Abstract
Background
Systemic inflammation and male hypogonadism are 2 increasingly recognized “nonconventional” risk factors for long‐QT syndrome and torsades de pointes (TdP). Specifically, inflammatory cytokines prolong, while testosterone shortens the heart rate–corrected QT interval (QTc) via direct electrophysiological effects on cardiomyocytes. Moreover, several studies demonstrated important interplays between inflammation and reduced gonad function in men. We hypothesized that, during inflammatory activation in men, testosterone levels decrease and that this enhances TdP risk by contributing to the overall prolonging effect of inflammation on QTc.
Methods and Results
We investigated (1) the levels of sex hormones and their relationship with inflammatory markers and QTc in male patients with different types of inflammatory diseases, during active phase and recovery; and (2) the association between inflammatory markers and sex hormones in a cohort of male patients who developed extreme QTc prolongation and TdP, consecutively collected over 10 years. In men with active inflammatory diseases, testosterone levels were significantly reduced, but promptly normalized in association with the decrease in C‐reactive protein and interleukin‐6 levels. Reduction of testosterone levels, which also inversely correlated with 17‐β estradiol over time, significantly contributed to inflammation‐induced QTc prolongation. In men with TdP, both active systemic inflammation and hypogonadism were frequently present, with significant correlations between C‐reactive protein, testosterone, and 17‐β estradiol levels; in these patients, increased C‐reactive protein and reduced testosterone were associated with a worse short‐term outcome of the arrhythmia.
Conclusions
During systemic inflammatory activation, interleukin‐6 elevation is associated with reduced testosterone levels in males, possibly deriving from an enhanced androgen‐to‐estrogen conversion. While transient, inflammatory hypotestosteronemia is significantly associated with an increased long‐QT syndrome/TdP risk in men.
Keywords: interleukin‐6, QTc interval, sudden cardiac arrest, systemic inflammation, testosterone, torsades de pointes
Subject Categories: Arrhythmias, Sudden Cardiac Death, Biomarkers, Inflammation
Nonstandard Abbreviations and Acronyms
- LQTS
long‐QT syndrome
- QTc
heart rate‐corrected QT interval
- SHBG
sex hormone–binding globulin
- TdP
torsades de pointes
Clinical Perspective
What Is New?
In male patients with active inflammatory diseases, testosterone levels were significantly reduced, but normalized within days in association with C‐reactive protein and interleukin‐6 decrease. Reduction of testosterone levels, which also inversely correlated with 17‐β estradiol over time, significantly contributed to mediate inflammation‐associated heart‐rate corrected QT prolongation.
In men with torsades de pointes, both active systemic inflammation and hypogonadism were frequently present, with significant correlations between C‐reactive protein, testosterone and 17‐β estradiol levels. In these patients, increased C‐reactive protein and reduced testosterone tended to be associated with worse arrhythmia outcomes.
What Are the Clinical Implications?
During systemic inflammatory activation, interleukin‐6 elevation correlates with reduced testosterone levels in males, possibly deriving from an enhanced androgen‐to‐estrogen conversion. While transient, inflammatory hypotestosteronemia significantly associates with heart rate–corrected QT interval prolongation and torsades de pointes risk.
In patients with inflammatory conditions, hypotestosteronemia and heart rate–corrected QT interval prolongation, a prompt and effective control of the underlying inflammatory disease might be a crucial measure to lower arrhythmic risk.
Administration of interleukin‐6 blocking drugs and testosterone replacement therapy could represent additional antiarrhythmic interventions in the short term, particularly in patients with torsades de pointes refractory to conventional treatments.
The heart rate–corrected QT interval (QTc), a surrogate of the average action potential duration in ventricular myocytes, is widely used in the clinical practice as an important marker of arrhythmic risk. 1 , 2 In fact, whenever QTc prolongs over 470 ms in men or 480 ms in women, a condition designated as long‐QT syndrome (LQTS), the vulnerability to malignant ventricular arrhythmias, particularly torsades de pointes (TdP), progressively increases (QTc >500 ms, high risk; QTc >600 ms, very high risk). 2 TdP is a polymorphic ventricular tachycardia characterized by a pattern of twisting points, which specifically develops in patients with LQTS, and that can degenerate in ventricular fibrillation (VF) and sudden cardiac death. 3 LQTS is traditionally classified as congenital, attributable to inherited mutations in genes encoding for cardiac ion channels and regulatory proteins, or acquired. 4 , 5 While congenital LQTS is relatively rare, 6 acquired LQTS is common 7 and recognizes many possible causes, primarily medications blocking the human ether‐à‐go‐go potassium channel, and electrolyte imbalances (hypokalemia, hypocalcemia, and hypomagnesemia), 2 , 5 but also structural heart diseases, bradyarrhythmias, endocrine and liver diseases, nervous system injuries, starvation, hypothermia, and toxins. 5 , 8 All these factors have in common the aptitude to induce the dysfunction of ≥1 of the channels regulating the action potential duration, leading to an inward shift in the balance of the total currents. However, all of the above “conventional” risk factors cannot explain all cases of LQTS/TdP development and recurrence observed in clinical practice. 2
In this scenario, an increasing number of “nonconventional” risk factors for LQTS/TdP are recently emerging, 9 , 10 , 11 , 12 among which systemic inflammation 13 and male hypogonadism 14 represent 2 of the most recognized. A large body of clinical and experimental evidence has demonstrated that inflammatory activation can prolong action potential duration/QTc 15 , 16 , 17 and increase TdP risk 15 , 17 via direct modulatory effects of interleukin‐6 and other cytokines on several cardiac ion currents, principally the rapid and the slow components of the delayed rectifier potassium current, the transient outward current, and the L‐type calcium current (inflammatory cardiac channelopathies). 13 , 16 , 18 , 19 , 20 , 21 Accumulating evidence also supports an important role for male hypogonadism in promoting LQTS/TdP, 22 , 23 , 24 , 25 mainly explained by the removal of the physiological action potential duration shortening effect of testosterone on the ventricular cardiomyocyte. 26 In fact, it is well demonstrated that testosterone can decrease QTc by both increasing the rapid and slow components of the repolarizing delayed rectifier potassium current, and decreasing the depolarizing L‐type calcium current. 14 Accordingly, it has long been known that women normally have a longer QTc than men and are at a greater risk of developing TdP. 2
In addition, several studies provided evidence for a direct interplay between inflammation and depressed gonad function in males. 27 Testosterone levels are frequently reduced in men with chronic inflammatory diseases, 28 and already in 1989 Rivier and Vale 29 had demonstrated how hypogonadism could be induced by injecting proinflammatory cytokines in rats. Consistent findings have been also recently reported in men with severe COVID‐19, 30 an acute inflammatory disease characterized by high‐level circulating cytokines along with an increased prevalence of QTc prolongation and life‐threatening ventricular arrhythmias. 31 , 32
Several mechanisms may account for these changes, both central on gonadotropin secretion and peripheral on androgen‐to‐oestrogen conversion. 28 In fact, inflammatory cytokines, such as interleukin‐6, interleukin‐1, and tumor necrosis factor‐α, can directly inhibit gonadotropin‐releasing hormone secretion in the hypothalamus, and luteinizing hormone (LH) release from anterior pituitary, 33 as well as stimulate in adipose stromal cells the enzyme aromatase, which catalyzes the biotransformation of testosterone to estradiol. 34 , 35
Based on this background, it is likely that, during inflammatory activation in men, testosterone levels decrease and that this contributes to the overall prolonging effect of inflammation on QTc. Thus, the aim of the present study was to analyze the acute impact of systemic inflammation on circulating levels of testosterone and other sex hormones in men, also defining whether these alterations play a role in increasing the risk of LQTS/TdP. To address this objective, we investigated (1) the levels of sex hormones and their relationship with inflammatory markers and QTc duration in male patients with different types of acute inflammatory diseases, during the active phase and recovery; and (2) the association between inflammatory markers and sex hormones in a cohort of male patients who developed extreme QTc prolongation and TdP, consecutively collected from the general population over 10 years.
METHODS
The authors declare that all supporting data are available within the article and its online supplemental files.
A local ethics committee approved the study, and patients from all groups gave their oral and written informed consent in accordance with the Principles of the Declaration of Helsinki.
