Abstract
Background
Currently, there is limited research on the prognostic value of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) as a biomarker in COVID‐19. We proposed the a priori hypothesis that an elevated NT‐proBNP concentration at admission is associated with increased in‐hospital mortality.
Methods and Results
In this prospective, observational cohort study of the American Heart Association’s COVID‐19 Cardiovascular Disease Registry, 4675 patients hospitalized with COVID‐19 were divided into normal and elevated NT‐proBNP cohorts by standard age‐adjusted heart failure thresholds, as well as separated by quintiles. Patients with elevated NT‐proBNP (n=1344; 28.7%) were older, with more cardiovascular risk factors, and had a significantly higher rate of in‐hospital mortality (37% versus 16%; P<0.001) and shorter median time to death (7 versus 9 days; P<0.001) than those with normal values. Analysis by quintile of NT‐proBNP revealed a steep graded relationship with mortality (7.1%–40.2%; P<0.001). NT‐proBNP was also associated with major adverse cardiac events, intensive care unit admission, intubation, shock, and cardiac arrest (P<0.001 for each). In subgroup analyses, NT‐proBNP, but not prior heart failure, was associated with increased risk of in‐hospital mortality. Adjusting for cardiovascular risk factors with presenting vital signs, an elevated NT‐proBNP was associated with 2‐fold higher adjusted odds of death (adjusted odds ratio [OR], 2.23; 95% CI, 1.80–2.76), and the log‐transformed NT‐proBNP with other biomarkers projected a 21% increased risk of death for each 2‐fold increase (adjusted OR, 1.21; 95% CI, 1.08–1.34).
Conclusions
Elevated NT‐proBNP levels on admission for COVID‐19 are associated with an increased risk of in‐hospital mortality and other complications in patients with and without heart failure.
Keywords: biomarker, COVID‐19, critical care, mortality/survival, NT‐proBNP
Subject Categories: Biomarkers, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Cardiopulmonary Arrest
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- MACEs
major adverse cardiac events
Clinical Perspective
What Is New?
In our prospective nationwide cohort study, an elevated NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) concentration on admission for COVID‐19 was associated with an increased risk of in‐hospital mortality, intubation, and intensive care unit admission in patients with and without heart failure.
After controlling for cardiovascular risk factors and presenting vital signs, risk for in‐hospital mortality demonstrated an increasing relationship with serum concentrations of NT‐proBNP; and after adjusting for other biomarkers, NT‐proBNP concentrations independently predicted the risk of death from COVID‐19.
What Are the Clinical Implications?
Measurement of NT‐proBNP at admission in patients hospitalized with COVID‐19 infection may be useful as a tool to prognosticate clinical course and guide resource management.
Further studies are needed in healthier patients with milder COVID‐19 symptoms and in outpatient settings to delineate the overall predictive power of natriuretic peptides in COVID‐19.
Future efforts examining the trend of NT‐proBNP during hospitalization may be helpful in tracking disease course and response to treatment.
Among patients hospitalized with COVID‐19, older patients and those with risk factors for cardiovascular disease (CVD) such as obesity, hypertension, diabetes, and coronary artery disease experience worse outcomes. 1 In line with this observation, cardiac injury as measured by elevation in cardiac troponin is associated with a higher risk of mortality in patients hospitalized with COVID‐19. 2 However, markers of myocardial stress, such as BNP (B‐type natriuretic peptide) and NT‐proBNP (N‐terminal proBNP), are not routinely assessed in patients admitted for COVID‐19, and limited data on the prognostic role of natriuretic peptides are available.
NT‐proBNP is an established biomarker for diagnosing and monitoring heart failure (HF), 3 , 4 , 5 , 6 ischemic heart disease, 7 , 8 and myocardial injury. 9 NT‐proBNP also carries prognostic value in noncardiac conditions such as lung disease. For example, in patients with chronic obstructive pulmonary disease without a history of HF, NT‐proBNP is associated with an increased risk of exacerbations and cardiopulmonary death. 10 , 11 , 12 A similar relationship may exist between COVID‐19 and NT‐proBNP.
Previous studies have suggested that elevated levels of NT‐proBNP correlate with worse outcomes from COVID‐19, but the small sample sizes and limited analyses in these studies reduce generalizability. 13 , 14 , 15 , 16 The aim of this study was to evaluate a large, nationwide database to better define the relationship between NT‐proBNP and clinical outcomes in patients hospitalized for COVID‐19. We defined an a priori hypothesis that elevated NT‐proBNP concentrations on admission would be associated with increased rates of in‐hospital mortality in those with and without HF.
Methods
Data and materials used within this study are available at https://precision.heart.org/ by request from the corresponding author and the American Heart Association (AHA).
This study was reviewed by our institutional review committee, which deemed the project to fall under a quality improvement exemption, like other AHA Get With The Guidelines program efforts.
Study Design
The present study is a prospective observational cohort study using the COVID‐19 CVD Registry Powered by the AHA’s Get With The Guidelines program. Inclusion criteria in the COVID‐19 CVD Registry were aged ≥18 years with SARS‐CoV‐2 infection confirmed by reverse transcriptase–polymerase chain reaction or positive IgM serology before or during the index hospitalization between January 23 and November 28, 2020, from 107 hospitals in the United States regardless of history of CVD (n=21 528). For the purpose of this analysis, we focused on patients who had NT‐proBNP measured as part of routine clinical care (n=4675; 21.7%). Seventy‐six of 107 participating hospitals nationwide (71.0%) regularly reported NT‐proBNP values. Patients with BNP data only were not included (n=4042; 18.7%). All patients without an NT‐proBNP or BNP value were treated as the reference cohort (n=13 039; 60.5%).
