Abstract
Background
Use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers (ACEi/ARB) is thought to affect COVID‐19 through modulating levels of angiotensin‐converting enzyme 2, the cell entry receptor for SARS‐CoV2. We sought to assess the association between ACEi/ARB, biomarkers of inflammation, and outcomes in patients hospitalized for COVID‐19.
Methods and Results
We leveraged the ISIC (International Study of Inflammation in COVID‐19), identified patients admitted for symptomatic COVID‐19 between February 1, 2020 and June 1, 2021 for COVID‐19, and examined the association between in‐hospital ACEi/ARB use and all‐cause death, need for ventilation, and need for dialysis. We estimated the causal effect of ACEi/ARB on the composite outcomes using marginal structural models accounting for serial blood pressure and serum creatinine measures. Of 2044 patients in ISIC, 1686 patients met inclusion criteria, of whom 398 (23.6%) patients who were previously on ACEi/ARB received at least 1 dose during their hospitalization for COVID‐19. There were 215 deaths, 407 patients requiring mechanical ventilation, and 124 patients who required dialysis during their hospitalization. Prior ACEi/ARB use was associated with lower levels of soluble urokinase plasminogen activator receptor and C‐reactive protein. In multivariable analysis, in‐hospital ACEi/ARB use was associated with a lower risk of the composite outcome of in‐hospital death, mechanical ventilation, or dialysis (adjusted hazard ratio 0.49, 95% CI [0.36–0.65]).
Conclusions
In patients hospitalized for COVID‐19, ACEi/ARB use was associated with lower levels of inflammation and lower risk of in‐hospital outcomes. Clinical trials will define the role of ACEi/ARB in the treatment of COVID‐19.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04818866.
Keywords: ACE inhibitors, angiotensin receptor blockers, COVID‐19, mortality, outcomes
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System, Biomarkers, Clinical Studies
Nonstandard Abbreviations and Acronyms
- ISIC
International Study of Inflammation in COVID‐19
- M2C2
Medicine COVID‐19 Cohort
- SuPAR
soluble urokinase plasminogen activator receptor
Clinical Perspective
What Is New?
Despite a larger burden of co‐morbidities, patients hospitalized for COVID‐19 on angiotensin‐converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) have significantly better outcomes compared with those not on ACEi or ARB.
This study used multiple approaches to evaluate the association between ACEi or ARB use and outcomes in patients hospitalized for COVID‐19, including time‐varying Cox modeling and marginal structural modeling accounting for important time‐varying confounders such as daily mean arterial blood pressure and daily measures of serum creatinine.
Use of ACEi or ARB were associated with significantly lower levels of inflammatory biomarkers after accounting for differences in clinical characteristics.
What Are the Clinical Implications?
ACEi and ARB may have beneficial effects on outcomes of patients with COVID‐19 and should not be discontinued unless clinically indicated.
A plausible explanation for the observed benefit of ACEi and ARBs in COVID‐19 is through the attenuation of inflammation that occurs via angiotensin II receptor blockade.
Whether initiation of ACEi or ARB in patients with COVID‐19 improves outcome merits study.
By June 2021, the COVID‐19 global pandemic had resulted in more than 170 million confirmed cases of infection and more than 3.7 million deaths worldwide. 1 The SARS‐CoV2—the pathogen behind this rampant disease 2 —has been shown to bear phylogenetic resemblance to the previous SARS‐CoV coronavirus responsible for 2002 to 2004 SARS epidemic. Due to the homology between the SARS‐CoV2 receptor binding domain with that of the previous strain, 3 it was postulated and then demonstrated that SARS‐CoV2 uses the same receptor for entry into host cells, angiotensin‐converting enzyme 2 (ACE2). 3 , 4 , 5
The ACE2 receptor is a transmembrane carboxypeptidase that metabolizes the vasoconstrictive angiotensin II to the more vasodilatory angiotensin, providing a counterregulatory effect to the proinflammatory renin‐angiotensin system cascade. 6 , 7 , 8 Activation of the renin‐angiotensin system cascade has been implicated in modulation of immune cell function involving the cytokines tumor necrosis factor‐α and interleukin‐6, and activation of the proinflammatory transcription factor NF‐kB in human monocytes. 9 , 10 , 11 , 12 ACE2 is found throughout the human body and is notably expressed by type I and type II pneumocytes. 13 Downregulation of ACE2 by SARS‐CoV is thought to promote the development of acute respiratory distress syndrome. 4 , 14 ACE inhibitors (ACEi) and angiotensin receptor blockers (ARB), drug classes commonly used for the treatment of hypertension and heart failure, have been hypothesized to worsen lung injury in COVID‐19 patients 4 , 15 through upregulation of ACE2 in various tissues. 16 , 17 , 18 However, recent studies have suggested that ACEi/ARB usage either before or during hospitalization was not associated with worse outcomes and may be beneficial in hospitalized patients with COVID‐19. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Conclusions related to these observations are limited by the high risk of selection bias afforded by unaccounted confounders that would guide the use of ACEi/ARB such as severity of the disease, hemodynamic instability, and kidney function.
To better understand the link between ACEi/ARB use and outcomes in COVID‐19, we leveraged the multicenter ISIC (International Study of Inflammation in COVID‐19) and the M2C2 (Michigan Medicine COVID‐19 Cohort) to assess whether the use of ACEi/ARB is associated with improved outcomes while accounting for inflammation and daily measures of blood pressure and kidney function.
