Abstract
Introduction:
Mechanically induced skin breakdown is a significant problem for many lower-limb prosthesis users. It is known that skin can adapt to the mechanical stresses of prosthesis use thereby reducing the risk of breakdown, yet little is understood about the biology behind skin adaptation. This is a proof-of-concept study for the use of novel, noninvasive optical coherence tomography (OCT) imaging techniques to investigate skin adaptation.
Methods:
Two OCT imaging-based tests were used to evaluate features of the skin that may be involved in adaptation to limb-socket interface stresses. The tests were used to assess the function and structure of the cutaneous microvasculature, respectively. Epidermal thickness was also quantified. Tests were run on three lower-limb prosthesis users in a region of the residual limb believed to be highly stressed within the prosthetic socket. The measurements were compared with measurements taken at a location-matched site on the contralateral limb.
Results:
Two of three participants demonstrated a faster time-to-peak and larger peak magnitude reactive hyperemia response in their residual limb compared with their contralateral limb. Two of three participants also demonstrated a larger magnitude vessel density at maximum dilation in their residual limb versus contralateral limb. The epidermal thickness was greater in the residual limb versus contralateral limb for all participants.
Conclusions:
This study demonstrated the utility of two novel OCT imaging techniques for investigating skin adaptation in users of lower-limb prostheses. If we are able to confirm these findings on a larger subject population, we will better understand the biology behind mechanically induced skin adaptation. These findings, along with the noninvasive OCT imaging methods introduced here, would have the potential to improve clinical practice by enabling the development of rehabilitation techniques and therapeutics to better strengthen skin, thereby reducing the incidence of harmful skin breakdown.
Keywords: optical coherence tomography, lower-limb amputation, prosthetics, residual limb, skin adaptation
INTRODUCTION
Skin breakdown is a problem that affects a majority of individuals with lower-limb loss.1 Skin breakdown is often painful and results in decreased use of the prosthesis. Furthermore, it can lead to more proximal revision amputation if wounds do not heal. Skin breakdown with use of a prosthesis is commonly caused by the repetitive mechanical stresses that are imposed on the residual limb at its interface with the prosthetic socket.
It is known that skin can adapt to become more tolerant to the stresses imposed on it, thus reducing the risk of breakdown. In clinical practice, skin adaptation occurs post-amputation when the prosthesis is first introduced. This period is known as “prosthetic training.” During prosthetic training, prosthesis use is gradually introduced to allow the residual limb tissues to adapt into a more load-tolerant state until, in many cases, the limb can eventually withstand full-day weight-bearing activities.2
Despite its important role in preventing skin breakdown, little is understood about the biology of skin adaptation. As such, our ability to leverage this phenomenon to improve clinical practice is severely limited. If the physiology of skin adaptation were better understood it may be possible to develop methods that clinicians could use to objectively monitor the progress of adaptation and to better understand the load tolerance state of their patients’ skin. Additionally, it may be possible to develop more effective methods for adapting skin so that it is better equipped to resist breakdown. In order to develop a better understanding of skin adaptation, new methods are needed that can safely and accurately probe the cutaneous physiology using methods that are applicable to people with lower-limb amputation.
Optical Coherence Tomography (OCT) is a noninvasive imaging technology that has recently shown promise for investigating the microvasculature of the skin at a level of detail that was not previously possible.3 OCT can be used to capture three-dimensional images of static skin structure and the skin microvasculature down to a depth of 2 mm with a resolution as detailed as 1 to 10 μm.4,5 Our research group recently developed two OCT-based test methods for the investigation of skin adaptation in prosthetics.6 The methods were designed to assess microvascular function (a Reactive Hyperemia [RH] Test) and structure (a Maximum Dilation Test).
The purpose of the current study was to use these newly developed methods to determine if potential signs of skin adaptation were present in the skin of transtibial prosthesis users. We studied chronically stressed regions of skin and compared these measurements to location-matched sites on the contralateral limb. Individuals without skin breakdown issues at the regions of interest (ROIs) were chosen to increase the likelihood that any differences identified would be indicative of skin that had adapted into a more load-tolerant state. We hypothesized that the residual limb skin of the prosthesis users would exhibit several differences compared to the contralateral limb that would support our hypotheses for functional and structural cutaneous microvascular adaptations. Following an occlusive load in the functional Reactive Hyperemia Test, we expected the residual limb would demonstrate a larger magnitude peak perfusion, a faster time-to-peak, and a faster recovery time. In the structural Maximum Dilation Test, we expected that residual limb skin would possess a greater vessel density, due to an increased number of vessels and an increase in vessel diameters. We also expected that residual limbs would have a thicker epidermis than the contralateral limb.
