Abstract
Background
Hypertriglyceridemia is as an independent risk factor for cardiovascular disease (CVD). Apolipoprotein C-III (ApoC-III) is known to regulate triglyceride (TG) metabolism. However, the causal association between ApoC-III and CVD development is unclear. The objectives were to examine the impact of ApoC-III concentration on TG and lipoproteins and investigate the role of known rare loss-of-function APOC3 variants for modulating ApoC-III, TG concentrations and CVD risk in different ethnic groups.
Methods
Plasma ApoC-III levels were measured in a multiethnic sample of 518 individuals comprising 271 Asian Indians (Sikhs), 87 Caucasians, 80 African Americans, and 80 Hispanics.
Results
ApoC-III levels showed a robust association with TG in Asian Indians (r = 0.5, p = 1.1 × 10−23), Caucasians (r = 0.4, p = 7.2 × 10−4), and Hispanics (r = 0.9, p = 2.7x × 10−28). African Americans had lowest ApoC-III and TG concentrations and highest (44%) prevalence of coronary artery disease (CAD). ApoC-III levels correlated with fasting blood glucose (r = 0.25, p = 6.1 × 10−5) in Asian Indians and central adiposity in Hispanics (waist: r = 0.22, p = 0.05; waist-hip ratio: r = 0.24, p = 0.04). The carriers of rare variants IVS1-2G-A (rs373975305); A43T (rs147210663) and IVS3 + 1G-T (rs140621530) showed high TG but not low ApoC-III levels in Asian Indians and Caucasians.
Conclusion
These results highlight the challenges of generalizing antisense ApoC-III inhibition for treating atherosclerotic disease in dyslipidemia that may benefit only specific sub-populations. The observed ethnic differences in ApoC-III concentrations and CAD risk factors, emphasize in-depth genetic and metabolomics evaluations on diverse ancestries.
Keywords: Circulating triglycerides, Apolipoprotein C-III concentration, Lipid metabolites, Rare variants, Ethnicity, Cardiovascular disease
1. Introduction
Cardiovascular disease (CVD) is the number one killer in the United States (US) and disproportionately affects underrepresented and minority communities of the US. The prevalence rate of CVD is highest in South Asians, approximately 60%, while the numbers are equally staggering for African Americans (47%) and Hispanics (30%) [1], [2], [3]. Elevated plasma triglyceride (TG) is a strong independent predictor for CVD [4], [5], [6]. Genome-wide association studies (GWAS) have identified more than 150 common genetic variants related to blood lipids and triglyceride-rich lipoproteins (TRLs) to be associated with increased risk for CVD [7], [8]. Of these loci, we and others have reported a chromosomal region (11q23.3) containing the APOA5-APOA4-APOC3-APOA1 gene cluster to have a robust association with TRLs with strong reproducibility [9], [10], [11], [12], [13]. Fine mapping of this region (Chr.11q23.3) revealed an aggregate of six rare loss-of-function (LoF)/reduced-function mutations in the APOC3 gene, including a nonsense mutation (R19X), three splice-site mutations (IVS1-2G → A, IVS2 + 1G → A, and IVS3 + 1G → T), and two missense mutation (A43T and D65N) to be associated with reduced plasma TG levels and decreased risk of CVD mainly in European populations [14]. However, whether these null or reduced function variants are cardioprotective in the general population (including non-Europeans) is still unclear and controversial. Apolipoprotein C-III (ApoC-III) is an inhibitor of LPL (lipoprotein lipase), which hydrolyses TRLs, their intermediates, and chylomicron remnants [15]. The increased circulating ApoC-III levels trigger an increase in blood TG levels and obstruct their hepatic uptake. However, the causal association between ApoC-III and CVD development is unclear. We earlier investigated the effects of these reported LoF variants in the APOC3 gene with TG levels in Asian Indians (residing in India, Singapore, and the UK) and other ethnic groups, including African Americans, Caucasians, and Chinese. Our study could not validate these variants as cardioprotective in Asian Indians and even in UK Caucasians [16]. Similarly, a study by Crawford et al. could not confirm the rare LoF variant to be protective from CVD in the European Americans from BioVU biobank [17]. Also, findings of Caucasians from Iceland could not support the reported effects of APOC3 LoF mutations on the risk of CVD [18]. On the other hand, our Mendelian randomization analysis of a common APOC3 variant (rs5128) showed a robust association with an increase in plasma TG level leading to a mild increase in the risk of coronary artery disease (CAD) in Asian Indians [16]. In addition to affecting TG metabolism, ApoC-III is an independent predictor of CVD and insulin resistance [19]. However, only a handful of these association studies have measured ApoC-III levels in humans. Moreover, how these reduced function variants correlate with ApoC-III concentrations is largely unknown, especially in non-Europeans. Therefore, we have measured plasma ApoC-III levels in this study to explore its distribution in multiethnic populations subsets and additionally we also included all earlier identified LoF rare variants from our earlier published study [16] to measure ApoC-III levels and determine their risk associated with high TG (HTG), type 2 diabetes (T2D), and CVD.