METHODS
The International Study of Inflammation in COVID‐19
The ISIC is an ongoing multicenter observational study with the primary purpose of characterizing levels of various biomarkers of inflammation and their association with in‐hospital outcomes of patients with COVID‐19. Participating centers include University of Michigan in Ann Arbor, MI; Rush University in Chicago, IL; Copenhagen University of Hospital in Hvidovre, Denmark; Attikon University Hospital in Athens, Greece; the University of Thessaly in Greece; the University Hospital of Dusseldorf in Germany; and Charité University Medicine Berlin in Germany (Table S1). Inclusion criteria were (1) adult (≥18 years old) patients hospitalized primarily for COVID‐19, (2) a confirmed SARS‐CoV‐2 infection diagnosed through reverse transcriptase polymerase chain reaction test of nasopharyngeal or oropharyngeal samples, and (3) at least 1 blood sample collected during the hospitalization and stored for biomarker testing. Patients with a positive test for SARS‐CoV‐2 who were asymptomatic or not requiring supplemental oxygen and who were hospitalized for non‐COVID‐19 reasons were excluded. Manual chart review and data mining tools were used to gather details of the presentation, demographics, past medical history, home medications, clinical characteristics, laboratory studies, inpatient medical therapy, hospitalization course, and outcomes. All patients were followed until hospital discharge or death. Institutional review board approval and consent procedures were obtained separately at each site according to local institutional policies. Data from ISIC can be made available upon request through a collaborative process. Please contact penegonz@med.umich.edu for additional information.
The Michigan Medicine COVID‐19 Cohort
The M2C2 is a prospective cohort study that systematically enrolled consecutive adults (≥18 years) with confirmed SARS‐CoV‐2 infection who were hospitalized specifically for COVID‐19 at the University of Michigan from February 1, 2020 to June 1, 2021. In addition to the variables collected for ISIC, all blood pressure measurements and laboratory testing were extracted from the electronic medical records for the purpose of this analysis.
Study Design and Definitions
For the purpose of this study, we included patients hospitalized for COVID‐19 (n=2044) during the period of February 1, 2020 to June 1, 2021, the date the database was locked for the purpose of this analysis. To limit the risk of selection bias, we excluded patients who were taking an ACEi/ARB before hospitalization but were discontinued during hospitalization (n=311), and those in whom an ACEi/ARB was initiated during hospitalization without a prior history of ACEi/ARB use (n=47) resulting in an analytic sample size of 1686 (Figure S1). Prior use of ACEi/ARB was determined through electronic medical record review of all active prescriptions and home medications noted in the chart. In‐hospital use of ACEi/ARB was defined as the administration of at least 1 dose of any ACEi/ARB during the hospital course. For this analysis, ACEi/ARB users were defined as those who had an ACEi or ARB listed on their home medications and received a ACEi or ARB during hospitalization. By contrast, nonusers were defined as those who did not have either ACEi orARB listed among their home medications and did not receive either medication during their hospitalization. Biomarker levels measured within 48 hours of admission included suPAR (soluble urokinase plasminogen activator receptor), interleukin‐6, C‐reactive protein, D‐dimer, ferritin, lactate dehydrogenase, and procalcitonin levels. The outcomes of interest were in‐hospital death or discharge to hospice, the need for mechanical ventilation, and the need for dialysis or continuous renal replacement therapy.
Statistical Analysis
We first report clinical characteristics stratified by use of ACEi/ARB using categorical variables expressed as a number and percentage and continuous variables expressed as means (±SD) and medians (25th–75th interquartile range) for normally and nonnormally distributed data, respectively. We used chi‐square or Fisher’s exact tests to compare categorical variables and 2‐sample t tests or Mann‐Whitney U tests to compare normally distributed and nonnormally distributed continuous variables across groups, respectively.
ACEi/ARB and Biomarkers
We used linear regression to determine whether ACEi/ARB use was independently associated with biomarker levels, adjusting for age, sex, race, body mass index, a history of diabetes, hypertension, coronary artery disease, congestive heart failure, admission estimated glomerular filtration rate, and reported standardized estimates to allow for comparison of the strength of the association. Each biomarker was log transformed to base 2, interpreted as per 100% increase. Covariates were chosen a priori based on their known roles as risk factors for COVID‐19 and indications for ACEi/ARB use.
ACEi/ARB and Outcomes
We represented the incidence of death, need for mechanical ventilation, and need for dialysis stratified by use of ACEi/ARB using bar graphs and examined the association between in‐hospital ACEi/ARB use and the aforementioned outcomes using binary logistic regression. Variables in the main model were chosen a priori based on clinical relevance and included age, sex, race, body mass index, a history of diabetes, hypertension, coronary artery disease, congestive heart failure, admission estimated glomerular filtration rate, and institution of enrollment. In a separate model, we further adjusted for the mean arterial pressure on presentation.
To address the risk of survivor bias attributed to the variability in timeline of in‐hospital ACEi/ARB administration, we used Cox proportional hazards models to examine the association of in‐hospital ACEi/ARB use as a time‐dependent covariate with the composite outcome of death, need for dialysis, and need for mechanical ventilation adjusting for the aforementioned covariates. Patients were censored at hospital discharge or June 1, 2021.
