using the enhanced chemiluminescence nonradioactive labeling and detection kit (Amersham Pharmacia Biotech). The probe consisted of a 564-bp ampD PCR fragment from plasmid pMS13 (Table 1) that contained the entire E. cloacae NOR-1 ampD gene.
Recombinant DNA techniques and complementation experiments.
Recombinant DNA techniques were performed mostly by standard procedures (36). Whole-cell DNAs from E. cloacae WT NOR-1 and ceftazidime-resistant E. cloacae NOR-1D mutants were prepared as previously described (27). The restriction enzymes, Klenow DNA polymerase, and ligase were from Amersham Pharmacia Biotech. The Pfu thermostable DNA polymerase was from Stratagene. Ligation products were subjected to electroporation into E. coli or E. cloacae strains according to the manufacturer's instructions (Gene Pulser II; Bio-Rad). Recombinant bacteria were plated onto TS agar plates containing the appropriate antibiotic. Recombinant plasmid DNAs were prepared using Qiagen Mini and Maxi columns (Qiagen) (34). Plasmids were subsequently electroporated into the appropriate host (Table 1). Fragment sizes were estimated by comparison to the molecular size standard 1-kb DNA ladder (Life Technologies).
The ampD genes of E. cloacae WT NOR-1 and of 11 ceftazidime-resistant E. cloacae NOR-1D strains were PCR amplified using the Pfu thermostable polymerase (Stratagene). The two primers used were AmpD-RBS, which created a consensus ribosomal binding site (RBS) 5 bp upstream of the ATG start codon (AmpD-RBS; 5′-AAGGAGGATAC CATGTTGTTAGAAAACGGATGGC-3′) and AmpD-H3, which matched to the end of the ampD gene (AmpD-H3; 5′-AAAAAGCTTTCATGTTA-TCTCCTTATCTGACG-3′). These PCR fragments were then cloned into pPCRScript, downstream of the lac promoter, yielding plasmids pMS1→12 (plasmids pMS1 to pMS12) that contained the mutated ampD1→12 (ampD1 to ampD12) alleles and pMS13 that contained the WT ampD allele (Table 1). The sequences of the cloned PCR-generated DNA fragments were confirmed by complete resequencing.
Plasmids pPTN-3, pPTN-7, and pPTN-9 were constructed by cloning a 2.2-kb NaeI fragment of plasmid pPTN-1 (26) into a SmaI-digested pK19 plasmid, a blunt-ended BamHI site of pACYC184 plasmid, and a SmaI-digested pGB2 plasmid, respectively (Table 1). The recombinant plasmids were then used in induction experiments in host E. coli strains.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the GenBank-EMBL-DDBJ nucleotide databases under the accession nos. AF298868 to AF298879.
RESULTS AND DISCUSSION
Mutant selection and analysis of their biochemical properties.
Twelve independent E. cloacae NOR-1 cultures were grown in 10-ml portions of TS broth for 16 h and were subsequently plated on ceftazidime-containing TS plates. An average of 10 to 100 colonies per 109 plated bacteria grew, resulting in a mutation frequency of 10−7 to 10−8. From each independent experiment, the antibiotic susceptibility of three colonies was checked on a routine antibiogram. In addition to the usual phenotype observed in E. cloacae of a derepressed cephalosporinase expression, the inhibition zone around the imipenem disk was reduced, suggesting that the expression of the carbapenem-hydrolyzing β-lactamase NmcA might also have been modified. Furthermore, clavulanic acid restored part of the susceptibility to imipenem. One clone was retained for further analysis from each independent experiment. Subsequently, the MICs of selected β-lactams for the 12 ceftazidime-resistant strains of E. cloacae NOR-1D1→12 (strains NOR-1D1 to NOR-1D12) and the E. cloacae WT NOR-1 strains were determined. The ceftazidime and imipenem MICs confirmed the antibiogram observations (see Table 4).
TABLE 4.