Study Populations
To evaluate the influence of systemic inflammatory activation on sex hormones in men and their relationship with QTc, we prospectively collected 22 men with elevated CRP (C‐reactive protein) levels attributable to different inflammatory diseases, including acute infections, chronic immune‐mediated diseases during flares, or acute microcrystalline arthritis (inflammatory cohort). These subjects underwent blood sample withdrawals and ECG recordings during the active phase (PRE), and after different pharmacological treatments resulting in a CRP decrease >75% when compared with baseline conditions (POST). None of the patients in this cohort was under treatment with drugs potentially influencing circulating levels of sex hormones, specifically androgen‐deprivation therapy (including gonadotropin‐releasing hormone receptor agonists/antagonists, cytochrome‐17 inhibitors, nonsteroidal androgen‐receptor antagonists) or opioids. 36 Moreover, we excluded subjects presenting with or developing electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia) or atrial fibrillation/flutter (needing more complicate methods for QT interval correction). Patients taking QT interval–prolonging medications with conditional, possible, or known risk of TdP (www.crediblemeds.org) were excluded only if they were treated with these drugs in PRE, but not in POST conditions, that is, those withdrawing liable drugs between PRE and POST (but keeping patients maintaining the same liable drug between PRE and POST). Detailed demography and clinical features of the inflammatory cohort are reported in Table S1.
A second cohort of 10 men, comparable in terms of age and comorbidities (comorbidity controls) but without signs of systemic inflammation, was enrolled as a confirmatory group to further compare laboratory and ECG findings of the inflammatory cohort, in both PRE and POST conditions (demography, clinical, and laboratory details depicted in Table S1).
Finally, male subjects belonging to a cohort of 75 consecutive TdP patients, prospectively collected in our institution in the period 2008 to 2020 independent of ongoing therapies and concomitant diseases, were also studied to analyze the putative relationship existing between sex hormones and inflammatory activation. During this 12‐year period, a total of 30 men with TdP came to our observation. However, 7 were excluded for ongoing treatments with drugs potentially affecting sex hormones (androgen‐deprivation therapy for prostatic cancer, n=5; methadone maintenance therapy, n=2). Moreover, in 4 additional subjects, a blood sample obtained within 24 hours from TdP to perform laboratory investigations was not available. As a result, 19 male patients with TdP were included in the study (TdP cohort), whose demographic, clinical, and laboratory characteristics are reported in Table S2. Notably, inflammatory cohort patients and comorbidity controls were selected to be comparable in terms of age with these 19 patients with TdP (TdP cohort: 76 [45–91] years; inflammatory cohort: 79 [36–93] years; comorbidity controls: 78.5 [67–85] years).
Laboratory Analysis
All subjects under study underwent a blood withdrawal in the morning (before 11:00 am) to measure circulating levels of inflammatory markers and sex hormones. Specifically, in patients with TdP, the blood sample was obtained within 24 hours of arrhythmia occurrence. In the other subjects (inflammatory cohort and controls), blood draws and ECG recordings were simultaneous. Measurements of CRP and cytokines (including interleukin‐6, interleukin‐1, interleukin‐10, and tumor necrosis factor‐α) are detailed in Data S1. The levels of the following sex hormones and related proteins were assessed: testosterone (total, free, and bioavailable), sex hormone–binding globulin (SHBG), 17β‐estradiol, progesterone, follicle‐stimulating hormone (FSH), and LH. All measurements were performed by an automatic chemiluminescent immunoassay system. Testosterone (total), LH, and FSH were measured by UniCel DxI 800 (Beckman Coulter), while SHBG, dehydroepiandrosterone sulfate, androstenedione, progesterone, and 17‐β estradiol levels by Immulite 2000 (Siemens). Following the current guidelines of the European Society of Endocrinology/European Academy of Andrology, 37 free and bioavailable testosterone were calculated according to a standard formula (available at http://www.issam.ch/freetesto.htm) based on SHBG and total testosterone concentration, considering albumin as a constant (average albumin concentration of 4.3 g/dL). This parameter more accurately reflects the level of bioactive testosterone than does the sole measurement of total serum testosterone. Testosterone circulates in plasma unbound (free, ≈2%–3%), bound to specific plasma proteins (SHBG), and weakly bound to nonspecific proteins such as albumin. The SHBG‐bound fraction is biologically inactive, because of the high binding affinity of SHBG for testosterone. Free testosterone measures the free fraction; bioavailable testosterone includes free plus weakly bound to albumin.
ECG Recordings
Measurements of heart rate, RR interval, QT interval, and QTc (Bazett’s formula) in patients with inflammatory diseases and controls was automated and obtained from 3 ECG strips consecutively recorded. A single investigator (cardiologist) blinded to the clinical and laboratory findings of the patients reviewed all ECGs to validate the measured intervals. Since the Bazett’s formula may over‐ or underestimate QTc at higher and lower heart rates, respectively, we additionally evaluated QTc using alternative correction formulas, ie Fridericia [QT/RR interval1/3], and Hodges [QT+1.75 (heart rate−60)], the latter being recognized as the correction formula showing the least heart rate dependence. 38 Since all the subjects in the study were men, QTc was considered prolonged if >470 ms, in accordance with the American Heart Association/American College of Cardiology recommendations. 2
Diagnosis of TdP was based on the presence of at least 1 episode of polymorphic ventricular arrhythmia with a rate ranging from 160 to 240 beats/min, associated with QTc prolongation. 9 In patients with TdP, the QT interval was manually measured on a standard 12‐lead ECG. 39
The details of QTc measurement in different groups are provided in Data S1.
Statistical Analysis
Descriptive statistics is reported as frequency count and percentage for qualitative data, and mean ± standard deviation (SD) or median and interquartile range for quantitative data.
We first performed a sample size and power analysis (more details are provided in Data S1) to define the number of subjects to include in the inflammatory cohort for comparison in PRE versus POST conditions, as well as in the control group. Specifically, by using a nonparametric approach based on the 2‐sided Wilcoxon test, and considering α=0.05, 1−β=0.85, and an effect size of 0.7, a sample size of 22 patients was obtained. Based on this first sample, a further power analysis was then performed to select the size of the control group. In this case, by considering the use of a 2‐sided Mann‐Whitney test, with α=0.05, 1−β=0.80, effect size=1.1, and a ratio between the size of groups of about 2:1, a control group size of 10 patients was calculated.
Because of sample size in each group and data distribution, the following nonparametric statistical analyses were carried out: the 2‐tailed Wilcoxon test or the 2‐tailed Mann‐Whitney test to evaluate differences in quantitative variables between 2 groups of data paired (inflammatory markers, sex hormones, and ECG parameters in patients with inflammatory disease, PRE versus POST) or unpaired (comparisons of sex hormones in inflammatory disease or patients with TdP versus controls; testosterone and 17‐β estradiol in patients with TdP with CRP >2 mg/dL versus CRP <2 mg/dL), respectively; the Spearman rank correlation to verify possible statistical association between quantitative variables in patients with inflammatory disease (sex hormones versus CRP, interleukin ‐6, or QTc; testosterone versus 17‐β estradiol; interleukin ‐6 versus QTc) or TdP (sex hormones versus CRP; testosterone versus 17‐β estradiol); and the 2‐sided McNemar test to evaluate statistical association between categorical variables in patients with inflammatory diseases (QTc prolongation, PRE versus POST). The Bonferroni correction for multiple tests was applied when patients with inflammatory diseases in PRE/POST conditions were compared with controls. Moreover, the Kruskal‐Wallis test, and Dunn multiple comparison post hoc test, were performed to evaluate differences in quantitative variables (age, total testosterone) among 3 groups (patients with inflammatory disease, patients with TdP, controls; patients with inflammatory disease, PRE, patients with TdP with CRP >2 mg/dL, controls; patients with inflammatory disease, POST, patients with TdP with CRP <2 mg/dL, controls). 17‐β estradiol assay had a detection limit of 20 pg/mL. Statistical handling of values <20 pg/mL is detailed in Data S1.
Finally, mediation analysis (R, version 4.0.1; R Foundation for Statistical Computing) was performed to evaluate the role of testosterone as mediator of part of the effects of interleukin‐6 on QTc. Three linear regressions were performed: one with QTc as outcome and interleukin‐6 as the independent variable to estimate the effect a; one with the addition of testosterone to estimate a′ and c; and one with testosterone as outcome and IL‐6 as independent variable (b). The resultant mediating effect of testosterone on the relationship between interleukin‐6 and QTc (a″) was tested using a mediation model by means of a bootstrap method.
P≤0.05 were considered significant (InStat, version 3.06 for Windows 2000; GraphPad Software).
