Abstract
Background
The optimal treatment strategy for patients with chronic limb‐threatening ischemia (CLTI) is often unclear. Frailty has emerged as an important factor that can identify patients at greater risk of poor outcomes and guide treatment selection, but few studies have explored its utility among the CLTI population. We examine the association of a health record‐based frailty measure with treatment choice and long‐term outcomes among patients hospitalized with CLTI.
Methods and Results
We included patients aged >65 years hospitalized with CLTI in the Medicare Provider Analysis and Review data set between October 1, 2009 and September 30, 2015. The primary exposure was frailty, defined by the Claims‐based Frailty Indicator. Baseline frailty status and revascularization choice were examined using logistic regression. Cox proportional hazards regression was used to determine the association between frailty and death or amputation, stratifying by treatment strategy. Of 85 060 patients, 35 484 (42%) were classified as frail. Frail patients had lower likelihood of revascularization (adjusted odds ratio [OR], 0.78; 95% CI, 0.75‒0.82). Among those revascularized, frailty was associated with lower likelihood of surgical versus endovascular treatment (adjusted OR, 0.76; CI, 0.72‒0.81). Frail patients experienced increased risk of amputation or death, regardless of revascularization status (revascularized: adjusted hazard ratio [HR], 1.34; CI, 1.30‒1.38; non‐revascularized: adjusted HR, 1.22; CI, 1.17‒1.27). Among those revascularized, frailty was independently associated with amputation or death irrespective of revascularization strategy (surgical: adjusted HR, 1.36; CI, 1.31‒1.42; endovascular: aHR, 1.29; CI, 1.243‒1.35).
Conclusions
Among patients hospitalized with CLTI, frailty is an important independent predictor of revascularization strategy and longitudinal adverse outcomes.
Keywords: chronic limb‐threatening ischemia, frailty, outcomes
Subject Categories: Peripheral Vascular Disease, Vascular Disease, Quality and Outcomes, Risk Factors, Aging
Nonstandard Abbreviations and Acronyms
- AFS
amputation‐free survival
- CFI
Claims‐based Frailty Indicator
- CLTI
chronic limb‐threatening ischemia
Clinical Perspective
What Is New?
Among patients hospitalized with chronic limb‐threatening ischemia, frailty is an important independent predictor of revascularization strategy and longitudinal adverse outcomes.
Frail patients with chronic limb‐threatening ischemia were less likely to receive aggressive treatments and more likely to experience death or amputation, regardless of treatment strategy.
What Are the Clinical Implications?
Clinicians should assess frailty in patients with chronic limb‐threatening ischemia and use such information to guide shared decision‐making with patients about their prognosis and the various treatment options available, ranging from invasive surgical revascularization to palliation.
Chronic limb‐threatening ischemia (CLTI) represents the most severe stage of peripheral artery disease and has devastating consequences if left untreated, with a 22% cardiovascular mortality rate and a 22% major amputation rate at 1 year. 1 Determining the optimal treatment strategy for each individual patient in the setting of these aggregate poor outcomes remains unclear. Although prompt revascularization is recommended in multiple major societal guidelines, 2 , 3 it is often uncertain whether an endovascular or surgical approach is superior for a particular patient, as each strategy carries its unique advantages and drawbacks. 4 Furthermore, there may be competing risks of morbidity and mortality that negate the benefit of revascularization altogether and may favor palliation. As such, it is critical that factors associated with prognosis are identified and used to guide clinical decision‐making to improve CLTI outcomes.
Prior attempts at creating clinical risk stratification tools have focused on traditional comorbidities and their cumulative burden. 5 , 6 , 7 , 8 , 9 , 10 However, these characteristics may not fully represent a patient’s risk or candidacy for a specific invasive strategy. Recently, frailty has emerged as an important prognostic factor that can guide treatment selection and help identify patients with cardiovascular disease at greater risk of poor outcomes. 11 , 12 , 13 Multiple methods have been adopted to measure frailty, including in‐person and health record‐based assessments. Despite the demonstrated utility of identifying patients with cardiovascular disease who are frail, the extension of these methods to larger CLTI populations is limited. 14 , 15
Therefore, this study involving patients hospitalized with CLTI aimed to examine the association of a health record‐based frailty measure with: (1) treatment choice and (2) outcomes. Such results can not only help inform shared decision‐making with patients on CLTI treatment options but can also help guide future studies examining the optimal treatment strategy for patients with CLTI.
