Abstract
Background
The Fontan circulation is a successful operative strategy for abolishing cyanosis and chronic volume overload in patients with congenital heart disease with single ventricle physiology. “Fontan failure” is a major cause of poor quality of life and mortality in these patients. We assessed the number and clinical characteristics of adult patients with Fontan physiology receiving pulmonary arterial hypertension (PAH) therapies across specialist centers in the United Kingdom.
Methods and Results
We identified all adult patients with a Fontan‐type circulation under active follow‐up in 10 specialist congenital heart disease centers in England and Scotland between 2009 and 2019. Patients taking PAH therapies were matched to untreated patients. A survey of experts was also performed. Of 1538 patients with Fontan followed in specialist centers, only 76 (4.9%) received PAH therapies during follow‐up. The vast majority (90.8%) were treated with a phosphodiesterase‐5 inhibitor. In 33% of patients, PAH therapies were started after surgery or during hospital admission. In the matched cohort, treated patients were more likely to be significantly limited, have ascites, have a history of protein‐losing enteropathy, or receive loop diuretics (P<0.0001 for all), also reflecting survey responses indicating that failing Fontan is an important treatment target. After a median of 12 months (11–15 months), functional class was more likely to improve in the treated group (P=0.01), with no other changes in clinical parameters or safety issues.
Conclusions
PAH therapies are used in adult patients with Fontan circulation followed in specialist centers, targeting individuals with advanced disease or complications. Follow‐up suggests stabilization of the clinical status after 12 months of therapy.
Keywords: adult congenital heart disease, case‐control study, Fontan, observational study, pulmonary hypertension
Subject Categories: Pulmonary Hypertension, Treatment, Congenital Heart Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- PAH
pulmonary arterial hypertension
- PLE
protein‐losing enteropathy
- TCPC
total cavopulmonary connection
Clinical Perspective
What Is New?
In most UK centers, pulmonary arterial hypertension therapy was reserved for a small minority of patients with Fontan circulation who have more advanced disease than untreated patients and led to an improvement in New York Heart Association functional class compared with untreated patients.
For most UK experts, the primary aim of pulmonary arterial hypertension therapy in patients after Fontan repair is to improve quality of life and control heart failure and protein‐losing enteropathy; improving exercise tolerance was another commonly reported aim.
What Are the Clinical Implications?
A shared, protocolized approach to the treatment and follow‐up of these patients is needed, along with collection of data in large registries that include patients from the entire spectrum of the Fontan physiology, many of whom have not been included in trials.
One of the great successes of congenital heart disease (CHD) surgery has been the Fontan procedure, an operative strategy that abolishes cyanosis and chronic volume overload in patients with single ventricle physiology. 1 The Fontan operation has evolved over the past few decades, leading to improved long‐term outcomes and a reduction in late complications, especially in patients after total cavopulmonary connection (TCPC). 2 , 3 However, outcomes in patients after Fontan‐type operations are far from optimal; a “failing Fontan” circulation is an almost inevitable long‐term consequence of the altered physiology apparent in an increasing number of patients. The ensuing complications of reduced exercise tolerance, leg edema, pleural effusions, ascites, liver disease, arrhythmia, and protein‐losing enteropathy (PLE) affect quality of life and increase mortality in these patients. 4 , 5 , 6 , 7 , 8
An important determinant of Fontan failure is an increasing pulmonary vascular resistance (PVR). 1 The pathophysiology of this remains uncertain but evidence suggests that it may occur because of a lack of pulsatile flow with endothelial dysfunction in the pulmonary vasculature, 9 as well as recurrent thromboembolic events. 10
Many experts advocate for the use of pulmonary arterial hypertension (PAH) therapies in patients with Fontan physiology to reduce PVR and optimize cardiac output and systemic venous pressure. Several studies have addressed this topic in adult patients with Fontan circulation, including 6 randomized trials, some of which have demonstrated small but significant improvements in exercise capacity and hemodynamics. 11 , 12 , 13 , 14 , 15 , 16 Despite this, the indications and timing of initiation of PAH therapies in the Fontan cohort are still debated. 17 , 18 , 19 Recent European and American adult CHD guidelines recommend an individualized approach and treatment in selected patients, although it is still unclear which patients with Fontan physiology are most likely to benefit.
In this study, we assessed contemporary patterns of PAH therapy prescription in adult patients after Fontan‐type surgery, with a focus on the clinical characteristics of patients receiving treatment across specialist services in the United Kingdom. A survey of experts was also conducted, assessing the therapeutic aims and current practice.
Methods
The study materials linked to this research are available from the corresponding author on reasonable request. To minimize the possibility of unintentionally sharing information that can be used to reidentify private information, in line with the conditions of the ethical approval, patient‐level data are not available for use outside of this study.