Data Collection
IQVIA (Parsippany, NJ) served as the data and coordination center for the nationwide data collection. Trained clinical personnel populated the registry data using standardized definitions for patient demographic characteristics, clinical comorbidities, inpatient laboratory data, treatments during admission, and in‐hospital outcomes.
Trained data abstracters recorded demographic data such as age, sex, body mass index, and past medical history such as diabetes; chronic kidney disease, atrial fibrillation, hypertension, HF, previous coronary artery bypass graft, percutaneous coronary intervention, and myocardial infarction. Presenting labs including NT‐proBNP, creatinine, troponin, C‐reactive protein, ferritin, d‐dimer, and procalcitonin were collected when performed as part of clinical care. Vitals signs on admission such as heart rate, respiratory rate, diastolic and systolic blood pressure, oxygen saturation, and need for supplemental oxygen were compiled.
Outcomes
The primary outcome for this analysis was in‐hospital mortality. Secondary outcomes included major adverse cardiovascular events (MACEs), which was defined as death, acute myocardial infarction, stroke, shock, new‐onset acute HF, or myocarditis; and individual rates of intubation, intensive care unit (ICU) admission, shock, cardiac arrest, new‐onset acute HF, and time from admission until death were examined.
Statistical Analysis
Data analysis was performed on the AHA Precision Medicine Platform (https://precision.heart.org/) using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) for reproducibility and public access. All patients with an NT‐proBNP value measured within the first 2 days of hospitalization were categorized into those with normal versus elevated NT‐proBNP using established age‐specific heart failure cutoffs (<50 years old, 450 pg/mL; 50–75 years old, 900 pg/mL; >75 years old, 1800 pg/mL). 17 Additionally, NT‐proBNP levels were analyzed categorically by quintile (n=935 per quintile) and continuously as a log‐transformed variable. Independent t‐ or Mann‐Whitney U tests were performed as applicable for continuous variables and χ 2 tests were used to compare categorical variables, respectively, between the age‐adjusted groups and quintiles. Kruskal‐Wallis rank‐sum and pairwise comparisons of Wilcoxon rank‐sum tests were used to determine differences in factors between the various groups of NT‐proBNP elevation as well as those patients without NT‐proBNP or BNP values. Continuous data were expressed as median (25th, 75th percentiles) values while categorical data were expressed as proportions. Variables found by univariable logistic regression to be associated with outcome were included in multivariable logistic regression models: male sex, age, body mass index, serum creatinine, and previous diagnoses of atrial fibrillation, HF, diabetes, and hypertension, as well as admission vital signs (heart rate, diastolic and systolic blood pressure, respiratory rate, and need for supplemental oxygen). A comprehensive multivariable regression model was constructed to include the above variables with other biomarkers, for which log transformations were performed for heart rate, diastolic blood pressure, systolic blood pressure, respiratory rate, troponin, d‐dimer, C‐reactive protein, procalcitonin, and NT‐proBNP to normalize the varying scales of continuous variables. The log‐transformed adjusted odds ratios (OR) for NT‐proBNP are relative to 2‐fold increases in the continuous NT‐proBNP value to provide clinical context. Receiver operating characteristic curves with area under the curve, specificity, and sensitivity were used to evaluate the performance of the regression models. To explore the nonlinear relationship between NT‐proBNP concentrations and the probability of death, we used restricted cubic spline modeling with logarithmic smoothing in the rms package in R with NT‐proBNP values >12 000 pg/mL truncated because of sparsity and instability in the data beyond that level. To illustrate the distribution of data in the cubic spline, a rug plot was overlaid on the x axis, which was restricted to 3000 pg/mL of NT‐proBNP to display the highest density of data and increase in probability of death. For all analyses, P<0.05 was considered significant.
Results
Patient Characteristics and Presenting Biomarker Levels
A total of 4675 patients (21.7%) hospitalized for COVID‐19 with an NT‐proBNP value measured on admission were included in our main analyses. The characteristics of admissions with an NT‐proBNP value included in this analysis are compared with those without either a BNP or NT‐proBNP value (n=13 039; 60.5%) in Table S1. Among patients with an admission NT‐proBNP, the median concentration was 299 pg/mL (25th, 75th percentiles: 75 and 1608) and, using age‐specific cut points, NT‐proBNP was elevated in 29% (n=1344). Patients with an elevated NT‐proBNP were older, had lower body mass index, and were more likely to have a history of diabetes, chronic kidney disease, or an underlying cardiac history including hypertension or HF (Table 1). Compared with patients with NT‐proBNP below the age‐specific cutoffs, patients with elevated NT‐proBNP had higher serum creatinine levels, increased cardiac troponin values, greater concentrations of inflammatory biomarkers such as C‐reactive protein, d‐Dimer, and procalcitonin (all P<0.001; Table 1). Patients with elevated NT‐proBNP had mildly lower heart rates and blood pressures, and more required oxygen on presentation than patients presenting with normal serum concentrations (all P<0.01; Table 1).
Table 1.
