The optimal anticoagulation and antiplatelet management in patients with atrial fibrillation (AF) and a recent acute coronary syndrome or percutaneous coronary intervention (PCI) was examined in AUGUSTUS (An Open‐Label, 2×2 Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban Versus Vitamin K Antagonist and Aspirin Versus Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome or Percutaneous Coronary Intervention). 1 Heart failure (HF) is frequent in this population, and patients with HF have an increased risk for thrombotic events thought to be driven by chronic neurohormonal hyperactivation causing platelet aggregation and reduced fibrinolysis. 2 We therefore examined the treatment effect on bleeding and ischemic outcomes of 2 antithrombotic strategies in patients with AF and a recent acute coronary syndrome or PCI, with and without a clinical history of HF.
The rationale and design of AUGUSTUS have been previously published. 3 Briefly, AUGUSTUS was a prospective, multicenter, 2×2 factorial trial that randomized patients with AF and recent acute coronary syndrome or PCI to apixaban or vitamin K antagonist and to aspirin or placebo for 6 months, on background P2Y12 inhibitors. The primary end point was time to first major or clinically relevant nonmajor bleeding as defined by the International Society on Thrombosis and Haemostasis. Secondary end points included the composite of time to death or time to first hospitalization and the composite of death or ischemic events (stroke, myocardial infarction, stent thrombosis, or urgent revascularization). All bleeding and ischemic events (except for urgent revascularization) were independently adjudicated by a clinical events committee blinded to randomized treatment allocation. The trial protocol was approved by appropriate ethics committees; patients provided written informed consent before participation in the study. The data that support the findings of this study are available from the corresponding author upon reasonable request.
All randomized patients were included and analyzed according to the intent‐to‐treat principle. History of HF was defined clinically by the treating physician upon study enrollment and was not based on left ventricular ejection fraction. Hazard ratios (HRs) were derived from Cox proportional hazard models using the time to the first event up to 6 months of follow up. All end points were adjusted for age, sex, creatinine clearance, hypertension, smoking status, race, weight, prior stroke, use of concomitant P2Y12 inhibitors at randomization, prior use of oral anticoagulation, qualifying index type, and use of β‐blockers, diuretics, or renin‐angiotensin‐aldosterone system blocker therapy at randomization. The interaction between randomized treatment and history of HF was tested.
Of 4614 randomized patients, 1973 (42.7%) had a history of HF. The median age was 70 years and 30% were women, which was comparable in patients with and without a history of HF. Patients with a history of HF had a higher CHA2DS2‐VASc score (4.5 versus 3.5), higher HAS‐BLED score (2.9 versus 2.8), and a lower median left ventricular ejection fraction (45% versus 55%), and were more commonly prescribed β‐blockers, renin‐angiotensin‐aldosterone system blockers, and diuretics (all P<0.001). Patients with a history of HF had a higher risk of cardiovascular death at 6 months compared with those without HF (adjusted HR, 1.73; 95% CI, 1.14–2.64 [P=0.011]), but had similar rates of stroke (adjusted HR, 1.44; 95% CI, 0.72–2.89 [P=0.303]), myocardial infarction (adjusted HR, 0.81; 95% CI, 0.57–1.15 [P=0.228]), definitive or probable stent thrombosis (adjusted HR, 0.5; 95% CI, 0.21–1.16 [P=0.106]), death or rehospitalization (adjusted HR, 1.10; 95% CI, 0.97–1.25 [P=0.124]), and major or clinically relevant nonmajor bleeding (adjusted HR, 1.02; 95% CI, 0.85–1.22 [P=0.832]).
There was no interaction between history of HF and the effect of apixaban versus warfarin on any end point (all P>0.05). In patients with a history of HF, apixaban reduced the risk of major or clinically relevant nonmajor bleeding (adjusted HR, 0.86; 95% CI, 0.52–0.88) and death or rehospitalization (adjusted HR, 0.86; 95% CI, 0.72–1.02) compared with warfarin (Figure [A]). Similarly, there was no interaction between history of HF and the effect of aspirin versus placebo on any end point (all P>0.05). In patients with HF, aspirin increased the risk of major or clinically relevant nonmajor bleeding (adjusted HR, 1.59; 95% CI, 1.23–2.07) without effecting the risk for death or rehospitalization (adjusted HR, 1.02; 95% CI, 0.86–1.21) (Figure [B]).