MICs of selected β-lactams and E. cloacae NmcA specific β-lactamase activities in different host strains
| Strain | Relevant genotype | MIC (μg/ml)a
|
β-Lactamase activityb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATM | ATM-CA | CAZ | CAZ-CA | IMP | IMP-CA | Basal | Inducedc | Ratio | ||
| E. cloacae | ||||||||||
| NOR-1 | WT | 16 | 0.12 | 0.5 | 0.5 | 8 | 1 | 0.85 | 27 | 31 |
| NOR-1D3 | CAZr mutant | 128 | 128 | 32 | 32 | 16 | 2 | 55 | 175 | 3 |
| NOR-1D1→12 | CAZr mutant | 256 | 128 | 256 | 256 | 32 | 2 | 475 | 480 | 1 |
| NOR-1D1/pNH5 | CAZr mutant with E. coli ampD | 128 | 64 | 32 | 32 | 16 | 1 | 29 | 285 | 10 |
| NOR-1D1/pSM13 | CAZr mutant with ampD from NOR-1 | 64 | 32 | 8 | 8 | 8 | 1 | 4.5 | 75 | 16 |
| NOR-1D1/pSM3 | CAZr mutant with ampD from NOR-1D3 | 128 | 128 | 64 | 64 | 16 | 2 | 320 | 350 | 1 |
| NOR-1D1/pSM1→12 | CAZr mutant with ampD from NOR-1D1→12 | 256 | 128 | 256 | 256 | 32 | 1 | 470 | 465 | 1 |
| MHN2d | ΔampD/Ee | 64 | 64 | 256 | 256 | 0.5 | 0.5 | 20,000 | 19,000 | 1 |
| MHN2/pNH5d | ΔampD/E, E. coli ampD | 32 | 32 | 32 | 32 | 0.5 | 0.5 | 685 | 4,890 | 7 |
| MHN2/pSM13d | ΔampD/E, ampD from NOR-1 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 0.75 | 20 | 27 |
| MHN2/pSM1→12d | ΔampD/E, ampD from NOR-1D1→12 | 128 | 64 | 256 | 256 | 0.5 | 0.5 | 20,500 | 18,300 | 1 |
| E. coli | ||||||||||
| MC4100 | WT | 0.06 | 0.06 | 0.25 | 0.12 | 0.12 | 0.12 | NDf | ND | |
| MC4100/pPTN-3 | WT strain with nmcA and nmcR (400 copies) | 512 | 128 | 2 | 0.25 | >128 | 8 | 1,220 | 1,435 | 1 |
| MC4100/pPTN-7 | WT strain with nmcA and nmcR (20–40 copies) | 128 | 32 | 1 | 0.25 | 128 | 4 | 455 | 910 | 2 |
| MC4100/pPTN-9 | WT strain with nmcA and nmcR (1–5 copies) | 64 | 4 | 0.5 | 0.25 | 64 | 2 | 200 | 710 | 4 |
| JRG582 | ΔampD mutant | 0.06 | 0.06 | 0.03 | 0.03 | 0.12 | 0.12 | ND | ND | |
| JRG582/pPTN-3 | ΔampD mutant with nmcA and nmcR (400 copies) | >512 | 256 | 1 | 0.25 | >128 | 32 | 2,720 | 1,690 | 1 |
| JRG582/pPTN-7 | ΔampD mutant with nmcA and nmcR (20–40 copies) | >512 | 128 | 2 | 0.5 | >128 | 32 | 2,400 | 2,090 | 1 |
| JRG582/pPTN-9 | ΔampD mutant with nmcA and nmcR (1–5 copies) | 256 | 64 | 2 | 0.5 | >128 | 16 | 1,150 | 1,020 | 1 |
| JRG582/pPTN-9/pSM13 | ΔampD mutant with nmcA and nmcR and ampDNOR-1 | 64 | 16 | 0.25 | 0.12 | 8 | 1 | 9.5 | 120 | 12 |
Drug abbreviations: ATM, aztreonam; CAZ, ceftazidime; IPM, imipenem; CA, clavulanic acid.
Specific activity (in micromoles per minute per milligram of protein). Imipenem was used as a substrate, unless indicated otherwise. The β-lactamase activities are geometric mean determinations for three independent cultures. The standard deviations were within 10% for each strain.
Induced with cefoxitin.
Cephalothin was used as the substrate for the determination of the specific activities for these strains.
ΔampD/E, deletion of ampD or ampE.
ND, not determined.