METHODS
OCT Imaging System
An OCT system (OCS1310V1, Thorlabs Inc., Newton, NJ, USA) was used to capture images of the skin in two tests: (1) a reactive hyperemia (RH) test to assess vascular function and (2) a Maximum Dilation Test to assess vessel structure, as described previously.6 The OCT system had a central wavelength of 1300 nm, a 100 nm bandwidth, an A-line scan rate of 100 kHz and imaging resolutions of 20 μm and 10 μm in the lateral and axial directions, respectively. The system was used to capture three-dimensional images of static skin tissue down to a depth of 1 mm. To enable vascular imaging, five repeated images were taken at each cross-section (B-scan) location. B-scan repetitions were registered to one another to reduce motion artifacts, and three-dimensional OCT Angiography (OCTA)7 vessel images were created using High-Sensitivity Speckle Variance, previously described.8 Maximum Dilation Test images were further enhanced by dividing each OCTA pixel intensity by the intensity of the corresponding pixel in the OCT static tissue image that was used to create the OCTA image. This reduced noise and normalized the intensity of vessels in low-intensity regions to those in higher-intensity regions. For both tests, the dermal-epidermal junction was then automatically detected and only the vessel-containing depths were retained. The vessel sections retained included a depth range of 125 pixels (0.70 mm) and 150 pixels (0.84 mm) for the RH Test and Maximum Dilation Test, respectively. The images were then converted to two-dimensional en face images using a maximum intensity projection and were further analyzed, as described for each imaging test below. Images had a field-of-view of 2.0 × 2.0 mm and 7.4 × 7.4 mm for the RH Test and Maximum Dilation Test, respectively, with a scanning pattern of 200 × 200 pixels and 925 × 740 pixels. All image post-processing was performed in MATLAB (R2016b, MathWorks, Inc., Natick, MA, USA), unless otherwise noted. To reduce the risk of altering the cutaneous blood flow during image collection, the OCT skin contact probe was open in the center (inner diameter (ID): 18 mm, outer diameter (OD): 30 mm) where imaging occurred. The contact probe also included a strain-gauged bending beam which was used to ensure no more than 4.5 kPa was applied, which is the minimum contact pressure that has been shown to alter cutaneous blood flow.9,10
Reactive Hyperemia OCT Test: Vessel Function Assessment
Microvascular function was assessed by using OCT to measure a response known as reactive hyperemia following the application of a cyclic stress that was designed to simulate limb-socket interface stresses during ambulation. RH is a protective mechanism of the microvasculature characterized by an increase in blood flow following an occlusion. Microvascular function is commonly assessed in vascular research by measuring various features of the RH response.11 The RH Test began with baseline OCT image capture at the ROI, followed by a controlled stress applied for 10 minutes using a biaxial load application device, described previously.12 Stress was applied to the ROI through a foam application pad (13.0 × 15.0 mm) using a 1 Hz sinusoidal waveform with pressure cycling from 5.1 kPa to 50.6 kPa and shear cycling from 0 kPa to 5.1 kPa. This stress profile was similar to waveforms previously measured at the limb-socket interface in transtibial sockets.13 Following the load application, the RH response that was induced was measured by capturing OCT images of the skin every 10–15 seconds for 10 minutes.
Perfusion in each OCT en face image was quantified as the vessel area density (VAD), reported in arbitrary units (a.u.).14 To calculate this, en face images were binarized into pixel values of 1 (vessel) or 0 (non-vessel) based on a manually selected threshold. For each RH Test data set, the VAD values of the images were plotted versus time, with the baseline image set to the 0 s time point, and a three-point moving average of VAD was calculated to smooth the data. From this plot the key RH features were determined. “Peak VAD” was the maximum VAD measured and indicated the intensity of the RH response. “Time-to-peak” VAD was the time that it took to reach the peak VAD following the stress removal, and it indicated how quickly the blood flow responded following the occlusive stress. “Recovery time” indicated how quickly it took for the entire RH response to resolve. It was defined as the time it took for the VAD to recover 75% of the way back to the baseline VAD from the peak VAD since a full recovery to baseline was uncommon within the test duration.
Maximum Dilation OCT Test: Vessel Structure Assessment
Microvascular structure was assessed using a Maximum Dilation Test, which consisted of OCT image capture following a localized skin heating protocol that was used to induce a repeatable vascular state. Consistent with prior cutaneous microvascular studies, maximum dilation was induced by heating the skin for at least 25 minutes at 42–44 °C using a local skin heater probe.15 The custom-built heater probe had an open inner diameter of 20.5 mm and was adhered to the skin with double-sided medical tape. The heater opening was filled with 2–3 ml of mineral oil which served as the heating medium through which OCT images were captured.
Following 25 minutes of heating, three sets of OCT images were captured. Of these, the image with the clearest vessels and least noise was selected for the vessel structure assessment. The three-dimensional vessel image was separated into two depth sections, the more superficial papillary dermis and the deeper reticular dermis. Each of these was converted into an en face image, from which the key Maximum Dilation Test measurements were calculated. “VAD” was determined using the same methods described for the RH Test. “Vessel count” (i.e., skeleton density) was determined by skeletonizing the binarized VAD image so that each vessel was reduced to a single pixel width. “Mean vessel diameter” was calculated by dividing VAD by vessel count.
Epidermal thickness was also calculated using the Maximum Dilation Test static tissue images by subtracting the mean pixel depth of the skin surface from the mean dermal-epidermal junction pixel depth, both of which were automatically identified. The mean epidermal thickness was calculated in all three maximum dilation images captured for each participant and the mean of the thickness found in each of the three images was used for the comparisons described below.
Participants
Study participants were chosen who had a unilateral transtibial amputation at least nine months prior. Inclusion criteria required that participants were 18 years of age or older, regularly visited a prosthetist, and wore their prosthesis at least seven hours per week. Participants were excluded if they had a history of skin breakdown at the ROI or if they had signs of skin breakdown anywhere on the limb at the time of study enrollment. All study procedures were approved by a University of Washington Institutional Review Board (#STUDY00006012). Written informed consent was obtained before test procedures were initiated.
Imaging Sessions
A prospective cross-sectional study was completed, where each participant visited the lab for a single imaging session. Test sessions began with the participants doffing their socket and completing a brief intake. The research prosthetist then performed a skin evaluation to verify no regions of breakdown were present. A residual limb skin ROI was selected by the research prosthetist on the medial aspect of the tibia that was believed to be in an area with high magnitude limb-socket interface stresses. This decision was based on the shape of the limb and socket and the presence of brief redness after doffing the socket. The skin was prepped with an alcohol prep pad and a surgical marker was used to mark the ROI and a location-matched ROI on the contralateral limb. Participants were maneuvered into a comfortable position with their limbs externally rotated and stabilized by a formable positioning cushion (Vac-Lok Cushion, CIVCO Radiotherapy, Orange City, IA, USA). Participants remained in the supine position for at least 15 minutes prior to any image collection in order to allow their cutaneous vasculature to acclimate to the room temperature and the position. Each OCT test was then performed on the residual limb, beginning with the RH Test, followed by the Maximum Dilation Test. This order was chosen so the RH response of the skin would not be altered by the skin heating protocol. The tests were then repeated at the contralateral ROI. Room temperature was maintained between 21–23°C.
