Abstract
Background
Data on the relative contribution of clinical and neuroimaging risk factors to acute ischemic stroke (AIS) versus intracerebral hemorrhage (ICH) occurring on oral anticoagulant treatment are scarce.
Methods and Results
Cross‐sectional study was done on consecutive oral anticoagulant–treated patients presenting with AIS, transient ischemic attack (TIA), or ICH from the prospective observational NOACISP (Novel‐Oral‐Anticoagulants‐In‐Stroke‐Patients)‐Acute registry. We compared clinical and neuroimaging characteristics (small vessel disease markers and atherosclerosis) in ICH versus AIS/TIA (reference) using logistic regression. Among 734 patients presenting with stroke on oral anticoagulant treatment (404 [55%] direct oral anticoagulants, 330 [45%] vitamin K antagonists), 605 patients (82%) had AIS/TIA and 129 (18%) had ICH. Prior AIS/TIA, coronary artery disease, dyslipidemia, and worse renal function were associated with AIS/TIA (adjusted odds ratio [aOR] [95% CI] 0.51 [0.32–0.82], 0.48 [0.26–0.86], 0.55 [0.34–0.89], and 0.82 [0.75–0.90] per 10 mL/min). Prior ICH, older age, higher admission blood pressure, and statin treatment were associated with ICH (aOR [95% CI] 6.33 [2.87–14.04], 1.37 [1.04–1.81] per 10 years, 1.19 [1.10–1.29] per 10 mm Hg, and 1.81 [1.09–3.03]). Cerebral microbleeds and moderate‐to‐severe white matter hyperintensities contributed more to ICH (aOR [95% CI] 2.77 [1.34–6.18], and 2.62 [1.28–5.63]). Aortic arch, common and internal carotid artery atherosclerosis, and internal carotid artery stenosis ≥50% contributed more to AIS/TIA (aOR [95% CI] 0.54 [0.31–0.90], 0.29 [0.05–0.97], 0.48 [0.30–0.76], and 0.32 [0.13–0.67]).
Conclusions
In patients presenting with stroke on oral anticoagulant, AIS/TIA was 5 times more common than ICH. A high atherosclerotic burden (indicated by cardiovascular comorbidities and extracranial atherosclerosis) and prior AIS/TIA contributed more to AIS/TIA, while small vessel disease markers and prior ICH were stronger determinants for ICH.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02353585.
Keywords: atherosclerosis, intracerebral hemorrhage, ischemic stroke, oral anticoagulants, small vessel disease
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Intracranial Hemorrhage
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- CMB
cerebral microbleeds
- CTA
computed tomography angiography
- DOAC
direct oral anticoagulant
- ICH
intracerebral hemorrhage
- NOACISP
Novel Oral Anticoagulants In Stroke Patients
- OAC
oral anticoagulant
- SVD
small vessel disease
- VKA
vitamin K antagonist
- WMH
white matter hyperintensities
Clinical Perspective
What Is New?
In a contemporary sample of consecutive patients presenting with stroke on oral anticoagulant treatment, ischemic stroke or transient ischemic attack was 5 times more common than intracerebral hemorrhage.
Regardless of anticoagulant type (vitamin K antagonists or direct oral anticoagulants), a high atherosclerotic burden (indicated by cardiovascular comorbidities and extracranial atherosclerosis) was a more important contributor to treatment failure in patients with ischemic stroke or transient ischemic attack despite anticoagulation, whereas small vessel disease contributed more to anticoagulant‐associated intracerebral hemorrhage.
What Are the Clinical Implications?
The findings of this study may inform future efforts to mitigate the risk of treatment failure and treatment complications in patients taking oral anticoagulants.
Oral anticoagulants (OAC) reduce the risk of acute ischemic stroke (AIS) in patients with atrial fibrillation (AF) but they put patients at risk of intracerebral hemorrhage (ICH). 1 Direct oral anticoagulants (DOAC) were proven to have a more favorable risk–benefit profile than vitamin K antagonists (VKA). Despite these recent advances, patients treated with OAC may still have cerebrovascular events: either AIS despite OAC treatment or ICH as a treatment complication. 2 , 3 , 4 , 5 As OAC use increases, a better understanding of the characteristics and risk factors associated with treatment failure versus treatment complications is crucial.
Many clinical characteristics have been identified as independent risk factors for AIS and ICH in patients taking OAC. 6 , 7 , 8 Imaging characteristics, including atherosclerotic lesions of the supracardiac arteries 6 , 9 and cerebral small vessel disease (SVD) markers, such as cerebral microbleeds (CMB) and white matter hyperintensities (WMH), 10 , 11 , 12 have also been associated with increased risk for cerebrovascular events in anticoagulated patients. So far, most of these studies focused on identifying risk factors for either AIS or ICH. However, these unidirectional risk evaluations are less informative in situations where overlapping risk factors predominate. Only few studies have directly compared anticoagulated patients with AIS to those with ICH. These were limited to the comparison of clinical characteristics and investigated exclusively VKA‐treated patients using data from the pre‐DOAC era. 13 , 14 To date, only 1 small study included the assessment of SVD markers but not atherosclerotic lesions in DOAC‐treated patients with AIS versus ICH. 15
With these considerations in mind, in this study, as a novel aspect, we investigated the relative contribution of both clinical and neuroimaging risk factors, including atherosclerotic lesions and SVD markers, to AIS versus ICH in a contemporary sample of patients presenting with acute cerebrovascular events while on VKA or DOAC treatment.