Study Design and Population
A survey of current practice among specialist centers was undertaken. Physicians from 10 adult CHD or PAH centers in England (Royal Brompton Hospital, London; Queen Elizabeth Hospital, Birmingham; Royal Hallamshire Hospital, Sheffield; Bristol Heart Institute; Liverpool Heart and Chest Hospital; Yorkshire Heart Centre, Leeds General Infirmary; Guy’s and St Thomas’ Hospital, London; Southampton General Hospital; Freeman Hospital, Newcastle; and Golden Jubilee National Hospital, Glasgow) were asked to answer a series of questions with regard to their center’s practice in the treatment of patients with Fontan circulation with PAH therapies. This included questions on the desired outcome of treatment, indications, and timing of the initiation of therapies, type of therapy used, and monitoring of such treatment with regard to efficacy and safety (see Data S1 for survey methodology and Figure S1 for full questionnaire).
We conducted a multicenter, retrospective longitudinal study. We identified all adult patients (aged ≥17 years) with a Fontan‐type circulation under active follow‐up between 2009 and 2019 in the participating centers listed above. Patients not undergoing PAH therapies were matched to those taking PAH therapies in a 1:1 or 2:1 fashion, where possible, by age (within 2 years in 93.4%), sex, and the CHD center providing care. Based on the type of Fontan operation, patients were classified as atriopulmonary Fontan, lateral tunnel TCPC, extracardiac TCPC, or others, including Fontan‐Björk modification. Patients with only a superior cavopulmonary (Glenn‐type) anastomosis were excluded.
Baseline data were collated from the last clinical assessment before starting PAH therapy for the therapy group, and from a routine clinical assessment within a 12‐month period for the matched, untreated group. This included detailed clinical characteristics, 12‐lead ECG, transthoracic echocardiography, and laboratory tests. Six‐minute walk or cardiopulmonary exercise testing and EmPHasis‐10 questionnaires were performed in a small minority of patients and so these data could not be meaningfully analyzed. Follow‐up data were then collected around 12 months from therapy initiation (treated group) or the concordant assessment (untreated group). Apart from the above variables, data on cardiac transplantation, heart failure admissions, new arrhythmia, or PLE were gathered and compared between groups.
Ethical approval was secured from the National Health Service’s Health Research Authority (Integrated Research Application System study ID 263589). This was a retrospective analysis based on anonymized data collected for routine clinical care and administrative purposes, hence individual informed consent was waived. The study was locally registered and approved at each study site.
Statistical Analysis
Statistical analyses were performed using R package version 3.6.3 (R Foundation for Statistical Computing). Continuous variables were assessed for normality using Q‐Q plots and the Shapiro‐Wilk test and presented as mean±SD or median (interquartile range), as appropriate. Categorical variables are presented as number and percentage. Comparisons of changes in clinical variables (baseline to follow‐up) between the treated and untreated groups were performed using a generalized linear model stratified by matched group for paired outcome data analysis of continuous variables. To assess the difference between treatment groups with respect to New York Heart Association functional class, conditional logistic regression analysis was performed using the clogit package, using improvement to functional class I/II as the response variable, stratified by matched group. Otherwise, comparisons were made using Student t test or Wilcoxon rank sum test for normally distributed and skewed continuous variables, respectively, and chi‐square test or Fisher exact test for categorical variables. The chi‐square test was used to compare the proportions of patients with Fontan circulation taking PAH therapy between the center with the greatest number of patients treated and the remainder as a pooled cohort. A 2‐tailed P value of <0.05 was used as the criterion for statistical significance.
Results
Survey of Experts
Sixteen of 20 experts who were invited responded to the survey, belonging to 10 specialist centers. In all centers, PAH therapies were considered part of the management of patients with Fontan circulation; however, none of the experts started PAH therapies across the spectrum of patients with Fontan circulation, but reserve these for specific indications. Strong indications for PAH therapy were PLE refractory to other therapy (69% of respondents), fluid overload with an inadequate response to diuretics (63%), and ≥2 “heart failure” admissions (63%). A minority of experts (25%) felt that Fontan‐associated liver disease was a strong indication for PAH therapy. Cardiac catheterization before initiation of therapy was not deemed essential by the majority of respondents (69%), but systemic ventricular dysfunction or atrioventricular valve regurgitation were seen as a strong contraindication by 56% of respondents (a summary of indications of PAH therapies in patients with Fontan physiology from the expert survey are shown in Table S1).
Half of the experts surveyed believed that liver dysfunction was of particular concern when starting an endothelin receptor antagonist in patients with a Fontan circulation, but all experts agreed that patients with Fontan circulation are not at particularly high risk of complications from PAH therapies compared with other forms of PAH. All experts would start therapy with a phosphodiesterase‐5 inhibitor. Factors affecting their choice of first‐line PAH therapy included national commissioning policy (69%), liver congestion (56%), history of anemia or thrombocytopenia (both 44%), or cost issues (19%).