Data Analysis
On a participant-by-participant basis, all measurements described above were compared between the ROI on the residual limb versus the ROI on the contralateral limb. Maximum dilation images were also qualitatively assessed, noting features such as vessel shape and orientation.
RESULTS
Participants
Three individuals with unilateral transtibial amputation participated in the study. Participant characteristics are described in Table 1. The ROI selected for imaging was located 4.0–5.5 cm distal of the mid-patellar tendon and between 2.5–3.0 cm medial of the tibial crest. Limb photographs including the ROI location are shown in Figure 1.
Table 1.
Study participant characteristics.
| CHARACTERISTIC | PARTICIPANT 1 | PARTICIPANT 2 | PARTICIPANT 3 |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Gender | Male | Male | Female |
| Age | 58 yr | 44 yr | 25 yr |
| Ethnicity | Not Hispanic/Latino | Not Hispanic/Latino | Not Hispanic/Latino |
| Race | White | African-American | White |
| Height | 193 cm | 175 cm | 168 cm |
| Weight (with prosthesis) | 130 kg | 93 kg | 57 kg |
| HEALTH INFORMATION | |||
| Diabetes | Type II | No | No |
| Hypertension | No | Yes | No |
| Smoker | No | No (> 1.5 yr) | No |
| Caffeine drinker | No | Yes | Yes |
| Vasodilator medication | No | Yes | No |
| RESIDUAL LIMB / PROSTHESIS | |||
| Residual limb side | Left | Right | Right |
| Years since amputation | 14 yr | 6 yr | 8 yr |
| Cause of amputation | Dysvascular | Trauma | Trauma |
| Socket type | PTB | PTB | TSB* |
| Suspension style | Lock & Pin | Lock & Pin | Elevated Vacuum* |
| Hours/day worn | 18 | 16–18 | 14 |
| MFCL K-level | K-3 | K-3 | K-3 |
| Skin health | Breakdown rare | Breakdown rare | Breakdown rare |
PTB: Patellar Tendon Bearing
TSB: Total Surface Bearing
MFCL: Medicare Functional Classification Level11
Participant 3 had previously used a PTB socket with lock & pin suspension but changed to a new elevated vacuum prosthesis 1 month prior to the study.
Figure 1.
Participant residual limbs. (a) Participant 1, (b) Participant 2, (c) Participant
3. Shown are: ROI locations where imaging occurred (dotted circle), tibial crest (dotted line), and mid-patellar tendon (“x”).
Reactive Hyperemia Test
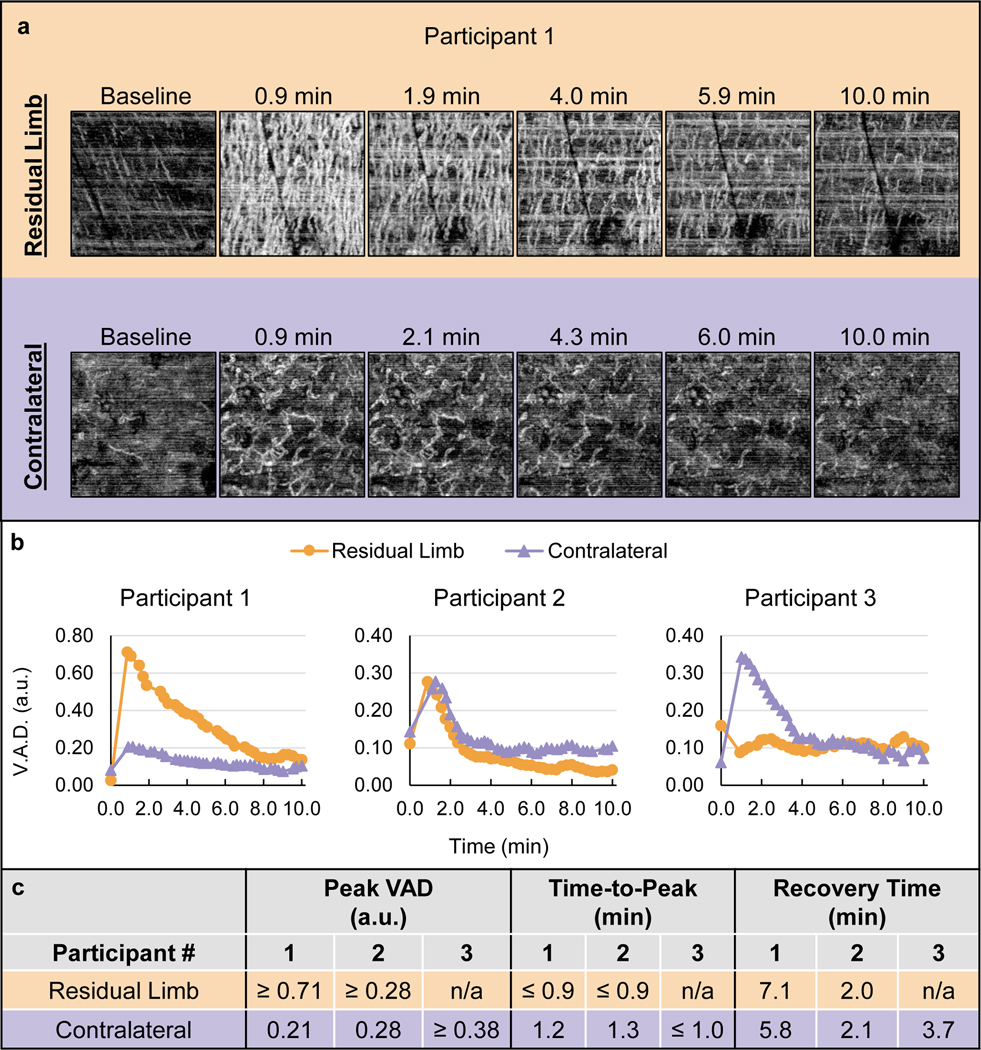
A representative RH Test image set is shown in Figure 2a. The images demonstrate that Participant 1 experienced notably more blood flow in the residual limb versus the contralateral limb throughout the duration of the RH response. The quantified RH response curves for all participants are shown in Figure 2b and the quantified RH features are summarized in Figure 2c. These data demonstrate that two of the three participants experienced a faster time-to-peak in the residual limb versus the contralateral limb. The peak VAD was also greater in the residual limb for one participant, and potentially for the other two; however, it was not possible to make this determination for Participants 2 and 3 since the peak of the response did not appear to have been captured.