METHODS
Study Design, Patient Population, and Data Collection
This was a cross‐sectional study using data from the observational, single‐center NOACISP‐(Novel Oral Anticoagulants In Stroke Patients)‐Acute registry (NCT02353585). The data used for this study are available from the corresponding author upon reasonable request. The detailed methodology of NOACISP‐Acute has been described previously. 16 In short, NOACISP‐Acute prospectively registers consecutive patients with an acute cerebrovascular event (AIS, transient ischemic attack [TIA], or intracranial hemorrhage) while treated with OAC (VKA or DOAC). Demographic, clinical, and neuroimaging characteristics are captured in an electronic database in a standardized manner.
In this study we included consecutive NOACISP‐Acute patients from December 2014 to May 2020 with (1) AIS (defined as a focal neurological deficit with acute onset and presence of a corresponding lesion on diffusion‐weighted magnetic resonance imaging [MRI] or, if no MRI was acquired, signs of early ischemic injury on computed tomography), TIA (defined as an acute‐onset focal neurological deficit of presumed ischemic origin without an MRI lesion or, if no MRI was acquired, lasting <24 hours) or spontaneous ICH (defined as a focal neurological deficit with acute onset and presence of intraparenchymal hemorrhage on computed tomography or MRI, without evidence of a secondary cause such as trauma, tumor, vascular malformation, or aneurysm) and (2) OAC treatment at event onset (either VKA or DOAC) for a label indication (AF, venous thromboembolism, or mechanical heart valve replacement).
The following variables were used in the analysis:
Demographic and clinical data: age, sex, event type (AIS, TIA, or ICH), antithrombotic treatment at event onset (VKA or DOAC, additional antiplatelet therapy), concomitant statin therapy, stroke severity on admission assessed by the National Institute of Health Stroke Scale, level of consciousness on admission assessed by the Glasgow Coma Scale, admission blood pressure and renal function (estimated glomerular filtration rate according to Cockcroft‐Gault), and the following risk factors applying predefined criteria in line with prior research 16 : hypertension, diabetes, dyslipidemia, prior AIS and/or TIA, prior ICH, prior gastrointestinal and/or other major bleeding, AF, congestive heart failure, peripheral artery disease, coronary artery disease, prosthetic heart valve replacement, and CHA2DS2‐VASc 17 and HAS‐BLED 18 score before event;
Neuroimaging data: (1) SVD markers including presence, number, and location of CMB and presence of superficial siderosis on susceptibility‐weighted MRI, as well as presence and extent of WMH on fluid‐attenuated inversion recovery MRI using the age‐related white matter changes rating scale 19 ; (2) presence, distribution, and severity of atherosclerotic lesions of the supracardiac arteries on computed tomography angiography (CTA). MRI scans were analyzed for markers of SVD by the investigators (F. S., L. H., N. P.), who were blinded to the history and outcome of the patient, as described previously. 12 Susceptibility‐weighted signal voids within the acute lesion or within nonacute (non‐diffusion‐restricted) lacunes were not considered. In cases with large ischemic lesions or large hemorrhage area and therefore only unilaterally assessable images, a symmetrical distribution of WMH on fluid‐attenuated inversion recovery was assumed. CTA images were analyzed by 2 authors (F. S., P. L.), blinded to the history and outcome of the patients and screened for presence of atherosclerosis on aortic arch, common and internal carotid artery, carotid siphon, M1 segment of the middle cerebral artery, vertebral artery, and basilar artery. In cases with atherosclerosis on the common and/or internal carotid artery, degree of stenosis was measured using the methods described by Bartlett et al. 20 The assessment of stenosis was completed by review of the neurovascular ultrasound, if available. In case of discrepancies for the degree of stenosis between CTA and neurovascular ultrasound, the degree measured by ultrasound was considered for the analysis. Only relevant atherosclerotic plaques were taken into consideration (either circular plaque or luminal stenosis). Consensus reading was performed for ambiguous cases for both MRI and CTA assessment.
Statistical Analysis
We present all patient characteristics stratified to event type (AIS/TIA versus ICH). Categorical data are presented as frequencies and percentages. For continuous variables, the mean, the SD, or (if skewed) the median and the interquartile range are presented. We assessed the association of clinical and neuroimaging characteristics with event type (ICH [as the outcome] versus AIT/TIA [reference group]) in univariable logistic regression models. For clinical data, we additionally fitted a multivariable logistic model including the following variables: sex, age, systolic blood pressure, renal function, hypertension, diabetes, coronary artery disease, heart failure, AF, peripheral artery disease, dyslipidemia, prior AIS/TIA, prior ICH, prior gastrointestinal and/or other major bleeding, prosthetic heart valve, type of OAC, concomitant antiplatelets, and statins. Because of expected collinearity, diastolic blood pressure, CHA2DS2‐VASc, and HAS‐BLED score were not included in the multivariable model. For the MRI and CTA imaging analyses, we additionally adjusted each univariable estimate for the CHA2DS2‐VASc and HAS‐BLED scores. The adjusted odds ratio (aOR), the (2‐sided 95% CI), and the P value based on likelihood ratio test are presented. OR >1 indicates that the respective variable favors ICH, while OR <1 favors AIS/TIA.
To assess for potential interactions between type of OAC (DOAC or VKA) and the rest of the clinical and neuroimaging characteristics on the odds of ICH versus AIS/TIA, we refitted each univariable logistic model by including type of OAC and the appropriate interaction term (type of OAC×characteristic), 1 model for 1 characteristic at a time.