METHODS
Study Population
We included all unique adults aged ≥66 years with an inpatient hospitalization for CLTI in the Centers for Medicare and Medicaid Services Medicare Provider Analysis and Review (MedPAR) database between October 1, 2009 and September 30, 2015. The cut‐off of the study corresponded with the transition to the International Classification of Diseases, Tenth Revision (ICD‐10) coding, as the frailty measure used in this study relies on the International Classification of Diseases, Ninth Revision (ICD‐9) claims codes and has yet to be validated in ICD‐10. CLTI hospitalizations were identified based on whether patients had a primary discharge diagnosis code for atherosclerosis of arteries of the extremities with rest pain (440.22), ulceration (440.23), or gangrene (440.24), as has been done previously (Table S1). 16 CLTI hospitalizations were excluded if patients did not have at least 1 continuous year of data within the MedPAR data set before the CLTI episode, since these data were used to ascertain comorbidities, or if patients had a previous diagnosis of CLTI in this 1‐year lookback period. We also excluded CLTI hospitalizations if patients died or received a major amputation during the index hospitalization, as we aimed to examine a population that was theoretically considered for a revascularization strategy as a definitive CLTI treatment. After exclusion criteria were applied, the first CLTI hospitalization for each patient was included in our study. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Exposure
The primary exposure in the study was frailty, as measured by the Claims‐based Frailty Indicator (CFI) developed by Segal et al, 17 based on administrative claims in the 12 months before CLTI admission. The CFI is a 21‐variable indicator derived from a lookback of primarily ICD‐9 administrative claims that was validated against the Fried Frailty Phenotype, a clinical tool for measuring frailty, in the Cardiovascular Health Study (Tables S2 and S3). We examined an ICD‐9 code in any position in any of the admissions in the 12‐month lookback period to identify frailty. The CFI has subsequently been externally validated, 18 and has been used extensively in studies of populations with cardiovascular disease. 12 , 13 Notably, this frailty measure does not directly include many of the traditional cardiovascular risk factors (eg, hypertension, hyperlipidemia, diabetes, obesity, smoking status), though it does consider congestive heart failure and stroke. For this study, we ascertained frailty status based on ICD‐9 codes from inpatient admissions in the year before the CLTI hospitalization. We used the established CFI threshold of 0.25 to define frailty. 17 We also performed multiple sensitivity analyses using different CFI score thresholds to supplement these results, as outlined below.
Characteristics
Patient characteristics included demographics (age, sex, race), Elixhauser comorbidities, 19 and current or prior tobacco use given known prior associations between these variables and outcomes. All comorbidities were ascertained during the 1‐year lookback period. Revascularization was defined as either endovascular or surgical using claims‐based ICD‐9 procedure codes from the index hospitalization (Table S1). 16 , 20 If a revascularization code was not found during the index admission, a patient was considered not to have had revascularization. Patients who underwent revascularizations with both surgical and endovascular types of procedures during the same index hospitalization contributed data to the surgical revascularization group.
Outcomes
The primary outcome was amputation‐free survival (AFS) measured from the discharge date. Amputation was defined as any major amputation and was identified through validated ICD‐9 and ICD‐10 coding algorithms (Table S1). 16 , 20 Mortality was determined using the vital status information from the Medicare Beneficiary Summary File. Secondary outcomes included the individual components of the composite outcome. Follow‐up data were available through December 2017.
