Abstract
Background
In the myocardium, pericytes are often confused with other interstitial cell types, such as fibroblasts. The lack of well‐characterized and specific tools for identification, lineage tracing, and conditional targeting of myocardial pericytes has hampered studies on their role in heart disease. In the current study, we characterize and validate specific and reliable strategies for labeling and targeting of cardiac pericytes.
Methods and Results
Using the neuron‐glial antigen 2 (NG2)DsRed reporter line, we identified a large population of NG2+ periendothelial cells in mouse atria, ventricles, and valves. To examine possible overlap of NG2+ mural cells with fibroblasts, we generated NG2DsRed; platelet‐derived growth factor receptor (PDGFR) αEGFP pericyte/fibroblast dual reporter mice. Myocardial NG2+ pericytes and PDGFRα+ fibroblasts were identified as nonoverlapping cellular populations with distinct transcriptional signatures. PDGFRα+ fibroblasts expressed high levels of fibrillar collagens, matrix metalloproteinases, tissue inhibitor of metalloproteinases, and genes encoding matricellular proteins, whereas NG2+ pericytes expressed high levels of Pdgfrb, Adamts1, and Vtn. To validate the specificity of pericyte Cre drivers, we crossed these lines with PDGFRαEGFP fibroblast reporter mice. The constitutive NG2Cre driver did not specifically track mural cells, labeling many cardiomyocytes. However, the inducible NG2CreER driver specifically traced vascular mural cells in the ventricle and in the aorta, without significant labeling of PDGFRα+ fibroblasts. In contrast, the inducible PDGFRβCreER line labeled not only mural cells but also the majority of cardiac and aortic fibroblasts.
Conclusions
Fibroblasts and pericytes are topographically and transcriptomically distinct populations of cardiac interstitial cells. The inducible NG2CreER driver optimally targets cardiac pericytes; in contrast, the inducible PDGFRβCreER line lacks specificity.
Keywords: aorta, fibroblast, lineage tracing, myocardium, pericyte
Subject Categories: Animal Models of Human Disease, Growth Factors/Cytokines, Myocardial Biology, Vascular Biology, Basic Science Research
Nonstandard Abbreviations and Acronyms
- α‐SMA
α smooth muscle actin
- CreERTM
tamoxifen‐inducible Cre recombinase
- Dsred
Discosoma species red
- EGFP
enhanced green fluorescent protein
- NG2
neuron‐glial antigen 2
- PCR
polymerase chain reaction
- PDGFR
platelet‐derived growth factor receptor
- tdTomato
tandem dimer Tomato
- VSMC
vascular smooth muscle cell
Clinical Perspective
What Is New?
We characterized mouse cardiac pericytes and demonstrated that myocardial neuron‐glial antigen 2 (NG2)+ pericytes and platelet‐derived growth factor receptor (PDGFR)α+ fibroblasts are nonoverlapping interstitial cell populations with distinct transcriptomic profiles.
We documented that the inducible NG2CreER mouse line optimally targets cardiac pericytes; in contrast, the germline NG2‐Cre mice and the inducible PDGFRβ‐Cre driver lack specificity.
What Are the Clinical Implications?
Our characterization and validation of genetic tools for labeling, tracing and targeting cardiac pericytes will greatly facilitate experimental investigations exploring the role of this understudied cell type in heart disease.
Blood vessels are composed of 2 distinct cell types: an inner layer of endothelial cells and mural cells that coat the endothelial cell tube. Mural cells can be further subdivided into vascular smooth muscle cells (VSMCs) and pericytes. VSMCs are associated with larger conduit vessels (such as arteries and veins), whereas pericytes enwrap the small caliber capillaries and are embedded within the endothelial basement membrane. 1 , 2 , 3 Pericytes are ubiquitously present in microvessels of all tissues, represent a critical interface between the circulating blood and the interstitium, 3 , 4 and have been implicated in both physiologic functions and pathophysiologic responses. Contractile pericytes contribute to the regulation of blood flow in the brain 5 and the kidney, 6 regulating basal blood flow resistance, 7 , 8 and have been implicated in blood pressure control. 9 Cerebral pericytes play a central role in the development and preservation of the blood‐brain‐barrier 10 , 11 and regulate vascular immune homeostasis in the central nervous system. 12 Pericytes may also be implicated in a wide range of pathologic responses, including inflammation, 13 fibrosis, 14 , 15 , 16 and neoplasia. 17 , 18
In the adult mammalian heart, maintenance of normal function and preservation of chamber geometry are not only dependent on contractile cardiomyocytes but also require the contribution of several other cell types, including macrophages, fibroblasts, and vascular cells. The myocardium contains a rich network of vessels necessary to provide perfusion in order to meet the high metabolic needs of cardiomyocytes. Considering the abundance of cardiac microvessels, it is not surprising that adult mammalian hearts contain a large population of pericytes. 19 , 20 , 21 , 22 , 23 , 24 Through their interactions with vascular endothelial cells, myocardial pericytes can regulate perfusion and vascular permeability. Moreover, in myocardial diseases, pericytes may contribute to the regulation of inflammatory, fibrogenic, and angiogenic responses. Despite their abundance and the diverse range of their functional properties, myocardial pericytes remain poorly understood. Most of the evidence implicating pericytes in cardiac pathophysiology is based on associative data. Studies in cells harvested from patients with heart failure have demonstrated that pericytes from cardiomyopathic hearts exhibit impaired mechanotransduction. 25 Moreover, animal model studies have suggested that pericytes may play a role in early postischemic microvascular injury, 26 may be involved in “no‐reflow” following ischemia and reperfusion, 27 , 28 and may contribute to the pathogenesis of the cardiomyopathy associated with receptor tyrosine kinase inhibitor treatment. 29 However, investigations documenting the involvement of pericytes in myocardial diseases using genetic approaches are lacking. Understanding the role of pericytes in heart disease is hampered by the absence of well‐characterized, specific, and reliable approaches for labeling and cell‐specific targeting of pericytes in vivo. A major source of confusion is the abundance of cardiac fibroblasts, 30 cells of mesenchymal origin that can be located in close proximity to vessels and may exhibit profiles that overlap with those of pericytes. Thus, study of the role of pericytes in myocardial disease requires validation and characterization of reporter lines and Cre drivers with specificity for pericytes and with no significant overlap with fibroblasts.
Published evidence on the molecular tools used for pericyte‐specific targeting generates confusion. Although the platelet‐derived growth factor receptor (PDGFR)β was found to be exclusively expressed in pericytes in the brain, 31 its specificity as a pericyte marker in other organs is controversial. Inducible PDGFRβ‐Cre lines were used for pericyte‐specific targeting in some studies 32 , 33 and for fibroblast‐specific targeting in other investigations. 34 , 35 The proteoglycan neuron‐glial antigen 2 (NG2) is a commonly used pericyte marker 36 ; however, the specificity and reliability of constitutive and inducible NG2‐Cre lines in targeting myocardial pericytes have not been studied. In the current investigation, we validated and characterized genetic tools for pericyte‐specific labeling and targeting. Using dual reporter mice, we identified phenotypically and transcriptomically distinct pericyte and fibroblast populations in the adult mouse heart. To study the reliability of pericyte‐specific Cre drivers, we used a fibroblast reporter system. Our findings suggest that inducible NG2‐Cre lines specifically target cardiac mural cells. In contrast, inducible PDGFRβ‐Cre drivers lack specificity, exhibiting recombination in both mural cells and the majority of interstitial fibroblasts.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mice
All animal experiments were performed according to the animal experimental guidelines issued by the Animal Care and Use Committee at Albert Einstein College of Medicine and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. NG2DsRed/+, constitutively active NG2‐Cre (NG2Cre/+), tamoxifen‐inducible NG2 Cre recombinase (NG2iCre/+), tamoxifen‐inducible PDGFRβ‐CreERT2 (PDGFRβiCre/+), reverse orientation splice acceptor (ROSA) 26tdTomato–/– (R26tdTomato), ROSA26EYFP–/– (R26EYFP), PDGFRαEGFP/+ mice were purchased from Jackson Laboratories (Table). NG2DsRed;PDGFRαEGFP double reporter mice were generated by breeding NG2DsRed/+ mice with PDGFRαEGFP/+. Both male and female 2‐ to 3‐month‐old mice were euthanized for histological end points and fluorescence‐activated cell sorting studies. NG2Cre/+ mice were crossed with R26EYFP mice to develop NG2Cre/+;R26EYFP mice. NG2iCre/+ or PDGFRβiCre/+ mice bred into R26tdTomato background were further crossed with PDGFRαEGFP fibroblast reporter mice to enable the simultaneous identification of mural cell–derived progeny, as well as fibroblasts. For lineage tracing studies, 10‐ to 12‐week‐old NG2iCre/+;R26tdTomato;PDGFRαEGFP and PDGFRβiCre/+;R26tdTomato;PDGFRαEGFP/+ mice received intraperitoneal injections of tamoxifen (Sigma‐T5648, CAS#10540‐29‐1) at a dosage of 100 mg/kg administered over a course of 5 consecutive days once every 24 hours. Mice were euthanized for histology 2 days after the last injection of tamoxifen.
Table .