Statistical analyses were performed using R version 3.6.2 (2019‐12‐12).
Ethics
The Ethics Committee of Northwestern and Central Switzerland approved the NOACISP‐Acute registry (EKNZ‐2014‐027), including this study (EKNZ‐2020‐02980). The committee waived the necessity to obtain informed consent from individual patients for this study. Patients who refused general consent for further use of health‐related personal data for research purposes at University Hospital Basel were excluded from the study.
RESULTS
A total of 734 patients (42% female, mean age 79.9 years) were eligible for analysis (for study flowchart see Figure S1). Of those, 605 patients (82%) had AIS or TIA and 129 (18%) had ICH. At event onset, 330 patients (45%) were taking VKA and 404 patients (55%) were on DOAC treatment. The distribution of OAC types (VKA versus DOAC) was balanced between the types of events. The clinical characteristics of all patients are displayed in Table 1. AF was the most common indication for OAC treatment (83.0%), followed by pulmonary embolism (8.3%), mechanical heart valve replacement (7.4%), and deep vein thrombosis (6.1%) (multiple indications may apply).
Table 1.
Patient Characteristics Stratified by Event Type
| Characteristic | All patients (n=734) | AIS/TIA (n=605) | ICH (n=129) | Missing values |
|---|---|---|---|---|
| Female sex, n (%) | 309 (42.1) | 255 (42.1) | 54 (41.9) | 0 |
| Age, y, median (IQR) | 82 (75–86) | 82 (75–86) | 82 (76–86) | 0 |
| NIHSS, median (IQR) | 5 (2–13) | 4 (1–10) | 13 (5–20) | 5 |
| GCS, median (IQR) | 15 (14–15) | 15 (14–15) | 14 (11–15) | 1 |
| Systolic blood pressure in mm Hg, mean (SD) | 152 (27) | 149 (26) | 163 (31) | 0 |
| Diastolic blood pressure in mm Hg, mean (SD) | 84 (17) | 83 (16) | 90 (17) | 0 |
| eGFR in mL/min per 1.73 m2, median (IQR) | 57.2 (41.9–77.2) | 55.1 (40.7–73.4) | 66.3 (47.6–84.1) | 2 |
| Medication | ||||
| VKA, n (%) | 330 (45.0) | 272 (45.0) | 58 (45.0) | 0 |
| DOAC, n (%) | 404 (55.0) | 333 (55.0) | 71 (55.0) | 0 |
| Any additional antiplatelets, n (%) | 87 (11.9) | 75 (12.4) | 12 (9.3) | 0 |
| Additional dual antiplatelets, n (%) | 3 (0.4) | 3 (0.5) | 0 (0.0) | 0 |
| Concomitant statins, n (%) | 322 (43.9) | 270 (44.6) | 52 (40.3) | 0 |
| Medical history | ||||
| Hypertension, n (%) | 616 (83.9) | 504 (83.3) | 112 (86.8) | 0 |
| Diabetes, n (%) | 170 (23.2) | 146 (24.1) | 24 (18.6) | 0 |
| Dyslipidemia, n (%) | 327 (44.6) | 284 (46.9) | 43 (33.3) | 0 |
| Atrial fibrillation, n (%) | 584 (79.6) | 485 (80.2) | 99 (76.7) | 0 |
| Heart failure, n (%) | 144 (19.6) | 126 (20.8) | 18 (14.0) | 0 |
| Prosthetic heart valve, n (%) | 69 (9.4) | 61 (10.1) | 8 (6.2) | 0 |
| Coronary artery disease, n (%) | 215 (29.3) | 194 (32.1) | 21 (16.3) | 0 |
| Peripheral artery disease, n (%) | 85 (11.6) | 74 (12.2) | 11 (8.5) | 0 |
| Prior AIS/TIA, n (%) | 276 (37.6) | 240 (39.7) | 36 (27.9) | 0 |
| Prior ICH, n (%) | 35 (4.8) | 19 (3.1) | 16 (12.4) | 0 |
| Prior gastrointestinal and/or other major bleeding, n (%) | 35 (4.8) | 28 (4.6) | 7 (5.4) | 0 |
| CHA2DS2‐VASc score, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) | 0 |
| HAS‐BLED score, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0 |
AIS indicates acute ischemic stroke; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IQR, interquartile range; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischemic attack; and VKA, vitamin K antagonist.
Clinical Characteristics
In univariable logistic regression models, prior AIS/TIA, coronary artery disease, dyslipidemia, higher CHA2DS2‐VASc score, and worse renal function were associated with AIS/TIA rather than ICH (OR <1). Prior ICH, higher blood pressure, higher National Institute of Health Stroke Scale, and lower Glasgow Coma Scale score on admission were associated with higher odds for ICH versus AIS/TIA (OR >1). The detailed results of the univariable logistic models are shown in Table S1.
In the multivariable model, prior AIS/TIA (aOR, 0.51 [95% CI, 0.32–0.82], P<0.01), coronary artery disease (aOR, 0.48 [95% CI, 0.26–0.86], P=0.012), dyslipidemia (aOR, 0.55 [95% CI, 0.34–0.89], P=0.014) and worse renal function (aOR, 0.82 per 10 mL/min lower estimated glomerular filtration rate [95% CI, 0.75–0.90], P<0.01) remained associated with AIS/TIA rather than ICH. Age (aOR, 1.37 per 10 years older age [95% CI, 1.04–1.81], P=0.024), prior ICH (aOR, 6.33 [95% CI, 2.87–14.04], P<0.01), higher admission blood pressure (aOR, 1.19 per 10 mm Hg higher systolic blood pressure [95% CI, 1.10–1.29], P<0.01), and concomitant statin therapy (aOR, 1.81 [95% CI, 1.09–3.03], P=0.022) were associated with ICH rather than AIS/TIA (Figure 1 and Table 2). A post hoc analysis using Lasso regression (with the regularization parameter λ determined based on 10‐fold cross‐validation) to reduce the multivariable model to a smaller set of independent variables yielded consistent results with the full multivariable model (Table S2).