Patient Characteristics According to Admission NT‐proBNP
|
Demographics, n or median (Q1 − Q3) |
Normal NT‐proBNP (n=3331) |
Elevated NT‐proBNP (n=1344) |
P value |
|---|---|---|---|
| Male | 1828 (54.9) | 735 (54.7) | 0.931 |
| Age, y | 63.0 (52.0–75.0) | 72.0 (61.0–83.0) | <0.001 |
| BMI, kg/m2 | 30.4 (25.9–35.7) | 27.6 (23.7–32.9) | <0.001 |
| CKD | 293 (8.8) | 463 (34.5) | <0.001 |
| Diabetes | 1224 (36.7) | 651 (48.4) | <0.001 |
| Heart failure | 301 (9.0) | 545 (40.6) | <0.001 |
| Hypertension | 2011 (60.4) | 1087 (80.9) | <0.001 |
| CABG/PCI/MI | 289 (8.7) | 103 (7.7) | 0.053 |
| Presenting labs | |||
| NT‐proBNP, pg/mL | 139 (50–366) | 4130 (2124–12 029) | <0.001 |
| Creatinine, mg/dL | 0.99 (0.76–1.30) | 1.60 (1.08–2.87) | <0.001 |
| Troponin, ng/L | 10.0 (0.0–30.0) | 50.0 (20.0–110.0) | <0.001 |
| CRP, mg/L | 70.0 (20.9–130.0) | 79.5 (22.3–159.0) | 0.011 |
| Ferritin, ng/mL | 567 (259–881) | 649 (307–1366) | <0.001 |
| d‐Dimer, ng/mL | 800 (400–1560) | 1428 (600–3094) | <0.001 |
| Procalcitonin, ng/mL | 0.14 (0.07–0.32) | 0.38 (0.14–1.50) | <0.001 |
| Vitals on admission | |||
| Heart rate, bpm | 93 (80–106) | 90 (76–105) | <0.001 |
| Systolic blood pressure, mm Hg | 132 (117–147) | 129 (111–148) | 0.003 |
| Diastolic blood pressure, mm Hg | 76 (67–85) | 72 (62–84) | <0.001 |
| Respiratory rate | 20 (18–24) | 20 (18–25) | 0.014 |
| SpO2% | 95 (92–97) | 95 (91–98) | 0.143 |
| Required supplemental oxygen | 916/2545 (36.0) | 562/1128 (49.8) | <0.001 |
BMI indicates body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CRP, C‐reactive protein; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; and SpO2%, oxygen saturation.
Elevated NT‐proBNP Associated With Worse Outcomes
The incidence of the primary outcome of in‐hospital mortality was significantly higher in patients with elevated NT‐proBNP (37% versus 16%; P<0.001; Table 2). Overall, the median time to death was shorter in the elevated NT‐proBNP cohort (7 versus 9 days; P<0.001; Table 2). Secondary outcomes, including the rates of MACEs, need for intubation, ICU admission, reported episodes of shock, cardiac arrest, and new‐onset acute HF, were each higher in patients with elevated as compared with normal NT‐proBNP (all P<0.001; Table 2).
Table 2.
Outcomes Stratified by Admission NT‐proBNP
|
Outcomes n (%) or median (Q1 − Q3) |
Normal NT‐proBNP (n=3331) |
Elevated NT‐proBNP (n=1344) |
P value |
|---|---|---|---|
| In‐hospital mortality | 543 (16.3) | 494 (36.8) | <0.001 |
| MACEs | 658 (19.8) | 603 (44.9) | <0.001 |
| Intubation | 689 (20.7) | 423 (31.5) | <0.001 |
| ICU admission | 1094 (32.8) | 632 (47.0) | <0.001 |
| Shock | 337 (10.1) | 271 (20.2) | <0.001 |
| Cardiac arrest | 178 (5.3) | 158 (11.8) | <0.001 |
| New acute heart failure | 72 (2.2) | 80 (6.0) | <0.001 |
| Time to death, d | 9.0 (5.0–11.8) | 7.0 (3.0–13.0) | <0.001 |
ICU indicates intensive care unit; MACEs, major adverse cardiac events as defined as death, acute myocardial infarction, stroke, shock, new onset heart failure, or myocarditis; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Additionally, analysis by quintiles of NT‐proBNP concentration revealed a significant increasing gradient in the risk of in‐hospital death from comparatively low risk (7%) in quintile 1 (NT‐proBNP <55 pg/mL) up to 40% in quintile 5 (>2385 pg/mL; Table 3 ). Analysis of MACEs, intubation, shock, and ICU admissions also revealed a progressive gradient with rising quintiles of NT‐proBNP (Table 3). The continuous relationship between admission NT‐proBNP level and the probability of in‐hospital mortality is illustrated in Figure 1.
Table 3.
Outcomes Stratified by Quintiles of NT‐proBNP
|
Outcomes n (%) or median (Q1 − Q3) |
Quintile 1 NT‐proBNP (10–55 pg/mL) (n=935) |
Quintile 2 NT‐proBNP (55–175 pg/mL) (n=935) |
Quintile 3 NT‐proBNP (175–545 pg/mL) (n=935) |
Quintile 4 NT‐proBNP (545–2385 pg/mL) (n=935) |
Quintile 5 NT‐proBNP (>2385 pg/mL) (n=935) |
|---|---|---|---|---|---|
| In‐hospital mortality | 66 (7.1) | 114 (12.2) | 192 (20.5) | 289 (30.9) | 376 (40.2) |
| MACEs | 83 (8.9) | 155 (16.6) | 224 (24.0) | 339 (36.3) | 460 (49.2) |
| Intubation | 125 (13.4) | 197 (21.1) | 237 (25.3) | 266 (28.4) | 287 (30.7) |
| ICU admission | 213 (22.9) | 316 (33.8) | 373 (39.9) | 392 (41.9) | 432 (46.2) |
| Shock | 49 (5.2) | 89 (9.5) | 126 (13.5) | 160 (17.1) | 184 (19.7) |
| Cardiac arrest | 32 (3.5) | 55 (5.9)** | 48 (5.1)* | 83 (8.9) | 118 (12.6) |
| New acute heart failure | 1.4% (13) | 2.4% (22)* | 2.5% (23)* | 3.5% (33)** | 6.5% (61) |
| Time to death, d | 12.0 (6.0–20.8) | 9.0 (4.0–14.3)* | 10.0 (6.0–17.0)* | 8.0 (4.0–15.0)** | 6.0 (3.0–13.7) |
ICU indicates intensive care unit; MACEs, major adverse cardiac events as defined as death, acute myocardial infarction, stroke, shock, new onset heart failure, or myocarditis; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide. Quintiles formed from whole NT‐proBNP cohort (n=4675). All comparisons to the referent quintile 1 were highly significant (P<0.001) with the exception of those marked as *P=NS, **P<0.05.