Figure 2.
RH Test results. (a) Representative image set showing select en face OCT images from the RH tests on each limb of Participant 1. Images are 2 × 2 mm. Horizontal white lines are motion artifacts. (b) Plots of VAD vs. time for each participant. (c) Quantified vascular response characteristics determined by the VAD vs. time data.
Participant 1’s residual limb demonstrated a much more extensive vascular response than the contralateral limb, with more vessel recruitment throughout the duration of the response (Figure 2a). The peak VAD appeared to occur prior to the first post-load image, thus the peak VAD was reported as ≥ 0.71 a.u. and the time-to-peak as ≤0.9 min. This peak VAD was notably larger than the contralateral limb (0.21 a.u.) and the time-to-peak was faster (1.2 min in the contralateral limb). The recovery was slower in the residual versus contralateral limb, with recovery times of 7.1 min and 5.8 min, respectively.
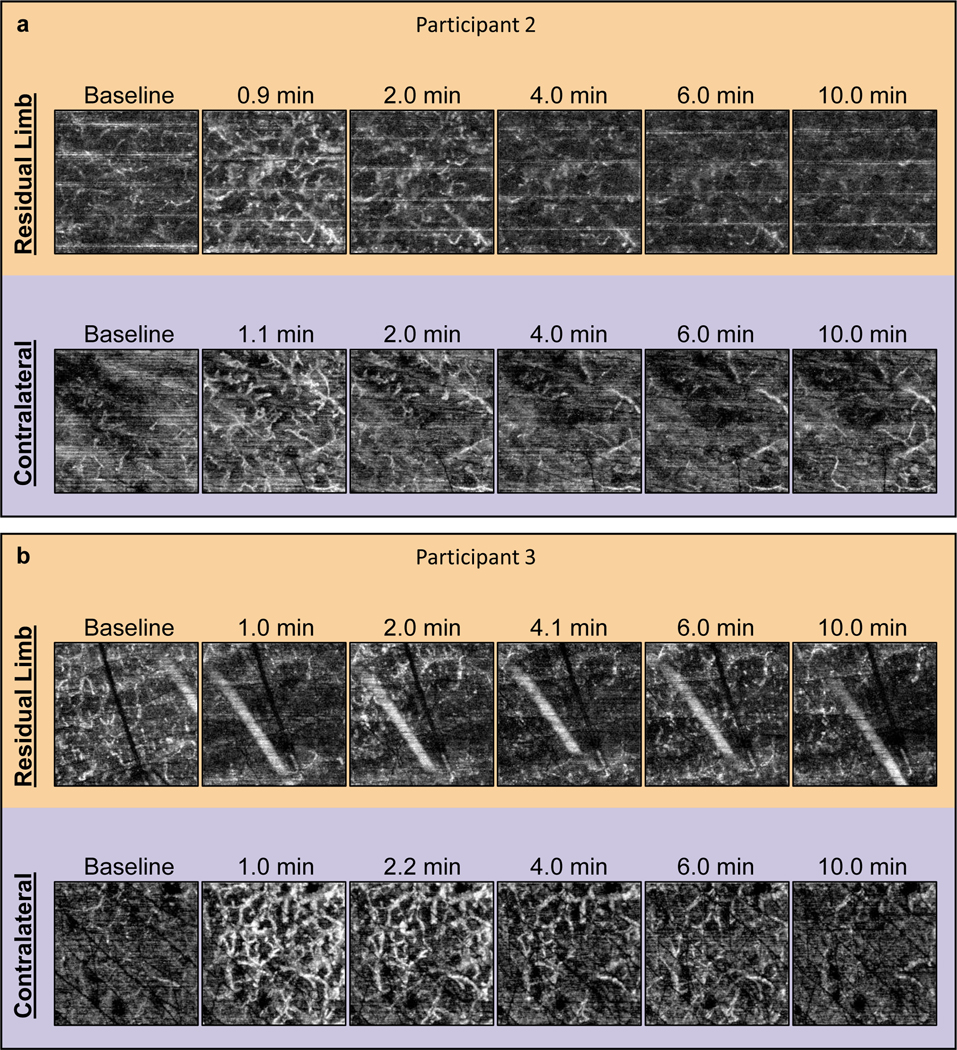
Participant 2’s time-to-peak was faster in the residual limb (≤0.9 min) compared to the contralateral limb (1.3 min). The peak VAD was ≥0.28 a.u. and 0.28 a.u. in the residual and contralateral limbs, respectively. As such, it was unclear if the physiological peak perfusion in the residual limb was greater or equal to the contralateral limb. The recovery time was similar in both limbs, with 2.0 min and 2.1 min in the residual and contralateral limbs, respectively. The en face images for Participant 2 are found in Figure 3a.