Mouse Lines Used in the Study
| Jackson strain number | |
|---|---|
| NG2DsRed/+ | 008241 |
| NG2Cre/+ | 008533 |
| NG2iCre/+ | 008538 |
| PDGFRβiCre/+ | 030201 |
| ROSA26tdTomato–/– | 007914 |
| ROSA26EYFP–/– | 006148 |
| PDGFRαEGFP/+ | 007669 |
EGFP indicates enhanced green fluorescent protein; EYFP, enhanced yellow fluorescent protein; NG2, neuron‐glial antigen 2; PDGFR, platelet‐derived growth factor receptor; ROSA, reverse orientation splice acceptor; and tdTomato, tandem dimer Tomato.
Immunohistochemistry
For histopathological analysis, mice were euthanized and hearts were fixed in Z‐fix (Anatech) and embedded in paraffin. Sequential 5‐μm sections were cut from base to apex at 250‐μm intervals. Following citrate buffer‐mediated antigen retrieval, sections were allowed to cool for close to an hour and then blocked with Tris‐buffered saline+0.1% Triton X containing 10% donkey serum. The following antibodies were used: mouse anti‐α‐smooth muscle actin antibody (dilution 1:100, Sigma F3777), rat anti‐Mac‐2 antibody (dilution 1:100, Cedarlane Laboratories), goat anti‐tdTomato antibody (dilution 1:300, Biorbyt orb334992), rabbit anti‐GFP antibody (dilution 1:100, D5.1‐2956, Cell Signaling Technology), rabbit anti‐CD31 (dilution 1:100, Cell Signaling Technology 89C2, 3528), and rabbit anti‐PDGFRβ antibody (1:50, ab32570). Primary antibody incubations were all performed at 4 °C overnight. Following washes in Tris‐buffered saline, secondary antibodies raised in donkey were used for 1 hour at room temperature. Sections were washed and then incubated with Trueblack (Biotium 23007) for 20 seconds to quench autofluorescence and then sealed with Fluoro‐Gel II mounting medium containing 4’,6’‐diamidino‐2‐phenylindole (EMS #17985‐50). Slides were then scanned using Zen 3.0 Pro software and a Zeiss Imager M2 microscope (Carl Zeiss Microscopy). Appropriate negative controls using isotype‐matched IgG were performed and confirmed the specificity of the immunofluorescence experiments (Figures S1 and S2).
Quantitative Analysis of Cell Density
Using default algorithms of the Intellesis Trainable Segmentation module of Zen 3.0 Pro software, an artificial intelligence–based model was trained on images representing atria, ventricles, valves, and the ascending aorta (in total, at least 4 images up to 8 images depending on the specific staining used) to identify and count mural cell profiles. Using the Image Analysis module, specific settings were incorporated in the trained model to count the segmented objects. For quantitative analysis, 5 to 10 fields were scanned from 2 to 3 different sections from each chamber under a magnification of 200×. Quantification of cell density was performed separately for the atria, ventricles, valves, and ascending aorta. The data were presented as the number of positive profiles per millimeters squared.
To assess the percentage of arteriolar and aortic medial α smooth muscle actin (α‐SMA)+ VSMCs expressing NG2, the number of double positive cells was counted in 4 to 7 fields (400× magnification) from a single level for each mouse using the count tool from Adobe Photoshop. To assess the extent of fibroblast labeling with the PDGFRβ tamoxifen‐inducible Cre recombinase (CreERTM) driver, we quantitatively assessed the percentage of PDGFRα+ fibroblasts that were labeled with the inducible PDGFRβ‐Cre in the ventricular myocardium as well as in the adventitia of the ascending aorta. A total of 8 to 12 fields were analyzed from the ventricular myocardium of each mouse, and 4 to 6 fields were analyzed from the ascending aorta of each mouse (400× magnification). Cells were counted using the count tool from Adobe Photoshop.
Fluorescence‐Activated Cell Sorting of Cardiac Pericytes and Fibroblasts
Single‐cell suspension for flow cytometry was prepared using a modified version of a previously described protocol. 17 Briefly, atria were removed from the myocardial tissue, and the ventricles were finely minced and suspended in digestion buffer cocktail of collagenase IV (2 mg/mL, Worthington Biochem) and dispase II (1.2 U/mL, Stemcell Technologies) in Dulbecco phosphate‐buffered saline. Tissue fragments were then incubated at 37 °C for 15 minutes with gentle rocking. After incubation, a tissue digestion buffer with tissue clusters was triturated by pipetting 10 times using a 10‐mL serological pipette. Tissue fragments were again incubated at 37 °C and triturated twice more (45 minutes of total digestion time). The final trituration was conducted by pipetting 30× with a p1000 pipette. Cell suspension was filtered through a 40‐μm cell strainer into 50‐mL tubes containing 40 mL of Dulbecco phosphate‐buffered saline and centrifuged for 20 minutes at 200g with centrifuge brakes deactivated to remove cell debris. Cells were then resuspended in 1× Red Blood Cell Lysis Buffer (eBioscience) and incubated for 5 minutes at room temperature. Cell suspension was then centrifuged at 500g for 5 minutes at room temperature and resuspended in Hanks Balanced Salt Solution containing 2% fetal bovine serum. Cells in single‐cell suspensions were blocked with anti‐mouse CD16/CD32 (1:250, BD Biosciences) for 30 minutes at 4 °C. To identify hematopoietic and endothelial cells, the cell suspension was incubated for 1 hour at 4 °C with anti‐CD31‐BV605 (1:100, BioLegend) and anti‐CD45‐APC‐Cy7 (2.5:100, BioLegend). Cell suspension was washed and labeled with calcein violet 450 (1.25 μmol/L, eBioscience, Invitrogen) to identify metabolically active cells and 7‐aminoactinomycin D (1:500, Invitrogen) to identify cells with compromised cell membranes. Nonhematopoietic/nonendothelial cells (CD45–/CD31–) were gated to identify NG2RFP+ pericytes and PDGFRαEGFP+ fibroblasts, which were sorted with a FACSAria Sorter (BD Biosciences). FlowJo software (BD) was used for data analysis. The gating strategy and controls for each marker using NG2DsRed and PDGFRαEGFP single reporter mice are shown in Figure S3.
RNA Isolation and Polymerase Chain Reaction Array
RNA isolation of sorted cells was performed using Dynabeads mRNA Direct Kit (61012, Invitrogen). Following isolation, RNA was converted to cDNA using a Qiagen RT2 First Strand Kit (330404). Quantitative polymerase chain reaction (PCR) was performed using the mouse RT2 Profiler extracellular matrix PCR array according to manufacturer’s protocol (PAMM‐013Z, Qiagen). Separately, quantitative PCR was also performed for the mural cell genes Pdgfrb (forward: 5' CACCTTCTCCAGTGTGCTGA 3’, reverse: 5’ GGAGTCCATAGGGAGGAAGC 3’) and Acta2 (forward: 5’ GAGTAATGGTTGGAATGG 3’, reverse: 5’ ATGATGCCGTGTTCTATC 3’). Primers were synthesized by Integrated DNA Technologies. The same thermal profile conditions were used for all primer sets: 95 °C for 10 minutes, 40 cycles at 95 °C for 15 seconds, and 60 °C for 1 minute on the CFX384™ Real‐Time PCR Detection System (Bio‐Rad). The data obtained were analyzed using the ΔCt method.
Statistical Analysis
For comparisons of 2 groups, an unpaired 2‐tailed Student t test using (when appropriate) Welch correction for unequal variances was performed. Mann‐Whitney test was used for comparisons between 2 groups that did not show Gaussian distribution. For comparisons of multiple groups, 1‐way ANOVA was performed followed by Sidak multiple comparison test for normal distributions or the Kruskal‐Wallis test for non‐Gaussian distributions. Data are expressed as mean±SE. Statistical significance was set at 0.05.
Results
Adult Mouse Heart Contains a Large Population of Periendothelial NG2+ Cells
To identify pericytes in the adult mouse myocardium, we used the NG2DsRed/+ reporter line. Dual immunofluorescence for NG2RFP and the endothelial cell marker CD31 identified a large population of myocardial NG2+ cells, located in close proximity to endothelial cells (Figure 1A). Quantitative analysis showed that the ventricles contained more NG2+ cells than the atria (Figure 1B). Significant numbers of NG2+ cells were also noted in the valve leaflets (Figure 1C). The aortic valve had a higher density of NG2+ cells than the mitral and tricuspid valves (Figure 1D).
Figure 1. The neuron‐glial antigen 2 (NG2)DsRed reporter model identifies a large population of pericytes in the mouse myocardium.