Figure 1. Probability of death by continuous baseline NT‐proBNP. Restricted cubic spline modeling for the probability of in‐hospital mortality across varying levels of NT‐proBNP admission value.
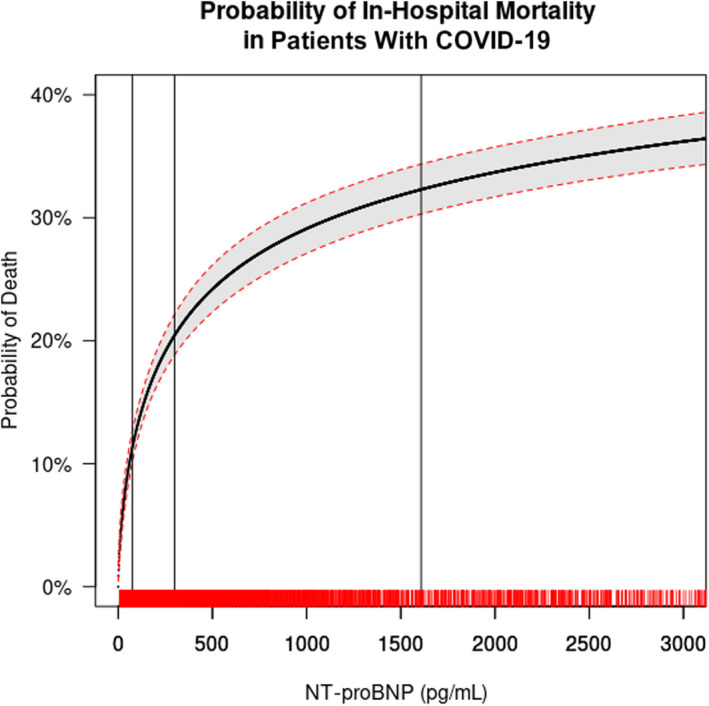
The solid black line indicating probability and the shaded area representing 95% CI. The vertical lines indicate the interquartile (IQR: 25th=74.55 pg/mL, 50th=299.0 pg/mL, and 75th=1608.0 pg/mL) of NT‐proBNP values. The C‐statistic for the model was 0.704, knots located at 200, 600 and 1000 pg/mL with logarithmic smoothing of the curve. A rug plot on the x axis displays the density of the data. The presentation of the x axis was restricted to NT‐proBNP values <3000 pg/mL to display the highest density of data and increased in probability of death. NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide.
In a subgroup analysis of patients without a history of HF, elevated NT‐proBNP levels were significantly associated with a higher rate of in‐hospital mortality (38% versus 16%; P<0.001; Table S2, Figure 2A). Those with a history of HF but normal NT‐proBNP had similar rates of mortality as those without HF and a normal NT‐proBNP (Figure 2A). Additionally, the rate of in‐hospital mortality progressively increased in patients without a HF history as the quintile of NT‐proBNP increased (Figure 2B). Rates of MACEs, intubation, ICU admission, shock, and cardiac arrest were higher in the patients without a history of HF but with an elevated admission NT‐proBNP (all P<0.001; Table S2).
Figure 2. In‐hospital mortality stratified by HF diagnosis and NT‐proBNP elevation.
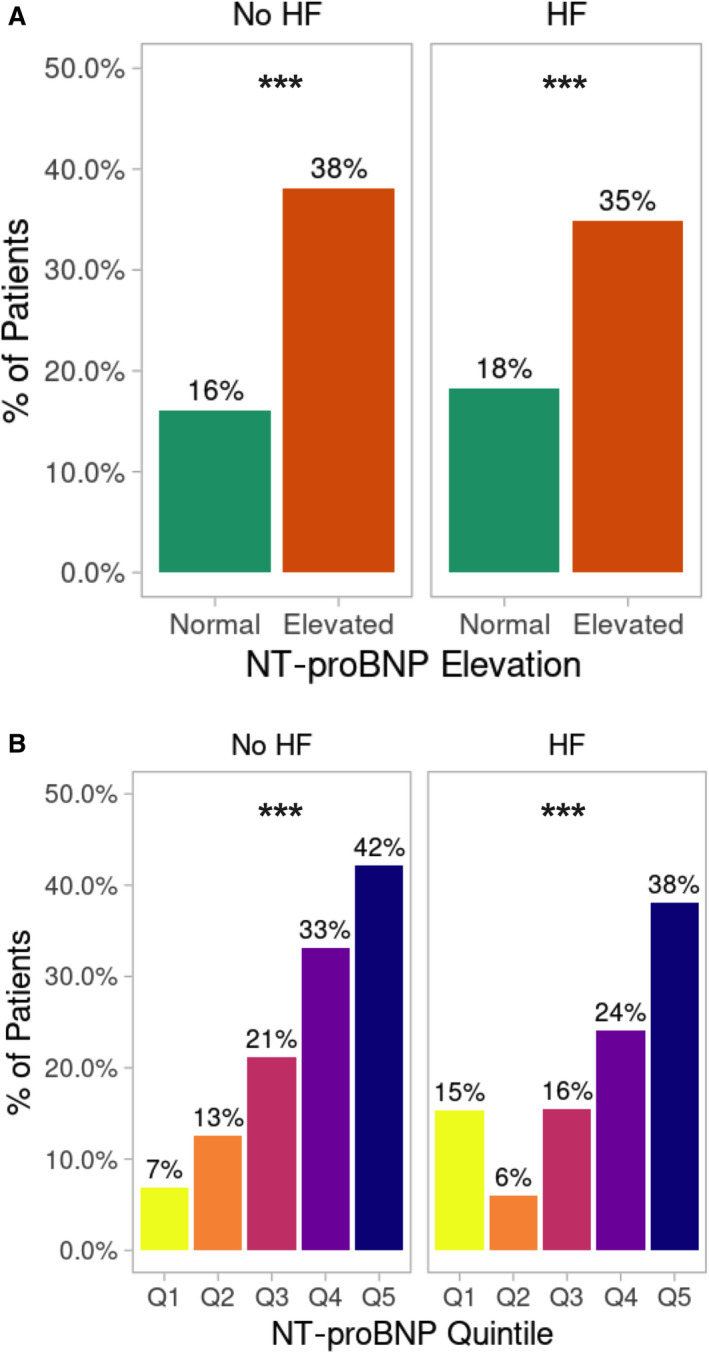
A, The percentage of patients with in‐hospital mortality stratified by previous HF diagnosis (no HF: n=3829) and known HF (n=846), which was analyzed by Kruskal‐Wallis χ2 test for normal vs elevated NT‐proBNP (no HF: normal NT‐proBNP, n=3030; elevated NT‐proBNP, n=799; HF: normal NT‐proBNP, n=301; elevated NT‐proBNP, n=545). B, The percentage of patients with in‐hospital mortality stratified by previous HF diagnosis (no HF: n=3829) and known heart failure (n=846), analyzed by χ2 test for the overall difference between quintiles (no HF: each quintile, n=766; HF: each quintile, n=169). ***P<0.001. HF indicates heart failure; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Multivariable Analysis of In‐Hospital Mortality