2. Methods
2.1. Study groups
Plasma ApoC-III levels were measured in 518 individuals comprising of 271 Asian Indians (Sikhs), 87 Caucasians, 80 African Americans, and 80 Hispanics from Oklahoma. The 271 Punjabi Sikh individuals were from the Asian Indian Diabetic Heart Study/Sikh Diabetes Study (AIDHS/SDS) recruited from the Northern part of India from 2002 to 2010 [20], [21], [22], [23]. The AIDHS/SDS has unique characteristics that are ideal for genetic studies. Sikhs are strictly a nonsmoking population, and ≈50% of participants are teetotalers and life-long vegetarians. All individuals in the study had a comprehensive clinical assessment, family history, baseline anthropometric, and metabolic assessment at the time of recruitment, as mentioned previously. All blood samples were obtained at the baseline visit [20], [22], [23]. Body mass index (BMI) was calculated as weight (kg) / height (m)2. New guidelines of the World Health Organization for the BMI thresholds for Asians were followed [24]. T2D was defined based upon their medical records for symptoms and use of diabetic medications and measuring fasting glucose levels following the American Diabetes Association guidelines as described previously [25]. CAD was considered if there was the use of nitrate medication (nitroglycerine), electrocardiographic evidence of angina pain, coronary angiographic evidence of severe (greater than 50%) stenosis, or echocardiographic evidence of myocardial infarction as described previously. The diagnosis was based on the date of coronary artery bypass graft (CABG) or angioplasty and medication usage obtained from patient records as described previously [22], [26]. The selection of controls was based on a fasting glucose of <100.8 mg/dL or a 2-hour glucose <141.0 mg/dL. All AIDHS/SDS protocols and consent documents were reviewed and approved by the University of Oklahoma Health Science Center's Institutional Review Board (IRB) as well as the Human Subject Protection (Ethics) committees at the participating hospitals and institutes in India [23], [27], [28].
We also measured plasma ApoC-III in 87 Caucasians, 80 African Americans, and 80 Hispanics from the Metabolome in Ischemic Stroke Study (MISS) and Oklahoma Multiethnic CV Health Disparity Study (OLIVER) [16]. The MISS is a prospective ongoing study that began in 2017, investigating ischemic stroke biomarker predictors by utilizing genomics, metabolomics, and Omics technologies. The OLIVER is a multiethnic population-based study investigating cardiovascular disease health disparity in Oklahoma populations residing in rural and urban areas and patients from Oklahoma University Medical Center (OUMC). Enrollment included children and adults and recording anthropometry, medical history, and collection of blood samples for measurement of fasting glucose, lipid profile, and genotyping as described previously [16], [29], [30]. Inclusion criteria for this study involved subjects with and without T2D or heart disease or dyslipidemia. An equal number of low (TG <100 mg/dL) and high (TG > 150 mg/dL), nearly matched for age, gender and ethnicity, were included. Excluded were the patients with any cancer or the issues with immunodeficiency. Study subjects with TG <100 mg/dL were considered as having low TG (LTG) and TG > 150 mg/dL as high TG (HTG) based on NCEP/ATP III criteria [31]. We also included all individuals who were carriers of LoF variants from all ethnic groups identified in our previous publication using 396,644 individuals of multiethnic ancestries by Goyal et al. [16] to study their correlation with ApoC-III levels.
2.2. Metabolic assays
Serum lipids (total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and TG) were measured using standard enzymatic methods (Roche, Basel, Switzerland) as described previously [32], [33]. To study the impact of rare variants on ApoC-III levels and TG, and for investigating the correlation between ApoC-III concentration with TG, for this study, we studied individuals with low and high TG.
2.3. Circulating plasma ApoC-III level measurements using ELISA
ApoC-III concentrations were measured using frozen plasma aliquots by enzyme-linked immunosorbent assay (ELISA) kits from Thermofisher Scientific (Waltham, MA, USA) in accordance to the manufacturer's instructions. A solid-phase sandwich ELISA technology was used with a target-specific antibody pre-coated onto 96-well plates, and 1 μL of test samples were added to it and followed by the addition of a biotinylated detection polyclonal antibody and subsequent washing with 1× PBS (phosphate buffer saline) buffer repeatedly up to 4 times. The streptavidin-biotin-peroxidase complex was further added, and the unbound conjugates were removed by repeating the washing step. Horseradish peroxidase (HRP) substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was used to visualize HRP enzymatic reaction. TMB was catalyzed by HRP to produce a blue color product that turned yellow on the addition of an acidic stop solution. The density of yellow color formed was proportional to the ApoC-III concentration of each sample, and the optical density (OD) absorbance was measured at 450 nm by SmartReader (ACCURIS Instruments) microplate reader. Samples were blinded for ApoC-III measurements, and a standard curve was generated to extrapolate the ApoC-III concentrations of the test plasma samples as described previously [16].
2.4. Statistical analysis
Continuous variables demonstrating non-normal distribution were normalized using log-transformation before the analysis. Group descriptive statistics for clinical parameters were expressed as mean ± standard deviation. Pearson correlation analysis was performed to determine the linear association between ApoC-III and TG and other traits in each ethnic group. General linear model analyses were used to adjust for the age, gender, BMI, T2D, and CAD to determine the association of plasma ApoC-III concentrations with TG and other traits. Statistical analyses were performed using SPSS for Windows statistical package (version 27.0) (SPSS Inc., Chicago, USA).