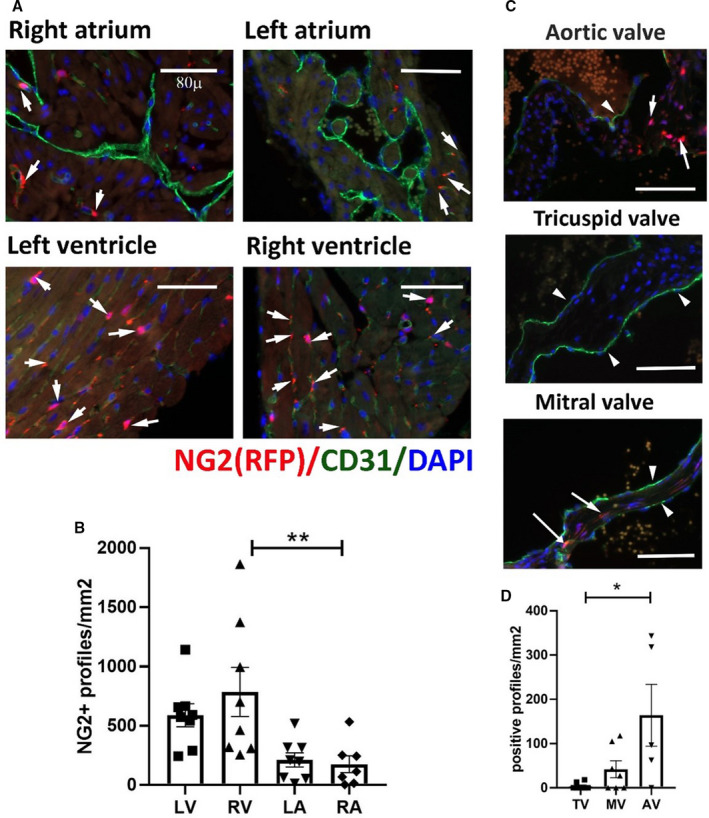
A, Dual immunofluorescence for red fluorescent protein (RFP) and CD31 identifies NG2+ pericytes in atria and ventricles (arrows), associated with CD31+ endothelial cells (A). B, Quantitative analysis shows that the density of pericytes is higher in the right ventricle (RV) than in the right atrium (RA). Moreover, there is a trend (P=0.08) toward a higher pericyte density in the left ventricle (LV), in comparison to the left atrium (LA). C, Dual immunofluorescence identifies NG2+ cells (arrows) in the valves. The arrowheads indicate the CD31+ valvular endothelial cells. D, The aortic valve (AV) has a higher density of pericytes than the mitral valve (MV) and tricuspid valve (TV) (*P<0.05, **P<0.01; n=5–8 per group). Scalebar=80 μm. Data are expressed as mean±SE. Statistical comparison was performed by ANOVA, followed by the Sidak post hoc test (B), or the nonparametric Kruskal‐Wallis test (D). DAPI indicates 4’,6’‐diamidino‐2‐phenylindole; and Dsred, Discosoma species red.
NG2 Labels a Subset of Arteriolar and Aortic VSMCs
Next, we examined the sensitivity of NG2 in the labeling of VSMCs, using dual fluorescence for NG2 and α‐SMA (Figure 2). In the myocardium, α‐SMA labeled only arteriolar VSMCs without staining NG2+ microvascular pericytes (Figure 2A and 2B). Two distinct populations of cardiac arteriolar VSMCs were identified on the basis of NG2 fluorescence intensity. The majority of arteriolar VSMCs (91.49%±3.02%, n=8) had high levels of NG2 expression, whereas a much smaller subpopulation (8.51%±3.02%, n=8) exhibited low NG2 expression levels (Figure 2A and 2B). In the mouse myocardium, NG2+/α‐SMA– cells (representing microvascular pericytes) were much more abundant than arteriolar NG2+/α‐SMA+ VSMCs (Figure 2C). In the ascending aorta, only 46.2%±8.0% (n=6) of the α‐SMA+ VSMCs in the media were positive for NG2 (Figure 2D through 2F). In the aortic adventitia, NG2 staining identified a significant number of α‐SMA– cells that may represent microvascular pericytes coating the vasa vasorum (Figure 2G through 2I).
Figure 2. Neuron‐glial antigen 2 (NG2) labels a subset of vascular smooth muscle cells (VSMCs) in the myocardial arterioles and the ascending aorta.
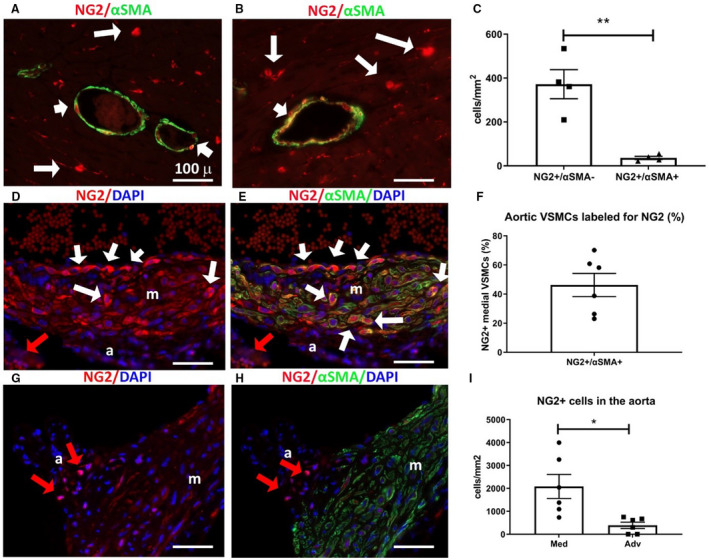
A and B, Dual fluorescence for red fluorescent protein (RFP) and α‐smooth muscle actin (α‐SMA) in the NG2DsRed model, identifies a subset of arteriolar α‐SMA+ VSMCs that express NG2 (short arrows). However, the majority of NG2+ profiles are α‐SMA– microvascular pericytes (long arrows). C, Quantitative analysis shows that the density of NG2+/α‐SMA– profiles (microvascular pericytes) is much higher than the density of NG2+/α‐SMA+ cells (arteriolar VSMCs) (**P<0.01; n=4 per group). D through F, In the ascending aorta, dual fluorescence for RFP and α‐SMA shows ≈40% of aortic VSMCs in the media (m) are NG2+ (long arrows). G and H, In the aortic adventitia (a), NG2+/α‐SMA– profiles (red arrows) represent the pericytes of the aortic vasa vasorum. I, The density of NG2+ cells in the aortic media is much higher than the number of adventitial NG2+ cells (*P<0.05; n=6 per group). Scalebar=100 μm. Data are expressed as mean±SE. Statistical comparison was performed using Welch t test. Adv indicates adventitia; DAPI, 4’,6’‐diamidino‐2‐phenylindole; Dsred, Discosoma species red; and Med, media.
Myocardial NG2+ Cells Express the Mural Cell Marker PDGFRβ But Not the Fibroblast Marker PDGFRα and the Macrophage Marker Mac2
We next investigated the specificity of NG2 labeling as a mural cell marker. Dual fluorescence for NG2 and the mural cell marker PDGFRβ showed that the majority of NG2+ cells were also PDGFRβ+ (Figure 3A through 3C). However, a large fraction of cardiac interstitial cells also exhibited PDGFRβ immunoreactivity in the absence of NG2 expression (Figure 3A through 3D). To examine whether a subset of the NG2+ cells were fibroblasts, we generated pericyte/fibroblast (NG2DsRed;PDGFRαEGFP) dual reporter mice. Dual fluorescence showed that NG2 does not label any PDGFRα+ fibroblasts in the mouse heart (Figure 3E through 3G). To examine whether NG2 may label a subset of macrophages, we performed dual fluorescence for NG2 and the macrophage marker Mac2. The Mac2+ population in the mouse heart was distinct from the NG2‐expressing cells (Figure 3H through 3J).
Figure 3. Myocardial neuron‐glial antigen 2 (NG2)+ pericytes are platelet‐derived growth factor receptor (PDGFR)β+ but do not express the fibroblast marker PDGFRα and the macrophage marker Mac2.
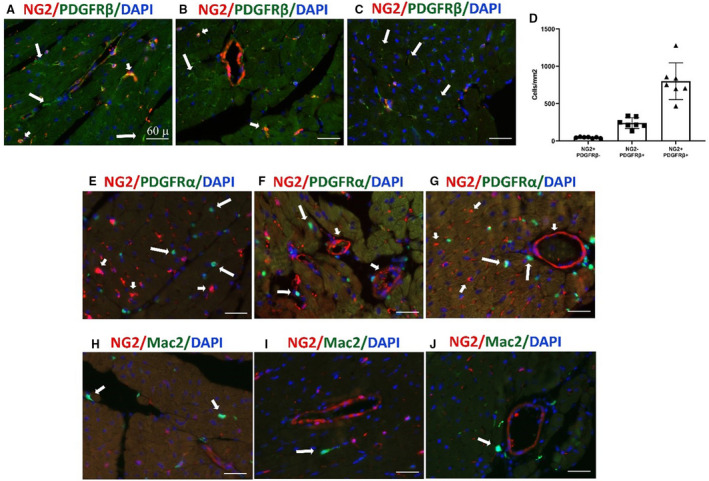
A through C, Dual immunofluorescence of NG2Dsred mouse cardiac sections shows that NG2+ pericytes coexpress PDGFRβ (short arrows). A large population of NG2– PDGFRβ+ interstitial cells is also identified (long arrows). D, Quantitative analysis (n=7 per group) shows that, although the majority of PDGFRβ+ profiles are also labeled for NG2, there is a large number of PDGFRβ+/NG2– cells. E through G, To examine potential overlap between NG2+ pericytes and PDGFRα+ fibroblasts, we generated NG2DsRed;PDGFRαEGFP dual reporter mice. Dual immunofluorescence shows no overlap of NG2+ pericytes (short arrows) and PDGFRα+ fibroblasts (long arrows). H through J, Staining of NG2DsRed mouse cardiac sections with the macrophage marker Mac2 was performed to examine whether NG2 labels macrophages. NG2+ pericytes do not express Mac2. A relatively small number of Mac2+ macrophages is noted in the mouse myocardium (arrows). Images are representative of 6 different experiments. Scale bar=60 μm. Data are expressed as mean±SE. Dsred indicates Discosoma species red; and EGFP enhanced green fluorescent protein.