Estimating a Causal Effect for ACEi/ARB on Outcomes
We used marginal structural modeling to estimate the causal effect of ACEi/ARB on the composite outcome (death, mechanical ventilation, and dialysis) and to account for potential confounding by blood pressure and serum creatinine. 32 Serial measurements of blood pressure and serum creatinine during hospitalization measurements were available for M2C2. Thus, this analysis was restricted to patients at the University of Michigan (n=1357). Model parameters were estimated through inverse‐probability‐of‐ACEi/ARB use weighting, allowing for appropriate adjustment for the time‐varying confounders blood pressure and creatinine, which are risk factors for the outcome and are affected by previous ACEi/ARB use. In the first step, we calculated a stabilized weight for each subject at each hospital day. The numerator of the weight is informally the probability that the subject had observed treatment of ACEi/ARB conditional on baseline covariates (age, sex, race, body mass index, admission estimated glomerular filtration rate, and history of diabetes, coronary artery disease, hypertension, and congestive heart failure) and days in hospital. The denominator of the weight is the probability that the subject had their own observed treatment of ACEi/ARB, adjusting of blood pressure and creatinine measurements and days in hospital. In the second step, we fitted a time‐dependent Cox model with baseline covariates (age, sex, race, body mass index, diabetes, hypertension, coronary artery disease, and congestive heart failure) and weighted each subject on each hospital day by the “stabilized” weight obtained from the first step. By weighting, we created, for a risk set on each hospital day, a pseudo‐population in which blood pressure and creatinine are no longer confounders. Weighted Kaplan‐Meier curves were plotted to visually compare survival free of the composite outcome by in‐hospital ACEi/ARB use.
Sensitivity Analyses
To explore the possibility of effect modification attributed to differences in baseline characteristics among patients, we computed the time‐dependent hazard ratios for the association between time to in‐hospital ACEi/ARB use and the combined outcome of death, need for mechanical ventilation, or need for dialysis in relevant subgroups and performed tests of interaction. To assess whether our initial exclusion criteria affected the findings, we repeated the analysis in the overall cohort.
Data analysis was performed using R software, version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Overall, the cohort had a mean age of 58.9 years (range 19–102) and consisted of 57.1% men, and 19.5% Black individuals. A total of 398 (23.6%) patients who were on ACEi/ARB before hospitalization for COVID‐19 received at least 1 dose during their hospitalization (Table 1). Patients on an ACEi/ARB were older (mean of 65 versus 57 years) and had significantly more comorbidities compared with the non‐ACEi/ARB group including diabetes (54.8% versus 22.0%), coronary artery disease (24.4% versus 9.2%), and chronic kidney disease (17.8% versus 12.3%) (Table 1). Laboratory testing was overall similar between both groups, except for a lower estimated glomerular filtration rate in patients taking an ACEi/ARB.
Table 1.
Clinical Characteristics and Laboratory Testing Stratified by In‐Hospital ACEi/ARB Use
| Variables | Did not receive ACEi/ARB (n=1288) | ACEi/ARB (n=398) | P value |
|---|---|---|---|
| Age, y, n (%) | <0.001* | ||
| <45 y | 300 (23.3) | 32 (8.0) | |
| 45–64 y | 549 (42.6) | 154 (38.7) | |
| 65–79 y | 313 (24.3) | 157 (39.4) | |
| ≥80 y | 126 (9.8) | 55 (13.8) | |
| Male sex, n (%) | 558 (43.3) | 165 (41.5) | 0.55 |
| Body mass index, kg/m2, mean (SD) | 31 (9) | 33 (11) | 0.001* |
| Black race, n (%) | 248 (19.3) | 80 (20.1) | 0.76 |
| History of tobacco use, n (%) | 429 (33.3) | 174 (43.7) | <0.001* |
| Hypertension, n (%) | 488 (37.9) | 371 (93.2) | <0.001* |
| Coronary artery disease, n (%) | 119 (9.2) | 97 (24.4) | <0.001* |
| Diabetes, n (%) | 284 (22.0) | 218 (54.8) | <0.001* |
| Congestive heart failure, n (%) | 107 (8.3) | 65 (16.3) | <0.001* |
| Chronic kidney disease, n (%) | 158 (12.3) | 71 (17.8) | 0.006* |
| End‐stage renal disease on dialysis, n (%) | 43 (3.3) | 7 (1.8) | 0.15 |
| Admission estimated glomerular filtration rate, mean (SD) | 77 (32) | 65 (28) | <0.001* |
| Presenting symptoms, n (%) | |||
| Fever | 831 (64.5) | 229 (57.5) | 0.014* |
| Shortness of breath | 933 (72.4) | 286 (71.9) | 0.87 |
| Diarrhea | 356 (27.6) | 108 (27.1) | 0.90 |
| Altered mental status | 107 (8.3) | 39 (9.8) | 0.41 |
| Hypoxia | 525 (41.4) | 147 (37.3) | 0.16 |
| Laboratory data, mean (SD) | |||
| Hemoglobin, g/dL | 12.9 (2.4) | 12.8 (2.2) | 0.49 |
| White blood cell count, k/µL | 7.4 (4.7) | 7.0 (4.3) | 0.19 |
| Absolute neutrophil, count, k/µL | 5.6 (3.6) | 5.4 (2.8) | 0.29 |
| Absolute lymphocyte count, k/µL | 1.2 (2.6) | 1.1 (2.9) | 0.70 |
| Aspartate aminotransferase, IU/L | 65.7 (221.4) | 55.3 (71.1) | 0.38 |
| Alanine aminotransferase, IU/L | 56.1 (304.6) | 43.9 (56.5) | 0.44 |
| Total bilirubin, mg/dL | 0.75 (1.3) | 0.66 (0.4) | 0.19 |
| Inflammatory markers, median (interquartile range) | |||
| Soluble urokinase plasminogen activator receptor, ng/mL | 7.0 (5.0, 10.7) | 7.7 (5.7, 10.3) | 0.05 |
| C‐reactive protein, mg/dL | 8.0 (4.1, 15.0) | 7.3 (3.8, 13.9) | 0.50 |
| Lactate dehydrogenase, IU/L | 1.4 (1.0, 1.9) | 1.4 (1.1, 1.9) | 0.25 |
| Interleukin‐6, pg/mL | 18.4 (12.5, 94.0) | 12.5 (12.5, 62.4) | 0.75 |
| Procalcitonin, ng/mL | 0.40 (0.17, 1.43) | 0.27 (0.12, 0.91) | 0.06 |
| Ferritin, ng/mL | 659 (273, 1367) | 636 (289, 1268) | 0.49 |
| D‐dimer, FEU mg/L | 0.92 (0.53, 1.91) | 0.87 (0.53, 1.56) | 0.31 |
ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; and IU, international units.