In order to investigate the molecular mechanism of this increased imipenem resistance, enzymatic assays were performed with and without induction. The results clearly indicated that when cephalothin, which is a substrate of both the AmpC and NmcA β-lactamases (Table 2) was used, an increase in the specific activity of more than 1,000-fold was observed in the mutant strains compared to that of the WT strain. Furthermore, the mutants lost their ability of induction and had a high level of constitutive β-lactamase expression. When imipenem was used as a substrate, merely the activity of the carbapenem-hydrolyzing β-lactamase NmcA was measured. Again, the mutants had an increased specific activity towards imipenem (500-fold) with a loss of induction. Interestingly, E. cloacae NOR-1D3 had only a partially derepressed expression of NmcA that was still induced (Table 2). Thus, the induction and derepression of NmcA seemed to follow the same regulation pathway as observed for cephalosporinase. In order to verify this hypothesis, the ampD genes of the mutants were sequenced and compared to the WT ampD gene.
TABLE 2.
β-Lactamase specific activities from cultures of WT and ceftazidime-resistant E. cloacae NOR-1 strains
| Substratea | Strainb | Basal β-lactamase activityc | Inducerd | Induced β-lactamase activityc | Fold increase in activity
|
|
|---|---|---|---|---|---|---|
| Induced/basal | Mutant/WT | |||||
| Cephalothin | NOR-1 | 6 | FOX | 170 | 28 | |
| IMP | 110 | 18 | ||||
| NOR-1D3 | 1,400 | FOX | 3,930 | 3 | 230 | |
| IMP | 2,950 | 2 | ||||
| NOR-1D1→12 | 10,000 ± 1,200 | FOX | 9,900 ± 970 | 1 | 1,670 | |
| IMP | 10,500 ± 950 | 1 | ||||
| Imipenem | NOR-1 | 0.85 | FOX | 27 | 31 | |
| IMP | 11 | 13 | ||||
| NOR-1D3 | 55 | FOX | 175 | 3 | 65 | |
| IMP | 170 | 3 | ||||
| NOR-1D1→12 | 475 ± 40 | FOX | 480 ± 70 | 1 | 560 | |
| IMP | 465 ± 70 | 1 | ||||
A substrate concentration of 100 μM was used.
NOR-1, the WT E. cloacae NOR-1 strain; NOR-1D3, E. cloacae NOR-1D mutant strain 3; NOR-1D1→12, E. cloacae NOR-1D mutant strains 1 to 12 but not including strains 3 and 7.
Specific activity (in micromoles per minute per milligram of protein). The β-lactamase activities are geometric mean determinations for three independent cultures. The standard deviations were within 10% for each individual strain. However, the standard deviations are shown whenever the values were those of cultures of several different strains.
FOX, cefoxitin (50 μg/ml); IMP, imipenem (10 μg/ml).
Cloning and sequencing of the ampD alleles.
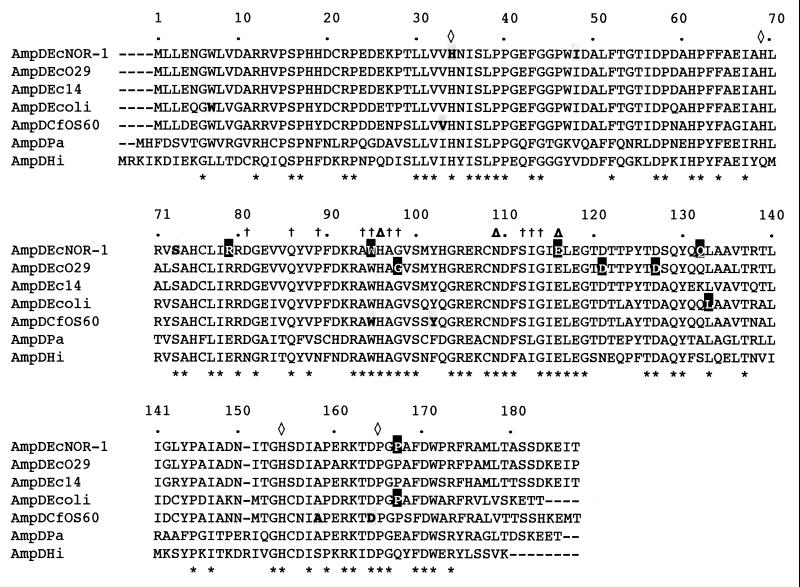
Nucleotide sequence comparison revealed significant divergence between E. cloacae ampD genes similar to that found among the Citrobacter freundii ampD genes (40). The deduced amino acid sequence of E. cloacae NOR-1 AmpD exhibited 95, 91, 83, 82, 56, and 52% identity with AmpD of E. cloacae O29 (9), E. cloacae 14 (18), E. coli (13), C. freundii OS60 (18), Pseudomonas aeruginosa (21) and with the putative Haemophilus influenzae AmpD protein (10), respectively. Amino acid sequence alignment of the AmpD proteins revealed several conserved motifs (Fig. 1). The conserved core region and the four strictly conserved residues outside this region, which relate the AmpD proteins of members of the family Enterobacteriaceae to the cell wall hydrolases of Bacillus spp. (16), were found in the E. cloacae NOR-1 AmpD. The high degree of identity of the AmpD proteins showed that AmpD of E. cloacae NOR-1 was closely related to its enterobacterial homologs and that they probably share a common mechanism of regulation of AmpC β-lactamase expression and murein metabolism.