Figure 3.
RH Test images for Participants 2 and 3. Select en face OCTA images from the RH Tests on each limb for (a) Participant 2 and (b) Participant 3. Images are 2 × 2 mm.
Participant 3 demonstrated a typical RH response in the contralateral limb but a lack of RH in the residual limb where an increase in perfusion was not captured (Figure 3b). Since skin redness was visually verified prior to placing the OCT probe for imaging, it is believed that RH did occur, but that it resolved prior to the first post-stress image. This would mean the time-to-peak and recovery time were much faster in the residual limb than the contralateral limb; however, since the peak was not captured no definitive comparisons could be made from this participant’s data.
Maximum Dilation Test
All participant vessel images and corresponding vessel measurements are shown in Figure 4 and Figure 5, respectively.
Figure 4.
Maximum Dilation Test images, all participants.
Figure 5.
Maximum Dilation Test measurements, all participants.
Participant 1 demonstrated notably different vascular morphologies between the residual limb and contralateral limb in each of the dermal layers. The residual limb contained a dense network of vessels that appeared aligned parallel to each other. This alignment was at approximately a 45° angle between the medial and proximal directions. The denser network was confirmed by greater VAD measurements in the papillary dermis (0.38 versus 0.27 a.u., residual versus contralateral limb) and the reticular dermis (0.58 versus 0.38 a.u., residual versus contralateral limb). This was mostly due to a larger vessel count in the residual limb compared to the contralateral limb: 11.90 versus 9.10 mm/mm2 (residual limb vessel count versus contralateral limb vessel count) in the papillary dermis and 9.93 versus 7.94 mm/mm2 (residual versus contralateral limb) in the reticular dermis. The mean vessel diameter was also slightly larger for the residual versus contralateral limb, with 32 versus 30 μm in the papillary dermis and 59 versus 47 μm in the papillary dermis.
Similar to Participant 1, the Maximum Dilation Test images for Participant 2 also showed a denser vascular network in both layers of the residual limb versus the contralateral limb. This was confirmed by VAD measurements in the papillary dermis (0.28 versus 0.20 a.u., residual versus contralateral limb) and in the reticular dermis (0.48 versus 0.35 a.u., residual versus contralateral limb). This appeared mostly due to a greater number of vessels in the residual versus contralateral limb (10.19 versus 8.02 mm/mm2 in the papillary dermis and 8.39 versus 6.57 mm/mm2 in the reticular dermis). The mean vessel diameter was marginally larger in the residual limb versus contralateral limb: 27 versus 25 μm in the papillary dermis and 57 versus 53 μm in the reticular dermis.
Participant 3 demonstrated notably different vessel morphologies in both layers of the residual limb compared to the contralateral limb, with shorter, less continuous vessel segments in both layers of the residual limb compared to the contralateral limb. Contrary to the other participants’ quantified vessel characteristics, Participant 3 demonstrated larger values in the contralateral limb compared to the residual limb for all vessel measurements. The vessel density difference was minimal in the papillary dermis (0.27 versus 0.30 a.u., residual versus contralateral limb), but more substantial in the reticular layer (0.34 versus 0.52 a.u., residual versus contralateral limb). This difference appeared to be driven by a larger vessel count and larger mean vessel diameter. The vessel count was 9.88 versus 10.19 mm/mm2 (residual versus contralateral limb) in the papillary dermis and 7.62 versus 9.68 mm/mm2 (residual versus contralateral limb) in the reticular dermis. The mean vessel diameter was 28 versus 30 μm (residual versus contralateral limb) in the papillary dermis and 44 versus 54 μm (residual versus contralateral limb) in the reticular dermis.
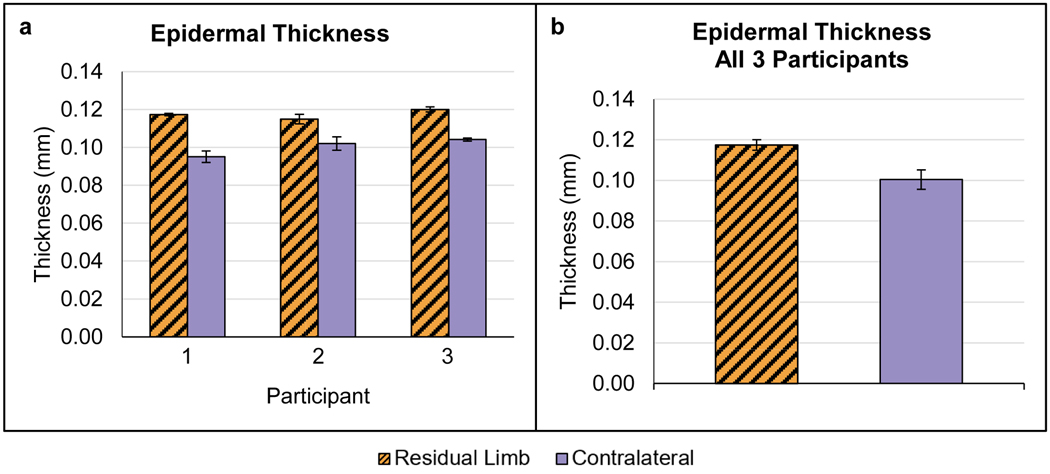
Epidermal Thickness
All participants demonstrated a thicker epidermal layer in the residual limb versus contralateral limb (Figure 6). Combining data for all three participants demonstrated a mean thickness of 0.117 ± 0.003 mm (mean ± SD) in the residual limb versus 0.100 ± 0.005 mm in the contralateral limb.