RESULTS
Levels of Sex Hormones in Male Patients With Inflammatory Diseases and Relationship With Inflammatory Markers
In male patients with active inflammatory diseases, total testosterone levels were significantly lower than in noninflamed comorbidity controls (Table 1; Table S2; Figure S1A), with a significant proportion of inflamed patients (11/22; 50%) showing levels ≤1.1 ng/mL, that is, falling into the female range according to our internal laboratory reference values. Free and bioavailable testosterone levels also showed a significant reduction in these subjects when compared with controls (Table 1; Table S1; Figure S1B and S1C). Circulating 17‐β estradiol was conversely increased, and in almost a quarter of cases (5/22; 23%) they reached values ≥100 pg/mL, as normally observed in premenopausal women only 40 (Table 1; Table S1; Figure S1D). The other sex hormones evaluated, including progesterone, FSH, and LH, as well as SHBG, did not show any significant difference when inflamed patients were compared with controls (all P>0.05; 2‐tailed Mann‐Whitney test) (Table 1; Table S1).
Table 1.
Laboratory, Electrocardiographic, and Echocardiography Parameters in Male Patients With and Without Inflammation
| Parameter | Male patients with inflammatory diseases (n=22) | Male patients without inflammation (controls) (n=10) | ||
|---|---|---|---|---|
| Before therapeutic intervention (PRE) | After therapeutic intervention (POST) | P value | ||
| CRP, mg/dL (r.v. <0.5) | 10.9 (7.9–23.2) | 1.6 (0.4–2.5) | <0.0001* | 0.18 (0.1–0.4) |
| Interleukin‐6, pg/mL (r.v. 0.49–1.25) | 14.0 (10.1–53.2) | 2.8 (0.8–12.4) | <0.0001* | 0.3 (0.1–7.2) |
| Interleukin‐1, pg/mL (r.v. 0.08–0.29) | 0.27 (0.2–0.4) | 0.26 (0.2–0.4) | 0.30 | 0.05 (0.0–0.1) |
| Tumor necrosis factor‐α, pg/mL (r.v. 0.60–3.24) | 0.75 (0.6–0.8) | 0.75 (0.6–0.9) | 0.68 | 0.42 (0.4–0.8) |
| Interleukin‐10, pg/mL (r.v. 0–3.60) | 0.56 (0.5–0.8) | 0.55 (0.4–1.6) | 0.59 | 0.46 (0.4–0.6) |
| Total testosterone, ng/mL | 1.10 (0.7–1.5) | 1.90 (1.5–2.6) | <0.001* | 2.22 (1.4–3.3) |
| Free testosterone, ng/mL | 0.021 (0.009–0.027) | 0.031 (0.019–0.043) | <0.001* | 0.044 (0.024–0.05) |
| Bioavailable testosterone, ng/mL | 0.50 (0.2–0.6) | 0.76 (0.5–1.0) | <0.001* | 1.02 (0.6–1.2) |
| SHBG, nmol/L | 56.8 (23.1–48.3) | 40.2 (30.0–56.2) | 0.46 | 39.8 (34.4–53.5) |
| 17‐β estradiol, pg/mL | 37.9 (10.8–103.4) | 15.5 (10.0–39.8) | 0.0052* | 16.7 (10.0–27.2) |
| Progesterone, ng/mL | 0.10 (0.1–0.1) | 0.10 (0.1–0.3) | 0.46 | 0.10 (0.1–0.2) |
| FSH, mIU/mL | 11.0 (4.8–19.0) | 10.2 (5.1–22.5) | 0.23 | 6.8 (4.9–14.6) |
| LH, mIU/mL | 6.9 (0.5–28.0) | 6.9 (1.9–26.7) | 0.54 | 4.0 (1.0–21.0) |
| QT, ms | 394 (361–423) | 398 (370–420) | 0.78 | 406 (368–421) |
| RR, ms | 758 (655–840) | 833 (731–992) | <0.001* | 995 (868–1074) |
| Heart rate, bpm | 79.0 (71.8–91.8) | 72.5 (60.0–82.0) | <0.001* | 60.6 (56.3–71.1) |
| QTc‐Bazett, ms | 472.0 (436–499) | 444.5 (426–452) | <0.001* | 417.0 (397–423) |
| Patients with prolonged QTc ‡ , n (%) | 12 (55) | 2 (9) | 0.004* | 0 (0) |
| Patients with QTc >500 ms, n (%) | 5 (23) | 1 (5) | 0.13 | 0 (0) |
| QTc‐Fridericia, ms | 436.5 (417–474) | 423.5 (407–443) | 0.0033* | 408.0 (386–422) |
| Patients with prolonged QTc ‡ , n (%) | 6 (27) | 1 (5) | 0.09 | 0 (0) |
| Patients with QTc >500 ms, n (%) | 2 (9) | 1 (5) | 1.0 | 0 (0) |
| QTc‐Hodges, ms | 436.5 (413–466) | 420.5 (406–439) | 0.0037* | 411.0 (384–422) |
| Patients with prolonged QTc * , n (%) | 5 (23) | 1 (5) | 0.13 | 0 (0) |
| Patients with QTc >500 ms, n (%) | 1 (5) | 1 (5) | 1.0 | 0 (0) |
Cytokine level ranges measured in an internal reference group of healthy controls. Therapeutic interventions resulted in a >75% CRP decrease when compared with the baseline. Data are expressed as median (interquartile range) or frequency (percentage). Differences in continue variables were evaluated by the 2‐tailed Wilcoxon matched pairs test. Difference in categorical variables were evaluated by McNemar test. CRP indicates C‐reactive protein; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; QTc, heart rate–corrected QT interval; r.v., reference values; and SHBG, sex hormone–binding globulin.
Statistically‐significant p‐values (<0.05).
QTc >470 ms.
Depending on the specific inflammatory disease present, treatment with antibiotics or anti‐inflammatory drugs was associated with a prompt (mean follow‐up time 14.7±18.1 days, median 10.0 [2–90] days) and marked decrease of CRP (median decrease, 85.3%), despite no complete return to control levels. In POST conditions, testosterone levels increased (total, free, bioavailable), while 17‐β estradiol decreased, in all cases reaching values not significantly different from controls (Figure 1A through 1D; Table 1; Table S1; Figure S1). In POST conditions, only 18% (4/22) and 5% (1/22) of the patients showed total testosterone levels ≤1.1 ng/mL, and 17‐β estradiol levels ≥100 pg/mL, respectively. A strong inverse correlation between testosterone and CRP levels was observed over time (Figure 2A through 2C). Moreover, total testosterone also inversely correlated with 17‐β estradiol concentration (Figure S2A). No significant variations of SHBG, progesterone, FSH, and LH levels were observed when PRE and POST conditions were compared (Table 1). In particular, it is important to highlight the absence of significant modifications in gonadotropin concentrations despite significant variations in testosterone levels.
Figure 1. Testosterone and 17‐β estradiol levels in male patients with inflammatory diseases, during active disease (PRE) and after therapeutic interventions resulting in a C‐reactive protein decrease >75% (POST) when compared with the baseline.
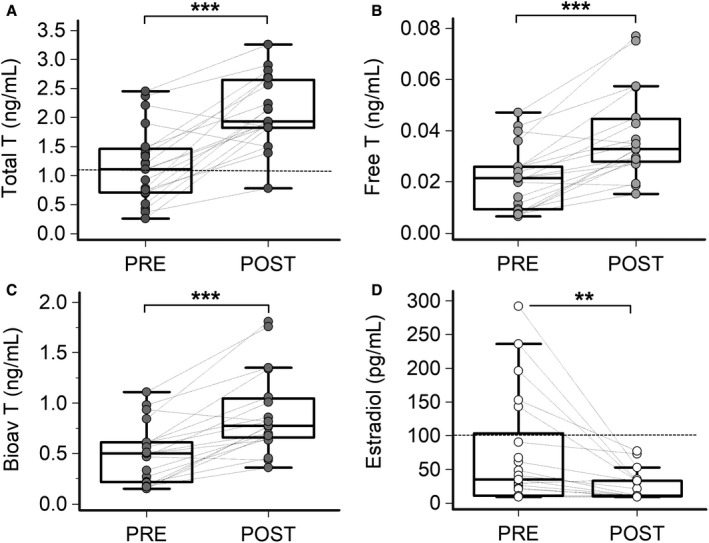
A, Total testosterone; 2‐tailed Wilcoxon matched‐pairs test, ***P<0.001. Values below the horizontal dotted line indicate a female range (upper limit of reference values in premenopausal women, ie, 1.1 ng/mL). B, Free testosterone; 2‐tailed Wilcoxon matched‐pairs test, ***P<0.001. C, Bioavailable testosterone; 2‐tailed Wilcoxon matched‐pairs test, ***P<0.001. D, 17‐β estradiol; 2‐tailed Wilcoxon matched‐pairs test, **P<0.01. Values above the horizontal dotted line indicate a female range (lower limit of reference values in premenopausal women, ie, 100 pg/mL). Patients, n=22. Bioav indicates bioavailable; Estradiol, 17‐β estradiol; and T, testosterone.