Statistical Analysis
All metrics and normally distributed variables were reported as mean±SD. Non‐normally distributed variables were presented as median (interquartile range). Categorical variables were presented as frequency and percentage. Baseline characteristics were compared between those who were frail and not frail using standardized differences. A standardized difference ≥10% was considered significant. 21
The association between frailty status and revascularization among patients with CLTI was then examined. Crude rates of revascularization versus no revascularization were compared by frailty status using chi‐squared tests. Multivariable logistic regression was used to determine the adjusted association between revascularization and frailty status. The first model evaluated the association between revascularization (compared with no revascularization) and frailty status among all patients with CLTI, adjusting for demographics, Elixhauser comorbidities, and tobacco use. The second model evaluated the association between surgical revascularization (compared with endovascular revascularization) and frailty status among all patients who underwent a revascularization procedure, again adjusting for demographics, Elixhauser comorbidities, and tobacco use.
Finally, the association between frailty status and outcomes was analyzed, stratified by treatment strategy. Kaplan‒Meier methods were used to estimate AFS by frailty status among patients who did not receive revascularization and, separately, among patients who received revascularization. In addition, among patients who received revascularization, patients were stratified by frailty status among those receiving endovascular treatment and, separately, surgical treatment. Cox proportional hazards regression was then used to evaluate the association between frailty status and outcomes, adjusted for patient characteristics. The proportional hazards assumption was evaluated by plotting the hazard ratios (HRs) between frail and non‐frail individuals with survival over time (Figure S1). Because of the possibility of unmeasured confounding and treatment selection bias, we elected to create separate models for patients who did not receive revascularization and for patients who received revascularization. Patients receiving revascularization were further stratified into those receiving endovascular or surgical treatment. To account for the competing risk of death, Fine‐Gray methods were used to analyze the outcome of major amputation. 22
As sensitivity analyses, the relationship between the degree of frailty and the risk of death or major amputation at 1 year was evaluated, considering frailty as a continuous variable using a cubic spline model with 4 knots. We additionally examined baseline characteristics and receipt of revascularization across quartiles of the frailty indicator, and repeated Kaplan‒Meier analyses of AFS by quartiles among patients who did not receive revascularization and, separately, among patients who received revascularization. Similarly, we repeated Kaplan‒Meier analyses of AFS by quartiles among patients who received surgical revascularization and, separately, among patients who received endovascular revascularization.
A 2‐sided P<0.05 was considered statistically significant without adjustment for multiple comparisons. All analyses were performed using SAS 9.4 (Cary, NC, USA). The study was approved by the institutional review board of Beth Israel Deaconess Medical Center, with a waiver of informed consent for retrospective data analysis.
RESULTS
During the study period, 85 060 patients met criteria and were included in the analysis, of which 35 484 (42%) were classified as frail (Figures S2 and S3). Median follow‐up in the whole cohort was 2.34 years (interquartile range, 3.32 years). Patients who were frail were more likely to be older, women, of Black or other race, and non‐smokers (Table 1). Frail patients also had a greater burden of both cardiovascular and non‐cardiovascular conditions, including congestive heart failure, valvular heart disease, pulmonary circulation disease, renal failure, and anemia. Mean number admissions before the index admission among non‐frail patients was 1.2 versus 2.1 for frail patients.
Table 1.