Statistically significant P values at α=0.05.
ACEi/ARB and Inflammatory Biomarkers
In unadjusted analysis, we found no significant differences in admission levels of biomarkers of inflammation between patients on ACEi/ARB and those who were not (Table 1). However, after accounting for the differences in clinical characteristics, ACEi/ARB use was associated with lower levels of suPAR and C‐reactive protein (Table 2).
Table 2.
ACEi/ARB Use and Biomarkers of Thrombo‐Inflammation
| Biomarker, standardized β, P value | |||||||
|---|---|---|---|---|---|---|---|
| SuPAR | C‐reactive protein | Lactate dehydrogenase | Interleukin‐6 | Procalcitonin | Ferritin | D‐dimer | |
| ACEi/ARB use | −0.080, P=0.001* | −0.055, P=0.038* | −0.008, P=0.76 | −0.032, P=0.45 | 0.003, P=0.93 | −0.001, P=0.96 | −0.044, P=0.11 |
| Age | −0.063, P=0.030 | −0.030, P=0.34 | −0.098, P=0.003 | −0.074, P=0.15 | 0.010, P=0.76 | −0.032, P=0.32 | −0.015, P=0.64 |
| Male sex | 0.001, P=0.95 | −0.030, P=0.23 | −0.084, P=0.001 | −0.056, P=0.17 | −0.007, P=0.78 | −0.116, P<0.001 | −0.027, P=0.31 |
| Body‐mass index | 0.078, P=0.002 | 0.065, P=0.015 | 0.081, P=0.004 | −0.004, P=0.94 | −0.025, P=0.37 | 0.015, P=0.57 | −0.022, P=0.43 |
| Black race | −0.021, P=0.38 | 0.049, P=0.05 | 0.099, P<0.001 | −0.026, P=0.52 | 0.012, P=0.66 | 0.077, P=0.003 | −0.034, P=0.20 |
| Diabetes | 0.046, P=0.07 | 0.033, P=0.23 | −0.037, P=0.20 | 0.044, P=0.33 | −0.015, P=0.60 | 0.006, P=0.83 | 0.035, P=0.22 |
| Hypertension | 0.014, P=0.61 | −0.009, P=0.76 | −0.051, P=0.11 | 0.044, P=0.37 | −0.046, P=0.15 | −0.026, P=0.40 | 0.039, P=0.22 |
| Coronary artery disease | 0.023, P=0.36 | 0.001, P=0.98 | −0.028, P=0.32 | −0.032, P=0.47 | −0.008, P=0.79 | −0.042, P=0.13 | −0.025, P=0.37 |
| Heart failure | 0.049, P=0.049 | −0.026, P=0.32 | 0.009, P=0.75 | 0.003, P=0.94 | −0.011, P=0.70 | −0.045, P=0.10 | −0.01, P=0.71 |
| Admission estimated glomerular filtration rate | −0.325, P<0.001 | −0.080, P=0.010 | −0.117, P<0.001 | −0.048, P=0.33 | −0.054, P=0.10 | −0.135, P<0.001 | −0.039, P=0.22 |
ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; and SuPAR, soluble urokinase plasminogen activator receptor.
Statistically significant P values at α=0.05 for associations between ACEi/ARB and biomarkers.
Outcomes
Overall, there were a total of 215 (12.8%) deaths, 407 (24.1%) patients who required mechanical ventilation, and 124 (7.4%) patients who required dialysis during their hospitalization. Patients on an ACEi/ARB during hospitalization had overall lower in‐hospital mortality (13.9% versus 9.9%, P=0.014) and incidence of requiring mechanical ventilation (25.4% versus 20.1%, P=0.037) compared with those who were not on an ACEi/ARB (Figure 1). Differences in the incidence of requiring dialysis between both groups were not statistically significant. In multivariable analysis using binary logistic regression, we found a significant decrease in the odds of in‐hospital death, requiring mechanical ventilation, and dialysis in patients who received ACEi/ARB during their hospitalization (Figure 2). This association was more pronounced after adjusting for age, sex, race, body mass index, and comorbidities. We similarly found ACEi/ARB use to be associated with lower odds of having prolonged hospitalization (>14 days), requiring admission to the intensive care unit, or experiencing acute respiratory distress syndrome (Table S2).
Figure 1. Cumulative incidence of outcomes by in‐hospital use of ACEi/ARB.