Figure 1. Multivariable logistic regression model for clinical characteristics with aOR favoring ICH vs AIS/TIA.
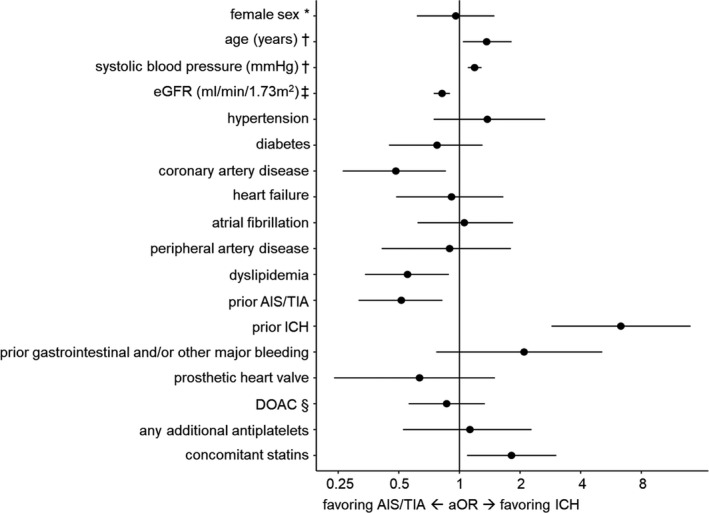
aOR and 95% CI are presented for each independent variable. *As opposed to male sex, †effect of an increase of 10 units, ‡effect of a decrease of 10 mL/min per 1.73 m2, §as opposed to VKA. AIS indicates acute ischemic stroke; aOR, adjusted odds ratio; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; ICH, intracerebral hemorrhage; TIA, transient ischemic attack; and VKA, vitamin K antagonist.
Table 2.
Multivariable Logistic Regression Model for Clinical Characteristics With aOR Favoring ICH Versus AIS/TIA
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| Female sex (vs male sex) | 0.96 | 0.62–1.49 | 0.853 |
| Age (per 10‐y increase) | 1.37 | 1.04–1.81 | 0.024 |
| Systolic blood pressure (per 10 mm Hg increase) | 1.19 | 1.10–1.29 | <0.01 |
| eGFR (per 10 mL/min per 1.73 m2 decrease) | 0.82 | 0.75–0.90 | <0.01 |
| Hypertension | 1.38 | 0.75–2.66 | 0.315 |
| Diabetes | 0.77 | 0.45–1.30 | 0.339 |
| Dyslipidemia | 0.55 | 0.34–0.89 | 0.014 |
| Atrial fibrillation | 1.06 | 0.62–1.84 | 0.840 |
| Heart failure | 0.91 | 0.49–1.65 | 0.770 |
| Prosthetic heart valve | 0.63 | 0.24–1.50 | 0.311 |
| Coronary artery disease | 0.48 | 0.26–0.86 | 0.012 |
| Peripheral artery disease | 0.89 | 0.41–1.80 | 0.762 |
| Prior AIS/TIA | 0.51 | 0.32–0.82 | <0.01 |
| Prior ICH | 6.33 | 2.87–14.04 | <0.01 |
| Prior gastrointestinal and/or other major bleeding | 2.09 | 0.77–5.12 | 0.141 |
| DOAC (vs VKA) | 0.86 | 0.56–1.34 | 0.510 |
| Any additional antiplatelets | 1.13 | 0.52–2.28 | 0.749 |
| Concomitant statins | 1.81 | 1.09–3.03 | 0.022 |
AIS indicates acute ischemic stroke; aOR, adjusted odds ratio; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; ICH, intracerebral hemorrhage; TIA, transient ischemic attack; and VKA, vitamin K antagonist.
SVD Assessment
MRI was available for assessment of SVD markers in 514/734 patients (70.0%; 477/605 AIS/TIA patients [78.8%] and 37/129 ICH patients [28.7%]). Patients who did not undergo MRI were generally more severely affected and had higher prevalence of cardiovascular risk factors. These imbalances were similar in patients with AIS/TIA and ICH (Table S3).
CMB were present in 237/477 patients with AIS/TIA (50%) and 27/37 patients with ICH (73%) with available MRI. The presence of CMB was strongly associated with ICH rather than AIS/TIA (OR, 2.73 [95% CI, 1.33–6.05], P<0.01) in univariable logistic regression. The detailed results of the CMB models are presented in Table 3. The association was strongest in patients with purely superficial and mixed location of CMB (OR, 2.74 [95% CI, 1.10–6.72] and OR, 3.50, [95% CI, 1.54–8.16, respectively). The higher the CMB burden was, the higher the odds for ICH compared with AIS/TIA (OR, 1.06 per 1 CMB higher count [95% CI, 1.02–1.09, P<0.01). These associations persisted after adjusting for comorbidities using the CHA2DS2‐VASc and HAS‐BLED scores (Table 3 and Figure 2).
Table 3.