Baseline Characteristics of Patients With CLTI by Frailty Status
| Subject characteristic | Not frail (n=49 576) | Frail (n=35 484) | Standardized difference* |
|---|---|---|---|
| Demographics | |||
| Age (y), mean (SD) | 75.77 (5.36) | 86.01 (6.01) | −1.798 |
| Men | 28 833 (58.16) | 13 655 (38.48) | 0.402 |
| Race | |||
| White | 41 773 (84.26) | 26 081 (73.50) | 0.266 |
| Black | 6043 (12.19) | 6777 (19.10) | −0.191 |
| Other † | 1760 (3.55) | 2626 (7.40) | −0.170 |
| Elixhauser comorbidity variables | |||
| Summary comorbidity index, mean (SD) | 3.79 (2.05) | 4.58 (2.40) | 0.35 |
| Acquired immune deficiency syndrome | 45 (0.09) | 13 (0.04) | 0.020 |
| Alcohol abuse | 147 (0.30) | 40 (0.11) | 0.042 |
| Chronic blood loss anemia | 893 (1.80) | 847 (2.39) | −0.041 |
| Chronic pulmonary disease | 15 984 (32.24) | 9881 (27.85) | 0.096 |
| Coagulopathy | 2436 (4.91) | 2422 (6.83) | −0.082 |
| Congestive heart failure | 6798 (13.71) | 12 670 (35.71) | −0.527 |
| Deficiency anemias | 10 665 (21.51) | 11 509 (32.43) | −0.248 |
| Depression | 2797 (5.64) | 4374 (12.33) | −0.236 |
| Diabetes w/ chronic complications | 10 659 (21.50) | 7570 (21.33) | 0.004 |
| Diabetes w/o chronic complications | 19 750 (39.84) | 12 933 (36.45) | 0.070 |
| Drug abuse | 34 (0.07) | 13 (0.04) | 0.013 |
| Fluid and electrolyte disorders | 12 826 (25.87) | 14 613 (41.18) | −0.329 |
| Hypertension | 39 953 (80.59) | 28 786 (81.12) | −0.013 |
| Hypothyroidism | 5640 (11.38) | 6529 (18.40) | −0.198 |
| Liver disease | 605 (1.22) | 352 (0.99) | 0.022 |
| Lymphoma | 452 (0.91) | 358 (1.01) | −0.010 |
| Metastatic cancer | 603 (1.22) | 336 (0.95) | 0.026 |
| Obesity | 3788 (7.64) | 1837 (5.18) | 0.101 |
| Other neurological disorders | 3189 (6.43) | 5895 (16.61) | −0.323 |
| Paralysis | 2031 (4.10) | 2677 (7.54) | −0.147 |
| Peptic ulcer disease without bleeding | 21 (0.04) | 24 (0.07) | −0.013 |
| Psychoses | 973 (1.96) | 1531 (4.31) | −0.135 |
| Pulmonary circulation disease | 1032 (2.08) | 1931 (5.44) | −0.177 |
| Renal failure | 12 660 (25.54) | 12 404 (34.96) | −0.206 |
| Rheum. arthritis/collagen vascular diseases | 1930 (3.89) | 2131 (6.01) | −0.098 |
| Solid tumor w/out metastasis | 1452 (2.93) | 1129 (3.18) | −0.015 |
| Valvular disease | 1607 (3.24) | 2968 (8.36) | −0.220 |
| Weight loss | 3482 (7.02) | 5160 (14.54) | −0.244 |
| Additional variables | |||
| Smoking | 25 386 (51.21) | 11 626 (32.76) | 0.381 |
| No. admissions preceding index (mean, SD) ‡ | 1.20 (1.79) | 2.14 (2.48) | 0.435 |
Standardized difference calculated as: for continuous variables and for categorical variables. Comorbidities were ascertained during both the index admission and the 1‐year lookback period.
†
Other race or ethnicity includes those who identify as Asian, Hispanic, North American Native, or Other and those with race unknown.
‡Number of admissions preceding index admission was not included in adjusted models given collinearity with frailty measure.
CLTI indicates chronic limb‐threatening ischemia.
Frailty and Treatment Choice
In unadjusted analyses, frail patients were less likely to receive revascularization than non‐frail patients (60.2% versus 76.4%, respectively; P<0.001) (Table 2). This relationship persisted after adjustment (adjusted OR, 0.78; 95% CI, 0.75‒0.82; P<0.001). Of patients who received revascularization, those who were frail were more likely to receive endovascular treatment when compared with non‐frail patients (59.5% versus 40.0%, respectively; P<0.001). Conversely, non‐frail patients who underwent revascularization were more likely to receive surgical treatment (60.0% versus 40.5% of frail patients; P<0.001). After adjustment, being classified as frail remained associated with a lower likelihood of receiving surgical treatment (adjusted OR, 0.76; 95% CI, 0.72‒0.81; P<0.001).
Table 2.