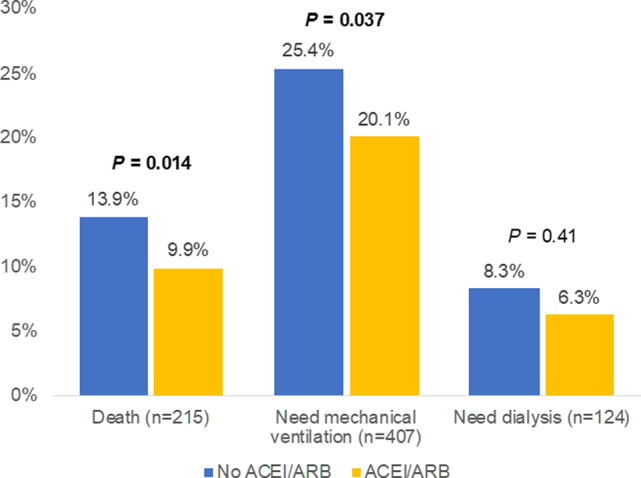
Bar graphs showing the cumulative incidence of death, need for mechanical ventilation, and need for renal replacement therapy by in‐hospital use of ACEi/ARB. ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker.
Figure 2. In‐hospital ACEi/ARB use and risk of death, need for mechanical ventilation, and need for renal replacement therapy.
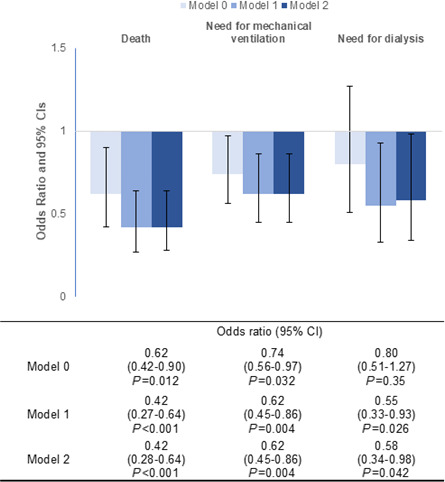
Bar graph depicting the odds ratio (OR) and 95% CI for the 3 different outcomes using 3 different models to calculate odds ratios. Model 0 was unadjusted. Model 1 was adjusted for age, sex, race, BMI, diabetes, hypertension, coronary artery disease, congestive heart failure, admission GFR, and institution. Model 2 incorporated the aforementioned variables in addition to mean arterial pressure on presentation. ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; and GFR, glomerular filtration rate.
When examining the outcomes combined, in‐hospital ACEi/ARB use was associated with lower odds (adjusted odds ratio [OR], 0.49; 95% CI, 0.36–0.66) of death, need for mechanical ventilation, or dialysis (Table 3). The effect size was similar when examining ACEi use (n=213, adjusted OR, 0.46; 95% CI, 0.31–0.67) and ARB use (n=185, adjusted OR, 0.53; 95% CI, 0.36–0.78) separately. The results were unchanged when using Cox proportional hazards modeling in‐hospital ACEi/ARB as a time‐dependent covariate (adjusted hazard ratio [HR], 0.48; 95% CI, 0.36–0.65) (Table 3). Further adjustment with inflammatory biomarkers in the models did not attenuate the association.
Table 3.
Multivariable Analysis of the Association Between In‐Hospital ACEi/ARB Use and Outcomes
| Variables | Death, mechanical ventilation, or dialysis (n=480) | |||
|---|---|---|---|---|
| Odds ratio* (95% CI) | P value | Hazard ratio † (95% CI) | P value | |
| In‐hospital ACEi/ARB use | 0.49 (0.36–0.66) | <0.001 ‡ | 0.48 (0.36–0.65) | <0.001 ‡ |
| Age, per 10 y | 1.00 (0.91–1.08) | 0.90 | 0.94 (0.88–1.00) | 0.06 |
| Male sex | 1.43 (1.14–1.80) | 0.002 ‡ | 1.21 (1.00–1.46) | 0.046 ‡ |
| Black race | 1.29 (0.98–1.71) | 0.07 | 1.27 (1.02–1.59) | 0.030 ‡ |
| Body mass index, per 5 kg/m2 | 1.13 (1.06–1.21) | <0.001 ‡ | 1.07 (1.03–1.11) | <0.001 ‡ |
| Diabetes | 1.12 (0.86–1.46) | 0.41 | 1.08 (0.88–1.33) | 0.46 |
| Hypertension | 1.22 (0.93–1.61) | 0.15 | 1.24 (1.00–1.55) | 0.05 |
| Coronary artery disease | 0.99 (0.69–1.41) | 0.93 | 1.12 (0.84–1.48) | 0.45 |
| Congestive heart failure | 0.96 (0.66–1.39) | 0.82 | 0.83 (0.62–1.11) | 0.21 |
| Admission eGFR, per 5 mL/min higher | 0.95 (0.93–0.98) | <0.001 ‡ | 0.95 (0.94–0.97) | <0.001 ‡ |
ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; and eGFR, estimated glomerular filtration rate.
Based on binary logistic regression model adjusted for age, sex, race, body mass index, diabetes, hypertension, coronary artery disease, congestive heart failure, and admission eGFR.
Based on time‐dependent Cox proportional hazards model adjusted for age, sex, race, body mass index, diabetes, hypertension, coronary artery disease, congestive heart failure, and admission eGFR.
Based on time‐dependent Cox proportional hazards model adjusted for age, sex, race, body mass index, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure, and admission eGFR.
Finally, in estimating a causal effect of ACEi/ARB and accounting for serial blood pressure and creatinine measurements, we found ACEi/ARB use was associated with a lower risk of the composite outcome of death, need for mechanical ventilation or dialysis (HR, 0.35; 95% CI, 0.28– 0.82) (Figure 3).