Figure 2. Correlation between testosterone levels and inflammatory markers in male patients with inflammatory diseases.
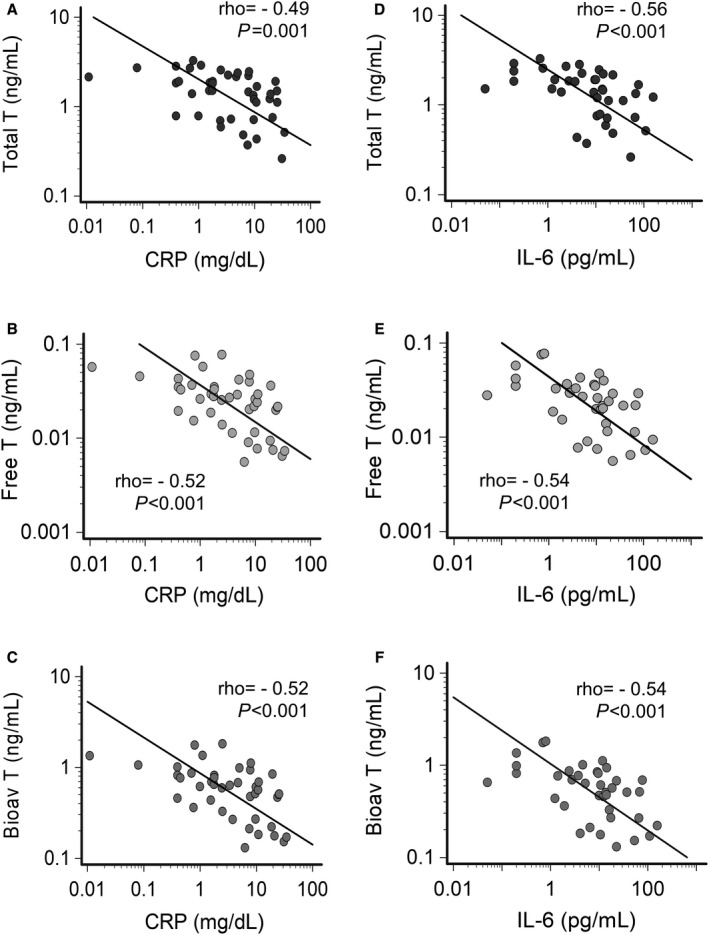
A, Relationship between total testosterone and CRP levels. B, Relationship between free testosterone and CRP levels. C, Relationship between bioavailable testosterone and CRP levels. D, Relationship between total testosterone and IL‐6 levels. E, Relationship between free testosterone and IL‐6 levels. F, Relationship between bioavailable testosterone and IL‐6 levels. Spearman test. Patients, n=22. Bioav indicates bioavailable; CRP, C‐reactive protein; IL‐6, interleukin‐6; and T, testosterone.
Circulating inflammatory cytokine concentration, specifically interleukin‐6, was elevated during active disease, and significantly decreased after treatment to near normal values (no complete return to control levels) (Table 1). Levels of interleukin‐6 inversely correlated over time with those of testosterone, even more strongly that observed for CRP (Figure 2D through 2F), as well as directly with those of 17‐β estradiol (Figure S2B). Interleukin‐1, tumor necrosis factor‐α, and interleukin‐10 were all within normal reference values in the PRE condition, and did not show any noticeable modification after therapeutic interventions (Table 1).
QTc in Male Patients With Inflammatory Diseases and Relationship With Testosterone and Interleukin‐6 Levels
In patients with active inflammatory diseases, median values of QTc were 472.0 (416–544), that is, over the currently recognized limit for QTc prolongation in men (470 ms). Accordingly, in PRE conditions, QTc prolongation was a common finding, with almost a quarter of the subjects even showing marked QTc prolongation (>500 ms) (Table 1).
Systemic inflammation recovery attributable to therapeutic interventions was associated with a significant reduction of median QTc duration, and QTc >470 ms prevalence (Table 1), inversely correlating with testosterone levels over time (Figure 3A through 3C). Other laboratory parameters, including electrolytes, renal function, or echocardiography findings, were normal at baseline and did not show significant changes throughout the study period (Table S3). Because heart rate was higher in our patients in PRE versus POST conditions because of inflammation‐induced sympathetic activation, 18 , 41 , 42 we repeated the same analysis with 2 alternative QT‐correction formulas (ie, Fridericia, and Hodges) to exclude that a QTc overestimation by Bazett’s formula at higher heart rates could have biased the results. Again, QTc‐Fridericia and QTc‐Hodges significantly reduced after treatment and maintained a significant inverse association with testosterone levels (Table 1; Figure S3A through S3F). No association was present between QTc and other sex hormones, including gonadotropins, regardless of the specific formula used (Table S4).
Figure 3. Correlation between QTc interval and testosterone levels in male patients with inflammatory diseases.
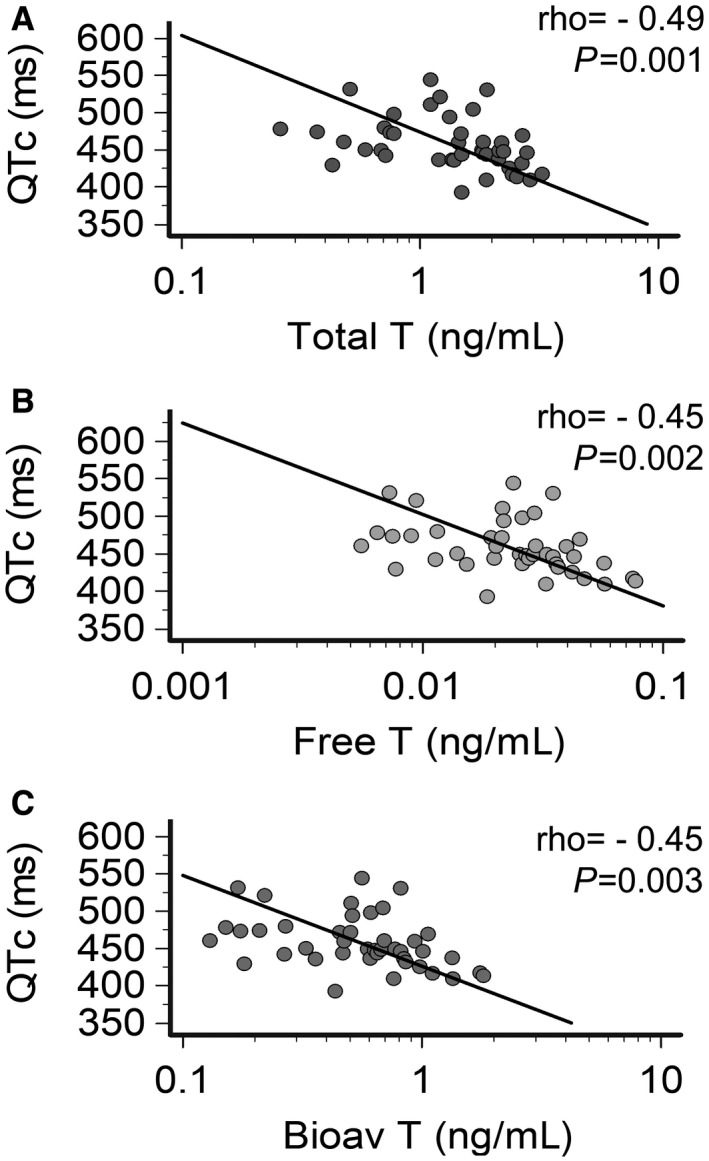
A, Relationship between QTc and total testosterone levels. B, Relationship between QTc and free testosterone levels. C, Relationship between QTc and bioavailable testosterone levels. Spearman test. Patients, n=22. Bioav indicates bioavailable; QTc, heart rate–corrected QT interval based on Bazett’s formula; and T, testosterone.