NG2+ Pericytes and PDGFRα+ Fibroblasts Have Distinct Transcriptomic Profiles
To compare the transcriptional signatures of myocardial pericytes and fibroblasts, we extracted RNA from sorted NG2+ and PDGFRα+ cells from adult mouse hearts. There was no significant overlap between these 2 populations (Figure 4A). A PCR array showed that PDGFRα+ and NG2+ cells had distinct transcriptomic profiles (Figure 4B through 4R, Figure S4). PDGFRα+ cells expressed much higher levels of fibrillar collagens (col1a1, col3a1, col5a1, and col6a1; Figure 4C through 4F), matrix metalloproteinases, tissue inhibitor of metalloproteinases (Mmp1a, Mmp2, Timp2, and Timp3; Figure 4G through 4J), and genes encoding matricellular proteins (Sparc, Thbs2, Thbs3, and Ccn2; Figure 4K through 4N). In contrast, NG2+ cells had higher levels of Adamts1 and Vtn. Quantitative PCR also showed that NG2+ cells had much higher levels of expression of the mural cell gene Pdgfrb and a trend towards higher expression of Acta2 (Figure 4O through 4R), Thus, the transcriptomic data suggest that NG2+ cells and PDGFRα+ cells represent 2 distinct cell types with characteristics of myocardial mural cells and fibroblasts, respectively.
Figure 4. Myocardial neuron‐glial antigen 2 (NG2)+ pericytes and platelet‐derived growth factor receptor (PDGFR)α+ fibroblasts have distinct transcriptomic profiles.
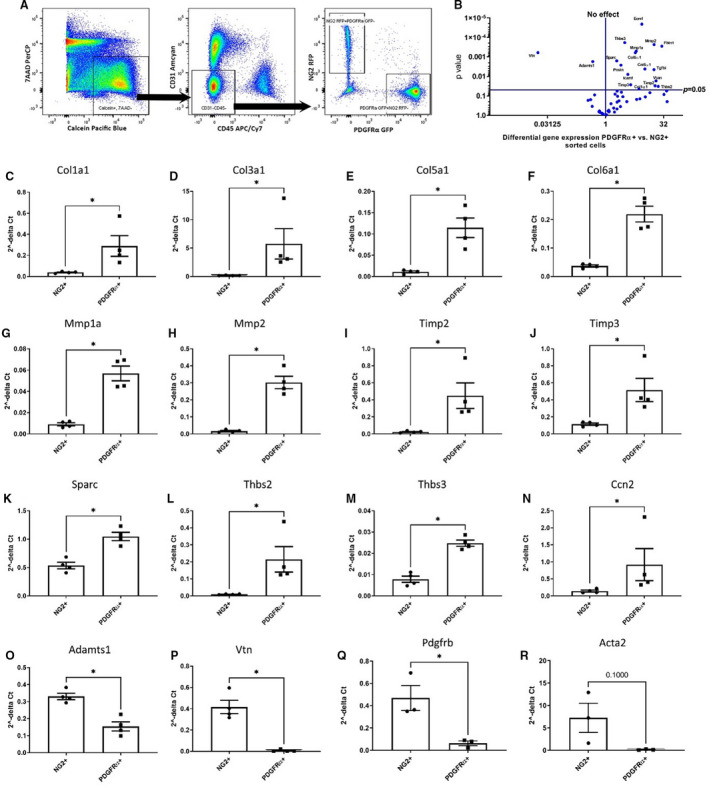
A, Single‐cell suspension was prepared from dual reporter NG2DsRed;PDGFRαEGFP hearts using a collagenase/dispase‐based enzymatic procedure. Viable (7‐aminoactinomycin D/7‐AAD) and metabolically active (calcein+) cells were gated and based on the expression of CD31 and CD45, the nonendothelial and nonhematopoietic cell population was identified and subgated. NG2+ and PDGFRα+ populations were sorted by fluorescence‐assisted cell sorter into cell lysis buffer to isolate RNA. B, The volcano plot summarizes the extracellular matrix array quantitative polymerase chain reaction (PCR) data. C through P, PDGFRα+ cells have higher levels of matrix‐related genes, such as Col1α1(C), Col3α1 (D), Col5α1 (E), Col6α1 (F), Mmp1α (G), Mmp2 (H), Timp2 (I), Timp3 (J), and genes encoding matricellular proteins, including Sparc (K), Thbs2 (L), Thbs3 (M), and Ccn2 (N). On the other hand, NG2+ cells expressed higher levels of Adamts1 (O) and Vtn (P) (*P=0.05; n=4). Q and R, Quantitative PCR showed that NG2+ pericytes had higher levels of Pdgfrb (Q) and a trend toward higher expression of Acta2 (P=0.1; R) (n=3; *P<0.05). Data are expressed as mean±SE. Statistical comparisons were performed using unpaired t test for normal distributions and Mann‐Whitney test for non‐Gaussian distributions. Dsred indicates Discosoma species red; and EGFP enhanced green fluorescent protein.
Tracing of NG2+ Cells Using a Germline NG2‐Cre Driver Labels Cardiomyocytes
We first used a germline NG2‐Cre line to label mural cells in mouse hearts. NG2Cre/+ animals were bred with R26EYFP mice. NG2Cre/+;R26EYFP mice were euthanized at 2 to 3 months of age and the hearts were harvested for histological studies. Dual fluorescence for enhanced yellow fluorescent protein and the endothelial cell marker CD31 showed that vascular mural cells could not be specifically identified. In contrast, there was intense cardiomyocyte labeling with individual variations between cells (Figure S5).
Inducible NG2CreERTM Line Specifically Labels Mural Cells in the Myocardium and in the Aorta
Because labeling of cardiomyocytes precluded identification of pericyte‐derived cells in the constitutive NG2Cre mice, we tested whether the inducible NG2CreERTM line can be used to trace pericytes in the myocardium. NG2CreERTM mice were crossed with R26tdTomato animals (Figure 5A). NG2iCre/+;R26tdTomato mice were injected with tamoxifen at 3 months of age. Heart and ascending aorta were harvested 2 days after the last injection and used for histological analysis. In both atria and ventricles, a large population of periendothelial mural cells was labeled, without staining of cardiomyocytes (Figure 5B). Although there was a trend towards increased density of NG2‐traced cells in the ventricles, in comparison to the atria, the differences did not reach statistical significance (Figure 5C). Lineage tracing using the NG2CreERTM line also labeled a significant population of valvular cells, which were more abundant in the aortic valve (Figure S6A and S6B). In the aorta, 60.7%±5.2% of aortic medial α‐SMA+VSMCs were labeled with the inducible NG2‐Cre driver (Figure S6C through S6H).
Figure 5. The inducible neuron‐glial antigen 2 (NG2) tamoxifen‐inducible Cre recombinase (CreERTM) line specifically labels myocardial pericytes.
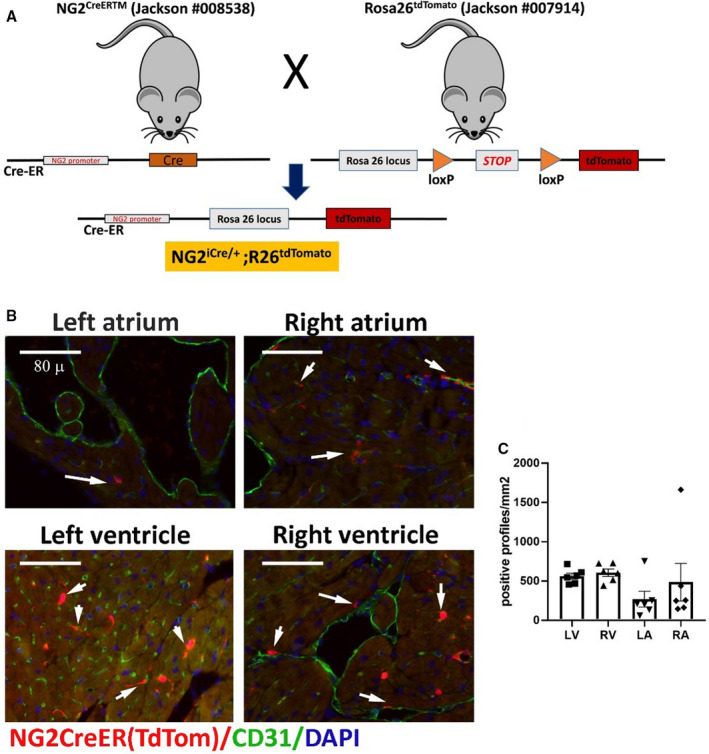
A, The schematic cartoon shows the breeding scheme used to develop NG2iCre/+; Rosa26tdTomato mice. NG2CreERTM mice were crossed with Rosa26tdTomato animals and the resultant NG2iCre/+;R26tdTomato mice were injected with tamoxifen at 3 months of age. B and C, NG2+ pericytes identified using the lineage tracing approach are closely associated with CD31+ endothelial cells. Quantitative analysis (n=6) shows the density of NG2‐derived cells in all cardiac chambers (C). Scalebar=80 μm. Data are expressed as mean±SE. Statistical comparison was performed using nonparametric ANOVA (Kruskal‐Wallis, P=not significant). LA indicates left atrium; LV, left ventricle; RA, right atrium; Rosa, reverse orientation splice acceptor; RV, right ventricle; and tdTom, tandem dimer Tomato.