The primary aim of PAH therapy most commonly reported by our experts was to improve quality of life in 44% and control heart failure or PLE in 25%. The most common secondary aim of therapy was to improve exercise capacity (50%).
Registry Results
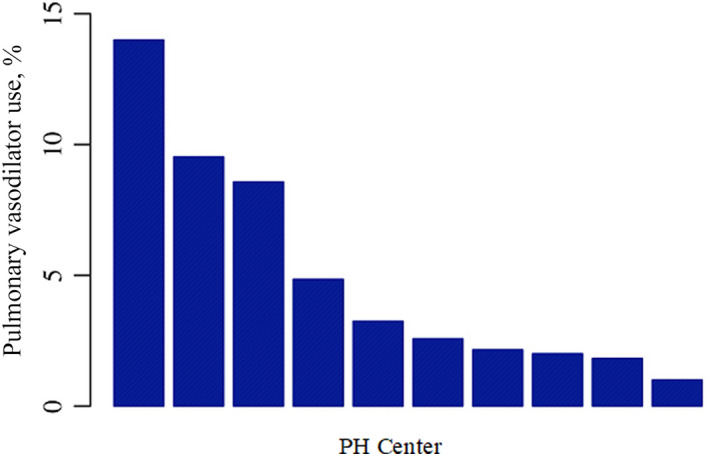
A total of 1538 patients with a Fontan circulation were managed in the study centers between 2009 and 2019. Over this period, 76 patients were started on PAH therapy (4.9% of patients with Fontan physiology under follow‐up). In one center, the proportion of patients treated was significantly higher than the remainder of our population (14% versus 3.3%, P<0.001; Figure).
Figure 1. Percentage of adult patients with Fontan started on pulmonary arterial hypertension therapies at each center, showing some variability in practice, with the majority of centers prescribing therapies in <10%. PH indicates pulmonary hypertension.
At the time of initiation of PAH therapy, patients had a median age of 29 years (23–33 years), and 57% were women. In the majority of patients (67%), the single ventricle was morphologically left. Median age at Fontan completion was 7 years (4–11 years). Following their original Fontan surgery but before the beginning of the study period, 12% of patients had undergone TCPC conversion. Hence, at baseline, 17 (22.4%) had a lateral tunnel and 29 (38.2%) had an extracardiac TCPC.
Choice, Indications, and Timing of Pulmonary Vasodilator Therapy
The majority of patients (n=69, 90.8%) were started on monotherapy with a phosphodiesterase‐5 inhibitor and 6 (7.9%) were started on an endothelin receptor antagonist. Only 1 (1.3%) patient received initial combination therapy. During the study period, only few patients (n=4, 5.3%) received sequential pulmonary vasodilator therapy, adding an endothelin receptor antagonist. In 1 patient, the endothelin receptor antagonist was replaced by an inhaled prostanoid, the only instance where a prostacyclin analogue was used. No patients received triple therapy.
Pulmonary vasodilator therapy was initiated electively in most cases (n=51, 67.1%). Conversely, 20 (29%) patients were initiated on a pulmonary vasodilator following urgent admission to hospital with signs of fluid overload. In 5 (6.8%) patients, sildenafil was started following elective TCPC conversion surgery. Reasons given for initiation of pulmonary vasodilator therapy included exercise intolerance, raised Fontan pressures at cardiac catheterization, and Fontan‐associated liver disease (Table 1).
Table 1.
Clinical Indications for Pulmonary Vasodilator Therapy in UK Patients With Fontan Circulation (≥1 Indication Allowed per Patient)
| Indication | No. (%) |
|---|---|
| Exercise intolerance | 28 (37) |
| Raised Fontan pressures at catheterization | 27 (36) |
| Liver disease | 18 (24) |
| Arrhythmia | 10 (13) |
| Fluid overload | 8 (11) |
| Protein‐losing enteropathy | 8 (11) |
| Postoperative | 7 (9) |
| Venous thromboembolism | 3 (4) |
| Low oxygen saturation | 3 (4) |
| Preoperative | 2 (3) |
| Other | 1 (1) |
Side Effects of PAH Therapies in Patients With Fontan Circulation
Pulmonary vasodilator therapy was changed or stopped because of side effects in 6 patients (7.9%). This included just under half (5 of 11, 45%) of the patients started on endothelin receptor antagonists in the acute setting: 1 patient with headache taking sildenafil, 1 patient with a constellation of side effects (headache, nausea, lethargy, and severe leg pain) taking sildenafil (and subsequently tadalafil), and 4 patients taking endothelin receptor antagonists for ankle edema, itching, arm and leg pain, and breathing difficulties at night. No patients stopped therapy because of new‐onset liver dysfunction.