At the same time, we found a robust direct correlation over time between QTc duration and interleukin‐6 levels (Figure 4A), again persisting also when QTc‐Fridericia or QTcHodges were alternatively considered (Figure S4). The strength of the association existing between QTc and interleukin‐6 was even stronger than that observed between QTc and testosterone levels (QTc‐Bazett/interleukin‐6: ρ=0.72 versus QTc‐Bazett/total testosterone: ρ=−0.49), thereby suggesting that testosterone lowering may represent, as anticipated, only 1 of the mechanisms by which inflammation, via interleukin‐6 increase, can promote QTc prolongation. To provide support to this premise, a mediation analysis was performed. The total effect of interleukin‐6 on QTc (a=0.66; P<0.001) was composed of a direct effect a′=0.55 (P<0.001), and an indirect effect a″=0.11 (P=0.039) mediated by the reduction of testosterone levels (Figure 4B). In practice, it means that for every 1 pg/mL increase of interleukin‐6, a mean QTc prolongation of 0.66 ms is observed, ≈20% of which is attributable to the testosterone‐lowering potential of this cytokine (on average, 0.11 ms every 1 ng/mL decrease of testosterone). Thus, although the direct effect explains most of the QT‐prolonging activity of interleukin‐6 (≈80%), concomitant testosterone reduction also plays a significant role in the determinism of interleukin‐6–associated QTc prolongation.
Figure 4. Correlation between IL‐6 levels and QTc interval in male patients with inflammatory diseases and relative contribution of direct and indirect effects.
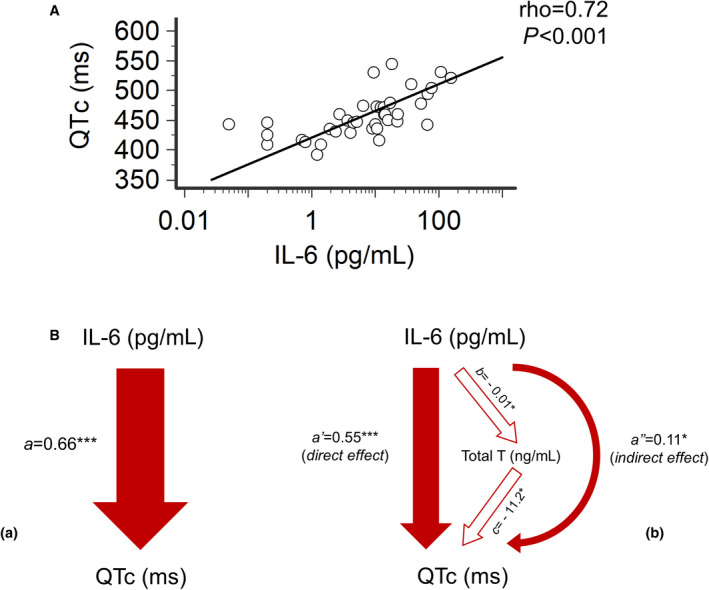
A, Relationship between heart rate‐corrected QT interval (QTc) and IL‐6 levels. Spearman test. B, Mediation analysis of the relationship between QTc and IL‐6. (a) Total effect of IL‐6 on QTc; (a=0.66). (b) Decomposition of IL‐6 effect on QTc: the direct effect of IL‐6 on QTc is expressed by the coefficient a′ (=0.55); the indirect effect (a″=0.11), mediated by testosterone reduction, is the product of path coefficients b (=−0.01) and c (=−11.2). Linear regression analysis, ***P<0.001, *P<0.05. Patients, n=22. IL‐6 indicates interleukin‐6; QTc, heart rate‐corrected QT interval based on Bazett’s formula; and T, testosterone.
Inflammation and Sex Hormones Levels in Male Patients With Torsades de Pointes
In our cohort of consecutive, unselected male patients with TdP, signs of systemic inflammatory activation, as demonstrated by elevated CRP levels (>0.5 mg/dL), were present in the large majority of cases. Specifically, systemic inflammation was significantly active (CRP >2 mg/dL) in approximately one‐half of subjects and highly active (CRP >5 mg/dL) in approximately one‐third (Table 2; Figure 5A). A definite inflammatory disease was identified in 10 of 19 patients (53%), most frequently acute infections, but also immune‐mediated diseases during flares or acute pancreatitis (Table 2).
Table 2.
Inflammatory Diseases, C‐Reactive Protein, and Sex Hormone Levels in Male Patients With Torsades de Pointes
| Patients, n | 19 |
| Mean CRP * , mg/dL | 4.49±6.23 |
| Median CRP*, mg/dL | 1.72 (0.91–5.77) |
| Patients with CRP >0.5 mg/dL | 16/19 (84) |
| Patients with CRP >2 mg/dL | 9/19 (47) |
| Patients with CRP >5 mg/dL | 6/19 (32) |
| Definite inflammatory diseases | 10/19 (53) |
| Acute infections | 7/10 (70) |
| Pneumonia | 4/7 (58) |
| Sepsis | 1/7 (14) |
| Endocarditis | 1/7 (14) |
| Acute bronchitis | 1/7 (14) |
| Immune‐mediated diseases | 2/10 (20) |
| Rheumatoid arthritis | 1/2 (50) |
| Undifferentiated arthritis | 1/2 (50) |
| Other | 1/10 (10) |
| Acute pancreatitis | 1/1 (100) |
| Total testosterone, ng/mL | 1.10 (0.8–2.6) |
| Free testosterone, ng/mL | 0.017 (0.009–0.043) |
| Bioavailable testosterone, ng/mL | 0.40 (0.2–1.0) |
| SHBG, nmol/L | 47.5 (28.8–74.3) |
| 17‐β estradiol, pg/mL | 39.6 (10.0–108.0) |
| Progesterone, ng/mL | 0.10 (0.1–0.2) |
| FSH, mIU/mL | 5.8 (3.7–11.8) |
| LH, mIU/mL | 4.5 (2.8–10.3) |
Data are expressed as frequency (percentage), mean±SD, or median (interquartile range). CRP indicates C‐reactive protein; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; and SHBG, sex hormone–binding globulin.
Reference values <0.5 mg/dL.
Figure 5. Correlation between testosterone, 17‐β estradiol and C‐reactive protein levels in male patients with Torsades de Pointes.
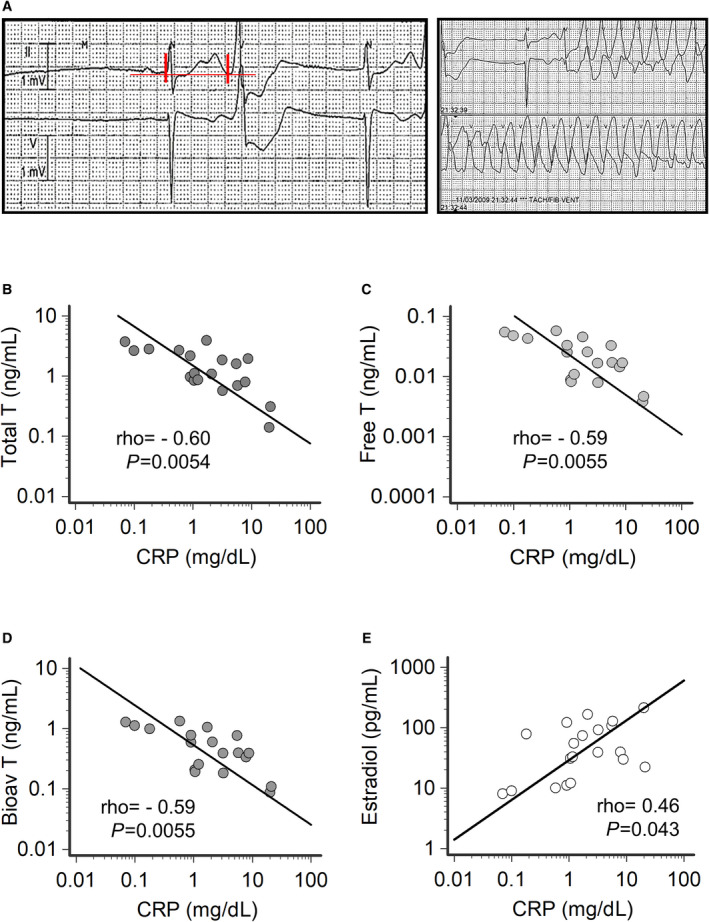
A, ECG strips during Torsades de Pointes from a patient with acute infective endocarditis (CRP, 20.2 mg/dL), low testosterone levels (total, 0.14 ng/mL; free, 0.0037 ng/mL; bioavailable, 0.09 ng/mL) and high 17‐β estradiol levels (214 pg/mL). In this patient, torsades de pointes degenerated in ventricular fibrillation and required electric shock. Vertical red lines show QT interval (620 ms). B, Relationship between total testosterone and CRP levels. C, Relationship between free testosterone and CRP levels. D, Relationship between bioavailable testosterone and CRP levels. E, Relationship between 17‐β estradiol and CRP levels. Spearman test. Patients, n=19. Bioav indicates bioavailable; CRP, C‐reactive protein; Estradiol, 17‐β estradiol; and T, testosterone.