3. Results
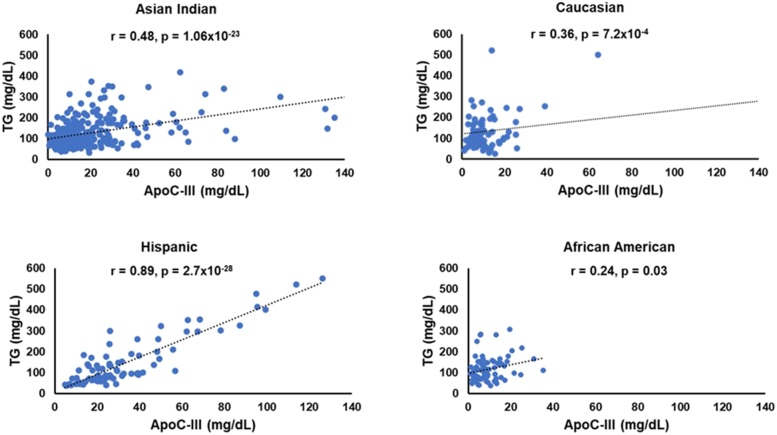
Clinical and demographic characteristics of study participants from all ethnic groups are described in Table 1. Hispanics were the youngest ethnic group with the lowest mean age (41.4 ± 14.2) compared to Asian Indians (50.0 ± 13.3), African Americans (61 ± 11.8), and Caucasians (66.4 ± 14.2) years. The Asian Indians had the lowest BMI (27.2 ± 4.2) in comparison to Caucasians (30.1 ± 7.6), African Americans (31.9 ± 16.2) and Hispanics (28.0 ± 5.2). Despite the youngest age group, Hispanics had a more atherogenic lipid profile with the highest TC, LDL-C, and TG among all ethnic groups, and Asian Indians had the lowest TC, HDL-C levels. At the same time, TG levels were lowest in African Americans (Supplementary Fig. 1 and 2) . As shown in Fig. 1, the mean plasma ApoC-III concentrations were lowest in African Americans (9.5 ± 6.8 mg/dL) and highest in Hispanics (34.0 ± 25.3 mg/dL). Despite with lowest ApoC-III concentrations, the prevalence of CAD was highest in African Americans (44%), followed by Caucasians (31%), Asian Indians (10%), and Hispanics had only 1% CAD cases.
Table 1.
Clinical attributes of study participants by ethnic group.
| Parameters | Asian Indian | Caucasian | African American | Hispanic |
|---|---|---|---|---|
| N | 271 | 87 | 80 | 80 |
| Male (%) | 56 | 54 | 55 | 63 |
| Age (years) | 50.01 ± 13.3 | 66.4 ± 14.2 | 61.0 ± 11.8 | 41.4 ± 14.2 |
| BMI (kg/m2) | 27.2 ± 4.2 | 30.1 ± 7.6 | 31.9 ± 16.3 | 28.0 ± 5.2 |
| Total cholesterol (mg/dL) | 152.6 ± 66.4 | 168.4 ± 47.5 | 177.4 ± 52.7 | 192.7 ± 43.2 |
| HDL-C (mg/dL) | 37.9 ± 14.9 | 44.4 ± 14.4 | 44.4 ± 12.7 | 42.1 ± 14.1 |
| LDL-C (mg/dL) | 99.04 ± 39.6 | 97.01 ± 37.3 | 109.4 ± 48.8 | 125.7 ± 35.6 |
| TG (mg/dL) | 135.0 ± 81.8 | 139.1 ± 109.9 | 116.2 ± 58.8 | 149.6 ± 118.2 |
| ApoC-III (mg/dL) | 22.5 ± 30.9 | 16.1 ± 34.7 | 9.5 ± 6.8 | 34.0 ± 25.3 |
| ApoC-III range (mg/dL) | 0.1–254.8 | 1.2–237.6 | 1.2–35.4 | 4.8–126.2 |
| CAD (%) | 10 | 31 | 44 | 1 |
| T2D (%) | 65 | 61 | 39 | 23 |
| Smokers (%) | 0 | 38 | 59 | 10 |
| Statin (%) | 0 | 18 | 31 | 0 |
Values are displayed in mean ± standard deviation; ApoC-III = Apolipoprotein C-III; BMI = body mass index; CAD = coronary artery disease; HDL-C = high density lipoproteins cholesterol; LDL-C = low density lipoproteins cholesterol; N = number of participants; TG = triglycerides; T2D = type 2 diabetes mellitus.
Fig. 1.
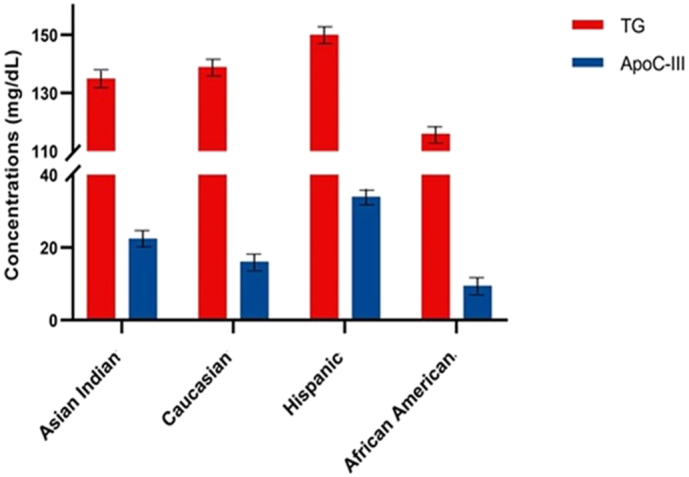
Distribution of mean levels of TG and ApoC-III concentrations (mg/dL) in different Ethnic groups.
ApoC-III = Apolipoprotein C-III; TG = triglycerides.