Compared with patients with an NT‐proBNP below the age‐specific cutoff, patients with an elevated NT‐pro‐BNP level on admission had a nearly 3‐fold higher unadjusted odds of death during the hospitalization (odds ratio, 2.98; 95% CI, 2.58–3.45; P<0.001; Table S3). After adjusting for potential confounding factors, an elevated NT‐proBNP remained associated with higher odds of death (adjusted OR, 2.23; 95% CI, 1.80–2.76; P<0.001; Figure 3). The increasing gradient of mortality risk across quintiles of NT‐proBNP concentration also remained significant beginning with quintile 2 when controlled for clinical characteristics and vitals (Figure 3). Moreover, after further adjusting for the continuous NT‐proBNP value with presenting vitals and other biomarkers by log transformation, every 2‐fold increase in the NT‐proBNP concentration was associated with an increase in the relative odds of death by 21% (adjusted OR, 1.21; 95% CI, 1.08–1.34; P<0.001; Figure 3). The receiver operating characteristic curves for the regression models performed better with NT‐proBNP included, where, for example, the area under the curve for the model with log‐transformed values for NT‐proBNP, vitals, and biomarkers improved to an area under the curve of 0.804 (95% CI, 0.764–0.844) with a sensitivity of 70.77% and specificity of 78.31% for predicting in‐hospital mortality (Figure S1).
Figure 3. Adjusted relative odds of in‐hospital death by category of admission NT‐proBNP.
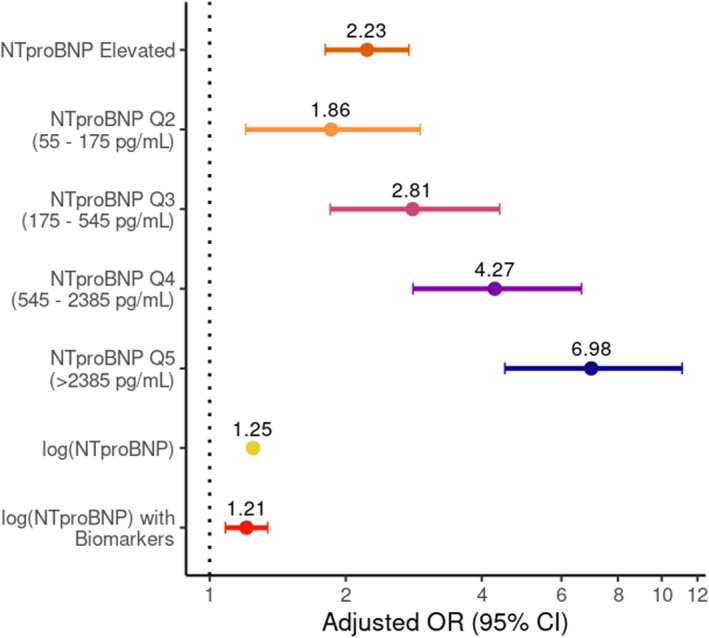
Multivariable logistical regressions all included adjustments for age, body mass index, sex, creatinine (mg/dL) and history of atrial fibrillation, chronic kidney disease, diabetes, hypertension, and previous heart failure diagnosis, in addition to presenting vital signs (heart rate, systolic and diastolic blood pressure, respiratory rate and need for supplemental oxygen). Elevated NT‐proBNP is compared with normal NT‐proBNP and NT‐proBNP quintiles are compared to quintile 1 (Q1) as the referent (n=3222). The same variables were included in models adjusting for log transformations of the continuous NT‐proBNP (pg/mL) values, presenting vitals (heart rate, systolic and diastolic blood pressure, and respiratory rate) (n=3222), and other COVID‐19 biomarkers (n=950) such as d‐dimer (ng/mL), C‐reactive protein (mg/L), procalcitonin (ng/mL), and troponin (ng/L) so the NT‐proBNP adjusted odds ratios can be interpreted per a 2‐fold increase in the NT‐proBNP concentration (pg/mL). The model area under the curve using elevated NT‐proBNP is 0.764 (95% CI, 0.745–0.784); NT‐proBNP quintiles, 0.774 (95% CI, 0.755–0.793); log (NT‐proBNP), 0.776 (95% CI, 0.758–0.795); and log (NT‐proBNP) with biomarkers, 0.804 (95% CI, 0.764–0.844). NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; and OR, odds ratio.