Figure 3. Weighted Kaplan‐Meier curve comparing survival by in‐hospital ACEi/ARB use.
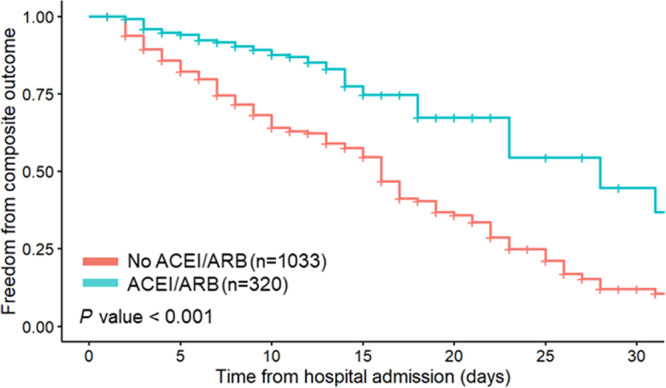
Weighted Kaplan‐Meier curve depicting survival probabilities of combined outcome of death, need for mechanical ventilation, or dialysis by in‐hospital ACEi/ARB over 30 days of hospitalization. Based on marginal structural model with weights accounting for age, sex, race, BMI, diabetes, hypertension, coronary artery disease, congestive heart failure, and serial measurements of blood pressure and serum creatinine. ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; and BMI, body mass index.
Sensitivity Analyses
In sensitivity analyses, the inclusion of patients with prior ACEi/ARB use but discontinued ACEi ARB during hospitalization and those without prior ACEi/ARB use but initiated ACEi/ARB during hospitalization did not influence the results (Table S2). Additionally, we found the association between ACEi/ARB and lower odds of the combined outcome was stronger in patients with chronic kidney disease (P interaction=0.037) but did not differ according to age, sex, race, or other comorbidities (Figure 4). Associations between ACEi/ARB use and outcomes in the overall cohort (n=2044) were consistent with the analysis in our defined subpopulation (Table S2).
Figure 4. Hazard ratio of the combined outcome of death, need for mechanical ventilation or dialysis for in‐hospital ACEi/ARB use stratified by subgroups.
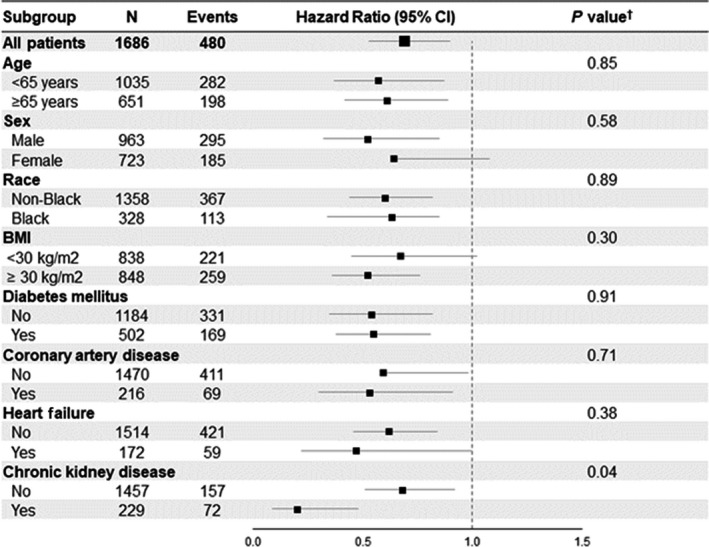
Forest plot showing the hazard ratios for the combined outcome of death, need for mechanical ventilation, or dialysis for in‐hospital ACEi/ARB use stratified by subgroups using a time‐dependent Cox proportional hazards model. † P value for test of interaction. ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; and BMI, body mass index.
DISCUSSION
In this multicenter observational study of patients hospitalized for COVID‐19, patients previously on ACEi/ARB who were treated with ACEi/ARB during their hospitalization had better in‐hospital outcomes compared with those not on ACEi/ARB despite having a significantly higher burden of comorbidities. Prior use of ACEi/ARB was associated with overall lower levels of inflammatory biomarkers on admission when accounting for comorbidities, suggesting an attenuated inflammatory response to SARS‐CoV‐2 in recipients of ACEi/ARB as a potential mechanism for the benefits observed in these patients. These findings support guidelines issued by various medical societies recommending the continued use of these medications as indicated. 33 , 34 Through marginal structural modeling estimating causal effects, we highlight the potential causal link between the use of ACEi/ARB and better COVID‐19 related outcomes, which is currently undergoing study in a randomized clinical trial setting. 26
We address limitations of prior observational studies examining the association between ACEi/ARB and outcomes in several ways. First, we do not limit our analysis to patients with hypertension as was done in most other studies, as there are other indications for the use of ACEi/ARB including diabetes, chronic kidney disease, and congestive heart failure. In sensitivity analysis we found improved outcomes across these relevant patient subgroups. Although other cohorts included all patients with a positive SARS‐CoV‐2 test, we included only patients presenting with and hospitalized primarily for symptomatic COVID‐19—a population that would be the target of a therapeutic trial. We have excluded patients who had ACEi/ARB completely discontinued during their hospitalization and those who were newly started on ACEi/ARB to minimize selection bias, as the former represents a higher risk patient group and the latter a lower risk patient group, which would both skew findings toward the benefits of ACEi/ARB. Additionally, our study is the first that incorporates granular and longitudinal data with daily in‐hospital creatinine and blood pressure values. Lastly, we examined the association between ACEi/ARB and outcomes using several approaches, including marginal structural modeling which allows us to estimate causal inferences while accounting for the most important confounders (serial blood pressure measurements and creatinine levels) and the risk of survivor bias.