Logistic Regression Models for Markers of Small Vessel Disease With OR Favoring ICH Versus AIS/TIA (Univariable Unadjusted Models [1–7] and Models Adjusted for CHA2DS2‐VASc and HAS‐BLED [1–7] for Each Small Vessel Disease Marker; Each Row Represents 1 Model for 1 Small Vessel Disease Marker)
| Model | Unadjusted estimates | Estimates adjusted for CHA2DS2‐VASc and HAS‐BLED | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | aOR | 95% CI | P value | |
| Model 1: CMB presence (vs absence) | 2.73 | 1.33–6.05 | <0.01 | 2.77 | 1.34–6.18 | <0.01 |
| Model 2: CMB location (vs no CMB) | ||||||
| Purely superficial | 2.74 | 1.10–6.72 | 0.012 | 2.90 | 1.16–7.20 | <0.01 |
| Purely deep | 1.08 | 0.16–4.21 | 0.98 | 0.15–3.89 | ||
| Mixed location | 3.50 | 1.54–8.16 | 3.72 | 1.60–8.85 | ||
| Model 3: CMB count (per 1 CMB increase) | 1.06 | 1.02–1.09 | <0.01 | 1.06 | 1.02–1.10 | <0.01 |
| Model 4: CMB count categorical (vs no CMB) | ||||||
| n=1 CMB | 1.56 | 0.47–4.53 | <0.01 | 1.50 | 0.45–4.39 | <0.01 |
| n=2–10 CMB | 2.89 | 1.29–6.76 | 3.03 | 1.34–7.18 | ||
| n>10 CMB | 5.33 | 1.70–15.56 | 5.69 | 1.78–17.06 | ||
| Model 5: presence of superficial siderosis (vs absence) | 0.80 | 0.04–4.10 | 0.826 | 1.01 | 0.05–5.38 | 0.993 |
| Model 6: ARWMC scale (vs ARWMC 0) | ||||||
| ARWMC 1 | 1.44 | 0.27–26.72 | 0.131 | 2.09 | 0.37–39.40 | 0.014 |
| ARWMC 2 | 1.99 | 0.37–36.96 | 3.75 | 0.65–71.71 | ||
| ARWMC 3 | 3.80 | 0.70–70.95 | 8.25 | 1.39–159.82 | ||
| Model 7: ARWMC scale 2–3 (vs ARWMC 0–1) | 1.88 | 0.95–3.88 | 0.071 | 2.62 | 1.28–5.63 | <0.01 |
AIS indicates acute ischemic stroke; aOR, adjusted odds ratio; ARWMC, age‐related white matter changes; CMB, cerebral microbleeds; ICH, intracerebral hemorrhage; and TIA, transient ischemic attack.
Figure 2. Logistic regression models for markers of small vessel disease on MRI with aOR favoring ICH vs AIS/TIA adjusted for CHA2DS2‐VASc and HAS‐BLED.
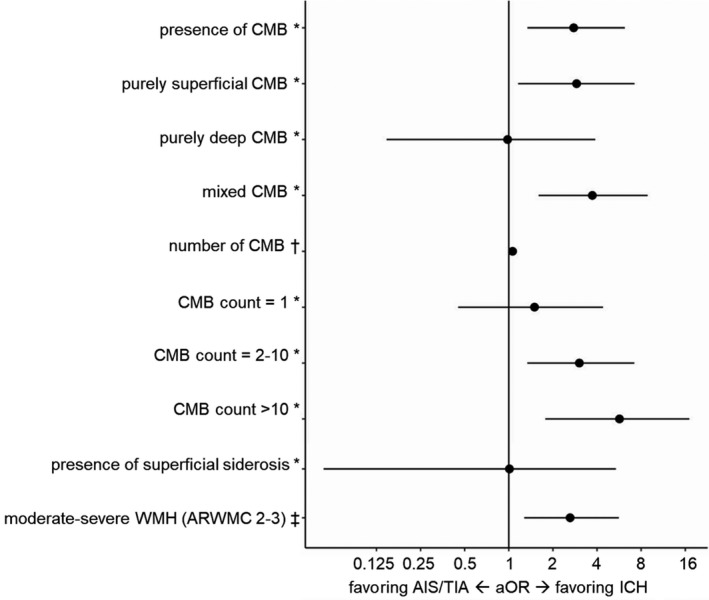
aOR and 95% CI are presented for each MRI marker from its respective model. *As opposed to absence, †effect per 1 CMB higher count, ‡as opposed to ARWMC 0 to 1. AIS indicates acute ischemic stroke; aOR, adjusted odds ratio; ARWMC, age‐related white matter changes; CMB, cerebral microbleeds; ICH, intracerebral hemorrhage; MRI, magnetic resonance imaging; TIA, transient ischemic attack; and WMH, white matter hyperintensities.
Superficial siderosis was present in 17/514 of patients with available MRI (3%) and was not associated with ICH or AIS/TIA (Table 3).
WMH were present in almost all patients with available MRI (452/477 [95%] patients with AIS/TIA and 36/37 [97%] patients with ICH). There was a tendency for an association of WMH with ICH versus AIS/TIA, with gradually increasing odds for more severe WMH (Table 3). Moderate‐to‐severe WMH (age‐related white matter changes 2–3) compared with no or mild WMH showed almost 2‐fold higher odds for ICH versus AIS/TIA (OR, 1.88 [95% CI, 0.95–3.88], P=0.071, aOR, 2.62 [95% CI, 1.28–5.63, P<0.01 after adjusting for CHA2DS2‐VASc and HAS‐BLED). The detailed results of the univariable and adjusted logistic models for WMH are shown in Table 3 and Figure 2.