Discussion
In this large national cohort study, elevated NT‐proBNP was associated with a steep rising gradient of in‐hospital mortality, as well as higher rates of MACEs, intubations, and ICU admission. After controlling for other known risk factors, we found that elevated NT‐proBNP was associated with 2‐fold higher odds of in‐hospital mortality. Findings were similar in patients with and without prior HF. These results suggest that NT‐proBNP may be useful to predict the risk of mortality and MACEs in hospitalized patients with COVID‐19.
The prognostic value of NT‐proBNP in COVID‐19 was first queried by Gao et al, 14 who showed that levels above 88 pg/mL were associated with increased mortality, but the reported threshold value falls within the normal range for the test, which made the application of this finding difficult. A prospective study of 135 patients in Norway by Omland et al 18 similarly showed higher rates of mortality and ICU admission with increased NT‐proBNP values; however, this relationship was no longer significant after multivariable adjustment for clinical characteristics. More recently, Caro‐Codón et al 15 also showed greater all‐cause mortality with elevated NT‐proBNP in patients with and without HF in Spain (n=396). However, these single‐center studies (n=54–396) with seemingly inconsistent results have left unanswered questions regarding the relationship of NT‐proBNP with other important outcomes in COVID‐19 and the spectrum of risk across clinically relevant cutoffs. In addition, these studies may have had limited generalizability to the diverse patient population in the United States.
The size and diversity of the AHA COVID‐19 Registry nationwide cohort contributes to the strengths of our study in characterizing the association between NT‐proBNP elevations and poor outcomes in COVID‐19. After controlling for presenting vital signs and clinical characteristics, NT‐proBNP elevations independently predicted a >2‐fold higher odds of death, which rose to almost a 7‐fold higher rate of mortality in quintile 5 (NT‐proBNP >2385 pg/mL). Further adjustments for inflammatory and cardiac biomarkers, NT‐proBNP independently predicted a 21% higher odds of death for each 2‐fold increase in the natriuretic peptide. When considered together with the association with increased rates of intubation and ICU admissions, our findings suggest that NT‐proBNP could be used as a tool to guide allocation of ICU beds.
Extending on prior COVID‐19 studies showing worse clinical outcomes in those with HF, regardless of ejection fraction, we demonstrate that mortality rates correlate between patients with and without HF on the basis of their admission NT‐proBNP level, and only a small proportion were diagnosed with new‐onset HF. 19 , 20 Recent, small, single‐center studies by Selçuk et al 21 , 22 and Yoo et al 21 , 22 have demonstrated increased risk of mortality from COVID‐19 with even “borderline elevations” of NT‐proBNP in patients without heart failure. These findings argue against the heightened risk associated with natriuretic peptides being driven solely by exacerbation of preexisting HF or presentations with new‐onset HF from COVID‐19 infection and suggests possible other mechanisms. 19 , 20 , 23 Echocardiographic analyses of cardiac function in patients with COVID‐19 by Bhatia et al 24 , 25 and Gibson et al, 24 , 25 both showed worse overall longitudinal strain patterns suggesting new subclinical dysfunction related to COVID‐19, but no clear mechanism was elucidated, and further studies are needed.
The possibility that alternative drivers to NT‐proBNP elevations exist is a novel concept. Fish‐Trotter et al 26 were the first to show that inflammatory cytokines such as interleukin‐6 can drive increases in NT‐proBNP outside of COVID‐19. Qin et al 27 demonstrated that dynamic changes in cardiac biomarkers such as NT‐proBNP coincided with inflammatory marker elevations in COVID‐19. Additionally, it has been previously shown that patients admitted for septic shock have elevations in serum natriuretic peptide concentrations similar to patients admitted with cardiogenic shock despite having significantly lower precapillary wedge pressures and higher cardiac outputs. 28 Whether elevated natriuretic peptide levels in septic shock are driven by acute changes in cardiac loading conditions or represent a biochemical response to sepsis is unknown. Together, these observations suggest a possible intriguing multifactorial mechanism for NT‐proBNP elevations with concomitant cardiac dysfunction in the extreme inflammatory state of COVID‐19. Further study is warranted before conclusions can be drawn and is outside the scope of the current study. However, future research that examines the relationship between inflammatory markers, natriuretic peptides, and cardiopulmonary function could provide additional insight into the pathophysiology of the cardiovascular complications of COVID‐19.