The mechanisms by which ACEi/ARB could improve COVID‐19 related outcomes are unclear. Initial fears regarding the risks of ACEi/ARB in COVID‐19 have largely been based on murine and human studies that have shown increased ACE2 expression in various tissues after ACEi/ARB administration 16 , 17 , 18 and the discovery that ACE2 serves as the SARS‐CoV‐2 host receptor. 5 , 35 Conversely, studies have demonstrated that ACE2 has a lung protective effect in patients with acute respiratory distress syndrome, further complicating the overall theoretical role that ACEi/ARB may have in patients with COVID‐19. 15 Our study provides supportive evidence for a beneficial impact of ACEi/ARB in patients with COVID‐19, which may be becauseof its purported lung protective mechanisms. In sensitivity analyses we found the association to be stronger in patients with chronic kidney disease, consistent with its known renoprotective effects in this patient subgroup.
Another potential mechanism for the benefits of ACEi/ARB is through the attenuation of the inflammatory response. ACEi/ARB have previously been shown to attenuate vascular microinflammation in hypertensive patients via angiotensin II receptor blockade and are associated with reduced levels of inflammatory cytokines such as C‐reactive protein, a mechanism that could theoretically counter the inflammatory state of COVID‐19. 12 , 36 , 37 We found use of ACEi/ARB was indeed associated with lower levels of suPAR and C‐reactive protein measured on admission, consistent with the prior observations of an anti‐inflammatory effect for ACEi/ARB. Serial biomarker measurements could shed further light on whether in‐hospital ACEi/ARB use affects the course of the inflammatory response. Lastly, the early diversion of the survival curves in our study suggests that the benefits of ACEi/ARB are likely derived from prior use rather than acute use of ACEi/ARB. Clinical trial evidence for the effectiveness of ACEi/ARB in improving COVID‐19‐related outcomes will spur experimental research to further delineate underlying mechanisms and perhaps identify new indications for the use of ACEi/ARB in the context of the COVID‐19 pandemic.
Limitations
The major limitation of the study is its observational nature. Although we have carefully characterized and adjusted for known confounders in this multipronged analysis, no amount of adjustment can fully account for all potential confounders, and ultimately a randomized study is needed to confirm the benefit of ACEi/ARB in COVID‐19. The University of Michigan M2C2 was unfortunately the only ISIC site with serial blood pressure and creatinine measurements available; however, it is the largest contributing site (n=1357) in which findings were consistent with the overall cohort.
CONCLUSIONS
Among patients hospitalized for symptomatic COVID‐19, use of ACEi/ARB was associated with lower levels of inflammatory markers and lower risk of in‐hospital outcomes after accounting for numerous confounders including serial blood pressure, creatinine measures, and survivor bias. In the absence of acute contraindications to ACEi/ARB such as hypotension or hemodynamic instability, ACEi/ARB should be continued or resumed. Whether patients hospitalized for COVID‐19 without an indication for ACEi/ARB would benefit from treatment warrants evaluation in a clinical trial setting.
Sources of Funding
Vasbinder is supported by a National Heart, Lung, and Blood Institute funded postdoctoral fellowship (T32HL007853). Hayek is funded by National Heart, Lung, and Blood Institute 1R01HL153384‐01, National Institute of Diabetes and Digestive and Kidney Diseases 1R01DK12801201A1, U01‐DK119083‐03S1, and the Frankel Cardiovascular Center COVID‐19: Impact Research Ignitor (U‐M G024231) award. Pop‐Busui is supported by NIDDK‐1‐R01‐DK‐107956‐01, U01 DK119083, the Juvenile Diabetes Research Foundation 5‐COE‐2019‐861‐S‐B, and by a Pilot and Feasibility Grant from the Michigan Diabetes Research Center (National Institutes of Health Grant P30‐DK020572). Tacke is supported through intramural funds from Charité Universitaetsmedizin Berlin and the Berlin Institute of Health. Vasbinder is supported by a National Heart, Lung, and Blood Institute funded postdoctoral fellowship (T32HL007853).
Disclosures
Eugen‐Olsen is a cofounder, shareholder, and chief scientific officer of Virogates. Reiser is cofounder of Trisaq, a biotechnology company developing drugs targeting suPAR. Hayek and Reiser are members of the scientific advisory board of Walden Biosciences. Giamarellos‐Bourboulis has received honoraria from Abbott CH, Angelini Italy, InflaRx GmbH, MSD Greece, XBiotech Inc., and B·R·A·H·M·S GmbH (Thermo Fisher Scientific); independent educational grants from AbbVie Inc, Abbott CH, Astellas Pharma Europe, AxisShield, bioMérieux Inc, Novartis, InflaRx GmbH, and XBiotech Inc; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie‐Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). The remaining authors have no disclosures to report.