Atherosclerosis Assessment
CTA was available for assessment of atherosclerotic lesions in 583/734 patients (79.4%; 490/605 patients with AIS/TIA [81.0%]; and 93/129 patients with ICH [72.1%]). The prevalence of atherosclerotic lesions on each vascular segment is presented in Figure 3.
Figure 3. Logistic regression models for atherosclerosis assessment on CTA with OR favoring ICH vs AIS/TIA adjusted for CHA2DS2‐VASc and HAS‐BLED.
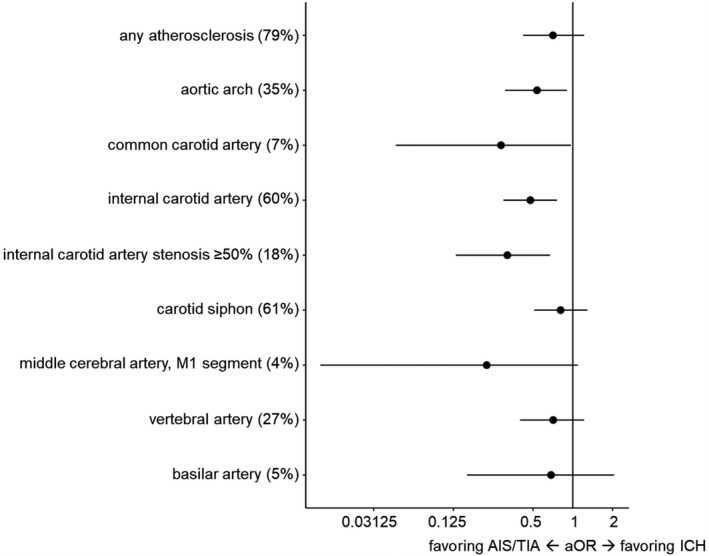
The prevalence (%), aOR, and 95% CI are presented for each CTA variable from its respective model. AIS indicates acute ischemic stroke; aOR, adjusted odds ratio; CTA, computed tomography angiography; ICH, intracerebral hemorrhage; OR, odds ratio; and TIA, transient ischemic attack.
In univariable logistic regression models, the presence of atherosclerotic lesions throughout the extra‐ and intracranial supracardiac arteries showed OR favoring AIS/TIA rather than ICH (OR <1). Extracranial atherosclerosis on aortic arch, common and internal carotid artery, and internal carotid artery stenosis ≥50% was associated with AIS/TIA rather than ICH in univariable logistic regression models (Table S4). These associations persisted after adjusting for CHA2DS2‐VASc and HAS‐BLED: Atherosclerotic lesions of the aortic arch (aOR, 0.54 [95% CI, 0.31–0.90], P=0.018), common and internal carotid artery (aOR, 0.29 [95% CI, 0.05–0.97], P=0.043 and aOR, 0.48 [95% CI, 0.30–0.76], P=0.002, respectively), as well as stenosis ≥50% of the internal carotid artery (aOR, 0.32 [95% CI, 0.13–0.67], P=0.002) showed a strong association with AIS/TIA (Figure 3, Table S4).
Type of OAC
There was no evidence for an interaction between type of OAC (DOAC versus VKA) and any of the clinical characteristics, SVD markers, and atherosclerotic lesions on their association with event type, as indicated by largely similar OR for ICH versus AIS/TIA in the subgroups of VKA‐ and DOAC‐treated patients in all models (Table S5).
DISCUSSION
This cross‐sectional study on the relative contribution of clinical and neuroimaging risk factors to ischemic versus hemorrhagic cerebrovascular events occurring on VKA or DOAC treatment yielded the following key findings: (1) AIS or TIA despite OAC was 5 times more common than OAC‐associated ICH. (2) Prior AIS/TIA, coronary artery disease, dyslipidemia, and worse renal function contributed more to AIS/TIA than ICH, whereas prior ICH, increasing age, higher blood pressure on admission, and concomitant statin therapy were associated with ICH rather than AIS/TIA. (3) The presence and severity of SVD markers, particularly CMB, had a larger contribution to ICH relative to AIS/TIA, while atherosclerotic lesions of the extracranial arteries were stronger contributors to AIS or TIA relative to ICH.
Our finding that ischemic cerebrovascular events despite treatment with VKA or DOAC were substantially more common than ICH occurring as a treatment complication in a contemporary sample of anticoagulated patients presenting with stroke confirms and expands on previous cross‐sectional reports on patients treated mostly with VKA. 13 , 14 , 15 Longitudinal investigations in anticoagulated patients with AF have also consistently demonstrated that the incidence of ischemic stroke is higher than the incidence of ICH. 10 , 12 , 21 Although the clinical impact of ICH is known to be more profound than that of AIS/TIA 22 (as demonstrated by the more severe neurological deficits [ie, higher National Institute of Health Stroke Scale and lower Glasgow Coma Scale scores] of patients with ICH in our study), our findings suggest that addressing the reasons behind OAC failure 7 , 23 and improving on the effectiveness of thromboembolic protection are pressing needs, just as important as safety considerations.