To determine the relationship of ApoC-III concentration with TG, we analyzed the ApoC-III distribution in data stratified by low TG (LTG <100 mg/dL) and high TG >150 mg/dL (HTG). ApoC-III levels were highest for Hispanics even in the low TG group (LTG = 21.5 mg/dL and HTG = 46.4 mg/dL) followed by Asian Indians (LTG = 14.2 mg/dL and HTG = 29.7 mg/dL) compared to Caucasians (LTG = 8.8 mg/dL and HTG = 22.7 mg/dL). And the African Americans showed the lowest ApoC-III levels in both low and high TG (LTG = 8.0 mg/dL and HTG = 11.0 mg/dL) (Supplementary Table 1). The strongest correlation of TG and ApoC-III levels was observed in Hispanics (r = 0.9, p = 2.7 × 10−28) followed by Asian Indians (r = 0.5, p = 1.1 × 10−23), Caucasians (r = 0.4, p = 7.2 × 10−4), and the weakest correlation between TG and ApoC-III levels was observed in African Americans (r = 0.2, p = 0.03) (Fig. 2). A linear regression analysis revealed a strong association of ApoC-III concentrations with plasma TG in all the ethnic groups. Even after adjusting for age, gender, BMI, T2D, and CAD, it remained significant. There was a nearly 2.5-fold increase in TG associated with ApoC-III levels in Hispanics showing (95% CI 1.9–2.9, p = 3.5 × 10−23) followed by Asian Indians (OR 1.8, 95% CI 1.5–1.9, p = 5.8 × 10−22) while Caucasians and African Americans had a nominal increase of (OR 1.4, 95% CI 1.3–1.5, p = 0.005) and (OR 1.3, 95% CI 1.2–1.4, p = 0.031) after adjusting for covariates, respectively (Supplementary Table 3). Although the African Americans had the highest prevalence of CAD in this study, there was a negative correlation between ApoC-III concentration with CAD (r = −0.3, p = 0.004) (Supplementary Table 2).
Fig. 2.
Scatter plot showing the correlation between the distribution of ApoC-III concentration (X-axis) vs.TG levels (Y-axis) among different ethnic groups.
ApoC-III = Apolipoprotein C-III; TG = triglycerides.
The increase in ApoC-III concentrations correlated significantly with fasting blood glucose (FBG) (r = 0.25, p = 6.1 × 10−5) and T2D (r = 0.20, p = 0.002) in Asians Indians while in Hispanics, ApoC-III levels correlated with marginal significance with waist (r = 0.22, p = 0.05) and waist-hip ratio (WHR) (r = 0.24, p = 0.04) and with T2D (r = 0.20, p = 0.04) (Supplementary Fig. 3). As several lipoproteins have shown differences in concentration among women and men, we evaluated sex differences in the distribution of lipoproteins and ApoC-III in this study. HDL cholesterol is usually much higher in women than men, and our data confirmed these patterns (Table 2).
Table 2.
Distribution of lipoprotein lipids and ApoC-III among various ethnicities segregated based on gender.
| Traits | Gender | Asian Indian (N = 271) |
Caucasian (N = 87) |
African American (N = 80) |
Hispanic (N = 80) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | Mean ± SD | p-Value | ||
| HDL-C (mg/dL) | Male | 36.2 ± 13.7 | −04 | 42.2 ± 12.5 | 0.24 | 42.1 ± 12.4 | 0.11 | 42.6 ± 14.4 | 0.71 |
| Female | 39.9 ± 16.1 | 46.7 ± 16.1 | 47.1 ± 12.7 | 41.1 ± 13.6 | |||||
| LDL-C (mg/dL) | Male | 96.1 ± 36.9 | 0.003 | 98.1 ± 33.5 | 0.53 | 103.2 ± 48.2 | 0.17 | 129.8 ± 39.2 | 0.30 |
| Female | 102.7 ± 42.6 | 95.8 ± 41.4 | 117.1 ± 49.1 | 117.9 ± 26.4 | |||||
| TC (mg/dL) | Male | 146.6 ± 65.1 | 0.006 | 169.3 ± 44.2 | 0.67 | 169.3 ± 50.7 | 0.12 | 195.5 ± 49.9 | 0.68 |
| Female | 160.1 ± 67.5 | 167.3 ± 51.6 | 187.2 ± 54.2 | 187.5 ± 27.03 | |||||
| TG (mg/dL) | Male | 142.8 ± 81.9 | 0.032 | 143.5 ± 118.2 | 0.46 | 117.4 ± 56.9 | 0.74 | 140.7 ± 117.5 | 0.27 |
| Female | 125.2 ± 81 | 134 ± 100.5 | 114.8 ± 61.7 | 164.3 ± 119.8 | |||||
| ApoC-III (mg/dL) | Male | 23.2 ± 31.9 | 0.32 | 12.2 ± 11.3 | 0.67 | 8.2 ± 5.9 | 0.21 | 32.4 ± 23.7 | 0.62 |
| Female | 21.5 ± 29.6 | 20.7 ± 49.7 | 11.1 ± 7.5 | 36.5 ± 28.04 | |||||
Values are displayed in mean ± standard deviation; ApoC-III = Apolipoprotein C-III; HDL-C = high density lipoproteins cholesterol; LDL-C = low density lipoproteins cholesterol; TC = total cholesterol; TG = triglycerides; N = number of individuals.a
The values marked in bold represents significant p-value (< 0.05) between genders for Asian Indian in HDL-C, LDL-C, TC and TG.