Figure 6.
Epidermal thickness results. (a) Data for each participant, shown as the mean (± SD) of the thickness calculated in each of three full field-of-view images captured during each test. (b) Mean thickness (± SD) for all participants combined.
DISCUSSION
Results from this study demonstrated the utility of two novel OCT-based imaging tests for the study of skin adaptation of lower-limb prosthesis users. To the authors’ knowledge, no such measurement methods exist that can noninvasively provide rich data sets of cutaneous vascular function and structure, including both qualitative vessel image assessments and quantified measurements. In the current study, the methods enabled comparisons of the magnitude and kinetics of reactive hyperemia in the skin following an occlusive cyclic stress and they also identified several structural differences in the skin’s microvasculature and tissue structure. While these methods were recently introduced to study skin adaptation in able-bodied participants using a model that simulated prosthetic stresses,6 the current study represents the first time the methods were used to investigate the skin of individuals with transtibial limb loss.
Consistent with our hypotheses for the functional vascular assessment—the Reactive Hyperemia Test—participants demonstrated a faster RH time-to-peak in the residual limb versus contralateral. Also consistent with our hypotheses, the peak VAD was much larger in the residual limb for one participant, and potentially for the other two, though it was not clear since the peak of the response was not captured in the residual limb test for these two participants. If these measured differences were the result of adaptation to repetitive stress from wearing a prosthetic socket, this change could have resulted from functional adaptations, structural adaptations, or a combination of the two. Functional adaptations refer to enhancements to the biomechanical and biochemical cascade of events that take place to induce vessel dilation; whereas structural adaptations refer to vascular remodeling that may enable more rapid and more extensive perfusion into the tissue. While definitively making this distinction was not within the scope of the current study, the latter explanation was supported by results from the Maximum Dilation Test which demonstrated that Participants 1 and 2 had denser vascular networks in their residual limb compared to the contralateral limb. Support also exists for the possibility of functional adaptations, largely in the field of exercise physiology. Researchers have found support for functional vascular adaptations following training programs that were used to elicit repetitive bouts of hyperemia in skin including exercise, arm heating, whole body heating, and repeated pressure cuff-induced occlusion.16,17 Researchers found that vessels could dilate more rapidly and closer to their maximum diameter after adaptation was induced. The studies also found support that multiple stimuli contribute to the initiation of this functional adaptation, namely the mechanical stress that endothelial and smooth muscle cells experience from repetitive bursts of reperfusion. A key mechanism that has been elucidated is that endothelial cells adapt by upregulating genes for NO and NO-precursor production, therefore more vasodilators become available for future RH responses.18,19
The structural vessel assessment—the Maximum Dilation Test—highlighted drastic differences in vessel morphologies between the residual and contralateral limbs of participants. Consistent with our hypotheses, two of three participants displayed a significantly larger vessel density in the residual limb compared to the contralateral limb, which was driven by an increase in vessel count and, to a lesser extent, an increase in mean vessel diameter. It is possible these differences represent vascular adaptations that have occurred in the skin due to several years of mechanical stresses imposed on the limb by the socket. Though this has not been studied in the context of prosthetics, support does exist in the literature for these vascular adaptations in response to stimuli that are similar to those present in the case of residual limb skin interacting with a prosthetic socket. Angiogenesis is the primary process by which new vessels are created. It is known to be initiated by tissue hypoxia, which occurs in the microvasculature when vessels are occluded.20,21 Thus, this angiogenic stimulus is likely to be present in many regions of the residual limb when the socket is worn, considering that cutaneous vessels can be occluded by as little as 11.6 kPa applied externally,9 far less than limb-socket interface stresses commonly seen.13 Angiogenesis in skin in response to a slightly different stress type—tension—has been shown to produce new vessels following only two days of a one-hour stress protocol. This was demonstrated in the mouse dorsum by Chin et al., where researchers determined that tension induced ischemia which initiated angiogenesis, as evidenced by upregulated hypoxia-stimulated angiogenic factors in the tissue (VEGF and HIF-1α).22 Similarly, Erba et al. found visual and biochemical evidence of increases in cutaneous microvascular vessel density in the dorsum of mice following a four-hour tension application.23 Vessel diameter increases are commonly initiated through a process known as arteriogenesis.24 The stimulus for arteriogenesis is believed to be increasing shear stress on vessel walls, which occurs through repeated bouts of considerably increased blood flow,25 such as during repetitive RH responses that are induced by limb-socket interface stresses. Arteriogenesis as a mechanism of adaptation has been demonstrated in exercise physiology research and tissue expansion research. Tinken et al. demonstrated increases in brachial and popliteal conduit artery diameters by the fourth week of an exercise training program that consisted of 30 minutes of running and cycling three times per week.26 Using a rat ear model to study tissue expansion, Pietramaggiori et al. demonstrated vessel diameter increases of 60% and 100% after a static tension protocol was used for two and four days, respectively.27
The notable vessel alignment seen in both dermal layers of Participant 1’s residual limb may have been induced by chronic shear stress acting through the skin. While the direction of shear force was not measured in this participant’s socket, it has been demonstrated previously in similarly designed PTB sockets that shear commonly acts approximately at 45° in the medial-proximal direction.28 This would mean Participant 1’s vessels aligned parallel to the direction of shear stresses. Erba et al. found a similar increase in vascular alignment in the cutaneous microvasculature of the dorsum of mice after 7 days of a 4-hour daily tension.23 Though their study did not investigate the cause of the alignment, the researchers hypothesized that aligned vessels may represent an adaptation of stressed tissue since aligned vessels may enable more efficient transport of blood into the stressed area compared to more tortuous vessels.