Inducible PDGFRβCreERT2 Driver Labels Abundant Myocardial Interstitial Cells, Including Mural Cells and a Significant Fraction of Cardiac Fibroblasts
Next, we used the inducible PDGFRβCreERT2 line to trace mural cells in the myocardium and the aorta. PDGFRβCreERT2 mice were bred with R26tdTomato animals. PDGFRβiCre/+;R26tdTomato mice (Figure 6A) were injected with tamoxifen and euthanized within a week. The heart and the ascending aorta were used for histological analysis. Dual immunofluorescence for tdTomato and CD31 showed that abundant myocardial interstitial cells were labeled for PDGFRβ, with both periendothelial and interstitial localizations. In comparison to the inducible NG2‐Cre driver, the inducible PDGFRβ‐Cre line labeled many more myocardial cells, the majority of which had interstitial nonperiendothelial localization (Figure 6B). In contrast to the NG2 reporter, no significant differences between the density of atrial and ventricular cells were noted, reflecting the abundance of atrial interstitial cells labeled with the inducible PDGFRβ‐Cre driver (Figure 6C).
Figure 6. The inducible platelet‐derived growth factor receptor (PDGFR)βCreERT2 driver labels not only periendothelial cells but also a large population of myocardial interstitial cells, which are not associated with vessels.
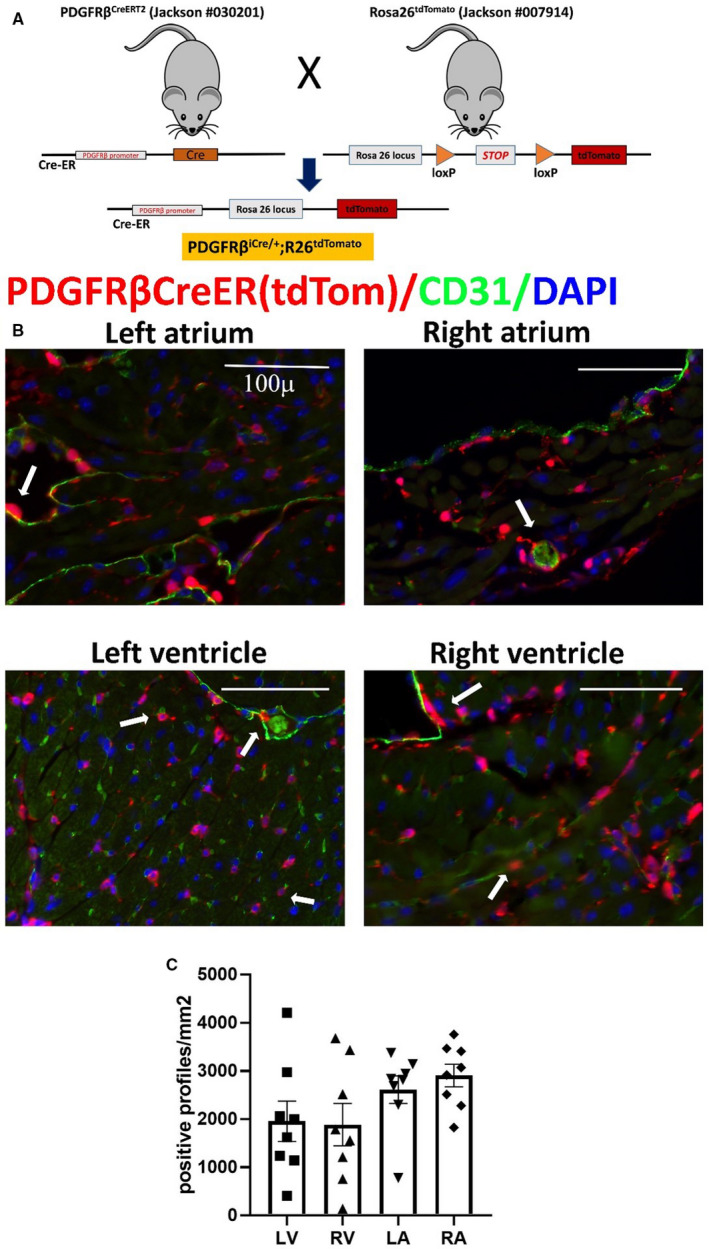
A, Breeding strategy shows the development of PDGFRβiCre/+;R26tdTomato model. B, Abundant PDGFRβ+ cells are identified in all cardiac chambers using the lineage tracing approach. Please note that the density of the PDGFRβ+ cells is much higher than the numbers of neuron‐glial antigen 2 (NG2)+ cells (shown in Figure 5). Dual fluorescence with CD31 shows that a significant proportion of these cells is not associated with endothelial cells. C, Quantitative analysis (n=8) of the density of PDGFRβ+ cells in atria and ventricles. Scalebar=100 µm. Data are expressed as mean±SE. Statistical comparison was performed using nonparametric ANOVA (Kruskal‐Wallis, P=not significant). LA indicates left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; and tdTom, tandem dimer Tomato.
In a similar manner, the PDGFRβ‐Cre driver labeled several times more valvular cells than the inducible NG2‐Cre driver (Figure S7A and S7B). In the ascending aorta, in addition to labeling a large fraction (69.6%±10.87%) of aortic medial VSMCs, the PDGFRβ‐Cre driver also stained a large number of adventitial α‐SMA– cells (Figure S7C through S7H).
Because of the interstitial nonperiendothelial localization of many myocardial cells labeled with the PDGFRβ‐Cre driver, we postulated that this driver may also label cardiac fibroblasts. To test this hypothesis, we crossed the NG2iCre/+;R26tdTomato and the PDGFRβiCre/+;R26tdTomato mice with fibroblast reporter PDGFRαEGFP mice (Figure 7A and 7B). Significant populations of perivascular and interstitial cells labeled with the inducible PDGFRβ‐Cre driver also expressed PDGFRα and were identified as fibroblasts (Figure 7C and 7D). In contrast, very few NG2‐derived cells exhibited PDGFRα staining in the ventricles or atria (Figure 7E). Quantitative analysis showed that the number of PDGFRα+ fibroblasts labeled with the inducible PDGFRβ‐Cre driver were much higher than the fibroblasts labeled with the inducible NG2‐Cre driver (Figure 7F). Also, 58.2%±3.42% (n=8) of ventricular PDGFRα+ fibroblasts were labeled for PDGFRβ; in contrast, only 2.3%±0.3% (n=6) of fibroblasts were traced with the inducible NG2‐Cre driver (Figure 7G). These findings demonstrated that PDGFRβ is a suboptimal marker to trace pericytes, as it also identifies a significant subset of resident cardiac fibroblasts at baseline. In a similar manner, in the ascending aorta, many of the adventitial cells traced with the inducible PDGFRβ‐Cre driver were identified as fibroblasts, on the basis of PDGFRα staining (Figure 8A through 8C). Quantitative analysis showed that 56.4%±6.8% of PDGFRα+ adventitial fibroblasts were also labeled for PDGFRβ (Figure 8D) In contrast, the inducible NG2‐driver did not label any adventitial fibroblasts (Figure 8E). Taken together, the findings demonstrate that the inducible PDGFRβ‐Cre driver is not specific for mural cells, but also tracks the majority of cardiac and aortic adventitial fibroblasts.
Figure 7. The neuron‐glial antigen 2 (NG2) tamoxifen‐inducible Cre recombinase (CreERTM) driver specifically labels mural cells, whereas the inducible platelet‐derived growth factor receptor (PDGFR)βCreERT2 line lacks specificity, also labeling a significant fraction of PDGFRα+ cardiac fibroblasts.