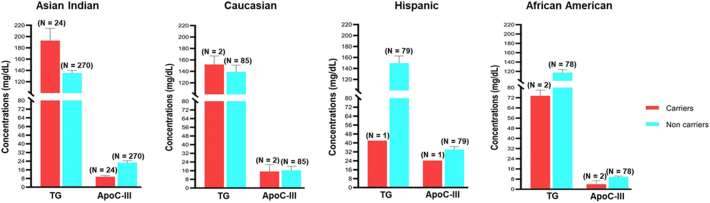
Next, we evaluated the genotype-phenotype association of rare/LoF variants between ApoC-III levels and TG in different ethnic groups. Even though, the carriers of IVS1-2G-A (rs373975305) splice acceptor; A43T (rs147210663) and IVS3 + 1G-T (rs140621530) splice donor in Asian Indians had low plasma ApoC-III levels (9.5 mg/dL, 9.8 mg/dL and 7.7 mg/dL), these individuals had high TG levels (184 mg/dL, 256 mg/dL and 178 mg/dL) respectively. Only IVS2 + 1G-A (rs138326449), a splice donor variant, showed both low ApoC-III and low TG levels in Asian Indians (1.879 mg/dL and 83 mg/dL). On the contrary, the same variant carriers in Hispanics showed high ApoC-III concentration (24.2 mg/dL) with low TG levels (42 mg/dL). The IVS3 + 1G-T (rs140621530) variant in Caucasians and African Americans showed relatively low ApoC-III levels (15.21 mg/dL and 6.71 mg/dL); however, despite low ApoC-III, the TG levels were strikingly high in Caucasians (152 mg/dL) than African Americans (69 mg/dL). A similar trend was observed for A43T (rs147210663) variant in Caucasian and African Americans showing relatively low levels of ApoC-III levels (15.21 mg/dL and 1.07 mg/dL), respectively, and high TG levels in Caucasians (152 mg/dL) than African Americans (78 mg/dL) (Fig. 3 and Supplementary Fig. 4).
Fig. 3.
Distribution of overall mean levels of TG and ApoC-III concentrations (mg/dL) among carriers and non-carriers of earlier known APOC3 loss-of-function (LoF) rare variants (IVS1-2G-A (rs373975305); IVS2 + 1G-A (rs138326449), IVS3 + 1G-T (rs140621530) and A43T (rs147210663)) in different ethnic groups.
ApoC-III = Apolipoprotein C-III; TG = triglycerides; N = number of individuals.
4. Discussion
Recent genetic and Mendelian randomization studies have provided robust evidence of the causal association between TG and CVD. Ever since some rare LoF variants in APOC3 were shown to be cardioprotective in Europeans, there has been a consensus to therapeutically inhibit ApoC-III to improve CV outcomes. However, inconsistent reports on the cardioprotective role of rare variants in the APOC3 by other groups urge a deeper evaluation of this critical protein in atherogenic dyslipidemia among different ethnic groups.
We observed a significant ethnic difference in the distribution of ApoC-III levels and their subsequent correlation with TG and other CV risk factors. Hispanic participants in this study had the highest ApoC-III concentrations, followed by Asian Indians. However, African Americans had the lowest ApoC-III levels (9.5 mg/dL), which are similar to earlier reported data on African American women (10.06 mg/dL) and African American Youth (9.4 mg/dL) [34], [35]. Likewise, plasma TG levels were highest in Hispanics, showing the highest prevalence of HTG in our study, despite being the youngest ethnic group in this study. These data support the earlier published findings of the NHANES 1999–2004 survey showing 85.9% prevalence of HTG in Hispanics compared to 31.2% in Caucasians with metabolic syndrome [36].
Caucasians and Asian Indians showed “dyslipidemia of insulin resistance” with a classic pattern of high TG with low HDL-C levels. However, African Americans had the highest prevalence of CAD despite having the lowest mean levels of ApoC-III and TG in this study and as reported earlier [37]. These results suggest that the increased prevalence of CAD and CVD in African Americans could be independent of ApoC-III and TG concentrations and may be regulated by pathways other than TG. Free fatty acids (FFA) in plasma are known to regulate ApoC-III concentrations positively; however, a study by Sacks et al. [38] on African American women with T2D showed lower plasma FFA, which might correlate or explain the low ApoC-III levels observed in African Americans [38], [39]. We also detected sex differences in some lipoproteins, but statistical significance was only seen in Asian Indians, perhaps because of the sample size. Women had higher HDL-C and lowered TG in all ethnic groups. Interestingly, this difference appears to reduce their CAD risk in African American women as the prevalence of CAD in women was 36% compared to 50% in African American men. Again, this variation seemed ethnicity-specific because no significant gender difference in CAD prevalence was observed in other ethnic groups (Table 2).
APOC3 expression is upregulated in the condition of insulin resistance, and glycemic control plays a central role in impacting ApoC-III secretion [40]. A significant positive correlation of ApoC-III concentrations with FBG was observed in Asian Indians and T2D in the case of both Asian Indians and Hispanics. Also, ApoC-III levels correlated significantly with central adiposity (waist or WHR) in Hispanics but not Asian Indians (Supplementary Fig. 3). Studies have shown high glucose concentrations induce APOC3 gene expression in rat and human hepatocytes with the help of transcription factors such as carbohydrate-responsive element-binding protein (Chrebp) and hepatocyte nuclear factor 4 alpha (Hnf4α). Forkhead box O1 (Foxo-1) via the insulin-responsive element during insulin resistance increases ApoC-III levels [41], [42], [43]. Interestingly, despite HTG and high ApoC-III, Hispanics had a low prevalence of CAD, as shown in earlier studies [44], [45], [46]. It is possible that in Hispanics, the elevated ApoC-III levels may predispose them to increased insulin resistance and T2D risk without increasing the risk for CAD.