FIG. 1.
Alignment of the AmpD amino acid sequence of E. cloacae NOR-1 (AmpDEcNOR-1) with those of E. cloacae O29 (AmpDEcO29) (9), E. cloacae 14 (AmpDEc14) (18), E. coli (AmpDEcoli) (13), C. freundii OS60 (AmpDCfOS600) (18), and P. aeruginosa (AmpDPa) (21) and a putative H. influenzae (AmpDHi) (10) AmpD protein. The identical amino acids are indicated by asterisks. The crosses and the open diamonds indicate the amino acids conserved in the core and outside region of the Bacillus cell wall hydrolases, respectively. The triangles show the amino acids strictly conserved in various cell wall hydrolases (16). The numbering used corresponds to the E. cloacae AmpD sequences. Amino acid substitutions that alter the activity of the AmpD protein (bold letters on gray shaded background) and mutated positions that yielded stop codons (white letters on black background) are indicated.
Of the 12 ceftazidime-resistant E. cloacae NOR-1D mutants, 3 had single amino acid changes in AmpD. Eight mutations resulted in premature termination of the protein by either creation of a stop codon at the site of mutation or by introducing a frameshift mutation leading to a stop codon located further downstream (Table 3 and Fig. 1). PCR amplification of the ampD gene of E. cloacae NOR-1D7 failed despite the use of different primer combinations. Dot blot hydridization experiments with an internal E. cloacae NOR-1 ampD probe indicated that E. cloacae NOR-1D7 still possessed an ampD gene. However, this gene may be mutated at the site of primer binding or may be partially deleted, thus lacking one primer-binding site. Interestingly, the amino acid substitutions were found in the N terminus of the protein, while the stop codons were mostly located within the C terminus. Sequence data from the literature indicated the importance of the carboxy portion of the AmpD protein for the inducible AmpC phenotype (11). Two mutations in the amino terminus which correlated with a fully derepressed phenotype have been characterized, a valine-to-glycine change at position 33 in E. cloacae (40) and a tryptophan-to-glycine change at position 7 in E. coli (13).
TABLE 3.
Amino acid differences among ampD gene products of ceftazidime-resistant E. cloacae NOR-1 mutants
| AmpD protein | Nucleotide and amino acid differences at positionsa:
|
Type of mutation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 | 143 | 218 | 235–236 | 284–285 | 346 | 394 | 496–502 | ||
| 34 | 48 | 73 | 79 | 95 | 116 | 132 | 166–167 | ||
| WT AmpD | C | T | C | C–G | GG | G | C | GGCCCGG | |
| H | I | S | R | W | E | Q | GP | ||
| AmpD1 | T | Missense | |||||||
| L | |||||||||
| AmpD2 | T | Missense | |||||||
| Y | |||||||||
| AmpD3 | G | Missense | |||||||
| S | |||||||||
| AmpD4 | T | Nonsense | |||||||
| ∗ | |||||||||
| AmpD5 | A | Nonsense | |||||||
| ∗ | |||||||||
| AmpD6 | G–––––G | 5-bp deletion | |||||||
| GL∗ | |||||||||
| AmpD7 | NDb | ||||||||
| AmpD8 | CAG | 1-bp insertion | |||||||
| QSRW∗ | |||||||||
| AmpD9 | T | Nonsense | |||||||
| ∗ | |||||||||
| AmpD10 | T | Nonsense | |||||||
| ∗ | |||||||||
| AmpD11 | A | Nonsense | |||||||
| ∗ | |||||||||
| AmpD12 | A | Nonsense | |||||||
| ∗ | |||||||||
The nucleotide and amino acid differences from the wild type at nucleotide and amino acid positions are shown in the pair of lines for each AmpD protein. For each pair of lines, the first line shows the nucleotide difference for nucleotide positions 100 to 502 (nucleotide numbering according to the E. cloacae NOR-1 WT ampD gene) and the second line shows the amino acid difference for amino acid positions 34 to 167 (in bold type) (amino acid numbering according to E. cloacae NOR-1 WT AmpD protein). ∗, stop codon; –, deletion.