Comparison With Untreated Patients
All 76 patients treated with a pulmonary vasodilator were matched with 108 untreated patients. The demographic and clinical characteristics of the matched cohort at the time of assessment are shown in Table 2, with no significant differences in demographic and anatomical characteristics between groups. Patients with an initial TCPC repair were as likely to be treated as those with an atriopulmonary Fontan (40.4% versus 42.2%, P=0.92), as were the 9.8% of patients who had undergone a Fontan conversion or revision procedure before study inclusion (50% versus 40.4%, P=0.59).
Table 2.
Baseline Characteristics
| Baseline variable |
PAH therapy (n=76) |
No therapy (n=108) |
P value |
|---|---|---|---|
| Men, % | 33 (43.4) | 54 (50) | 0.47 |
| Age (IQR), y | 28.7 (22.9–33.1) | 27.9 (22–33.1) | 0.42 |
| Age at first Fontan, median (IQR), y | 6.9 (4.4–10.7) | 6 (3.8–9.1) | 0.22 |
| Body mass index (IQR), kg/m2 | 23 (20.3–28) | 24.6 (21.3–26.8) | 0.41 |
| Resting saturations (IQR), % | 92 (89–94) | 93 (90–95) | 0.08 |
| Left ventricular morphology, n (%) | 51 (67.1) | 71 (65.7) | 0.39 |
| Cavopulmonary connection, n (%) | |||
| Atriopulmonary Fontan | 30 (39.5) | 43 (39.8) | 0.16 |
| Lateral tunnel TCPC | 17 (22.4) | 36 (33.3) | |
| Extracardiac TCPC | 29 (38.2) | 29 (26.9) | |
| Initial fenestration | 37 (54.4) | 46 (50.5) | 0.75 |
| Patent fenestration | 24 (35.3) | 26 (31) | 0.69 |
| Bilateral SVC connections | 9 (11.8) | 11 (10.2) | 0.91 |
| NYHA classification, n (%) | |||
| I | 4 (5.5) | 65 (65) | <0.0001* , † |
| II | 25 (34.2) | 29 (29) | |
| III | 42 (57.5) | 6 (6) | |
| IV | 2 (2.7) | 0 (0) | |
| History of atrial arrhythmia, n (%) | 42 (56) | 50 (46.3) | 0.25 |
| Previous ablation procedure, n (%) | 24 (32) | 24 (22.4) | 0.2 |
| Pacemaker, n (%) | 20 (26.7) | 26 (24.1) | 0.82 |
| Laboratory | |||
| Creatinine, median (IQR), µmol/L | 74 (64.5–82.5) | 74 (65–85) | 0.96 |
| Sodium, mean (SD), mmol/L | 140 (138–142.5) | 140 (138–141) | 0.21 |
| Albumin, median (IQR), g/L | 43 (38–46.8) | 45 (42–48) | 0.02 † |
| Bilirubin, median (IQR), µmol/L | 16 (9.25–27.5) | 15.5 (10–22.3) | 0.84 |
| ALT, median (IQR), IU/L | 26 (19–37.75) | 28 (23.75–37) | 0.29 |
| Medication | |||
| Loop diuretics, n (%) | 38 (50.7) | 22 (20.8) | <0.0001 † |
| Diuretic dose, median (IQR), mg ‡ | 40 (20–110) | 30 (20–40) | 0.06 |
| ACEI or ARB, n (%) | 37 (48.7) | 45 (41.7) | 0.43 |
| β‐Blocker, n (%) | 35 (46.1) | 28 (25.9) | 0.007 † |
| MRA, n (%) | 22 (29.3) | 8 (7.5) | 0.0002 † |
| Antiarrhythmic, n (%) | 21 (27.6) | 22 (20.6) | 0.35 |
| Warfarin, n (%) | 62 (81.6) | 80 (74.1) | 0.31 |
| NOAC, n (%) | 3 (3.9) | 2 (1.9) | 0.69 |
| Antiplatelet, n (%) | 9 (11.8) | 18 (16.7) | 0.48 |
| New/worsening fluid overload, n (%) | 19 (25.3) | 2 (1.9) | <0.0001 † |
| Ascites, n (%) | 12 (16) | 0 (0) | <0.0001 † |
| PLE (any history), n (%) | 12 (16.2) | 1 (0.9) | 0.0003 † |
| PLE (new or recurrent within 12 mo), n (%) | 7 (9.3) | 0 (0) | 0.004 † |
| Fontan failure, n (%) | 42 (56) | 8 (7.4) | <0.0001 † |
| Prior transplant assessment, n (%) | 15 (19.7) | 3 (2.8) | 0.0004 † |
ACEI indicates angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NOAC, nonvitamin K anticoagulant; PAH, pulmonary arterial hypertension; PLE, protein‐losing enteropathy; SVC, superior vena cava; and TCPC, total cavopulmonary connection.