Supporting information
Appendix S1. ISIC Investigators
Tables S1–S2
Figure S1
Acknowledgments
The authors acknowledge the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication. The authors are grateful to the services of the Microbiome Core supported by U2CDK110768, especially Chris Blair; the Michigan Clinical Research Unit including Wrenn Woodard and Dexter Hobdy, and the University of Michigan Medical School Central Biorepository for providing biospecimen storage, management, and distribution services in support of the research reported in the publication.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023535
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. WHO COVID‐19 Dashboard. World Health Organization; 2020. Available at: https//covid19.who.int/. Accessed June 30, 2021. [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, Niu P, Yang BO, Wu H, Wang W, Song H, Huang B, Zhu NA, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94:e00127‐20. doi: 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira‐dos‐Santos AJ, da Costa J, Zhang L, Pei Y, et al. Angiotensin‐converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786 [DOI] [PubMed] [Google Scholar]
- 7. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:e1–e9. doi: 10.1161/01.RES.87.5.e1 [DOI] [PubMed] [Google Scholar]
- 8. Rudemiller NP, Crowley SD. Interactions between the immune and the renin‐angiotensin systems in hypertension. Hypertension. 2016;68:289–296. doi: 10.1161/HYPERTENSIONAHA.116.06591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sriramula S, Haque M, Majid DSA, Francis J. Involvement of tumor necrosis factor‐α in angiotensin II‐mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin‐6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1999;34:118–125. doi: 10.1161/01.HYP.34.1.118 [DOI] [PubMed] [Google Scholar]
- 11. Kranzhöfer R, Browatzki M, Schmidt J, Kübler W. Angiotensin II activates the proinflammatory transcription factor nuclear factor‐κB in human monocytes. Biochem Biophys Res Commun. 1999;257:826–828. doi: 10.1006/bbrc.1999.0543 [DOI] [PubMed] [Google Scholar]
- 12. Ferrario CM, Strawn WB. Role of the renin‐angiotensin‐aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059 [DOI] [PubMed] [Google Scholar]
- 13. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sodhi CP, Wohlford‐Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB, Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des‐Arg9 bradykinin/BKB1R axis and facilitates LPS‐induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imai Y, Kuba K, Rao S, Huan YI, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong‐Poi H, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vuille‐dit‐Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, Hamie QM, Meier CF, Hunziker S, Forras‐Kaufmann Z, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE‐inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6 [DOI] [PubMed] [Google Scholar]
- 17. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin‐converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a [DOI] [PubMed] [Google Scholar]
- 18. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- 19. Zhang P, Zhu L, Cai J, Lei F, Qin J‐J, Xie J, Liu Y‐M, Zhao Y‐C, Huang X, Lin L, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, Cai J, Yang R, Han J, Huang Y, et al. Angiotensin II receptor blockers and angiotensin‐converting enzyme inhibitors usage is associated with improved inflammatory status and clinical outcomes in COVID‐19 patients with hypertension [Internet]. 2020. doi: 10.1101/2020.03.31.20038935 [DOI] [PubMed]
- 21. Bean D, Kraljevic Z, Searle T, Bendayan R, Pickles A, Folarin A, Roguski L, Noor K, Shek A, O’gallagher K, et al. Treatment with ACE‐inhibitors is associated with less severe disease with SARS‐Covid‐19 infection in a multi‐site UK acute Hospital Trust. medRxiv. 2020:2020.04.07.20056788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fosbøl EL, Butt JH, Østergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168–177. doi: 10.1001/jama.2020.11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of COVID‐19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin–angiotensin–aldosterone system inhibitors and risk of COVID‐19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, Spatz ES, Murugiah K, Lin Z, Omer SB, et al. Association of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease‐19. medRxiv Prepr Serv Heal Sci. 2020:2020.05.17.20104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ajmera V, Thompson WK, Smith DM, Malhotra A, Mehta RL, Tolia V, Yin J, Sriram K, Insel PA, Collier S, et al. RAMIC: design of a randomized, double‐blind, placebo‐controlled trial to evaluate the efficacy of ramipril in patients with COVID‐19. Contemp Clin Trials. 2021;103:106330. doi: 10.1016/j.cct.2021.106330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, Mallipattu SK, Skopicki HA, Singer AJ, Duong TQ. Continued In‐hospital angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID‐19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222:1256–1264. doi: 10.1093/infdis/jiaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen JB, Hanff TC, William P, Sweitzer N, Rosado‐Santander NR, Medina C, Rodriguez‐Mori JE, Renna N, Chang TI, Corrales‐Medina V, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID‐19: a prospective, randomised, open‐label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll‐Bernardes RJ, dos Santos TM, Mazza L, Feldman A, D’Andréa Saba Arruda G, de Albuquerque DC, Camiletti AS, et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baral R, Tsampasian V, Debski M, Moran B, Garg P, Clark A, Vassiliou VS. Association between renin‐angiotensin‐aldosterone system inhibitors and clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. JAMA Netw Open. 2021;4:e213594. doi: 10.1001/jamanetworkopen.2021.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 33. American Heart Association (AHA), Heart Failure Society of America (HFSA) and American College of Cardiology (ACC) Patients taking ACE‐I and ARBs who contract COVID‐19 should continue treatment, unless otherwise advised by their physician. 2020. Available at: https://newsroom.heart.org/news/patients‐taking‐ace‐i‐and‐arbs‐who‐contract‐covid‐19‐should‐continue‐treatment‐unless‐otherwise‐advised‐by‐their‐physician. Accessed May 5, 2021.
- 34. International Society of Hypertension A statement from the International Society of Hypertension on COVID‐19. 2020. 22. Available at: https://ish‐world.com/a‐statement‐from‐the‐international‐society‐of‐hypertension‐on‐covid‐19/. Accessed May 5, 2021.
- 35. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N‐H, Nitsche A, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E [DOI] [PubMed] [Google Scholar]
- 37. Molnar MZ, Kalantar‐Zadeh K, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Quarles DL, Kovesdy CP. Angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63:650–658. doi: 10.1016/j.jacc.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. ISIC Investigators
Tables S1–S2
Figure S1