ND, not determined. No PCR product obtained. Hybridization positive.
Here, we present further evidence that point mutations within the amino terminus lead to a fully derepressed phenotype for AmpC and NmcA expression. The histidine 34 residue, which is conserved among the AmpD proteins and also among the hydrolases, when replaced by tyrosine led to a fully derepressed phenotype (Fig. 1). A serine-to-leucine replacement at position 73 gave a similar phenotype. This last serine, even though conserved among the AmpD proteins, lies outside the conserved boxes shared with the hydrolases and thus may represent an AmpD-specific amino acid important for its activity. The isoleucine-to-serine substitution at position 48 gave a high basal level and still inducible phenotype. This position is not strictly conserved among AmpD proteins but contains a neutral amino acid residue, which may be involved in the proper folding of the enzyme.
The remaining mutations led to premature termination of AmpD proteins. Even though these mutations were scattered throughout the entire protein sequence, three were located at amino acid position 95. Indeed, of 12 independent mutations, 3 were located at this position. In C. freundii, this position was also found to be a site of mutation (40). The last 16 amino acids are important for the activity of AmpD, since the deletion of these amino acids led to a derepressed phenotype. This observation has been made with an AmpD mutant of E. coli (13). Most mutations identified in the present work were located at positions that have never been reported and were mostly located outside of the known conserved motifs. The 11 ampD mutations identified in this work expand the number of known mutations leading to altered Bush group 1 and 2e β-lactamase expression (4). They support previous findings demonstrating the essential nature of the carboxy terminus of the mature protein but also revealed key positions at the amino terminus of the protein that are also important for the activity. Mutations introducing stop codons are primarily encountered in the C-terminal portion, while mutations introducing amino acid substitutions are mostly encountered in the N-terminal portion.
In clinical and laboratory isolates of E. cloacae, C. freundii, and P. aeruginosa, several phenotypes of altered β-lactamase expression have also been described (11, 37). In enterobacteria, three of four phenotypes of altered β-lactamase expression have been associated with mutations in ampD (3, 9, 13, 18, 23, 40). They include wild-type (normal induction), hyperinducible (higher basal level of AmpC expression and high-level induction in the presence of low levels of inducing drugs), and stably derepressed. In our study, these three phenotypes were found. Kuga et al. have shown that mutations in ampR may also lead to high-level AmpC expression, and the mutation frequency was 10−6 as tested from a plasmid carrying ampC or ampR in an ampD-deficient E. coli strain (20). Since the mutation frequencies of ampD were 10−7 to 10−8, mutations in nmcR and/or ampR of E. cloacae NOR-1 also should have been detected. However, of the 12 mutants studied, all had a mutation in the ampD sequence. Sequencing of nmcR from E. cloacae NOR-1D1 and NOR-1D3 revealed WT sequences (data not shown). nmcR mutations may occur only after ampD mutations and thus may increase the resistance of the strains even more. The frequency of mutation to beta-lactam resistance via mutations in nmcR is probably always at least 10-fold lower than mutations in ampD, since the former type of mutation must not affect the ability of the regulator to interact properly with its target sequence, while any mutations affecting the amidase activity of AmpD will have a phenotype. This issue will be addressed in future investigations. In addition, it is not known whether nmcR mutations have an impact on the expression of a single copy of the nmcA gene present on the chromosome in the absence of an ampD mutation. Using the E. coli host strains and the induction assay based on a low-copy-number plasmid carrying nmcA and nmcR, we will be able to investigate the role of nmcR mutations.
Complementation of ceftazidime-resistant E. cloacae NOR-1 strains.