Comparison between New York Heart Association (NYHA) class I/II and III/IV.
P‐value < 0.05 is indicative of statistical significance.
Reported for patients taking a loop diuretic, in milligrams of furosemide or dose equivalent.
However, treated patients were significantly more functionally limited (higher New York Heart Association functional class) compared with their matched counterparts, with 60.3% of the former in New York Heart Association functional class III or IV compared with 6% of the latter (P<0.0001). New or worsening fluid overload over the past 12 months was present in a quarter of patients starting therapy and was significantly more common in the treated group (25.3% versus 1.9%, P<0.0001). Ascites was only encountered in the treated group (12% versus 0%, P<0.0001). Significantly more patients in the treated group had a history of PLE (16.2% versus 0.9%, P<0.0001) at baseline, with a recent diagnosis or recurrence (within the past year) in 9.3% of treated patients. Moreover, treated patients were more likely to have lower albumin levels (43 g/L [38–47 g/L] versus 45 g/L [42–48 g/L], P=0.02). A diagnosis of “Fontan failure,” defined as a decline in exercise tolerance to functional class III or IV, ≥1 heart failure admission, new fluid overload, ascites, or PLE diagnosis in the past 12 months, was significantly more common in the treated compared with the untreated group (56% versus 7%, P<0.0001).
There were no differences between the 2 groups in terms of a history of atrial tachyarrhythmia or liver or renal function (see Table 2 for data).
Treated patients were more likely to be taking a loop diuretic, eg, furosemide (P<0.0001), or a mineralocorticoid receptor antagonist, eg, spironolactone (29.3% versus 7%, P=0.0002), than the untreated cohort. Moreover, treated patients were more likely to be receiving a β‐blocker (P=0.007). Finally, patients starting PAH therapies were significantly more likely to have undergone prior transplant assessment compared with the controls (19.7% versus 2.8%, P=0.0002). Of those assessed, 11 patients (58%) had been rejected for transplantation. Reasons given for formal rejection for transplantation included a decision to proceed to Fontan conversion instead, the need for 3‐organ transplantation (heart‐kidney‐liver), renal dysfunction, poorly controlled diabetes, and high human leukocyte antigen antibody titers.
Effects of Therapy
After a median follow‐up period of 12 months (interquartile range, 11–15 months), 4 patients (5.3%) underwent escalation of therapy for ongoing PLE or incomplete clinical response to therapy. Another 4 patients underwent late dose escalation >12 months after starting therapy. New York Heart Association functional class was more likely to improve in the pulmonary vasodilator compared with the untreated group (odds ratio [OR], 13.3; 95% CI, 1.7–106.5 [P=0.01]), but no difference was detected between groups in the change in oxygen saturation, hemoglobin, or albumin concentration (Table 3). There was no significant improvement in resting oxygen saturation prepulmonary versus postpulmonary vasodilator therapy (92% [interquartile range, 89%–94%] versus 92% [interquartile range, 89%–95%], P=0.5). Whereas at baseline a history of hospitalization with heart failure in the past 12 months was more common in the treated than the untreated cohort (20.3% versus 0.9%, P<0.0001), there was no significant difference between groups in heart failure admissions in the 12 months after initiation of treatment (12.0% versus 5.8%, P=0.17). During the follow‐up period, there were 3 (3.9%) deaths in the treatment group and 2 (2.6%) underwent transplantation (1 orthotopic heart transplant, 1 heart‐liver transplant). None of the patients in the nontreatment group died or underwent transplantation during the study period.
Table 3.
Clinical Measures Pre‐PAH and 12 Months Post‐PAH Therapy With Comparison to the Matched, Untreated Group
| Clinical measures | PAH therapy | No therapy | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Change | Baseline | Follow‐up | Change | ||
| Oxygen saturation, median (IQR), % | 92 (89–94) | 92 (89–95) | 0 (−2 to 2.3) (68) | 93 (90–95) | 93 (90–94) | 0 (−1.3 to 1) (84) | 0.1 |
| NYHA functional class I/II, n (%) | 28 (41.2) | 33 (48.5) | 12 (17.6) (68) | 5 (5.2) | 9 (9.4) | 1 (1) (96) | 0.01* |
| Hemoglobin, median (IQR), g/L | 149 (138–165) | 148 (135–159) | −1.5 (−12 to 8) (64) | 154 (143.5–164.5) | 155 (138–164) | −3 (−7 to 5) (63) | 0.5 |
| Albumin, median (IQR), g/L | 43 (37–46) | 44 (36.5–47) | 0 (−2 to 3) (59) | 46 (42–48) | 45 (40–47) | 0 (−2 to 2) (54) | 0.6 |
P values were derived for the comparison of the changes in clinical variables (baseline to follow‐up) between the pulmonary arterial hypertension (PAH) therapy and no therapy groups. A generalized linear model stratified by matched group was used for paired outcome data analysis. P value <0.05 indicative of statistical significance. IQR indicates interquartile range; and NYHA, New York Heart Association.