To that end, investigating the relative contribution of comorbidities and neuroimaging characteristics to the occurrence of ischemic events versus ICH can provide insights into the underlying mechanisms of OAC failure versus OAC complications. In line with previous reports using large data sets from the pre‐DOAC era, 13 , 14 but contrary to a smaller study on DOAC‐treated patients, 15 we found comorbidities indicative of atherosclerotic disease, including coronary artery disease, dyslipidemia, and worse renal function, to be overrepresented in patients with AIS/TIA compared with those with ICH. As in previous research, 14 prior AIS/TIA was another consistent determinant for AIS/TIA rather than ICH in OAC‐treated patients. As a novelty, in this study we also examined the importance of atherosclerotic lesions of the supracardiac arteries as contributors to AIS/TIA relative to ICH. We found that atherosclerotic lesions across the entire extra‐ and intracranial vasculature were overrepresented in patients with AIS/TIA, with lesions of the aortic arch and common and internal carotid arteries showing the strongest associations. Taken together, these findings indicate that atherosclerotic large artery disease, which might be less responsive to anticoagulation, may represent an important contributor to OAC failure. This is crucial, because the risk of recurrence in AF patients with AIS despite OAC is known to be high, 23 and efforts to mitigate it might have to address large artery disease as a stroke mechanism in addition to cardioembolism.
The strongest contributor to ICH relative to AIS among clinical characteristics was prior ICH in our study. This confirms and expands on previous findings showing an association of prior ICH and prior major extracranial bleeding with ICH versus AIS/TIA in DOAC‐treated 15 and VKA‐treated patients, 13 respectively. In our study, concomitant statin use was not associated with either event type in univariable analysis, but was overrepresented in patients with ICH compared with AIS/TIA in the multivariable model including adjustment for dyslipidemia. Considering that this might have been confounded through collinearity between statin use and dyslipidemia in the multivariable model and the known controversy regarding the effect of statins on ICH risk, 24 , 25 this finding should be interpreted cautiously. The underrepresentation of statin use in patients with AIS or TIA compared with ICH might also reflect the known beneficial effect of statins in the prevention of ischemic stroke. 26 Besides clinical characteristics, in this study we also examined the contribution of SVD markers to AIS/TIA relative to ICH. Although both CMB and WMH were highly prevalent in OAC‐treated patients with both ischemic and hemorrhagic cerebrovascular events, these SVD markers, in particular CMB, showed a larger relative contribution to ICH than AIS/TIA. This is in line with a previous smaller study on DOAC‐treated patients presenting with AIS or ICH 15 and complements recent evidence that SVD is an important contributor to OAC‐associated ICH, 27 with a larger relative contribution to ICH than AIS risk longitudinally. 10 Of note, the HAS‐BLED score in our study did not differ between OAC‐treated patients presenting with AIS/TIA versus ICH, confirming previous observations on its poor performance in identifying patients at high ICH risk and discriminating between that and AIS risk. 13 , 14 , 15 Recently, the superior performance of risk scores incorporating SVD markers in predicting ICH risk was demonstrated in large data sets. 27 , 28
The strengths of our study include (1) its large, prospectively collected, homogeneous sample of consecutive patients from a single center, which minimizes selection bias; (2) the balanced representation of both VKA and DOAC in our contemporary sample; and (3) the detailed clinical and neuroimaging characterization and high data completeness (see Table 1 for missing values).
We acknowledge the following limitations: (1) Our study is cross‐sectional and included exclusively patients with either AIS/TIA or ICH but no controls without stroke. We can therefore only report on the relative contribution of risk factors to AIS/TIA versus ICH, but not on competing absolute risks. (2) The strength of conclusions in the analyses of imaging features is limited by the lower number of patients with available MRI and CTA in the ICH subgroup, disallowing precise estimates, which is a potential source of bias. (3) Since our analyses comprised almost exclusively White patients, generalizability across different ethnicities is limited.
CONCLUSIONS
In conclusion, among consecutive patients presenting with acute cerebrovascular events on OAC treatment, AIS/TIA was 5 times more common than ICH. Our data suggest that atherosclerotic disease is a more important contributor to treatment failure in patients who have AIS/TIA despite OAC treatment, while SVD contributes more to OAC‐associated ICH. These findings, which did not differ according to OAC type (VKA or DOAC), may inform future efforts to mitigate the risk of treatment failure and treatment complications in patients taking OAC.
Sources of Funding
The NOACISP registry was supported by grants from the Swiss Heart Foundation and the Science Funds of the University Hospital Basel. This project was supported by a grant from the Neurology Department at University Hospital Basel.