In order to know whether the ampD mutations were responsible for the observed phenotype, the ampD genes were cloned onto a high-copy-number plasmid, resulting in plasmids pMS1→12 and tested in transcomplementation experiments. The WT ampD gene of E. coli and E. cloacae NOR-1, as expressed from plasmids pNH5 (13) and pMS-13, respectively, transcomplemented the ceftazidime-resistant E. cloacae NOR-1D strains to low-level β-lactam resistance (decrease in ceftazidime and imipenem MICs) and restored WT β-lactamase expression (low basal level and inducibility) (Table 4). The induced/noninduced ratio of β-lactamase activity was 9.5 in cells producing the E. coli AmpD protein, while in cells expressing the E. cloacae NOR-1 AmpD protein, this ratio was 16. This difference could reflect differences in expression levels of the AmpD proteins or species-specific differences in activity.
The 11 mutated ampD alleles failed to restore a low-level and inducible phenotype, indicating that the single ampD mutation was sufficient to inactivate AmpD. The mutated ampD3 allele was capable of partial recovering of an inducible phenotype. These results showed that the cloned WT ampD allele is sufficient to restore the WT NmcA expression level, thus confirming that AmpD is involved in the induction of NmcA.
Similarly, the WT ampD genes of E. coli and E. cloacae NOR-1, as expressed from plasmids pNH5 and pMS-13, respectively, transcomplemented the derepressed E. cloacae MNH2 mutants to low-level β-lactam resistance and WT β-lactamase expression (low basal level and inducibility). Taken together, these results clearly showed that the WT AmpD protein was sufficient to restore basal and inducible β-lactamase expression of NmcA and AmpC β-lactamases.
Complementation of E. coli ampD mutants.
To determine the ability of the E. cloacae NOR-1 ampD gene to complement E. coli ampD mutations, plasmids pSM13 and pSM1→12 were transformed into E. coli JRG582 (ΔampDE) and E. coli MC4100 (ampDE+) (13) containing plasmid pPTN-9. pPTN-9 is a low-copy-number plasmid that carries the nmcA and nmcR genes from E. cloacae NOR-1. E. coli MC4100/pPTN-9 exhibits a high basal β-lactamase activity and is inducible, while E. coli JRG582/pPTN-9 has a fully derepressed phenotype (Table 4). These two strains were resistant to aztreonam and imipenem (Table 4). The ampD genes of E. coli and E. cloacae, as expressed from pNH5 and pMS-13, respectively, transcomplemented the E. coli ampDE mutant to low-level β-lactam resistance and WT β-lactamase expression (low basal level and inducibility) (Table 4). In addition, the 11 mutated alleles of ampD failed to restore an inducible phenotype of carbapenem-hydrolyzing β-lactamase (data not shown). These results showed that the cloned E. cloacae NOR-1 ampD gene expresses a functional AmpD protein in E. coli cells and that the mutations observed in the ampD gene also account for the observed phenotype in E. coli.
Recombinant plasmids pPTN-3, -7, and -9 carry the same nmcA and nmcR genes but at different copy numbers (Table 1). Induction assays and MICs (Table 4) revealed that β-lactamase activity was directly related to the plasmid copy number measured. However, the activity increase was not linearly related to the theoretical copy number of the plasmid. Furthermore, the induced/noninduced ratio is conversely related to its theoretical copy number.
Conclusions.
In order to determine whether genetic alterations leading to a derepressed NmcA expression phenotype of E. cloacae NOR-1 could be linked to ampD mutations, 12 independent ceftazidime-resistant E. cloacae NOR-1D strains were investigated. Of these 12 strains, 11 had point mutations in the ampD coding sequence leading to nonfunctional AmpD. These results along with the high percentage of identity observed among enterobacterial AmpD proteins strongly suggest that E. cloacae NOR-1 AmpD acts as an N-acetyl-anhydromuramyl-l-alanine amidase, which leads to a decreased amount of anhydromuropeptide (MTp), the signal molecule for β-lactamase expression (17).
Our data indicate that controls of the induction process are similar for NmcA and AmpC β-lactamases, suggesting that different structural genes may be under the control of identical regulatory systems. Such an observation has already been made for Aeromonas sobria, where three different β-lactamase genes are under the same two-component regulatory pathway that is not related to LysR-type regulation (1). It would be interesting to study the regulation of β-lactamase expression in Yersinia enterocolitica, an enterobacterial species that naturally contains an AmpC-type cephalosporinase and an Ambler class A β-lactamase (38, 39).