P‐value < 0.05 indicative of statistical significance.
Discussion
Only a small proportion of adult patients with Fontan circulation followed in specialist centers in the United Kingdom receive PAH therapies. In this setting, physician expectations of these therapies focus on managing complications of Fontan failure and improving overall quality of life. UK specialists appear to reserve therapy for patients with more advanced disease, a higher degree of limitation, and other evidence of Fontan failure, although centers can vary in their practice. Pulmonary vasodilator therapy appeared to be safe and improved functional class compared with the untreated Fontan group.
PVR is a critical determinant of pulmonary blood flow in patients with Fontan physiology, who lack a subpulmonary ventricle and rely on a passive pressure gradient for maintaining an efficient Fontan circulation. Even a marginal rise in PVR is poorly tolerated in this setting, reducing cardiac output and raising central venous pressure, the hallmarks of Fontan failure. 9 , 20 In our study, the small proportion of patients after Fontan‐type surgery in the United Kingdom who receive pulmonary vasodilator therapy were more likely to exhibit features of a failing Fontan circulation compared with their age‐ and sex‐matched counterparts, including greater exercise intolerance, fluid overload, congestive heart failure, and PLE. Indeed, one fifth of these patients had already undergone transplant assessment, even though few had been listed.
Our study represents real‐world practice encompassing patients with Fontan circulation across the entire clinical spectrum. PAH therapies were started on a nonelective basis in a third of cases in which pulmonary vasodilators were used, either postoperatively or following urgent hospital admission. This is in stark contrast to the population of patients with Fontan physiology included in randomized controlled trials of PAH therapy, who were clinically stable, often asymptomatic, and had good exercise capacity. Indeed, a peak oxygen consumption ≥50% predicted was a requirement for inclusion in large trials in this area. 16 Providing that such therapies are safe in this population, it is conceivable that patients with more advanced disease will derive a greater benefit and therefore are targeted by specialists. Indeed, the critical role of a low PVR in the normal functioning of the Fontan circulation supports the notion that patients with a failing Fontan may benefit from therapies designed to lower PVR. 1 Furthermore, patients undergoing heart transplant for Fontan failure have a higher PVR posttransplant, suggesting that pulmonary vascular remodeling is a feature of late Fontan failure in certain patients. 21
Current guidelines support this individualized approach of targeting selected patients, especially those with symptoms and signs of raised Fontan pressures. 18 , 19 Ideally, there should be a physiological relationship between the indications for PAH therapies and the medication provided, supported by invasive hemodynamics. The largest randomized trials in this area that excluded unstable patients and those with advanced disease have either shown no benefit or small improvements in exercise capacity of uncertain clinical relevance. 11 , 15 Perhaps more importantly, exercise is a surrogate of more significant morbidity and mortality outcomes and therefore persistent stabilization of exercise capacity might be associated with better long‐term outcomes. However, demonstrating a long‐term benefit in a study is a substantial challenge because of the practical realities of a clinical trial. Furthermore, other indications for the use of PAH therapies in patients with Fontan circulation, such as raised Fontan pressures and clinical manifestations of Fontan failure, either require an invasive procedure to gather data or cannot be easily measured in the setting of a clinical trial. Multicenter registries examining contemporary practice can provide evidence for these outcomes, which are difficult to study in prospective clinical trials. The data from our study and the expert survey show that the individualized approach in the United Kingdom consists of a series of clinical criteria to identify patients experiencing ≥1 consequences of a deteriorating Fontan physiology not explained by other disease modifiers, eg, systemic ventricular dysfunction or atrioventricular valve regurgitation, and often not responsive to other therapies. From the expert survey, it appears that PAH therapy in patients after Fontan‐type repair is used by UK centers with the aim of reducing complications and improving quality of life, representing a possible indication for PAH therapies beyond a small, short‐term improvement in peak oxygen uptake in current practice and according to expert opinion. Our registry study could not, however, provide evidence for an improvement in quality of life or reduction in complications following PAH therapies. This needs to be explored in future studies.
Our study findings support the safety of pulmonary vasodilator therapy in selected patients with Fontan circulation, even when used for urgent indications and in those with advanced disease. There were no instances of pulmonary vasodilator therapy causing clinical worsening or liver dysfunction requiring discontinuation. This was reflected in the survey responses, where all experts agreed that patients with Fontan physiology were not at increased risk of complications from pulmonary vasodilator therapies compared with PAH cohorts. In our registry, few patients received endothelin receptor antagonists, and, in almost half of these, the agent had to be stopped or changed because of poor tolerability. However, randomized trials including larger numbers of participants started on endothelin receptor antagonists have confirmed the safety of pulmonary vasodilators, including endothelin receptor antagonists, in the Fontan population, noting a high rate of nonsevere side effects in both the endothelin receptor antagonist and placebo groups. 14 , 16 Further randomized controlled trials of endothelin receptor antagonists in patients with Fontan circulation will establish the safety profile of these agents in this population.