Disclosures
Thilemann has received travel grants from Pfizer. Traenka has received funding for travel from Bayer. Seiffge served on scientific advisory boards for Bayer and Pfizer and received compensation for educational efforts by Stago. De Marchis has received consultant honoraria by Bayer and speaker honoraria by Medtronic and BMS/Pfizer. Bonati received a research grant from AstraZeneca, consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, BMS, and Claret Medical and travel grants from Amgen and Bayer. Engelter has received travel‐compensation and speaker honoraria from Bayer, Boehringer, and Daiichi‐Sankyo. He has served on advisory boards for Bayer, Boehringer, and BMS/Pfizer. Peters has served on scientific advisory boards for AstraZeneca, Bayer, Boehringer, BMS/Pfizer, and Daiichi‐Sankyo and has received speaker honoraria from Vifor. Lyrer has served on scientific advisory boards for Bayer, Boehringer, and BMS/Pfizer. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023345
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 4. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 6. Paciaroni M, Agnelli G, Ageno W, Caso V, Corea F, Lanari A, Alberti A, Previdi P, Fedele M, Manina G, et al. Risk factors for cerebral ischemic events in patients with atrial fibrillation on warfarin for stroke prevention. Atherosclerosis. 2010;212:564–566. doi: 10.1016/j.atherosclerosis.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 7. Paciaroni M, Agnelli G, Caso V, Silvestrelli G, Seiffge DJ, Engelter S, De Marchis GM, Polymeris A, Zedde ML, Yaghi S, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non‐vitamin K antagonist oral anticoagulants for stroke prevention. Stroke. 2019;50:2168–2174. doi: 10.1161/STROKEAHA.119.025350 [DOI] [PubMed] [Google Scholar]
- 8. Hughes M, Lip GY. Risk factors for anticoagulation‐related bleeding complications in patients with atrial fibrillation: a systematic review. QJM. 2007;100:599–607. doi: 10.1093/qjmed/hcm076 [DOI] [PubMed] [Google Scholar]
- 9. Becattini C, Dentali F, Camporese G, Sembolini A, Rancan E, Tonello C, Manina G, Padayattil S, Agnelli G. Carotid atherosclerosis and risk for ischemic stroke in patients with atrial fibrillation on oral anticoagulant treatment. Atherosclerosis. 2018;271:177–181. doi: 10.1016/j.atherosclerosis.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 10. Wilson D, Ambler G, Lee K‐J, Lim J‐S, Shiozawa M, Koga M, Li L, Lovelock C, Chabriat H, Hennerici M, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. 2019;18:653–665. doi: 10.1016/S1474-4422(19)30197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al‐Shahi Salman R, Lip GYH, Cohen H, Banerjee G, Houlden H, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS‐2): a multicentre observational cohort study. Lancet Neurol. 2018;17:539–547. doi: 10.1016/S1474-4422(18)30145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hert L, Polymeris AA, Schaedelin S, Lieb J, Seiffge DJ, Traenka C, Fladt J, Thilemann S, Gensicke H, De Marchis GM, et al. Small vessel disease is associated with an unfavourable outcome in stroke patients on oral anticoagulation. Eur Stroke J. 2020;5:63–72. doi: 10.1177/2396987319888016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lehtola H, Hartikainen J, Hartikainen P, Kiviniemi T, Nuotio I, Palomaki A, Ylitalo A, Airaksinen KEJ, Mustonen P. How do anticoagulated atrial fibrillation patients who suffer ischemic stroke or spontaneous intracerebral hemorrhage differ? Clin Cardiol. 2018;41:608–614. doi: 10.1002/clc.22935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGrath ER, Kapral MK, Fang J, Eikelboom JW, ó Conghaile A, Canavan M, O'Donnell MJ; Investigators of the Registry of the Canadian Stroke N . Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke. 2012;43:2048–2054. doi: 10.1161/STROKEAHA.112.654145 [DOI] [PubMed] [Google Scholar]
- 15. Purrucker JC, Wolf M, Haas K, Siedler T, Rizos T, Khan S, Heuschmann PU, Veltkamp R. Microbleeds in ischemic vs hemorrhagic strokes on novel oral anticoagulants. Acta Neurol Scand. 2018;138:163–169. doi: 10.1111/ane.12934 [DOI] [PubMed] [Google Scholar]
- 16. Seiffge DJ, Traenka C, Polymeris AA, Thilemann S, Wagner B, Hert L, Müller MD, Gensicke H, Peters N, Nickel CH, et al. Intravenous thrombolysis in patients with stroke taking rivaroxaban using drug specific plasma levels: experience with a standard operation procedure in clinical practice. J Stroke. 2017;19:347–355. doi: 10.5853/jos.2017.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 18. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 19. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, et al. A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.STR.32.6.1318 [DOI] [PubMed] [Google Scholar]
- 20. Bartlett ES, Walters TD, Symons SP, Fox AJ. Quantification of carotid stenosis on CT angiography. AJNR Am J Neuroradiol. 2006;27:13–19. [PMC free article] [PubMed] [Google Scholar]
- 21. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 22. Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, Macha Md K, Tsivgoulis G, Ambler G, Arihiro S, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87:677–687. doi: 10.1002/ana.25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pandit AK, Kumar P, Kumar A, Chakravarty K, Misra S, Prasad K. High‐dose statin therapy and risk of intracerebral hemorrhage: a meta‐analysis. Acta Neurol Scand. 2016;134:22–28. doi: 10.1111/ane.12540 [DOI] [PubMed] [Google Scholar]
- 25. Ziff OJ, Banerjee G, Ambler G, Werring DJ. Statins and the risk of intracerebral haemorrhage in patients with stroke: systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry. 2019;90:75–83. doi: 10.1136/jnnp-2018-318483 [DOI] [PubMed] [Google Scholar]
- 26. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, et al. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 27. Seiffge DJ, Wilson D, Ambler G, Banerjee G, Hostettler IC, Houlden H, Shakeshaft C, Cohen H, Yousry TA, Al‐Shahi Salman R, et al. Small vessel disease burden and intracerebral haemorrhage in patients taking oral anticoagulants. J Neurol Neurosurg Psychiatry. 2021;92:805–814. doi: 10.1136/jnnp-2020-325299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Best JG, Ambler G, Wilson D, Lee K‐J, Lim J‐S, Shiozawa M, Koga M, Li L, Lovelock C, Chabriat H, et al. Development of imaging‐based risk scores for prediction of intracranial haemorrhage and ischaemic stroke in patients taking antithrombotic therapy after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. 2021;20:294–303. doi: 10.1016/S1474-4422(21)00024-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