The most consistent difference found between the residual and contralateral limbs in the current study was a thicker epidermis in the residual limb compared to the contralateral limb. This result was consistent with our hypotheses for adapted skin. It is possible that the participants in this study gained a thicker epidermis through skin adaptation to the chronic mechanical stresses of using a lower-limb prosthesis. A thicker epidermis may be beneficial to absorb more of the energy caused by the imposed shear forces, thereby limiting the shear that is able to act on the more fragile, vascularized tissues below. Based on the appearance of the participants’ limbs it seemed the thicker epidermis was not simply due to callusing of the skin. Callus formation is generally undesirable on the residual limb since the stiff callus mass can lead to increased stress concentrations in tissues below or at the edges of the callus and calluses are more prone to skin breaks than non-callused skin. Furthermore, callusing has been shown to increase the risk of pressure ulceration to the underlying tissue in load-bearing regions of skin.29 While it is unknown whether this finding represents a beneficial adaptation or not, it deserves further investigation.
While this study demonstrated the ability of novel noninvasive imaging techniques to identify differences in cutaneous microvascular function in lower-limb prosthesis users, the small subject number limited the statistical power of the tests. Rather, this study served a proof-of-concept for the potential value of the developed test methods for investigating skin adaptation in individuals with lower-limb loss. And, while the methods used here proved able to identify some interesting findings, including multiple potential biomarkers for skin adaptation, the study also highlighted some of the current limitations of the methods that need to be addressed.
The lack of consistent RH peak capture during the RH Test resulted in gaps in the data collected. While we were still able to determine several relationships for Participants 1 and 2 since the peak was captured in their contralateral limb, no comparisons could be made for Participant 3 since no residual limb response was captured. If imaging could have been initiated sooner for all participants, more complete data sets could have been obtained. This lack of peak capture was due to the RH peak occurring faster than the time it took to remove the stress application device and prepare for OCT image capture. In the future, this transition could be hastened by automating several of the steps required to prepare for imaging. This could be accomplished through integrating the OCT probe into the load application device or by incorporating motors and a control system to quickly set the OCT probe pressure and imaging depth.
In this study, we chose to focus on a few possible skin adaptations based on the measurements that could be made given the test methods used. It should be noted, however, that support exists for additional mechanisms of adaptation that are worth considering for further investigation. For one, support exists for structural adaptations in the extracellular matrix of the dermis that may lead to decreased deformation to mechanical stress. Using a porcine model and daily stress protocol designed to replicate stresses experienced by a transtibial residual limb, Sanders et al. previously demonstrated an increase in collagen fibril diameters in the stressed tissue.30 In that study, measurements were taken of biopsied tissues, whereas noninvasive methods are needed to study this in a population of prosthesis users. While such methods are still sparse, polarization-sensitive OCT has shown promise. Polarization-sensitive OCT detects birefringent tissue components, such as collagen in the skin, providing measurements of density and alignment of the molecules. Polarization-sensitive OCT has been used to study skin collagen density in burn scars,31 collagen denaturation due to burns,32 and burn depth.33 Insight into extracellular matrix adaptations could also be gained by taking biomechanical measurements of skin. Suction-based skin measurement devices, such as the Cutometer Skin Elasticity Meter (Cutometer SEM575, Courage and Khazaka, Cologne, Germany), have been used in skin research to take noninvasive, in-vivo biomechanical measurements of skin.
CONCLUSION
The results of this study demonstrated the utility of OCT imaging as a noninvasive tool to better understand skin adaptation and skin health as a whole in individuals whose skin experiences chronic mechanical stress. Further research is needed to confirm the findings of this study using a larger subject population and to determine if the findings are indicative of changes that represent beneficial adaptations or if they are merely passive changes that do not impart a benefit to the tissue. Improvements to the test methods such as enabling faster RH Test image capture following load removal or by combining OCT imaging with other structural and functional skin measurement techniques could provide an even richer data set that could be used in future studies to further enhance our understanding of skin adaptation to limb-socket interface stresses. Using these methods to further improve the understanding of skin adaptation in people with lower-limb amputations could lead to improved rehabilitation techniques and therapeutics to adapt skin or to tools that could be used by clinicians to better assess skin health and help to guide skin adaptation when desired.
Acknowledgments
This research was funded by the National Institutes of Health grant number R01 HD060585. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institutes of Health. The authors declare no conflict of interest.
Contributor Information
Eric C. Swanson, Department of Bioengineering, University of Washington, Seattle.
Janna L. Friedly, Department of Rehabilitation Medicine, University of Washington, Seattle.
Ruikang K. Wang, Department of Bioengineering, University of Washington, Seattle.
Joan E Sanders, Department of Bioengineering, University of Washington, Seattle.