In these subjects, significantly lower testosterone levels were observed when compared with controls (1.10 [0.14–3.89] versus 2.22 [0.66–4.29] ng/mL; P=0.035; 2‐tailed Mann‐Whitney test). Hypogonadism (testosterone levels <2.5 ng/mL) was present in most patients (14/19; 74%), frequently a profound hypogonadism reaching very low values as per the female range (≤1.1 ng/mL in 9/19; 47%) (Table 2; Table S2). Free and bioavailable testosterone levels were consistently reduced (both P=0.031, 2‐tailed Mann‐Whitney test), while circulating 17‐β estradiol was higher than in controls (39.6 [10.0–214.0] versus 16.7 [10.0–44.0] pg/mL; P=0.034, two‐tailed Mann‐Whitney test), with 5 of 19 patients with TdP (26%) showing values ≥100 pg/mL, as usually observed in premenopausal women 40 (Table 2; Table S2). Progesterone, FSH, LH, and SHBG levels were not significantly different when patients with TdP were compared with controls (all P>0.05; 2‐tailed Mann‐Whitney test) (Table 2; Table S2).
Circulating levels of testosterone (total, free, and bioavailable) showed a robust inverse correlation with CRP, in turn directly associated with 17‐β estradiol concentration (Figure 5B through 5E). Moreover, an inverse association between total testosterone and 17‐β estradiol levels was also found (ρ=−0.46; P=0.045; Spearman test). To further analyze the relationship between sex hormones and the degree of inflammatory activation in men with TdP, we divided the subjects in 2 groups based on the presence or not of significantly active systemic inflammation (cutoff: CRP, 2 mg/dL). Despite no significant age difference between the 2 groups (78 [53–91] versus 74 [45–89] years; P=0.97; 2‐tailed Mann‐Whitney test), patients with TdP with CRP>2 mg/dL displayed total, free, and bioavailable testosterone levels significantly lower (>2 times) and 17‐β estradiol levels significantly higher (>4 times), respectively, when compared with those with CRP <2 mg/dL (Figure 6A through 6D). Testosterone concentrations in patients with TdP with CRP >2 mg/dL were comparable with those observed in males with active inflammatory diseases (PRE), both being significantly lower when compared with controls (Figure S5A); conversely, no difference was found between TdP subjects with CRP <2 mg/dL, patients with inflammatory diseases in the recovery phase (POST), and controls (Figure S5B).
Figure 6. Comparison of testosterone and 17‐β estradiol levels in male torsades de pointes patients with medium‐high vs absent‐low degree systemic inflammation (C‐reactive protein >2 mg/dL vs <2 mg/dL).

A, Total testosterone; 2‐tailed Mann‐Whitney test,*P<0.05. Values below the horizontal dotted line indicate a female range (upper limit of reference values in premenopausal women, ie, 1.1 ng/mL). B, Free testosterone; 2‐tailed Mann‐Whitney test,*P<0.05. C, Bioavailable testosterone; 2‐tailed Mann‐Whitney test, *P<0.05. D, 17‐β estradiol; 2‐tailed Mann‐Whitney test, *P<0.05. Values above the horizontal dotted line indicate a female range (lower limit of reference values in premenopausal women, ie, 100 pg/mL). Patients with CRP <2 mg/dL, n=10; patients with CRP >2 mg/dL, n=9. Bioav indicates bioavailable; Estradiol, 17‐β estradiol; and T, testosterone.
In a significant proportion of cases (8/19; 42%), patients with TdP developed VF/sudden cardiac arrest and/or underwent electric shock (TdP rapidly degenerated to VF/sudden cardiac arrest [n=2]; out‐of‐hospital VF/sudden cardiac arrest followed by direct current shock, only later revealing to be a manifestation of TdP episodes [n=2]; direct current shock for sustained TdP not responsive to medical therapy [n=4]). Most cases of complicated TdP occurred in the group with significantly active systemic inflammation (CRP >2 mg/dL: 6/9, 67% versus CRP <2 mg/dL: 2/10, 20%; 2‐sided Fisher’s exact test, P=0.069). Moreover, patients with complicated TdP, considered as a whole, showed significantly lower testosterone levels (≈2.5 times) when compared with those with uncomplicated TdP (0.76 [0.14–2.62] versus 1.94 [0.79–3.89] ng/mL; 2‐tailed Mann‐Whitney test, P=0.033).
Finally, as anticipated, most patients with TdP were burdened by many concomitant traditional QT‐prolonging risk factors (>5 on average), more commonly cardiac and extracardiac diseases, electrolyte abnormalities, anti‐Ro/SSA antibodies, and drugs (Table S2). Indeed, based on the multihit theory, >1 factor/hit usually needs to be present in a specific patient to disturb ventricular repolarization so critically to provoke the extreme QTc prolongation required for TdP occurrence. 19 In this regard, the prevalence of severe hypogonadism (total testosterone ≤1.1 ng/mL, 47% of cases) was unpredictably high in our TdP cohort, as it was similar to that found for other well‐recognized TdP risk factors, such as electrolyte imbalances (53%) or use of QT‐prolonging medications (47%) (Table S2).
DISCUSSION
The most important findings of the present study are the following: (1) In male patients with active inflammatory diseases, regardless of specific etiology and organ localization, testosterone levels were significantly reduced, but promptly normalized within days in association with CRP and interleukin‐6 level decrease; reduction of testosterone levels, which also inversely correlated with 17‐β estradiol levels over time, significantly contributed to mediate the observed inflammation‐induced QTc prolongation; and (2) in consecutive men with TdP, both active systemic inflammation and hypogonadism were frequently present, with significant correlations between CRP, testosterone, and 17‐β estradiol levels; in these patients, increased CRP and reduced testosterone tended to be associated with a worse outcome for the arrhythmia, that is, degeneration to VF/sudden cardiac arrest and/or requirement of electric shock.
In recent years, there was a growing interest in understanding the impact of acute inflammation on the risk of arrhythmic events in general 13 , 19 , 43 , 44 and of LQTS/TdP in particular, 15 , 18 an awareness further emphasized by the current COVID‐19 outbreak. 31 , 45 , 46 Several underlying mechanisms have been identified to date, mostly mediated by the pleiotropic effects of inflammatory cytokines able to enhance myocardial electric instability both directly, modulating cardiac ion channels function, and indirectly, increasing the sympathetic drive on the heart. 13 , 19 , 20 , 41 However, given the wide spectrum of cytokine‐induced multisystem changes during inflammatory activation, which deeply affects, among others, the endocrine system, 35 , 47 , 48 it is plausible that additional mechanisms of hormonal nature may also contribute to explain the strong link between inflammation and LQTS/TdP risk. Indeed, a key role of testosterone in regulating ventricular repolarization is increasingly recognized, 14 , 23 and male hypogonadism was recently demonstrated to be a reversible cause of TdP in men. 22 , 24 , 49 Moreover, several studies have demonstrated that gonadic function is significantly suppressed in men affected with inflammatory diseases. 27 , 28 Thus, it could be anticipated that inflammation‐associated hypogonadism in males is a significant contributor of the increased LQTS/TdP risk observed during the inflammatory response. The present study provides support to this hypothesis, along with important details regarding time‐scale and size of the arrhythmogenic effects mediated by testosterone changes in the course of inflammation.