As with any observational study, our study is limited by the scope and depth of data collected. A source of bias in our study is that a majority of the patients in the COVID‐19 CVD Registry did not routinely have an NT‐proBNP level drawn on admission, which selected for an older, sicker presenting population with more comorbidities as demonstrated in Table S1. Additionally, differences in clinical practice were evident as some facilities measured NT‐proBNP in >80% of admissions for COVID‐19, while other facilities measured NT‐proBNP in 1% of cases contributed to the COVID‐19 CVD Registry. Each of the 76 participating sites contributed an average of 1.4% (95% CI, 0.2–1.8%) of the total NT‐proBNP cases in the database and no one facility significantly weighted the results. The large, national scale of the database should theoretically reduce other sampling biases.
Given the strength of the signal in this large nationwide data set, we believe our study demonstrates the utility and prognostic value of elevated NT‐proBNP levels in predicting mortality in patients hospitalized with severe COVID‐19. However, further prospective studies defining the prognostic value of NT‐proBNP in COVID‐19 are needed. Studies routinely measuring NT‐proBNP in patients with mild disease and fewer comorbidities would help to fully define the predictive power of NT‐proBNP in COVID‐19 as well as offer mechanistic insights behind the drivers of elevation. Additionally, longitudinal analyses of the trend of NT‐proBNP concentration during hospitalization could provide interesting predictive value for the response to treatment, resolution of symptoms, or worsening cardiopulmonary strain predicting death. Finally, considering the increasing recognition of prolonged COVID‐19 symptoms, identifying NT‐proBNP elevation as a feature of severe disease suggests a possible role for longitudinal screening for HF symptoms following COVID‐19 and treating with guideline‐directed medications.
Conclusions
Elevations in NT‐proBNP on admission to the hospital for COVID‐19 predict worse clinical outcomes, including increased risk of death and major cardiovascular complications, and may serve as a biomarker to help guide clinical decision making and resource allocation.
Sources of Funding
American Heart Association (AHA) Rapid Response Grant COVID‐19 and Its Cardiovascular Impact: AHA’s suite of registries is funded by multiple industry sponsors. AHA’s COVID‐19 CVD Registry is partially supported by the Gordon and Betty Moore Foundation. Dr Lu is funded by the Stanford Center for Clinical & Translational Education and Research (Spectrum) from the National Health Institutions, 3UL1TR003142‐02S2. Dr Wang is funded by American Heart Association: 20SFRN35360189; 18SFRN34120036; Stanford University co–principal investigator of BAROS (Bariatric Atrial Restoration of Sinus Rhythm), NCT04050969, the John R. and Ai Giak L. Singleton co‐director of the Stanford Center for Arrhythmia Research. Dr Rodriguez is funded by a career development award from the National Heart, Lung, and Blood Institute (K01 HL 144607) and the American Heart Association/Robert Wood Johnson Harold Amos Medical Faculty Development Program.
Disclosures
Dr Lu reports grant support from Merck Sharp & Dohme Corp. and Abeona Therapeutics Inc., has served on clinical trial data monitoring committees for Nektar and Gilead, and has received consulting income from United Health Care. Dr Wang is on the Varian Medical Systems Advisory Board (unpaid) and is a EndoEpiAF cofounder. Dr Daniels reports consulting income from Quidel, Roche, and Siemens; and has served on a clinical end points adjudication committee for Abbott and Siemens. Dr de Lemos reports grant support from Roche Diagnostics and Abbott Diagnostics, and consulting income from Siemen’s Health Care Diagnostics, Ortho Clinical Diagnostics, and Quidel, Inc. Dr Morrow is a member of the TIMI Study group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, Anthos Therapeutics, Arca Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi‐Sankyo, Eisai, Intarcia, Janssen, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron, Roche, Siemens, The Medicines Company, and Zora Biosciences. Dr Morrow has received consulting fees from Bayer Pharma, InCarda, Merck, Novartis, and Roche Diagnostics. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figure S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022913
For Sources of Funding and Disclosures, see page 8.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi S, Qin MU, Shen BO, Cai Y, Liu T, Yang F, Gong W, Liu XU, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Hanlon R, O'Shea P, Ledwidge M, O’Loughlin C, Lange S, Conlon C, Phelan D, Cunningham S, McDonald K. The biologic variability of B‐type natriuretic peptide and N‐terminal pro‐B‐type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13:50–55. doi: 10.1016/j.cardfail.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 4. Lai M‐Y, Kan W‐C, Huang Y‐T, Chen J, Shiao C‐C. The predictivity of N‐terminal pro b‐type natriuretic peptide for all‐cause mortality in various follow‐up periods among heart failure patients. J Clin Med. 2019;8:357. doi: 10.3390/jcm8030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maries L, Manitiu I. Diagnostic and prognostic values of B‐type natriuretic peptides (BNP) and N‐terminal fragment brain natriuretic peptides (NT‐pro‐BNP). Cardiovasc J Afr. 2013;24:286–289. doi: 10.5830/CVJA-2013-055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olsson LG, Swedberg K, Cleland JG, Spark PA, Komajda M, Metra M, Torp‐Pedersen C, Remme WJ, Scherhag A, Poole‐Wilson P, et al. Prognostic importance of plasma NT‐pro BNP in chronic heart failure in patients treated with a β‐blocker: results from the Carvedilol Or Metoprolol European Trial (COMET) trial. Eur J Heart Fail. 2007;9:795–801. doi: 10.1016/j.ejheart.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 7. Bibbins‐Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B‐type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation. 2003;108:2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carvalho LSF, Bogniotti LAC, de Almeida OLR, e Silva JCQ, Nadruz W, Coelho OR, Sposito AC. Change of BNP between admission and discharge after ST‐elevation myocardial infarction (Killip I) improves risk prediction of heart failure, death, and recurrent myocardial infarction compared to single isolated measurement in addition to the GRACE score. Eur Hear J Acute Cardiovasc Care. 2019;8:643–651. doi: 10.1177/2048872617753049 [DOI] [PubMed] [Google Scholar]