Our study also could not validate the cardioprotective role of LoF variants analyzed. The rare variants evaluation was an extension of our earlier published study [16], where we evaluated the role of all earlier published rare (LoF) variants of ApoC-III with TG in 396,644 individuals of multiethnic ancestries. Our study could not confirm the correlation of these variants with low TG in Asian Indians and other ethnic groups [16]. In this study, we also evaluated all available rare variant carriers for their serum ApoC-III levels. With few exceptions, the LoF carriers did not have low ApoC-III levels, which correlated with their high TG levels (Fig. 3; Supplementary Fig. 4). Also, our data revealed that African Americans had lower TG and ApoC-III levels irrespective of their genotype. Similarly, the cardioprotective effect of some of these rare variants also could not be confirmed in Icelanders [18] and US Caucasians [17]. Most individuals carrying these known rare variants specifically (rs140621530; IVS3 + 1G-T) and (A43T; rs147210663 identified initially in Yucatan Indians) had low ApoC-III but showed high TG levels in Asian Indians and did not reveal any cardioprotective effects in Asian Indians and Caucasians (Supplementary Fig. 4). The insulin resistance may offset the lowering impact of LoF variants in some ethnic groups. It is also possible that the other variants within the APOC3 and other genes in the APOA5-APOA4-APOC3-APOA1 gene cluster may influence the ApoC-III concentrations. Thus, due to possible pleiotropic effects of different genes within the LD region, the same rare (LoF or splice variant) may not show similar phenotypic effects in multiple carriers [13]. Perhaps, because of these differences, we and other studies cannot confirm the LoF variants cardioprotective role [16], [17].
Like every study, this study has strengths and limitations. Strengths include well-characterized and ethnically diverse patient cohorts. Our study fills the gap in knowledge for evaluating circulating ApoC-III concentrations with TG and CV risk factors in various ethnic cohorts. The observed ethnic differences in this study further substantiate the existence of ‘TG paradox’ in African Americans, and the gender differences in lipoproteins (specifically HDL) seem to reduce the CAD risk only in African American women and not in other women. These results highlight the need to understand these differences more deeply at the molecular level. Among the limitations, one main limitation is the small sample size in each ethnic group. Secondly, one could argue that the statin treatment could have impacted TG levels as 31% of African Americans were on statins compared to 18% Caucasians. However, observed differences in the unique atherogenic lipid profiles among African Americans have been reported by other investigators [35]. African Americans are more insulin resistant with lower TG and low ApoC-III and lower ApoB levels [35], [47]. Overall, these results highlight the challenges of the generalizability of antisense ApoC-III inhibition for treating atherosclerotic disease in dyslipidemia. This treatment may only be beneficial to specific sub-populations. Notably, African Americans do not show high ApoC-III levels or HTG despite having a high prevalence, and mortality due to CVD should be evaluated differently. Our study also emphasizes the need to perform in-depth genetic and metabolomics/proteomic evaluations on diverse ancestries to identify race-specific biomarkers, risk factors, and therapies to effectively address cardiometabolic disease health disparities.
Funding
AIDHS/SDS: The Sikh Diabetes Study/Asian Indian Diabetic Heart Study was supported by NIH grants-R01DK082766; R01DK118427 (NIDDK), NOT-HG11-009 (NHGRI), and grants from Presbyterian Health Foundation of Oklahoma. Sequencing services were provided through the RS&G Service by the Northwest Genomics Center at the University of Washington, Department of Genome Sciences, under US Federal Government contract number HHSN268201100037C from the National Heart, Lung, and Blood Institute of the NIH.
MISS-OLIVER: The OLIVER and MISS are partly supported by NIH grants-R01DK082766 and R01DK118427 funded by the National Institute of Health (NIDDK), Presbyterian Health Foundation Grants, the College of Medicine Alumni Association grant, and Leinbach Seed Grant from the University of Oklahoma Health Sciences Center.
Ethics approval and consent to participate
All AIDHS/SDS protocols and consent documents were reviewed and approved by the University of Oklahoma Health Science Center's Institutional Review Board (IRB) and the Human Subject Protection (Ethics) committees at the participating hospitals and institutes in India.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank all the AIDHS/SDS and MISS-OLIVER study participants and are grateful for their contribution to this study.
This manuscript is dedicated to the memory of Co-Author Dr. Marvin Peyton, who passed away in August 2021.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2022.100128.