First, we confirmed that inflammation‐driven perturbations of sex hormones in men are relevant, and for the first time demonstrated that they appear rapidly but just as quickly reverse. In fact, in our cohort of men with active inflammatory diseases of different origin and etiology, testosterone levels were significantly reduced, in one‐half of the cases even reaching very low concentrations as physiologically found in premenopausal women. In these patients, disease‐specific treatments leading to marked CRP lowering resulted in a prompt normalization of testosterone levels, in the course of a few days/weeks only. Overall, circulating testosterone showed a strict inverse correlation with CRP and interleukin‐6 over time. While transient, these modifications were relevant, given that during the active phase median testosterone concentration was nearly half, and the rate of marked hypogonadism 3 times more frequent when compared with the recovery period. An increased androgen‐to‐estrogen conversion seems to be the main underlying mechanism, likely attributable to cytokine‐dependent enhancement of aromatase activity in the adipose tissue. This is supported by the evidence here provided that 17‐β estradiol levels markedly increased in PRE conditions and then normalized in the POST phase, with a significant correlation with both testosterone (inverse) and interleukin‐6 (direct) levels over time. Nevertheless, the fact that, despite signs of peripheral hypogonadism, no compensatory increase in gonadotropins was observed (normal levels in absolute, but inappropriately low for circulating testosterone) suggests that an additional central component may also contribute to the phenomenon. Accordingly, the inhibitory effects of cytokines on gonadoliberin and gonadotropin secretion are well recognized, 33 as well as that of the 17‐β estradiol increase on LH release in men. 50
While these hormonal alterations, in a teleological perspective, sound understandable since reproduction is not convenient during active inflammatory illnesses, it is also conceivable that such a transient “feminization” may increase the predisposition of men to LQTS/TdP, to a similar extent to what is usually observed in women. The validity of his hypothesis is demonstrated for the first time by our results, representing the other major finding of the present study. In fact, during active inflammation, men showed a transient but significant prolongation of the QTc, which over time correlated directly with circulating interleukin‐6 and inversely with testosterone levels, regardless of the specific QT‐correction formula used. Mediation analysis provided evidence that interleukin‐6 is a primary factor involved in inflammation‐driven QTc prolongation, both directly and indirectly, by reducing testosterone. While direct effects, probable expression of the impact of interleukin‐6 on the cardiomyocyte electrophysiology, 13 , 16 , 19 seems to be predominant, testosterone‐mediated indirect effects may also add a significant contribution accounting for ≈20% of the overall changes.
A support to the clinical and epidemiological relevance of these mechanisms in promoting arrhythmogenesis is provided by the second part of the study, in which we demonstrated how in a consecutive cohort of men with TdP both inflammatory activation and hypogonadism are common and interconnected findings. In fact, at the moment of arrhythmia occurrence, active inflammatory diseases and/or significant CRP elevation along with female‐range testosterone levels were found in ≈50% of subjects, with a robust inverse association between CRP and testosterone serum concentrations. In men with TdP with significantly active systemic inflammation, testosterone levels overlapped with those found in male patients of the inflammatory cohort during the active phase, thereby pointing to shared mechanisms. This view is further strengthened by the evidence that circulating 17‐β estradiol significantly correlated with CRP (directly) and testosterone (inversely) levels also in patients with TdP, a finding suggestive of an increased inflammation‐driven androgen‐to‐estrogen conversion in peripheral tissues.
Our results not only confirm in a larger cohort the datum first reported by Salem et al 22 that hypogonadism is very common in men who develop TdP, but also provide a new insight in the underlying mechanisms and prognostic significance. Specifically, the present study suggests that a concomitant inflammatory activation may be the main cause of apparently unexplained testosterone deficiency in most male patients with TdP. Indeed, by reviewing the data reported by Salem et al, 22 , 51 a concomitant inflammatory disease was present in 6 of 7 men with TdP with hypogonadism, that is, sepsis (n=3), endocarditis (n=2), or lung infection (n=1). In addition, the present finding that inflammation‐induced male hypogonadism tends to be associated with a worse prognosis of arrhythmia could reinforce the potential importance of a prompt diagnosis and treatment of this condition. Finally, it is intriguing to speculate how such mechanisms might contribute to explain the higher COVID‐19 mortality observed in men, 52 given that in this disease high levels of circulating cytokines 31 coexist with low testosterone concentrations, 30 and increased prevalence of QTc prolongation and life‐threatening ventricular arrhythmias. 31 , 32
In conclusion, the present findings provide evidence that during systemic inflammatory activation, interleukin‐6 elevation is associated with reduced testosterone levels in men, possibly deriving from an enhanced androgen‐to‐estrogen conversion. While transient, inflammatory hypotestosteronemia may significantly contribute to acutely increase the risk of LQTS and TdP in men. The epidemiological and prognostic impact of these arrhytmogenic mechanisms seems to be relevant, as suggested by the fact that they are actively involved in a high portion of men with TdP consecutively enrolled from the general population, particularly in those with a poorer immediate outcome.
From a therapeutic perspective, our data indicate that a specific treatment of the underlying inflammatory disease might be the crucial step to interrupt the entire pathogenic cascade from the beginning. Nevertheless, they also intriguingly suggest that administration of interleukin‐6–blocking drugs (tocilizumab, sarilumab) 45 , 53 and testosterone replacement therapy could represent additional important antiarrhythmic interventions in the short term, particularly in patients with TdP complicated by or refractory to conventional treatments.
Sources of Funding
This work was funded by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Progetti di Rilevante Interesse Nazionale (PRIN), and Bando 2017, protocollo 2017XZMBYX.
Disclosures
Dr Lazzerini received a grant from Roche Italia S.p.A in 2018, unrelated to this submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S4
Figures S1–S5
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023371
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081 [DOI] [PubMed] [Google Scholar]
- 2. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology tCoCN, and the American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El‐Sherif N, Turitto G. Torsade de pointes. Curr Opin Cardiol. 2003;18:6–13. doi: 10.1097/00001573-200301000-00002 [DOI] [PubMed] [Google Scholar]
- 4. El‐Sherif N, Turitto G, Boutjdir M. Congenital long QT syndrome and torsade de pointes. Ann Noninvasive Electrocardiol. 2017;22:e12481. doi: 10.1111/anec.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El‐Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin Electrophysiol. 2018;41:414–421. doi: 10.1111/pace.13296 [DOI] [PubMed] [Google Scholar]
- 6. Schwartz PJ, Stramba‐Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long‐QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tisdale JE. Prevalence and significance of acquired QT interval prolongation in hospitalized patients. Heart Rhythm. 2017;14:979–980. doi: 10.1016/j.hrthm.2017.03.036 [DOI] [PubMed] [Google Scholar]
- 8. Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8 [DOI] [PubMed] [Google Scholar]
- 9. Lazzerini PE, Capecchi PL, Laghi‐Pasini F, Boutjdir M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat Rev Cardiol. 2017;14:521–535. doi: 10.1038/nrcardio.2017.61 [DOI] [PubMed] [Google Scholar]
- 10. Brouillette J, Cyr S, Fiset C. Mechanisms of arrhythmia and sudden cardiac death in patients with HIV infection. Can J Cardiol. 2019;35:310–319. doi: 10.1016/j.cjca.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 11. Cho JH, Zhang R, Kilfoil PJ, Gallet R, de Couto G, Bresee C, Goldhaber JI, Marbán E, Cingolani E. Delayed repolarization underlies ventricular arrhythmias in rats with heart failure and preserved ejection fraction. Circulation. 2017;136:2037–2050. doi: 10.1161/CIRCULATIONAHA.117.028202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woosley RL. Arrhythmogenic foods—a growing medical problem. Trends Cardiovasc Med. 2020;30:310–312. doi: 10.1016/j.tcm.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 13. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. [DOI] [PubMed] [Google Scholar]