Figure 1. Efficacy and safety end points stratified by a history of heart failure, use of apixaban vs vitamin K antagonist, and aspirin vs placebo.
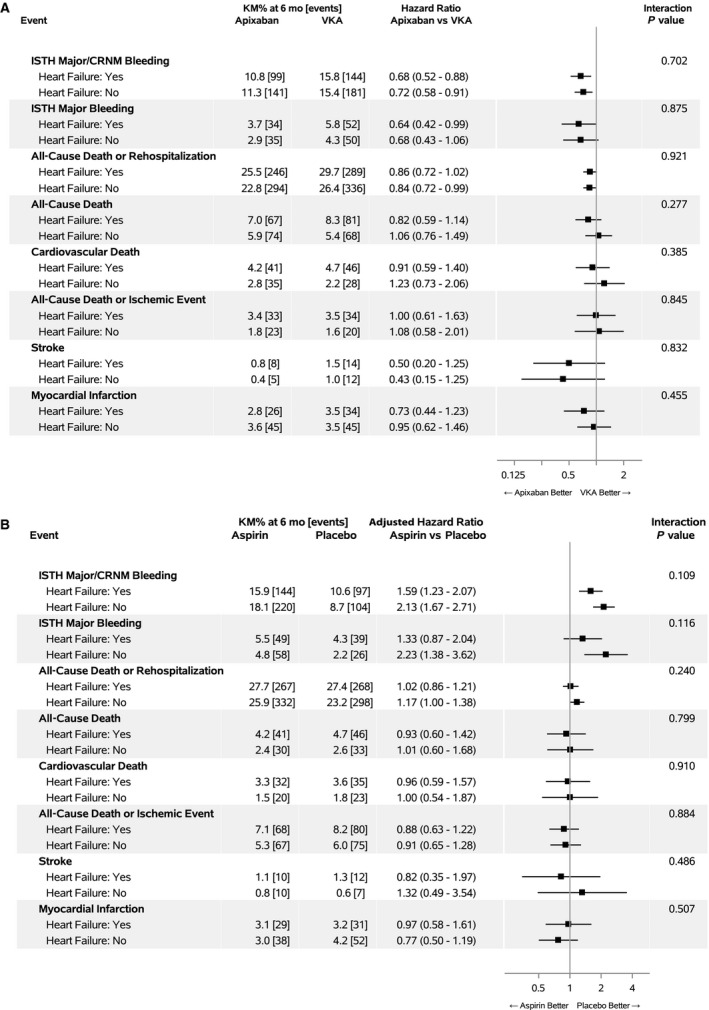
A, Apixaban vs vitamin K antagonist (VKA). B, Aspirin vs placebo. CRNM indicates clinically relevant nonmajor bleeding; ISTH, International Society for Thrombosis and Hemostasis; and KM, Kaplan‐Meier.
Several limitations warrant consideration. History of HF was clinically defined based on presence of signs and symptoms but not independently adjudicated. However, patients classified with HF had obvious evidence for a clinically sicker phenotype. The open‐label treatment with apixaban versus vitamin K antagonist could lead to unintentional bias to admit patients treated with vitamin K antagonists compared with apixaban, especially for bleeding or cardiovascular causes. Bias would not be expected for the blinded aspirin/placebo comparison, which did not demonstrate an overall difference in hospitalization. The increased risk of bleeding with aspirin was reflected by the increase in bleeding‐related hospitalizations.
In AUGUSTUS, HF was the second most commonly documented comorbidity after hypertension. Patients with AF and HF are among the highest risk for stroke and bleeding.
Our findings expand on 2 prior studies that investigated the safety and efficacy of triple therapy with novel oral anticoagulants in patients with AF undergoing PCI (RE‐DUAL PCI [Evaluation of Dual Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting] 4 and PIONEER‐AF PCI [A Study Exploring Two Strategies of Rivaroxaban (JNJ39039039; BAY‐59‐7939) and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention] 5 ). The prespecified analysis of AUGUSTUS demonstrates that a history of HF is common in patients with AF and a recent acute coronary syndrome or PCI, and is associated with a higher risk for cardiovascular death. The novel finding is that irrespective of a history of HF, treatment with a P2Y12 inhibitor and an antithrombotic regimen that included apixaban without aspirin resulted in less major or clinically relevant nonmajor bleeding and fewer deaths and hospitalizations than regimens that included a vitamin K antagonist, aspirin, or both.