Finally, from a clinical point of view, our results showed that treatment with ceftazidime might select strains that are resistant to all available β-lactams through a single genetic event affecting the expression of two unrelated broad-spectrum β-lactamases.
ACKNOWLEDGMENTS
This work was funded in part by grants from the Ministères de l'Education Nationale et de la Recherche and the Université Paris XI (grant UPRES-JE 2227), Paris, France.
REFERENCES
- 1.Alksne L E, Rasmussen B A. Expression of the AsbA1, OXA-12, and AsbM1 β-lactamases in Aeromonas jandaei AER 14 is coordinated by a two-component regulon. J Bacteriol. 1997;179:2006–2013. doi: 10.1128/jb.179.6.2006-2013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambler R P, Coulson A F, Frère J-M, Ghuysen J M, Joris B, Forsman M, Lévesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett P M, Chopra I. Molecular basis of β-lactamase induction in bacteria. Antimicrob Agents Chemother. 1993;37:153–158. doi: 10.1128/aac.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 7.Datz M, Joris B, Azab E A M, Galleni M, Van Beeumen J, Frère J-M, Martin H H. A common system controls the induction of very different genes: the class-A β-lactamase of Proteus vulgaris and the enterobacterial class-C β-lactamase. Eur J Biochem. 1994;226:149–157. doi: 10.1111/j.1432-1033.1994.tb20036.x. [DOI] [PubMed] [Google Scholar]
- 8.Dietz H, Pfeifle D, Wiedemann B. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob Agents Chemother. 1997;41:2113–2120. doi: 10.1128/aac.41.10.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrhardt A F, Sanders C C, Romero J R, Leser J S. Sequencing and analysis of four new Enterobacter ampD alleles. Antimicrob Agents Chemother. 1996;40:1953–1956. doi: 10.1128/aac.40.8.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Hanson N D, Sanders C C. Regulation of inducible AmpC beta-lactamase expression among Enterobactericeae. Curr Pharm Des. 1999;5:881–894. [PubMed] [Google Scholar]
- 12.Höltje J-V, Kopp U, Ursinus A, Wiedemann B. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-L-alanine amidase. FEMS Microbiol Lett. 1994;122:159–164. doi: 10.1111/j.1574-6968.1994.tb07159.x. [DOI] [PubMed] [Google Scholar]
- 13.Honoré N, Nicolas M H, Cole S T. Regulation of enterobacterial cephalosporinase production: the role of a membrane-bound sensory transducer. Mol Microbiol. 1989;3:1121–1130. doi: 10.1111/j.1365-2958.1989.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs C, Frère J-M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs C, Huang L J, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs C, Joris B, Jamin M, Klarsov K, Van Beeumen J, Mengin-Lecreulx D, van Heijenoort J, Park J T, Normark S, Frère J-M. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-L-alanine amidase. Mol Microbiol. 1995;15:553–559. doi: 10.1111/j.1365-2958.1995.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones M E, Bennett P M. Inducible expression of the chromosomal cdiA from Citrobacter diversus NF85, encoding an Ambler class A beta-lactamase, is under similar genetic control to the chromosomal ampC, encoding an Ambler class C enzyme, from Citrobacter freundii OS60. Microb Drug Resist. 1995;1:285–291. doi: 10.1089/mdr.1995.1.285. [DOI] [PubMed] [Google Scholar]
- 18.Kopp U, Wiedemann B, Lindquist S, Normark S. Sequences of wild-type and mutant ampD genes of Citrobacter freundii and Enterobacter cloacae. Antimicrob Agents Chemother. 1993;37:224–228. doi: 10.1128/aac.37.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korfmann G, Sanders C C. ampG is essential for high-level expression of AmpC β-lactamase in Enterobacter cloacae. Antimicrob Agents Chemother. 1989;33:1946–1951. doi: 10.1128/aac.33.11.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuga A, Okamoto R, Inoue M. ampR gene mutations that greatly increase class C β-lactamase activity in Enterobacter cloacae. Antimicrob Agents Chemother. 2000;44:561–567. doi: 10.1128/aac.44.3.561-567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langaee T Y, Dargis M, Huletsky A. An ampD gene in Pseudomonas aeruginosa encodes a negative regulator of AmpC β-lactamase expression. Antimicrob Agents Chemother. 1998;42:3296–3300. doi: 10.1128/aac.42.