Association of Treatment Choice for CLTI by Frailty Status
| Cohort | Not frail | Frail | Chi‐squared P value | Adjusted OR for treatment modality for frailty vs non‐frail individuals* (95% CI) | P value |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| All patients | 49 576 | 35 484 | <0.0001 | 0.784 (0.746‒0.824) † | <0.0001 |
| Revascularization | 37 887 (76.4) | 21 379 (60.2) | |||
| No revascularization | 11 689 (23.6) | 14 105 (39.8) | |||
| Patients treated with revascularization | 37 887 | 21 379 | <0.0001 | 0.764 (0.724‒0.808) ‡ | <0.0001 |
| Surgical | 22 734 (60.0) | 8654 (40.5) | |||
| Endovascular | 15 153 (40.0) | 12 725 (59.5) |
CLTI indicates chronic limb‐threatening ischemia; and OR, odds ratio.
Multivariable logistic regression models controlling for all covariates listed in Table 1 unless otherwise specified.
OR of revascularization (relative to no revascularization).
OR of surgical treatment (relative to endovascular treatment).
Frailty and Outcomes
Overall, the median AFS was 2.5 years (interquartile range, 0.7 years‒4.1 years) for non‐frail patients and 0.8 years (interquartile range, 0.2 years‒2.6 years) for frail patients. The Kaplan‒Meier cumulative estimate of AFS at 8.25 years of follow‐up was greater among non‐frail patients compared with frail patients for both those who were revascularized (17.9% non‐frail versus 4.0% frail, P<0.001) and those who were not revascularized (10.8% non‐frail versus 1.8% frail, P<0.001) (Figure 1). The association between frailty and worse AFS also persisted when all patients were considered in aggregate (Figure S4). Of those who received revascularization, non‐frail patients also had a greater cumulative AFS than frail patients, irrespective of revascularization strategy (surgical: 19.9% non‐frail versus 5.0% frail, P<0.001; endovascular: 15.1% non‐frail versus 3.4% frail, P<0.001) (Figure 2).
Figure 1. Amputation‐free survival among patients with chronic limb‐threatening ischemia by frailty status, stratified by treatment choice.
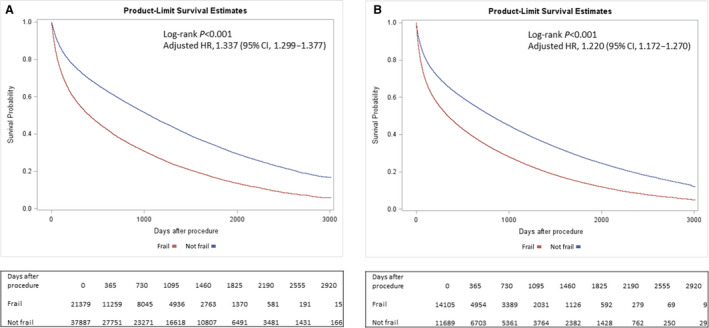
A, Among patients undergoing revascularization. B, Among patients not undergoing revascularization. HR indicates hazard ratio.
Figure 2. Amputation‐free survival among patients with chronic limb‐threatening ischemia undergoing revascularization by frailty status, stratified by revascularization strategy.

A, Surgical revascularization. B, Endovascular revascularization. HR indicates hazard ratio.
In adjusted analysis, frailty remained associated with an increased risk of death or major amputation, regardless of whether revascularization was performed (revascularized cohort: hazard ratio [HR], 1.34; 95% CI, 1.30‒1.38; P<0.001; non‐revascularized cohort: HR, 1.22; 95% CI, 1.17‒1.27; P<0.001) (Figure 1). The difference in death or major amputation was primarily driven by an increased risk of death among those that were frail (Figures S5 and S6). Notably, frailty was a stronger independent predictor of death or major amputation compared with many traditional risk factors (including tobacco use, diabetes, and obesity), among both the revascularized and non‐revascularized cohorts (Figure 3, Table S4).
Figure 3. Top predictors of amputation or death for patients with chronic limb‐threatening ischemia using Cox proportional hazards.