Sources of Funding
AUGUSTUS was funded by Bristol Myers Squibb and Pfizer Inc.
Disclosures
Dr Fudim was supported by the National Heart, Lung, and Blood Institute (K23HL151744), the American Heart Association (grant no. 20IPA35310955), Mario Family Award, Duke Chair’s Award, Translating Duke Health Award, Bayer, and BTG Specialty Pharmaceuticals. He receives consulting fees from AxonTherapies, Bodyport, CVRx, Daxor, Edwards LifeSciences, Fire1, NXT Biomedical, Zoll, and Viscardia. Dr Alexander received institutional research grants and consulting fees/honoraria from Bristol Myers Squibb and CSL Behring; institutional research grants from AstraZeneca, CryoLife, US Food and Drug Administration, National Institutes of Health, Sanofi, VoluMetrix, and Boehringer Ingelheim; and consulting fees/honoraria from Pfizer, AbbVie Pharmaceuticals, NovoNordisk, Portola Pharmaceuticals, Quantum Genetics, Teikoku Pharmaceuticals, VA Cooperative Studies Program, and Zafgen. Dr Mehran received institutional research grants from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol‐Myers Squibb/Sanofi, CSL Behring, Eli Lilly/Daiichi Sankyo, Medtronic, Novartis, and OrbusNeich; consulting fees from Boston Scientific, Abbott Vascular, Medscape, Siemens Medical Solutions, Roivant Sciences Inc, and Sanofi; consulting (no fees) from Regeneron Pharmaceuticals Inc; institutional consulting fees from Abbott Vascular, Spectranetics/Phillips/Volcano Corporation, Bristol‐Myers Squibb, Novartis, and Watermark Research; is an executive committee member for Janssen Pharmaceuticals and Bristol‐Myers Squibb; and has <1% equity in Claret Medical and Elixir Medical. Dr Granger received research grants from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Pfizer, Armetheon, AstraZeneca, US Food and Drug Administration, GlaxoSmithKline, The Medicines Company, Medtronic Foundation, Medtronic Inc, and Novartis; consulting fees from Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Pfizer, Abbvie, Armetheon, Astra Zeneca, Eli Lilly, Gilead, GlaxoSmithKline, Hoffmann‐La Roche, The Medicines Company, National Institutes of Health, Novartis, Sirtex, Verseon, Apple, Medscape, LLC, Merck, Novo Nordisk, Roche Diagnostics, and Rho Pharmaceuticals. Dr Bahit received lecture fees from Bristol Myers Squibb and Pfizer; and consulting fees from Merck Sharp & Dohme and CSL Behring. Dr Halvorsen reports speaker fees from Bayer, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Sanofi, and Merck. Dr Vinereanu reports research grants and honoraria from Pfizer, Boehringer Ingelheim, Bayer, Amgen, and CSL Behring. Dr Windecker received institutional research and educational grants from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. Dr Aronson is an employee of Bristol Myers Squibb. Dr Lopes received institutional research grants and consulting fees from Bristol Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and consulting fees from Amgen, Bayer, and Boehringer Ingelheim. Dr Wojdyla has no disclosures to report.
For Sources of Funding and Disclosures, see page 3.
REFERENCES
- 1. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 2. Jug B, Vene N, Salobir BG, Sebestjen M, Sabovic M, Keber I. Procoagulant state in heart failure with preserved left ventricular ejection fraction. Int Heart J. 2009;50:591–600. doi: 10.1536/ihj.50.591 [DOI] [PubMed] [Google Scholar]
- 3. Lopes RD, Vora AN, Liaw D, Granger CB, Darius H, Goodman SG, Mehran R, Windecker S, Alexander JH. An open‐label, 2 x 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: rationale and design of the AUGUSTUS trial. Am Heart J. 2018;200:17–23. doi: 10.1016/j.ahj.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Bhatt DL, Oldgren J, Lip GY, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 5. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594 [DOI] [PubMed] [Google Scholar]