In view of the complexity of the anatomy and physiology, indications for the use of pulmonary vasodilators in this population should only be provided by specialist centers in CHD and PAH, and who have a strong understanding of Fontan physiology and the mechanisms of a failing Fontan circulation. We would recommend a shared protocolized approach to the management of these patients, with prospective data capture in national or international registries, to provide robust evidence on long‐term efficacy and safety.
Study Limitations
This is a retrospective analysis of current UK practice in a small number of patients with Fontan physiology treated with pulmonary vasodilators. A control group matched for age, sex, and treating center was used, and, even though there were no significant differences in baseline anatomical characteristics between groups, this cannot be compared with the rigor of a randomized trial. The retrospective analysis of the impact of PAH medication on clinical parameters was exploratory and, while it may provide valuable information in this real‐life cohort, it carries significant limitations.
The primary scope of this work was to provide an overview of current practice in relation to PAH therapies in patients with a Fontan circulation, as a result of a growing yet conflicting body of evidence in this area, with some positive but mostly negative randomized studies. We also explored differences in practice between the 10 centers, but this analysis was not the focus of the study. Finally, we conducted an expert survey to summarize the opinions of experts in the field. Even though the number of experts surveyed was small, there was representation from most expert centers in the United Kingdom to reflect differing practices. Moreover, the responses to the survey closely reflected the findings of the registry study and provide a clear idea of to which patients experts target pulmonary vasodilators.
Conclusions
An individualized approach to pulmonary vasodilator therapy for patients with Fontan circulation is the current practice in the United Kingdom, predominantly targeting patients with a failing Fontan. Further work is required to better identify patients who will benefit from these therapies and define outcomes for monitoring response, especially in patients with advanced disease. Ongoing studies will hopefully shed more light onto the indications, timing, and choice of pulmonary vasodilator therapies in this population.
Appendix
List of Study Group Investigators
The CHAMPION (Congenital Heart Disease and Pulmonary Arterial Hypertension: Improving Outcomes Through Education and Research Networks) Steering Committee is a panel of experts in pulmonary hypertension and congenital heart disease: Dr Paul Clift, Dr Robin Condliffe, Professor Konstantinos Dimopoulos, Dr Katrijn Jansen, and Dr Shahin Moledina. Dr Andrew Constantine is the CHAMPION PhD Research Fellow.
Sources of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. The CHAMPION group is funded by Janssen‐Cilag Limited.
Disclosures
Drs Jansen, Chung, Oliver, Parry, Fitzsimmons, Walker, Papaioannou, and Von Klemperer have received nonfinancial support from Janssen‐Cilag Limited. Dr Constantine has received educational grants, personal fees, and nonfinancial support from Janssen‐Cilag Limited. Professor Dimopoulos has received nonfinancial support from Janssen‐Cilag Limited, and has been a consultant to and received grants and personal fees from Janssen‐Cilag Limited, Pfizer, GlaxoSmithKline, and Bayer/MSD. Dr Jenkins has received nonfinancial support and conference attendance support from Janssen‐Cilag Limited. Professor Tulloh has received nonfinancial support from Janssen‐Cilag Limited, and has received personal fees from Janssen‐Cilag Limited, Pfizer, Abbott International, GlaxoSmithKline, and Bayer. Dr Condliffe has received nonfinancial support from Janssen‐Cilag Limited, and has received personal fees from Janssen‐Cilag Limited, Bayer, and GlaxoSmithKline. Dr Wort has received nonfinancial support from Janssen‐Cilag Limited, has received grants and personal fees from Janssen‐Cilag Limited and Bayer, and has received personal fees from GlaxoSmithKline. Dr Clift has received nonfinancial support from Janssen‐Cilag Limited, has received grants and personal fees from Janssen‐Cilag Limited, and has received personal fees from Bayer.
Supporting information
Data S1
Table S1
Figure S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023035
For Sources of Funding and Disclosures, see page xxx.