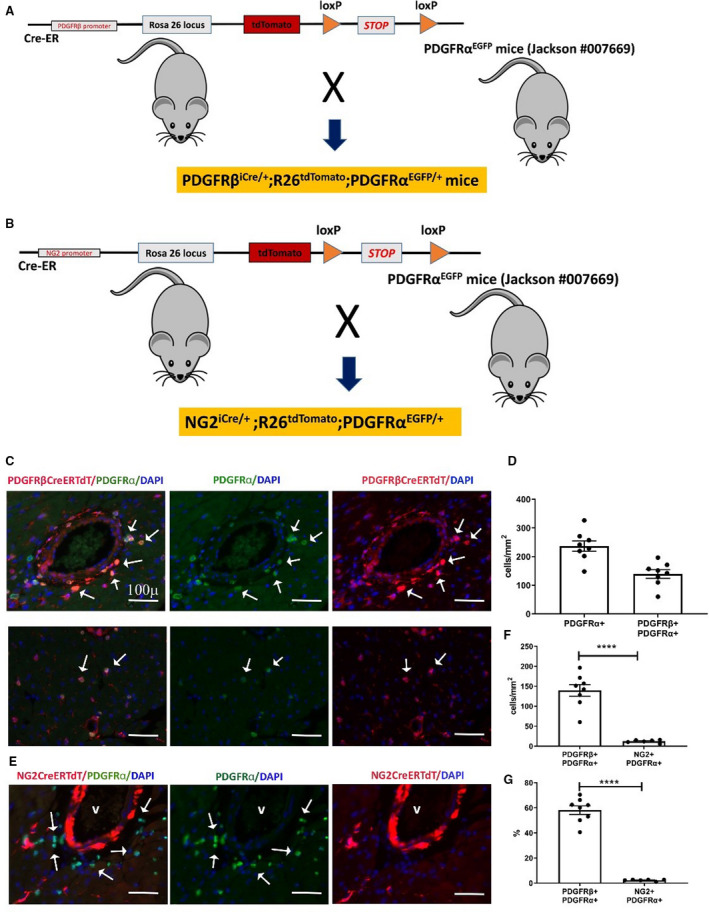
A and B, To test the specificity of the inducible PDGFRβ and NG2‐Cre drivers for myocardial pericytes, we bred PDGRβiCre/+;R26tdTomato mice (A), and NG2iCre/+;R26tdTomato animals (B) with fibroblast reporter PDGFRαEGFP mice. Schematic cartoons show the breeding scheme. C, Dual immunofluorescence of left ventricular sections shows that the inducible PDGFRβ‐Cre driver lacks specificity, labeling a large fraction of perivascular fibroblasts (C, top row) and interstitial fibroblasts (C, lower row) in the myocardium. PDGFRβ+/PDGFRα+ fibroblasts are indicated with arrows. D, Quantitative analysis (n=8) shows the density of PDGFRα+ fibroblasts and PDGFRα+/PDGFRβ+ fibroblasts in the myocardium. E, In NG2iCre/+;R26tdTomato;PDGFRαEGFP mice, staining for tandem dimer Tomato (tdTom) and green fluorescent protein shows that cardiac fibroblasts are not labeled for NG2, supporting the specificity of the inducible NG2‐Cre line. F, Quantitative analysis shows that the number of PDGFRβ+ cells identified as fibroblasts is markedly higher than the number of NG2+ cells that exhibit PDGFRα expression (****P<0.0001; n=6–8). G, Approximately 60% of PDGFRα+ fibroblasts are also labeled for PDGFRβ, whereas the percentage of fibroblasts labeled for NG2 is low (****P<0.0001; n=6–8). Scalebar=100 μm. Data are expressed as mean±SE. Statistical comparisons were performed using unpaired t test. DAPI indicates 4’,6’‐diamidino‐2‐phenylindole; and EGFP, enhanced green fluorescent protein.
Figure 8. In the ascending aorta, the neuron‐glial antigen 2 (NG2) tamoxifen‐inducible Cre recombinase (CreERTM) driver is specific for mural cells, whereas the inducible platelet‐derived growth factor receptor (PDGFR)βCreERT2 driver lacks specificity, also labeling PDGFRα+ aortic adventital fibroblasts.
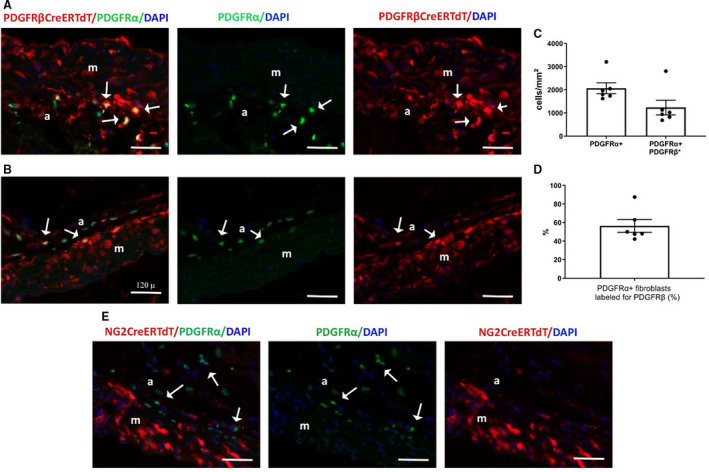
A through D, To test the specificity of the inducible PDGFRβ‐Cre driver for aortic mural cells, we bred PDGRβiCre/+;R26tdTomato mice with fibroblast reporter PDGFRαEGFP mice. Dual immunofluorescence of aortic sections shows that the inducible PDGFRβ‐Cre driver lacks specificity, labeling a large fraction of PDGFRα+ adventitial fibroblasts (A, C: arrows). Quantitative analysis shows (n=6) the density of adventitial PDGFRα+ fibroblasts and the density of fibroblasts labeled for PDGFRβ in the ascending aorta (C). D, More than 50% of adventitial fibroblasts (PDGFRα+ cells) were labeled for PDGFRβ (n=6). E, To test the specificity of the inducible NG2‐Cre driver, NG2iCre/+;R26tdTomato were bred into the PDGFRαEGFP reporter mouse line. Sections from the ascending aorta were stained for tandem dimer Tomato (tdTomato) and green fluorescent protein. Dual fluorescence shows that aortic adventitial fibroblasts (arrows) are not labeled for NG2, supporting the specificity of the inducible NG2‐Cre line. Images are representative of 6 different experiments. Scalebar=120 μm. Data are expressed as mean±SE. a indicates adventitia; DAPI, 4’,6’‐diamidino‐2‐phenylindole; EGFP, enhanced green fluorescent protein; and m, media.
Discussion
We report the first systematic characterization of genetic tools for identification, labeling, and tracing of mouse cardiac pericytes. Using NG2DsRed;PDGFRαEGFP dual reporter mice, we identified morphologically and transcriptomically distinct myocardial populations of fibroblasts (PDGFRα+/NG2–) and mural cells (NG2+/PDGFRα–). To evaluate the specificity of pericyte Cre drivers, we crossed 3 different mouse lines that have been extensively used to trace and target pericytes with the well‐documented and specific PDGFRαEGFP fibroblast reporter. 37 , 38 We found that only the inducible NG2‐CreER line is specific for mural cells. In contrast, the germline NG2‐Cre line is of limited value, as it also labels cardiomyocytes, whereas use of the inducible PDGFRβCreERT2 results in Cre‐mediated recombination not only in mural cells, but also in the majority of cardiac fibroblasts. Our findings establish optimal strategies for identification and targeting of cardiac pericytes in mice.