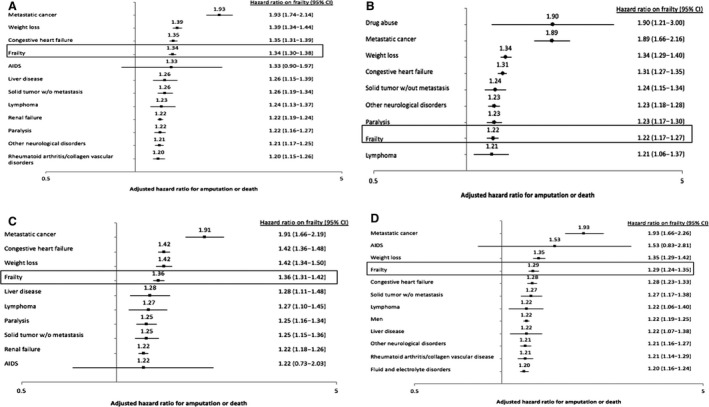
A, Among patients undergoing revascularization. B, Among patients not undergoing revascularization. C, Among patients undergoing surgical revascularization. D, Among patients undergoing endovascular revascularization. Figures include predictors with hazard ratio >1.2. See Tables S4 and S5 for full model results.
Of patients who were revascularized, frailty was also a strong independent predictor of AFS, irrespective of revascularization strategy (surgical cohort: adjusted HR, 1.36; 95% CI, 1.31‒1.42; P<0.001; endovascular cohort: adjusted HR, 1.29; 95% CI, 1.24‒1.35; P<0.001) (Figure 2). The difference in death or major amputation was again primarily driven by an increased risk of death among those that were frail (Figures S7 and S8). Frailty was a stronger predictor of death or major amputation compared with many traditional risk factors, including age, race, sex, tobacco use, diabetes, and obesity (Figure 3, Table S5).
Frailty as a Continuous Measure
When frailty was examined as a continuous variable, higher values of frailty were associated with a higher risk of death or major amputation at 1 year among the total cohort, as well as when stratified by revascularization status (Figure S9). When frailty was examined by quartiles, patients with greater degree of frailty showed greater burden of both cardiovascular and non‐cardiovascular conditions as well as a sequentially lower likelihood of receiving any revascularization, as well as surgical revascularization, if revascularized (Tables S6 and S7). Patients with a greater degree of frailty had sequentially worse outcomes, regardless of whether revascularization was pursued or the type of revascularization (Figures S10 and S11).
DISCUSSION
In this retrospective cohort study of Medicare beneficiaries with long‐term follow‐up, we evaluated the impact of a claims‐based measurement of frailty on treatment selection and outcomes among patients hospitalized with CLTI. We found the following notable results. Patients hospitalized for CLTI were, on average, more frail than the community‐dwelling Medicare beneficiary population. 18 Frailty was a useful tool to discriminate whether a patient underwent a revascularization strategy versus a non‐invasive treatment approach, irrespective of most traditional comorbidities. The presence of frailty also impacted who underwent an endovascular revascularization strategy versus surgical. Furthermore, regardless of treatment strategy, frailty was independently associated with worse outcomes, even after adjusting for an array of demographics and comorbidities. This association was primarily driven by an increased risk of death and not necessarily by differences in amputation. These results suggest that frailty can be used to prognosticate future outcomes and guide shared decision‐making with patients about the various treatment options available.
We found evidence that treatment selection for patients hospitalized with CLTI is influenced by frailty, a multidimensional syndrome characterized by decreased reserve and diminished resistance to stressors. 23 Previous studies have shown that patients with CLTI who receive conservative or endovascular therapy are likely to have more comorbidities than those who receive surgical therapy. 5 , 6 Guidelines currently recommend endovascular over surgical treatment for CLTI in patients with substantial comorbidities, which may place them at higher risk of postoperative complications from surgical revascularization. 3 Our finding that frail patients are more likely to receive endovascular intervention, despite controlling for comorbidities, reflects how clinicians may already consider frailty in the selection of appropriate treatment options for patients with CLTI. However, a frailty assessment may be one method to improve communication about what characteristics of the patient may make them a better or worse candidate for a particular revascularization strategy, thereby improving patient counseling and comprehension.