Appendix A. Supplementary data
Supplementary material
References
- 1.Martinez-Amezcua P., Haque W., Khera R., Kanaya A.M., Sattar N., Lam C.S.P. The upcoming epidemic of heart failure in South Asia. Circ. Heart Fail. 2020;13(10) doi: 10.1161/CIRCHEARTFAILURE.120.007218. [DOI] [PubMed] [Google Scholar]
- 2.Mensah G.A. Cardiovascular diseases in african americans: fostering community partnerships to stem the tide. Am. J. Kidney Dis. 2018;72(5 Suppl. 1):S37–S42. doi: 10.1053/j.ajkd.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez F., Hastings K.G., Hu J., Lopez L., Cullen M., Harrington R.A., et al. Nativity status and cardiovascular disease mortality among hispanic adults. J. Am. Heart Assoc. 2017;6(12) doi: 10.1161/JAHA.117.007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hokanson J.E., Austin M.A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 5.Morrison A., Hokanson J.E. The independent relationship between triglycerides and coronary heart disease. Vasc. Health Risk Manag. 2009;5(1):89–95. [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg J.J., Tybjaerg-Hansen A., Jensen J.S., Nordestgaard B.G. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300(18):2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 7.Do R., Willer C.J., Schmidt E.M., Sengupta S., Gao C., Peloso G.M., et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013;45(11):1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenson R.S., Davidson M.H., Hirsh B.J., Kathiresan S., Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2014;64(23):2525–2540. doi: 10.1016/j.jacc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Boren J., Packard C.J., Taskinen M.R. The roles of ApoC-III on the metabolism of triglyceride-rich lipoproteins in humans. Front. Endocrinol. 2020;11:474. doi: 10.3389/fendo.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun T.R., Been L.F., Singhal A., Worsham J., Ralhan S., Wander G.S., et al. A replication study of GWAS-derived lipid genes in asian indians: the chromosomal region 11q23.3 harbors loci contributing to triglycerides. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui G., Tian M., Hu S., Wang Y., Wang D.W. Identifying functional non-coding variants in APOA5/A4/C3/A1 gene cluster associated with coronary heart disease. J. Mol. Cell. Cardiol. 2020;144:54–62. doi: 10.1016/j.yjmcc.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Mar R., Pajukanta P., Allayee H., Groenendijk M., Dallinga-Thie G., Krauss R.M., et al. Association of the APOLIPOPROTEIN A1/C3/A4/A5 gene cluster with triglyceride levels and LDL particle size in familial combined hyperlipidemia. Circ. Res. 2004;94(7):993–999. doi: 10.1161/01.RES.0000124922.61830.F0. [DOI] [PubMed] [Google Scholar]
- 13.Talmud P.J., Hawe E., Martin S., Olivier M., Miller G.J., Rubin E.M., et al. Relative contribution of variation within the APOC3/A4/A5 gene cluster in determining plasma triglycerides. Hum. Mol. Genet. 2002;11(24):3039–3046. doi: 10.1093/hmg/11.24.3039. [DOI] [PubMed] [Google Scholar]
- 14.Tg, Hdl Working Group of the Exome Sequencing Project NHL, Blood I, Crosby J., Peloso G.M., Auer P.L. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks F.M., Alaupovic P., Moye L.A., Cole T.G., Sussex B., Stampfer M.J., et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the cholesterol and recurrent events (CARE) trial. Circulation. 2000;102(16):1886–1892. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 16.Goyal S., Tanigawa Y., Zhang W., Chai J.F., Almeida M., Sim X., et al. APOC3 genetic variation, serum triglycerides, and risk of coronary artery disease in asian indians, europeans, and other ethnic groups. Lipids Health Dis. 2021;20(1):113. doi: 10.1186/s12944-021-01531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford D.C., Restrepo N.A., Diggins K.E., Farber-Eger E., Wells Q.S. Frequency and phenotype consequence of APOC3 rare variants in patients with very low triglyceride levels. BMC Med. Genet. 2018;11(Suppl. 3):66. doi: 10.1186/s12920-018-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudbjartsson D.F., Helgason H., Gudjonsson S.A., Zink F., Oddson A., Gylfason A., et al. Large-scale whole-genome sequencing of the icelandic population. Nat. Genet. 2015;47(5):435–444. doi: 10.1038/ng.3247. [DOI] [PubMed] [Google Scholar]
- 19.Kohan A.B. Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2015;22(2):119–125. doi: 10.1097/MED.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanghera D.K., Bhatti J.S., Bhatti G.K., Ralhan S.K., Wander G.S., Singh J.R., et al. The khatri sikh diabetes study (SDS): study design, methodology, sample collection, and initial results. Hum. Biol. 2006;78(1):43–63. doi: 10.1353/hub.2006.0027. [DOI] [PubMed] [Google Scholar]
- 21.Sanghera D.K., Nath S.K., Ortega L., Gambarelli M., Kim-Howard X., Singh J.R., et al. TCF7L2 polymorphisms are associated with type 2 diabetes in khatri sikhs from North India: genetic variation affects lipid levels. Ann. Hum. Genet. 2008;72(Pt 4):499–509. doi: 10.1111/j.1469-1809.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 22.Saxena R., Bjonnes A., Prescott J., Dib P., Natt P., Lane J., et al. Genome-wide association study identifies variants in casein kinase II (CSNK2A2) to be associated with leukocyte telomere length in a Punjabi Sikh diabetic cohort. Circ. Cardiovasc. Genet. 2014;7(3):287–295. doi: 10.1161/CIRCGENETICS.113.000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxena R., Saleheen D., Been L.F., Garavito M.L., Braun T., Bjonnes A., et al. Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in sikhs of punjabi origin from India. Diabetes. 2013;62(5):1746–1755. doi: 10.2337/db12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consultation WHOE Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl. 