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langaee T Y, Gagnon L, Huletsky A. Inactivation of the ampD gene in Pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible AmpC β-lactamase expression. Antimicrob Agents Chemother. 2000;44:583–589. doi: 10.1128/aac.44.3.583-589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindquist S, Galleni M, Lindberg F, Normark S. Signalling proteins in enterobacterial AmpC β-lactamase regulation. Mol Microbiol. 1989;3:1091–1102. doi: 10.1111/j.1365-2958.1989.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto Y, Inoue M. Characterization of SFO-1, a plasmid-mediated inducible class A β-lactamase from Enterobacter cloacae. Antimicrob Agents Chemother. 1999;43:307–313. doi: 10.1128/aac.43.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miro E, Barthélémy M, Peduzzi J, Reynaud A, Morand A, Prats G, Labia R. Properties of a cephalosporinase produced by Proteus penneri inhibited by clavulanic acid. Pathol Biol. 1994;42:487–490. [PubMed] [Google Scholar]
- 26.Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc Natl Acad Sci USA. 1994;91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naas T, Vandel L, Sougakoff W, Livermore D M, Nordmann P. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A β-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob Agents Chemother. 1994;38:1262–1270. doi: 10.1128/aac.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 29.Nicolas M-H, Honoré N, Jarlier V, Philippon A, Cole S T. Molecular genetic analysis of cephalosporinase production and its role in β-lactam resistance in clinical isolates of Enterobacter cloacae. Antimicrob Agents Chemother. 1987;31:295–299. doi: 10.1128/aac.31.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordmann P, Mariotte S, Naas T, Labia R, Nicolas M-H. Biochemical properties of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and cloning of its gene into Escherichia coli. Antimicrob Agents Chemother. 1993;37:939–946. doi: 10.1128/aac.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Normark S. β-Lactamase induction in Gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb Drug Resist. 1995;1:111–114. doi: 10.1089/mdr.1995.1.111. [DOI] [PubMed] [Google Scholar]
- 32.Normark S, Bartowsky E, Erickson J, Jacobs C, Lindberg F, Lindquist S, Weston-Hafer K, Wikström M. Mechanisms of chromosomal β-lactamase induction in Gram-negative bacteria. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 485–503. [Google Scholar]
- 33.Peduzzi J, Farzaneh S, Reynaud A, Barthélémy M, Labia R. Characterization and amino acid sequence analysis of a new oxyimino cephalosporin-hydrolyzing class A beta-lactamase from Serratia fonticola CUV. Biochim Biophys Acta. 1997;1341:58–70. doi: 10.1016/s0167-4838(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 34.Poirel L, Guibert M, Girlich D, Naas T, Nordmann P. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob Agents Chemother. 1999;43:769–776. doi: 10.1128/aac.43.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pridmore R D. Versatile cloning vectors with kanamycin resistance marker. Gene. 1987;56:309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sanders C C, Sanders W E., Jr β-Lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin Infect Dis. 1992;15:825–839. doi: 10.1093/clind/15.5.824. [DOI] [PubMed] [Google Scholar]
- 38.Seoane A, Garcia Lobo J M. Nucleotide sequence of a new class A β-lactamase gene from the chromosome of Yersinia enterocolitica: implications for the evolution of class A β-lactamases. Mol Gen Genet. 1991;228:215–220. doi: 10.1007/BF00282468. [DOI] [PubMed] [Google Scholar]
- 39.Seoane A, Francia M V, Garcia Lobo J M. Nucleotide sequence of the ampC-ampR region from the chromosome of Yersinia enterocolitica. Antimicrob Agents Chemother. 1992;36:1049–1052. doi: 10.1128/aac.36.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton P, Shannon K, Phillips I. DNA sequence differences of ampD mutants of Citrobacter freundii. Antimicrob Agents Chemother. 1995;39:2494–2498. doi: 10.1128/aac.39.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trepanier S, Prince A, Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob Agents Chemother. 1997;41:2399–2405. doi: 10.1128/aac.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]