- 14. Salem JE, Alexandre J, Bachelot A, Funck‐Brentano C. Influence of steroid hormones on ventricular repolarization. Pharmacol Ther. 2016;167:38–47. doi: 10.1016/j.pharmthera.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 15. Lazzerini PE, Laghi‐Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, Selvi E, Bacarelli MR, Finizola F, Vanni F, et al. Systemic inflammation as a novel QT‐prolonging risk factor in patients with torsades de pointes. Heart. 2017;103:1821–1829. doi: 10.1136/heartjnl-2016-311079 [DOI] [PubMed] [Google Scholar]
- 16. Aromolaran AS, Srivastava U, Alí A, Chahine M, Lazaro D, El‐Sherif N, Capecchi PL, Laghi‐Pasini F, Lazzerini PE, Boutjdir M. Interleukin‐6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One. 2018;13:e0208321. doi: 10.1371/journal.pone.0208321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lazzerini PE, Acampa M, Laghi‐Pasini F, Bertolozzi I, Finizola F, Vanni F, Natale M, Bisogno S, Cevenini G, Cartocci A, et al. Cardiac arrest risk during acute infections: systemic inflammation directly prolongs QTc interval via cytokine‐mediated effects on potassium channel expression. Circ Arrhythm Electrophysiol. 2020;13:e008627. doi: 10.1161/CIRCEP.120.008627 [DOI] [PubMed] [Google Scholar]
- 18. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovasc Med. 2015;2:26. doi: 10.3389/fcvm.2015.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazzerini PE, Capecchi PL, El‐Sherif N, Laghi‐Pasini F, Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7:e010595. doi: 10.1161/JAHA.118.010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazzerini PE, Laghi‐Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. doi: 10.1038/s41577-018-0098-z [DOI] [PubMed] [Google Scholar]
- 21. Capecchi PL, Laghi‐Pasini F, El‐Sherif N, Qu Y, Boutjdir M, Lazzerini PE. Autoimmune and inflammatory K. Heart Rhythm. 2019;16:1273–1280. [DOI] [PubMed] [Google Scholar]
- 22. Salem J‐E, Waintraub X, Courtillot C, Shaffer CM, Gandjbakhch E, Maupain C, Moslehi JJ, Badilini F, Haroche J, Gougis P, et al. Hypogonadism as a reversible cause of torsades de pointes in men. Circulation. 2018;138:110–113. doi: 10.1161/CIRCULATIONAHA.118.034282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salem J‐E, Yang T, Moslehi JJ, Waintraub X, Gandjbakhch E, Bachelot A, Hidden‐Lucet F, Hulot J‐S, Knollmann BC, Lebrun‐Vignes B, et al. Androgenic effects on ventricular repolarization: a translational study from the international pharmacovigilance database to iPSC‐cardiomyocytes. Circulation. 2019;140:1070–1080. doi: 10.1161/CIRCULATIONAHA.119.040162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lazzerini PE, Bertolozzi I, Acampa M, Cantara S, Castagna MG, Pieragnoli L, D’Errico A, Rossi M, Bisogno S, El‐Sherif N, et al. Androgen deprivation therapy for prostatic cancer in patients with torsades de pointes. Front Pharmacol. 2020;11:684. doi: 10.3389/fphar.2020.00684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasegawa K, Ito H, Kaseno K, Miyazaki S, Shiomi Y, Tama N, Ikeda H, Ishida K, Uzui H, Ohno S, et al. Impact of medical castration on malignant arrhythmias in patients with prostate cancer. J Am Heart Assoc. 2021;10:e017267. doi: 10.1161/JAHA.120.017267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gutierrez G, Wamboldt R, Baranchuk A. The impact of testosterone on the QT interval: a systematic review. Curr Probl Cardiol. 2021:100882. Epub ahead of print. doi: 10.1016/j.cpcardiol.2021.100882 [DOI] [PubMed] [Google Scholar]
- 27. Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur‐Farhana MF, Ima‐Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129–140. doi: 10.1080/13685538.2018.1482487 [DOI] [PubMed] [Google Scholar]
- 28. Cutolo M, Straub RH. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol. 2020;16:628–644. doi: 10.1038/s41584-020-0503-4 [DOI] [PubMed] [Google Scholar]
- 29. Rivier C, Vale W. In the rat, interleukin‐1 alpha acts at the level of the brain and the gonads to interfere with gonadotropin and sex steroid secretion. Endocrinology. 1989;124:2105–2109. [DOI] [PubMed] [Google Scholar]
- 30. Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, Mani K, Randolph GJ, Edwards JR, Mudd PA, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID‐19. JAMA Netw Open. 2021;4:e2111398. doi: 10.1001/jamanetworkopen.2021.11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lazzerini PE, Boutjdir M, Capecchi PL. COVID‐19, arrhythmic risk and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293 [DOI] [PubMed] [Google Scholar]
- 32. Coromilas EJ, Kochav S, Goldenthal I, Biviano A, Garan H, Goldbarg S, Kim JH, Yeo I, Tracy C, Ayanian S, et al. Worldwide survey of COVID‐19 associated arrhythmias. Circ Arrhythm Electrophysiol. 2021;14:e009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomaszewska‐Zaremba D, Herman A. The role of immunological system in the regulation of gonadoliberin and gonadotropin secretion. Reprod Biol. 2009;9:11–23. doi: 10.1016/S1642-431X(12)60091-6 [DOI] [PubMed] [Google Scholar]
- 34. Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor necrosis factor‐alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein‐1 binding site upstream of promoter 1.4. Mol Endocrinol. 1996;10:1350–1357. [DOI] [PubMed] [Google Scholar]
- 35. Herrmann M, Scholmerich J, Straub RH. Influence of cytokines and growth factors on distinct steroidogenic enzymes in vitro: a short tabular data collection. Ann N Y Acad Sci. 2002;966:166–186. [DOI] [PubMed] [Google Scholar]
- 36. Antony T, Alzaharani SY, El‐Ghaiesh SH. Opioid‐induced hypogonadism: pathophysiology, clinical and therapeutics review. Clin Exp Pharmacol Physiol. 2020;47:741–750. doi: 10.1111/1440-1681.13246 [DOI] [PubMed] [Google Scholar]
- 37. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. doi: 10.1210/jc.2018-00229 [DOI] [PubMed] [Google Scholar]
- 38. Chiladakis J, Kalogeropoulos A, Arvanitis P, Koutsogiannis N, Zagli F, Alexopoulos D. Heart rate‐dependence of QTc intervals assessed by different correction methods in patients with normal or prolonged repolarization. Pacing Clin Electrophysiol. 2010;33:553–560. doi: 10.1111/j.1540-8159.2009.02657.x [DOI] [PubMed] [Google Scholar]
- 39. Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug‐induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–899. doi: 10.1016/j.ahj.2007.01.040 [DOI] [PubMed] [Google Scholar]
- 40. Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471 [DOI] [PubMed] [Google Scholar]
- 41. Lazzerini PE, Acampa M, Hammoud M, Maffei S, Capecchi PL, Selvi E, Bisogno S, Guideri F, Galeazzi M, Pasini FL. Arrhythmic risk during acute infusion of infliximab: a prospective, single‐blind, placebo‐controlled, crossover study in patients with chronic arthritis. J Rheumatol. 2008;35:1958–1965. [PubMed] [Google Scholar]
- 42. Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321 [DOI] [PubMed] [Google Scholar]
- 43. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2 [DOI] [PubMed] [Google Scholar]
- 44. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8 [DOI] [PubMed] [Google Scholar]
- 45. Lazzerini PE, Laghi‐Pasini F, Acampa M, Boutjdir M, Leopoldo CP. IL‐6 (interleukin 6) blockade and heart rate corrected QT interval prolongation in COVID‐19. Circ Arrhythm Electrophysiol. 2020;13:e008791. doi: 10.1161/CIRCEP.120.008791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, Tedrow U. Arrhythmias and COVID‐19: a review. JACC Clin Electrophysiol. 2020;6:1193–1204. doi: 10.1016/j.jacep.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wasyluk W, Wasyluk M, Zwolak A. Sepsis as a pan‐endocrine illness‐endocrine disorders in septic patients. J Clin Med. 2021;10:2075. doi: 10.3390/jcm10102075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turnbull AV, Rivier CL. Regulation of the hypothalamic‐pituitary‐adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1 [DOI] [PubMed] [Google Scholar]
- 49. Hasegawa K, Morishita T, Miyanaga D, Hisazaki K, Kaseno K, Miyazaki S, Uzui H, Ohno S, Horie M, Tada H. Medical castration is a rare but possible trigger of torsade de pointes and ventricular fibrillation. Int Heart J. 2019;60:193–198. doi: 10.1536/ihj.18-127 [DOI] [PubMed] [Google Scholar]
- 50. Finkelstein JS, O'Dea LS, Whitcomb RW, Crowley WF. Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin‐releasing hormone‐deficient men. J Clin Endocrinol Metab. 1991;73:621–628. doi: 10.1210/jcem-73-3-621 [DOI] [PubMed] [Google Scholar]
- 51. Salem J‐E, Bretagne M, Lebrun‐Vignes B, Waintraub X, Gandjbakhch E, Hidden‐Lucet F, Gougis P, Bachelot A, Funck‐Brentano C; Centres FNoRP . Clinical characterization of men with long QT syndrome and torsades de pointes associated with hypogonadism: a review and pharmacovigilance study. Arch Cardiovasc Dis. 2019;112:699–712. doi: 10.1016/j.acvd.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 52. Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID‐19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lazzerini PE, Acampa M, Capecchi PL, Fineschi I, Selvi E, Moscadelli V, Zimbone S, Gentile D, Galeazzi M, Laghi‐Pasini F. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken). 2015;67:332–339. doi: 10.1002/acr.22455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
Figures S1–S5