Contributor Information
Konstantinos Dimopoulos, Email: k.dimopoulos02@gmail.com.
the CHAMPION steering committee members:
Paul Clift, Robin Condliffe, Konstantinos Dimopoulos, Katrijn Jansen, Shahin Moledina, and Andrew Constantine
References
- 1. Egbe AC, Connolly HM, Miranda WR, Ammash NM, Hagler DJ, Veldtman GR, Borlaug BA. Hemodynamics of Fontan failure: the role of pulmonary vascular disease. Circ Heart Fail. 2017;10:e004515. doi: 10.1161/CIRCHEARTFAILURE.117.004515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Leval MR, Kilner P, Gewillig M, Bull C, McGoon DC. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–695. doi: 10.1016/S0022-5223(19)35174-8 [DOI] [PubMed] [Google Scholar]
- 3. d’Udekem Y, Iyengar AJ, Cochrane AD, Grigg LE, Ramsay JM, Wheaton GR, Penny DJ, Brizard CP. The Fontan procedure: contemporary techniques have improved long‐term outcomes. Circulation. 2007;116:I157–164. doi: 10.1161/CIRCULATIONAHA.106.676445 [DOI] [PubMed] [Google Scholar]
- 4. Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein‐losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg. 1998;115:1063–1073. doi: 10.1016/S0022-5223(98)70406-4 [DOI] [PubMed] [Google Scholar]
- 5. Rychik J. Protein‐losing enteropathy after Fontan operation. Congenit Heart Dis. 2007;2:288–300. doi: 10.1111/j.1747-0803.2007.00116.x [DOI] [PubMed] [Google Scholar]
- 6. Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward‐Sadler H, Haw M, Cope R, Salmon AP, Sivaprakasam MC, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–584. doi: 10.1136/hrt.2006.094516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hebert A, Jensen AS, Mikkelsen UR, Idorn L, Sørensen KE, Thilen U, Hanseus K, Søndergaard L. Hemodynamic causes of exercise intolerance in Fontan patients. Int J Cardiol. 2014;175:478–483. doi: 10.1016/j.ijcard.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 8. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. doi: 10.1161/CIRCULATIONAHA.115.017202 [DOI] [PubMed] [Google Scholar]
- 9. Henaine R, Vergnat M, Bacha EA, Baudet B, Lambert V, Belli E, Serraf A. Effects of lack of pulsatility on pulmonary endothelial function in the Fontan circulation. J Thorac Cardiovasc Surg. 2013;146:522–529. doi: 10.1016/j.jtcvs.2012.11.031 [DOI] [PubMed] [Google Scholar]
- 10. Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta‐analysis. Heart. 2015;101:1731–1737. doi: 10.1136/heartjnl-2015-307930 [DOI] [PubMed] [Google Scholar]
- 11. Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–1687. doi: 10.1093/eurheartj/ehn215 [DOI] [PubMed] [Google Scholar]
- 12. Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double‐blind, placebo‐controlled, crossover trial. Circulation. 2011;123:1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhodes J, Ubeda‐Tikkanen A, Clair M, Fernandes SM, Graham DA, Milliren CE, Daly KP, Mullen MP, Landzberg MJ. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. Int J Cardiol. 2013;168:2435–2440. doi: 10.1016/j.ijcard.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hebert A, Mikkelsen UR, Thilen U, Idorn L, Jensen AS, Nagy E, Hanseus K, Sørensen KE, Søndergaard L. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo‐Controlled, Double‐Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130:2021–2030. doi: 10.1161/CIRCULATIONAHA.113.008441 [DOI] [PubMed] [Google Scholar]
- 15. Cedars AM, Saef J, Peterson LR, Coggan AR, Novak EL, Kemp D, Ludbrook PA. Effect of ambrisentan on exercise capacity in adult patients after the Fontan procedure. Am J Cardiol. 2016;117:1524–1532. doi: 10.1016/j.amjcard.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 16. Goldberg DJ, Zak V, Goldstein BH, Schumacher KR, Rhodes J, Penny DJ, Petit CJ, Ginde S, Menon SC, Kim SH, et al.; Pediatric Heart Network Investigators . Results of the Fontan Udenafil Exercise Longitudinal (FUEL) Trial. Circulation. 2020;141:641–651. doi: 10.1161/CIRCULATIONAHA.119.044352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al.; Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 18. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;139:e698–e800. doi: 10.1161/CIR.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 19. Baumgartner H, De Backer J, Babu‐Narayan SV, Budts W, Chessa M, Diller GP, lung B, Kluin J, Lang IM, Meijboom F, et al.; ESC Scientific Document Group . 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554 [DOI] [PubMed] [Google Scholar]
- 20. Ridderbos FJ, Wolff D, Timmer A, van Melle JP, Ebels T, Dickinson MG, Timens W, Berger RM. Adverse pulmonary vascular remodeling in the Fontan circulation. J Heart Lung Transplant. 2015;34:404–413. doi: 10.1016/j.healun.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 21. Mitchell MB, Campbell DN, Ivy D, Boucek MM, Sondheimer HM, Pietra B, Das BB, Coll JR. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004;128:693–702. doi: 10.1016/j.jtcvs.2004.07.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figure S1