- 9. Irfan A, Reichlin T, Twerenbold R, Fischer C, Ballarino P, Nelles B, Wildi K, Zellweger C, Rubini Gimenez M, Mueller M, et al. Cardiomyocyte injury induced by hemodynamic cardiac stress: differential release of cardiac biomarkers. Clin Biochem. 2015;48:1225–1229. doi: 10.1016/j.clinbiochem.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 10. Medina AM, Marteles MS, Sáiz EB, Martínez SS, Laiglesia FR, Rodríguez JA, Pérez‐Calvo JI. Prognostic utility of NT‐proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med. 2011;22:167–171. doi: 10.1016/j.ejim.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 11. Patel ARC, Donaldson GC, Mackay AJ, Wedzicha JA, Hurst JR. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest. 2012;141:851–857. doi: 10.1378/chest.11-0853 [DOI] [PubMed] [Google Scholar]
- 12. Adrish M, Nannaka VB, Cano EJ, Bajantri B, Diaz‐Fuentes G. Significance of NT‐pro‐BNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int J COPD. 2017;12:1183–1189. doi: 10.2147/COPD.S134953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, You LN, Lei P, Tan XW, Qin S, et al. Prognostic value of NT‐proBNP in patients with severe COVID‐19. Respir Res. 2020;21:83. doi: 10.1186/s12931-020-01352-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caro‐Codón J, Rey JR, Buño A, Iniesta AM, Rosillo SO, Castrejon‐Castrejon S, Rodriguez‐Sotelo L, Martinez LA, Marco I, Merino C, et al. Characterization of NT‐proBNP in a large cohort of COVID‐19 patients. Eur J Heart Fail. 2021;23:456–464. doi: 10.1002/ejhf.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunningham JW, Claggett BL, Jering KS, Vaduganathan M, Bhatt AS, Rosenthal N, Solomon SD. Prognostic value of natriuretic peptides and cardiac troponins in COVID‐19. Circulation. 2021;144:177–179. doi: 10.1161/CIRCULATIONAHA.121.054969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes‐Genis A, Ordonez‐Llanos J, Santalo‐Bel M, Pinto YM, Richards M. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the international collaborative of NT‐proBNP study. Eur Heart J. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631 [DOI] [PubMed] [Google Scholar]
- 18. Omland T, Prebensen C, Røysland R, Søvik S, Sørensen V, Røsjø H, Svensson M, Berdal JE, Myhre PL. Established cardiovascular biomarkers provide limited prognostic information in unselected patients hospitalized with COVID‐19. Circulation. 2020;142:1878–1880. doi: 10.1161/CIRCULATIONAHA.120.050089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, Signorovitch J, Thune JJ, Vardeny O, Solomon SD. Clinical outcomes in patients with heart failure hospitalized with COVID‐19. JACC Hear Fail. 2021;9:65–73. doi: 10.1016/j.jchf.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez‐Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas‐Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID‐19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Selçuk M, Keskin M, Çınar T, Günay N, Doğan S, Çiçek V, Kılıç Ş, Asal S, Yavuz S, Keser N, et al. Prognostic significance of N‐terminal pro‐BNP in patients with COVID‐19 pneumonia without previous history of heart failure. J Cardiovasc Thorac Res. 2021;13:141–145. doi: 10.34172/jcvtr.2021.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoo J, Grewal P, Hotelling J, Papamanoli A, Cao K, Dhaliwal S, Jacob R, Mojahedi A, Bloom ME, Marcos LA, et al. Admission NT‐proBNP and outcomes in patients without history of heart failure hospitalized with COVID‐19. ESC Hear Fail. 2021;8:4278–4287. doi: 10.1002/ehf2.13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rey JR, Caro‐Codón J, Rosillo SO, Iniesta ÁM, Castrejón‐Castrejón S, Marco‐Clement I, Martín‐Polo L, Merino‐Argos C, Rodríguez‐Sotelo L, García‐Veas JM, et al. Heart failure in COVID‐19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205–2215. doi: 10.1002/ejhf.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatia HS, Bui QM, King K, Demaria A, Daniels LB. Subclinical left ventricular dysfunction in COVID‐19. IJC Hear Vasc. 2021;34:100770. doi: 10.1016/j.ijcha.2021.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibson LE, Fenza RD, Lang M, Capriles MI, Li MD, Kalpathy‐Cramer J, Little BP, Arora P, Mueller AL, Ichinose F, et al. Right ventricular strain is common in intubated COVID‐19 patients and does not reflect severity of respiratory illness. J Intensive Care Med. 2021;36:900–909. doi: 10.1177/08850666211006335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fish‐Trotter H, Ferguson JF, Patel N, Arora P, Allen NB, Bachmann KN, Daniels LB, Reilly MP, Lima JAC, Wang TJ, et al. Inflammation and circulating natriuretic peptide levels. Circ Hear Fail. 2020;13:e006570. doi: 10.1161/CIRCHEARTFAILURE.119.006570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qin J‐J, Cheng XU, Zhou F, Lei F, Akolkar G, Cai J, Zhang X‐J, Blet A, Xie J, Zhang P, et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID‐19. Hypertension. 2020;76:1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudiger A, Gasser S, Fischler M, Hornemann T, Von Eckardstein A, Maggiorini M. Comparable increase of B‐type natriuretic peptide and amino‐terminal pro‐B‐type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34:2140–2144. doi: 10.1097/01.CCM.0000229144.97624.90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