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 26.Sapkota B.R., Hopkins R., Bjonnes A., Ralhan S., Wander G.S., Mehra N.K., et al. Genome-wide association study of 25(OH) vitamin D concentrations in punjabi sikhs: results of the asian indian diabetic heart study. J. Steroid Biochem. Mol. Biol. 2016;158:149–156. doi: 10.1016/j.jsbmb.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanghera D.K., Hopkins R., Malone-Perez M.W., Bejar C., Tan C., Mussa H., et al. Targeted sequencing of candidate genes of dyslipidemia in punjabi sikhs: population-specific rare variants in GCKR promote ectopic fat deposition. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0211661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanghera D.K., Ortega L., Han S., Singh J., Ralhan S.K., Wander G.S., et al. Impact of nine common type 2 diabetes risk polymorphisms in asian indian sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med. Genet. 2008;9:59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamani P., Proto E.A., Mazurek J.A., Prenner S.B., Margulies K.B., Townsend R.R., et al. Peripheral determinants of oxygen utilization in heart failure with preserved ejection fraction: central role of adiposity. JACC Basic Transl. Sci. 2020;5(3):211–225. doi: 10.1016/j.jacbts.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidorov E., Bejar C., Xu C., Ray B., Reddivari L., Chainakul J., et al. Potential metabolite biomarkers for acute versus chronic stage of ischemic stroke: a pilot study. J. Stroke Cerebrovasc. Dis. 2020;29(4) doi: 10.1016/j.jstrokecerebrovasdis.2019.104618. [DOI] [PubMed] [Google Scholar]
- 31.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 32.Schierer A., Been L.F., Ralhan S., Wander G.S., Aston C.E., Sanghera D.K. Genetic variation in cholesterol ester transfer protein, serum CETP activity, and coronary artery disease risk in Asian Indian diabetic cohort. Pharmacogenet. Genomics. 2012;22(2):95–104. doi: 10.1097/FPC.0b013e32834dc9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanghera D.K., Been L.F., Ralhan S., Wander G.S., Mehra N.K., Singh J.R., et al. Genome-wide linkage scan to identify loci associated with type 2 diabetes and blood lipid phenotypes in the sikh diabetes study. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.Y., Hong H.R., Kang H.S. Ethnicity differences in plasma apoC-III levels between african american and caucasian youths. World J. Pediatr. 2011;7(2):136–142. doi: 10.1007/s12519-011-0266-8. [DOI] [PubMed] [Google Scholar]
- 35.Sumner A.E., Furtado J.D., Courville A.B., Ricks M., Younger-Coleman N., Tulloch-Reid M.K., et al. ApoC-III and visceral adipose tissue contribute to paradoxically normal triglyceride levels in insulin-resistant african-american women. Nutr. Metab. (Lond.) 2013;10(1):73. doi: 10.1186/1743-7075-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinueza R., Boissonnet C.P., Acevedo M., Uriza F., Benitez F.J., Silva H., et al. Dyslipidemia in seven latin american cities: CARMELA study. Prev. Med. 2010;50(3):106–111. doi: 10.1016/j.ypmed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Sumner A.E. "Half the dsylipidemia of insulin resistance" is the dyslipidemia [corrected] of insulin-resistant blacks. Ethn Dis. 2009;19(4):462–465. [PMC free article] [PubMed] [Google Scholar]
- 38.Sacks F.M., Liang L., Furtado J.D., Cai T., Davidson W.S., He Z., et al. Protein-defined subspecies of HDLs (High-density Lipoproteins) and differential risk of coronary heart disease in 4 prospective studies. Arterioscler. Thromb. Vasc. Biol. 2020;40(11):2714–2727. doi: 10.1161/ATVBAHA.120.314609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller B.V., III, Patterson B.W., Okunade A., Klein S. Fatty acid and very low density lipoprotein metabolism in obese African American and Caucasian women with type 2 diabetes. J. Lipid Res. 2012;53(12):2767–2772. doi: 10.1194/jlr.P030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adiels M., Taskinen M.R., Bjornson E., Andersson L., Matikainen N., Soderlund S., et al. Role of apolipoprotein C-III overproduction in diabetic dyslipidaemia. Diabetes Obes. Metab. 2019;21(8):1861–1870. doi: 10.1111/dom.13744. [DOI] [PubMed] [Google Scholar]
- 41.Caron S., Verrijken A., Mertens I., Samanez C.H., Mautino G., Haas J.T., et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2011;31(3):513–519. doi: 10.1161/ATVBAHA.110.220723. [DOI] [PubMed] [Google Scholar]
- 42.Hertz R., Bishara-Shieban J., Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. J. Biol. Chem. 1995;270(22):13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 43.Qu S., Su D., Altomonte J., Kamagate A., He J., Perdomo G., et al. PPAR{alpha} mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am. J. Physiol. Endocrinol. Metab. 2007;292(2):E421–E434. doi: 10.1152/ajpendo.00157.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balfour P.C., Jr., Ruiz J.M., Talavera G.A., Allison M.A., Rodriguez C.J. Cardiovascular disease in Hispanics/Latinos in the United States. J. Lat. Psychol. 2016;4(2):98–113. doi: 10.1037/lat0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferdinand K.C. Coronary artery disease in minority racial and ethnic groups in the United States. Am. J. Cardiol. 2006;97(2A):12A–19A. doi: 10.1016/j.amjcard.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Saab K.R., Kendrick J., Yracheta J.M., Lanaspa M.A., Pollard M., Johnson R.J. New insights on the risk for cardiovascular disease in african americans: the role of added sugars. J Am Soc Nephrol. 2015;26(2):247–257. doi: 10.1681/ASN.2014040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ukegbu U.J., Castillo D.C., Knight M.G., Ricks M., Miller B.V., III, Onumah B.M., et al. Metabolic syndrome does not detect metabolic risk in African men living in the U.S. Diabetes Care. 2011;34(10):2297–2299. doi: 10.2337/dc11-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material