Cardiac Pericytes: Abundant Yet Enigmatic
Early ultrastructural studies have identified a large population of pericytes in mammalian hearts. Myocardial pericytes were described as extensively branched cells that form an incomplete layer around the endothelium 39 and express contractile proteins, 40 findings consistent with their proposed role in regulation of microvascular blood flow. Data on the relative abundance of pericytes in mammalian hearts are conflicting and are dependent on the species studied, and on the methodology used for their identification. A highly systematic study that isolated myocardial pericytes from several different species reported that pericytes may be more abundant than cardiomyocytes, thus representing the second most frequent myocardial cell type in the rat heart, outnumbered only by endothelial cells. 24 In contrast, other investigations using single markers for flow cytometric cell identification suggested that in adult mouse hearts, pericytes are vastly outnumbered by fibroblasts. 19 Data from human hearts are scarce. An ultrastructural study suggested that mural cells (both pericytes and VSMCs) in the left ventricle may be more abundant than fibroblasts, representing ≈22% to 28% of interstitial cells. 41 A recent single‐cell transcriptomic study of myocardial samples harvested from deceased organ donors also suggested an abundant population of myocardial pericytes and smooth muscle cells, identifying ≈21% of ventricular cells and ≈17% of atrial cells as mural cells. 20
Pericytes and Fibroblasts
It should be emphasized that a large fraction of the fibroblast‐like interstitial cells in the myocardium exhibit a perivascular location. 42 These cells may share certain common characteristics with pericytes; in fact, in some studies, pericytes have been referred to as “fibroblast‐like cells.” 43 The most rigorous and accepted definition of a mature pericyte requires demonstration of the localization of the cell within the microvascular basement membrane, a feature that can only be assessed using electron microscopy. Single‐cell omics have revealed the remarkable heterogeneity of myocardial interstitial populations in health and disease. 44 , 45 , 46 Despite the unquestionable value of high‐resolution definition of the diversity of interstitial cell populations at the single‐cell level, identification and classification of cells into traditionally defined types remain critical for understanding the cell biology of myocardial disease. In the current study, we demonstrate that use of the NG2DsRed;PDGFRαEGFP dual reporter mouse line allows identification of mural cells and fibroblasts in the mouse heart. No significant overlap between the 2 populations was noted (Figure 3, Figure 4A). Moreover, PDGFRα+/NG2– cells exhibited a transcriptomic profile typical of matrix‐synthetic fibroblasts, expressing high levels of fibrillar collagens, matrix metalloproteinases, tissue inhibitor of metalloproteinases, and matricellular genes (Figure 4C through 4N). In contrast, NG2+/PDGFRα– cells expressed low levels of matrix genes but exhibited much higher levels of Adamts1, Vtn, and Pdgfrb expression (Figure 4O through 4Q). High levels of vitronectin expression were previously reported in brain pericytes. 47 In contrast, Adamts1 has not been previously found to be expressed in normal kidney pericytes but has been reported to be highly upregulated upon injury. 48
Contractile Filament Proteins Lack Specificity for Pericyte Identification
Several “pericyte‐specific” markers have been used in attempts to identify pericytes and distinguish them from other interstitial cell types. 49 , 50 Some of these markers are clearly unreliable, lacking specificity and sensitivity. CD146, also known as melanoma cell adhesion molecule, has been used to identify pericytes 51 but is also highly expressed in endothelial cells. 52 , 53 , 54 On the other hand, vimentin, α‐SMA, and desmin are expressed in contractile filaments and have been used to label pericytes in some studies. Vimentin is highly expressed in all mesenchymally derived cells, including fibroblasts and all mural cells. 55 Desmin expression has been variably reported in subsets of fibroblasts and pericytes in several different tissues but is not expressed by myocardial pericytes. 51 α‐SMA also has low specificity and sensitivity as a pericyte marker. In comparison to VSMCs, pericytes have low levels of α‐SMA expression 56 but upregulate α‐SMA synthesis in response to stimulation with growth factors, such as transforming growth factor β. 57 However, the specificity of α‐SMA as a marker for activated pericytes is limited by its marked induction in injury‐site myofibroblasts. 58 , 59
NG2 and PDGFRβ as Pericyte Markers
The proteoglycan NG2 and the growth factor receptor PDGFRβ are the most commonly used markers for pericytes in mouse studies. 50 Anti‐NG2 antibodies, NG2DsRed fluorescent reporter mice, and constitutive and inducible NG2‐Cre drivers have been extensively used to identify, trace, and target pericytes in vivo. 60 , 61 , 62 , 63 PDGFRβ staining has also been used to specifically label pericytes in mouse tissues, 31 and inducible PDGFRβ‐Cre drivers have been considered in some studies optimal genetic tools for pericyte‐specific targeting. 32 , 33 However, the frequent use of the same inducible PDGFRβ‐Cre drivers to target fibroblast‐like interstitial cells 34 , 35 in other studies generates confusion regarding the specificity of these tools. To evaluate the specificity of NG2 and PDGFRβ‐Cre drivers, we crossed these lines with fibroblast‐specific PDGFRα reporter mice. Our findings demonstrate that only the inducible NG2‐CReERTM line induces recombination specifically in mural cells (Figure 5), showing no significant overlap with myocardial interstitial PDGFRα+ fibroblasts (Figure 7E through 7G). In contrast, the inducible PDGFRβ‐Cre driver labeled, in addition to mural cells, ≈60% of PDGFRα+ myocardial perivascular and interstitial fibroblasts (Figure 7G). Similar observations were noted in the aorta. The inducible PDGFRβ‐Cre driver targeted not only mural cells in the media and the adventitia but also a large fraction of aortic adventitial fibroblasts (Figure 8A through 8D). In contrast, the inducible NG2‐Cre driver specifically labeled mural cells (Figure 8E).
Is There an Optimal Tool for Labeling and Targeting Cardiac Pericytes in Mice?
Single‐cell transcriptomic studies indicate that no single transcript qualifies as a specific pan‐pericyte marker. 64 The differences in pericyte populations residing in various tissues and the distinct patterns of diversity of fibroblasts and other interstitial cell types in different organs suggest the need for organ‐specific approaches for tracing and targeting pericytes. Our study shows that myocardial mural cells are optimally labeled and targeted by using an inducible NG2‐Cre driver. In contrast, the inducible PDGFRβ‐Cre driver lacks specificity and may be useful only for manipulation of a broader population of interstitial cells, including a large fraction of fibroblasts.
Conclusions
Unfortunately, the field of myocardial biology has neglected the pericyte, a cell type of high potential significance in cardiac homeostasis and disease. Associative evidence suggests that pericytes may be involved in early myocardial ischemic injury, 26 may respond to myocardial mechanical stress 25 and neurohumoral stimulation, 9 and may be implicated in fibrosis of the remodeling heart. 65 , 66 Moreover, some studies have suggested that subpopulations of pericytes may have reparative, angiogenic, and even regenerative potential in models of myocardial injury. 67 , 68 Thus, experimental studies investigating the role of pericytes in mouse models of heart disease, using well‐characterized and validated tools, are critical for understanding cardiac pathophysiology.
Sources of Funding
Dr Frangogiannis’ laboratory is supported by National Institutes of Health grants R01 HL76246, R01 HL85440, and R01 HL149407 and by U.S. Department of Defense grants PR151029 and PR181464. Dr Tuleta is supported by a postdoctoral grant from the Deutsche Forschungsgemeinschaft (TU 632/1‐1).
Disclosures
None.
Supporting information
Figures S1–S7
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023171
For Sources of Funding and Disclosures, see page 15.
References
- 1. Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial‐mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- 2. Sweeney M, Foldes G. It takes two: endothelial‐perivascular cell cross‐talk in vascular development and disease. Front Cardiovasc Med. 2018;5:154. doi: 10.3389/fcvm.2018.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 4. Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol. 2012;44:1800–1812. doi: 10.1016/j.biocel.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9:165–170. doi: 10.1159/000052608 [DOI] [PubMed] [Google Scholar]
- 7. Hartmann DA, Berthiaume AA, Grant RI, Harrill SA, Koski T, Tieu T, McDowell KP, Faino AV, Kelly AL, Shih AY. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat Neurosci. 2021;24:633–645. doi: 10.1038/s41593-020-00793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzales AL, Klug NR, Moshkforoush A, Lee JC, Lee FK, Shui B, Tsoukias NM, Kotlikoff MI, Hill‐Eubanks D, Nelson MT. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc Natl Acad Sci USA. 2020;117:27022–27033. doi: 10.1073/pnas.1922755117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Špiranec Spes K, Chen W, Krebes L, Völker K, Abeßer M, Eder Negrin P, Cellini A, Nickel A, Nikolaev VO, Hofmann F, et al. Heart‐microcirculation connection: effects of ANP (Atrial Natriuretic Peptide) on pericytes participate in the acute and chronic regulation of arterial blood pressure. Hypertension. 2020;76:1637–1648. doi: 10.1161/HYPERTENSIONAHA.120.15772 [DOI] [PubMed] [Google Scholar]
- 10. Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood‐brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 11. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood‐brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Török O, Schreiner B, Schaffenrath J, Tsai HC, Maheshwari U, Stifter SA, Welsh C, Amorim A, Sridhar S, Utz SG, et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc Natl Acad Sci USA. 2021;118:e2016587118. doi: 10.1073/pnas.2016587118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pellowe AS, Sauler M, Hou Y, Merola J, Liu R, Calderon B, Lauridsen HM, Harris MR, Leng L, Zhang YI, et al. Endothelial cell‐secreted MIF reduces pericyte contractility and enhances neutrophil extravasation. FASEB J. 2019;33:2171–2186. doi: 10.1096/fj.201800480R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw I, Rider S, Mullins J, Hughes J, Peault B. Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol. 2018;14:521–534. doi: 10.1038/s41581-018-0032-4 [DOI] [PubMed] [Google Scholar]