REFERENCES
- 1.Meulenbelt HE, Geertzen JH, Jonkman MF, Dijkstra PU. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90(1):74–81. doi: 10.1016/j.apmr.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 2.Sanders JE, Goldstein BS, Leotta DF. Skin response to mechanical stress: Adaptation rather than breakdown — A review of the literature. J Rehabil Res Dev. 1995;32(2):214–226. [PubMed] [Google Scholar]
- 3.Tomlins PH, Wang RK. Theory, developments and applications of optical coherence tomography. Appl Phys. 2005;38(15):2519–2535. [Google Scholar]
- 4.Deegan AJ, Wang W, Men S, et al. Optical coherence tomography angiography monitors human cutaneous wound healing over time. Quant Imaging Med Surg. 2018;8(2):135–150. doi: 10.21037/qims.2018.02.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deegan AJ, Talebi-Liasi F, Song S, et al. Optical coherence tomography angiography of normal skin and inflammatory dermatologic conditions. Lasers Surg Med. 2018;(December):1–11. doi: 10.1002/lsm.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanson EC, Friedly JL, Wang RK, Sanders JE. Optical Coherence Tomography for the investigation of skin adaptation to mechanical stress. Ski Res Technol. 2020;26(5):627–638. doi: 10.1111/srt.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CL, Wang RK. Optical coherence tomography based angiography. Biomed Opt Express. 2017;8(2):1056–1082. doi: 10.1364/BOE.8.001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi WJ, Reif R, Yousefi S, Wang RK. Improved microcirculation imaging of human skin in vivo using optical microangiography with a correlation mapping mask. J Biomed Opt. 2014;19(3):036010. doi: 10.1117/1.JBO.19.3.036010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens RH, Zegers R, Hoek van Dijke GA, Snijders CJ. Influence of shear on skin oxygen tension. Clin Physiol. 1994;14(1):111–118. doi: 10.1111/j.1475-097X.1994.tb00495.x [DOI] [PubMed] [Google Scholar]
- 10.Holloway G, Daly C, Kennedy D, Chimoskey J. Effects of external pressure loading on human skin blood flow measured by 133Xe clearance. J Appl Physiol. 1976;40(4):597–600. doi: 10.1166/jnn.2012.6247 [DOI] [PubMed] [Google Scholar]
- 11.Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation. 2012;19(1):47–64. doi: 10.1111/j.1549-8719.2011.00129.x [DOI] [PubMed] [Google Scholar]
- 12.Sanders JE, Garbini JL, Leschen JM, Allen MS, Jorgensen JE. A bidirectional load applicator for the investigation of skin response to mechanical stress. IEEE Trans Biomed Eng. 1997;44(4):290–296. [DOI] [PubMed] [Google Scholar]
- 13.Sanders JE, Daly CH, Burgess EM. Clinical measurement of normal and shear stresses on a trans-tibial stump: characteristics of wave-form shapes during walking. Prosthet Orthot Int. 1993;17(1):38–48. [DOI] [PubMed] [Google Scholar]
- 14.Chu Z, Lin J, Gao C, et al. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt. 2016;21(6):066008. doi: 10.1117/1.JBO.21.6.066008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JM, Kellogg DL. Local thermal control of the human cutaneous circulation. J Appl Physiol. 2010;109(4):1229–1238. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DHJ. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. 2014;27(7):918–925. doi: 10.1093/ajh/hpu004 [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;97(2):495–528. doi: 10.1152/physrev.00014.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75(3):1354–1363. [DOI] [PubMed] [Google Scholar]
- 19.Chen HI, Li HT. Physical conditioning can modulate endothelium-dependent vasorelaxation in rabbits. Arter Thromb. 1993;13(6):852–856. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Swerlick RA. Structure and function of the cutaneous vasculature. In: Freinkel RK, Woodley DT, eds. The Biology of the Skin. New York: The Parthenon Publishing Group; 2001:177–189. [Google Scholar]
- 21.Logsdon EA, Finley SD, Popel AS, MacGabhann F. A systems biology view of blood vessel growth and remodelling. J Cell Mol Med. 2014;18(8):1491–1508. doi: 10.1111/jcmm.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin MS, Ogawa R, Lancerotto L, et al. In vivo acceleration of skin growth using a servo-controlled stretching device. Tissue Eng Part C-Me. 2010;16(3):397–405. doi: 10.1089/ten.tec.2009.0185 [DOI] [PubMed] [Google Scholar]
- 23.Erba P, Miele LF, Adini A, et al. A morphometrical study of mechanotransductively induced dermal neovascularization. Plast Reconstr Surg. 2011;128(4):288e–299e. doi: 10.1016/j.jsbmb.2011.07.002.Identification [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651 [DOI] [PubMed] [Google Scholar]
- 25.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97(3):1119–1128. doi: 10.1152/japplphysiol.00035.2004 [DOI] [PubMed] [Google Scholar]
- 26.Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586(20):5003–5012. doi: 10.1113/jphysiol.2008.158014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietramaggiori G, Liu P, Scherer SS, et al. Tensile forces stimulate vascular remodeling and epidermal cell proliferation in living skin. Ann Surg. 2007;246(5):896–902. doi: 10.1097/SLA.0b013e3180caa47f [DOI] [PubMed] [Google Scholar]
- 28.Sanders JE, Daly CH, Burgess EM. Interface shear stresses during ambulation with a below-knee prosthetic limb. J Rehabil Res Dev. 1992;29(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabet Med. 1996;13(11):979–982. doi:10.1002 [DOI] [PubMed] [Google Scholar]
- 30.Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34(12):1581–1587. doi: 10.1016/s0021-9290(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 31.Jaspers MEH, Feroldi F, Vlig M, de Boer JF, van Zuijlen PPM. In vivo polarization-sensitive optical coherence tomography of human burn scars: birefringence quantification and correspondence with histologically determined collagen density. J Biomed Opt. 2017;22(12):121712. doi: 10.1117/1.JBO.22.12.121712 [DOI] [PubMed] [Google Scholar]
- 32.Pierce MC, Sheridan RL, Hyle Park B, Cense B, De Boer JF. Collagen denaturation can be quantified in burned human skin using polarization-sensitive optical coherence tomography. Burns. 2004;30(6):511–517. doi: 10.1016/j.burns.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 33.Srinivas SM, de Boer JF, Park H, et al. Determination of burn depth by polarization-sensitive optical coherence tomography. J Biomed Opt. 2004;9(1):207–212. [DOI] [PubMed] [Google Scholar]