- 15. Greenhalgh SN, Iredale JP, Henderson NC. Origins of fibrosis: pericytes take centre stage. F1000prime Reports. 2013;5:37. doi: 10.12703/P5-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol. 2014;34:374–383. doi: 10.1016/j.semnephrol.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garza Trevino EN, Gonzalez PD, Valencia Salgado CI, Martinez GA. Effects of pericytes and colon cancer stem cells in the tumor microenvironment. Cancer Cell Int. 2019;19:173. doi: 10.1186/s12935-019-0888-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paiva AE, Lousado L, Guerra DA, Azevedo PO, Sena IF, Andreotti JP, Santos GS, Goncalves R, Mintz A, Birbrair A. Pericytes in the premetastatic niche. Cancer Res. 2018;78:2779–2786. doi: 10.1158/0008-5472.CAN-17-3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Litviňuková M, Talavera‐López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee LL, Khakoo AY, Chintalgattu V. Cardiac pericytes function as key vasoactive cells to regulate homeostasis and disease. FEBS Open Bio. 2021;11:207–225. doi: 10.1002/2211-5463.13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avolio E, Madeddu P. Discovering cardiac pericyte biology: from physiopathological mechanisms to potential therapeutic applications in ischemic heart disease. Vascul Pharmacol. 2016;86:53–63. doi: 10.1016/j.vph.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 23. Alex L, Frangogiannis NG. Pericytes in the infarcted heart. Vasc Biol. 2019;1:H23–H31. doi: 10.1530/VB-19-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nees S, Weiss DR, Senftl A, Knott M, Forch S, Schnurr M, Weyrich P, Juchem G. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol. 2012;302:H69–H84. doi: 10.1152/ajpheart.00359.2011 [DOI] [PubMed] [Google Scholar]
- 25. Rolle IG, Crivellari I, Zanello A, Mazzega E, Dalla E, Bulfoni M, Avolio E, Battistella A, Lazzarino M, Cellot A, et al. Heart failure impairs the mechanotransduction properties of human cardiac pericytes. J Mol Cell Cardiol. 2021;151:15–30. doi: 10.1016/j.yjmcc.2020.10.016 [DOI] [PubMed] [Google Scholar]
- 26. Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen‐Gould L, et al. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J Exp Med. 2012;209:2291–2305. doi: 10.1084/jem.20111749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Farrell FM, Attwell D. A role for pericytes in coronary no‐reflow. Nat Rev Cardiol. 2014;11:427–432. doi: 10.1038/nrcardio.2014.58 [DOI] [PubMed] [Google Scholar]
- 28. O'Farrell FM, Mastitskaya S, Hammond‐Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no‐reflow after myocardial ischaemia. Elife. 2017;6. doi: 10.7554/eLife.29280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chintalgattu V, Rees ML, Culver JC, Goel A, Jiffar T, Zhang J, Dunner K, Pati S, Bankson JA, Pasqualini R, et al. Coronary microvascular pericytes are the cellular target of sunitinib malate‐induced cardiotoxicity. Sci Transl Med. 2013;5:187ra169. doi: 10.1126/scitranslmed.3005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tallquist MD. Cardiac fibroblast diversity. Annu Rev Physiol. 2020;82:63–78. doi: 10.1146/annurev-physiol-021119-034527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winkler EA, Bell RD, Zlokovic BV. Pericyte‐specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cuervo H, Pereira B, Nadeem T, Lin M, Lee F, Kitajewski J, Lin CS. PDGFRbeta‐P2A‐CreER(T2) mice: a genetic tool to target pericytes in angiogenesis. Angiogenesis. 2017;20:655–662. doi: 10.1007/s10456-017-9570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Špiranec K, Chen W, Werner F, Nikolaev VO, Naruke T, Koch F, Werner A, Eder‐Negrin P, Diéguez‐Hurtado R, Adams RH, et al. Endothelial C‐type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation. 2018;138:494–508. doi: 10.1161/CIRCULATIONAHA.117.033383 [DOI] [PubMed] [Google Scholar]
- 34. Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sciurba JC, Gieseck RL, Jiwrajka N, White SD, Karmele EP, Redes J, Vannella KM, Henderson NC, Wynn TA, Hart KM. Fibroblast‐specific integrin‐alpha V differentially regulates type 17 and type 2 driven inflammation and fibrosis. J Pathol. 2019;248:16–29. doi: 10.1002/path.5215 [DOI] [PubMed] [Google Scholar]
- 36. Stallcup WB. The NG2 proteoglycan in pericyte biology. Adv Exp Med Biol. 2018;1109:5–19. doi: 10.1007/978-3-030-02601-1_2 [DOI] [PubMed] [Google Scholar]
- 37. Ivey MJ, Kuwabara JT, Riggsbee KL, Tallquist MD. Platelet derived growth factor receptor alpha is essential for cardiac fibroblast survival. Am J Physiol Heart Circ Physiol. 2019;317:H330–H344. doi: 10.1152/ajpheart.00054.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial‐derived cell epithelial‐to‐mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forbes MS, Rennels ML, Nelson E. Ultrastructure of pericytes in mouse heart. Am J Anat. 1977;149:47–70. doi: 10.1002/aja.1001490105 [DOI] [PubMed] [Google Scholar]
- 40. Joyce NC, Haire MF, Palade GE. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol. 1985;100:1379–1386. doi: 10.1083/jcb.100.5.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Popescu LM, Curici A, Wang E, Zhang H, Hu S, Gherghiceanu M. Telocytes and putative stem cells in ageing human heart. J Cell Mol Med. 2015;19:31–45. doi: 10.1111/jcmm.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Di Carlo SE, Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest. 2018;128:54–63. doi: 10.1172/JCI93558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsia LT, Ashley N, Ouaret D, Wang LM, Wilding J, Bodmer WF. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc Natl Acad Sci USA. 2016;113:E2162–E2171. doi: 10.1073/pnas.1603534113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub‐Lis K, Ho JW, Nordon RE, Harvey RP. Single‐cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife. 2019;8:e43882. doi: 10.7554/eLife.43882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McLellan MA, Skelly DA, Dona MSI, Squiers GT, Farrugia GE, Gaynor TL, Cohen CD, Pandey R, Diep H, Vinh A, et al. High‐resolution transcriptomic profiling of the heart during chronic stress reveals cellular drivers of cardiac fibrosis and hypertrophy. Circulation. 2020;142:1448–1463. doi: 10.1161/CIRCULATIONAHA.119.045115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang LI, Yang Y, Ma H, Xie Y, Xu J, Near D, Wang H, Garbutt T, Li Y, Liu J, et al. Single cell dual‐omics reveals the transcriptomic and epigenomic diversity of cardiac non‐myocytes. Cardiovasc Res. 2021. doi: 10.1093/cvr/cvab134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, Lebouvier T, Ando K, Hofmann J, Keller A, Betsholtz C. Analysis of the brain mural cell transcriptome. Sci Rep. 2016;6:35108. doi: 10.1038/srep35108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamazaki T, Mukouyama YS. Tissue specific origin, development, and pathological perspectives of pericytes. Front Cardiovasc Med. 2018;5:78. doi: 10.3389/fcvm.2018.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hartmann DA, Underly RG, Watson AN, Shih AY. A murine toolbox for imaging the neurovascular unit. Microcirculation. 2015;22:168–182. doi: 10.1111/micc.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen WC, Baily JE, Corselli M, Diaz ME, Sun B, Xiang G, Gray GA, Huard J, Peault B. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015;33:557–573. doi: 10.1002/stem.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Z, Xu Q, Zhang N, Du X, Xu G, Yan X. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Signal Transduct Target Ther. 2020;5:148. doi: 10.1038/s41392-020-00259-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–79. doi: 10.1177/002215540205000108 [DOI] [PubMed] [Google Scholar]
- 54. Anfosso F, Bardin N, Francès V, Vivier E, Camoin‐Jau L, Sampol J, Dignat‐George F. Activation of human endothelial cells via S‐Endo‐1 antigen (CD146) stimulates the tyrosine phosphorylation of focal adhesion kinase p125(FAK). J Biol Chem. 1998;273:26852–26856. doi: 10.1074/jbc.273.41.26852 [DOI] [PubMed] [Google Scholar]
- 55. Barron L, Gharib SA, Duffield JS. Lung pericytes and resident fibroblasts: busy multitaskers. Am J Pathol. 2016;186:2519–2531. doi: 10.1016/j.ajpath.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alarcon‐Martinez L, Yilmaz‐Ozcan S, Yemisci M, Schallek J, Kilic K, Can A, Di Polo A, Dalkara T. Capillary pericytes express alpha‐smooth muscle actin, which requires prevention of filamentous‐actin depolymerization for detection. Elife. 2018 Mar 21;7:e34861. doi: 10.7554/eLife.34861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verbeek MM, Otte‐Holler I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha‐smooth muscle actin expression in cultured human brain pericytes by transforming growth factor‐beta 1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- 58. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor‐beta 1 induces alpha‐smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 60. Zhao G, Joca HC, Lederer WJ. Dynamic measurement and imaging of capillaries, arterioles, and pericytes in mouse heart. J Vis Exp. 2020. doi: 10.3791/61566 [DOI] [PubMed] [Google Scholar]
- 61. Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10:67–84. doi: 10.1016/j.scr.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type‐1 pericytes accumulate after tissue injury and produce collagen in an organ‐dependent manner. Stem Cell Res Ther. 2014;5:122. doi: 10.1186/scrt512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Horiuchi K, Kano K, Minoshima A, Hayasaka T, Yamauchi A, Tatsukawa T, Matsuo R, Yoshida Y, Tomita Y, Kabara M, et al. Pericyte‐specific deletion of ninjurin‐1 induces fragile vasa vasorum formation and enhances intimal hyperplasia of injured vasculature. Am J Physiol Heart Circ Physiol. 2021;320:H2438–H2447. doi: 10.1152/ajpheart.00931.2020 [DOI] [PubMed] [Google Scholar]
- 64. Muhl L, Genové G, Leptidis S, Liu J, He L, Mocci G, Sun Y, Gustafsson S, Buyandelger B, Chivukula IV, et al. Single‐cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun. 2020;11:3953. doi: 10.1038/s41467-020-17740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Su H, Zeng H, Liu B, Chen JX. Sirtuin 3 is essential for hypertension‐induced cardiac fibrosis via mediating pericyte transition. J Cell Mol Med. 2020;24:8057–8068. doi: 10.1111/jcmm.15437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury‐induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alvino VV, Fernández‐Jiménez R, Rodriguez‐Arabaolaza I, Slater S, Mangialardi G, Avolio E, Spencer H, Culliford L, Hassan S, Sueiro Ballesteros L, et al. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction. J Am Heart Assoc. 2018;7:e006727. doi: 10.1161/JAHA.117.006727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez‐Arabaolaza I, Dang Z, Mitchell K, et al. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116:e81–e94. doi: 10.1161/CIRCRESAHA.115.306146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S7