This study extends existing investigation about factors affecting CLTI outcomes. Several risk scores have been developed to predict 30‐day or 1‐year amputation‐free survival among patients with CLTI undergoing surgical revascularization, 8 , 9 or mortality after endovascular or surgical revascularization at 2 or 5 years. 7 , 10 However these scores only capture select comorbidities and are not frequently used in clinical practice. 24 We include many of these risk score components in our multivariate analysis and show that frailty remains a strong, independent factor that improves patient risk stratification for future clinically important events, regardless of treatment strategy. As such, frailty may be a final common pathway that integrates information from other established factors and risk scores. Several studies using varied frailty metrics have shown an association between frailty and adverse functional outcomes after peripheral artery disease procedures more broadly. 25 Additionally, small, single‐center studies have found that frailty was associated with a lower AFS or overall survival at 2 years among patients with CLTI who received revascularization. 14 , 15 We found similar results in a large national sample of >85 000 Medicare beneficiaries with CLTI with follow‐up available up to 8 years. Additionally, we found that frailty was associated with worse outcomes among hospitalized patients who do not receive revascularization, which comprises a large proportion of Medicare patients with CLTI. Thus, our study further establishes the role of phenotypic frailty as a strong, independent secular predictor of CLTI outcomes, independent of physician management.
These results have important implications for the treatment of patients hospitalized with CLTI and for future studies evaluating CLTI treatment options. These findings suggest that physicians should routinely assess frailty in clinical management of patients hospitalized with CLTI. While multiple different conceptualizations of frailty exist, 26 the one used for the current study, which is anchored to a physical phenotype, can be easily quantified using a comprehensive geriatric assessment, and is thus readily translatable into practice. Therefore, clinicians can use such information to guide shared decision‐making with patients about their prognosis and the various treatment options available, ranging from invasive surgical revascularization to palliation. For instance, if a frail patient hospitalized with CLTI is informed of an expected overall median AFS of 0.8 years (versus 2.5 years in a non‐frail patient) in considering treatment options and recovery times, less invasive treatment may be chosen in shared decision‐making. These results highlight the need for team‐based care of patients with CLTI as well as the need for additional patient‐centered outcomes to enhance the shared‐decision making process. Additionally, the additive prognostic value of frailty suggests that observational studies comparing treatment options among patients hospitalized with CLTI may be confounded if frailty is not considered. Finally, our evidence that frailty affects CLTI outcomes suggests that future randomized trials of CLTI treatment should consider examining outcomes among frail patients separately, to individualize treatment for this high‐risk subgroup.
Our analysis must be interpreted in light of its limitations. First, for the primary analysis, we considered frailty as a dichotomous exposure for pragmatism. However, in supplemental analysis, similar relationships between quartiles of frailty and AFS were observed, and a continuous measure of frailty was associated with increased 1‐year mortality in a dose‐dependent manner, further emphasizing the importance of frailty as a predictor of outcomes. Second, given evidence that frailty likely impacts treatment selection, we chose not to compare outcomes among frail patients across different treatment strategies to avoid confounding by indication. Third, the frailty scale used is based on longitudinal ICD‐9 claims data. As such, the application of this specific scale for future investigation or clinical utility is limited, both by the availability of these data and by the transition to the ICD‐10 coding system, though work to crosswalk these claims to ICD‐10 codes is ongoing. Furthermore, we studied a more historic cohort of patients; although this enabled us to capture long‐term follow‐up of these patients, treatment options may have since evolved. Nonetheless, the utility of this analysis is supported by the correlation between the claims‐based frailty scale used in this study and the widely adopted in‐person Fried frailty assessment. 27 Fourth, we do not account for patients who may become frail in the follow‐up time; however, such analysis pertains primarily to the treatment decision faced at time of CLI and time‐updating is of modest benefit with a non‐frail median survival of 2.4 years. Fifth, we only included patients hospitalized for CLTI, which may represent a more severe form of the disease and did not include the minority of patients who were discharged with plans for outpatient intervention or treated primarily in the outpatient setting. Lastly, the MedPAR data set lacks granular anatomical and procedural characteristics or medications that may also be prognostic of future adverse events.
CONCLUSIONS
In this large nationwide analysis of hospitalized Medicare beneficiaries with CLTI, we find that frailty is an important independent predictor of revascularization strategy and longitudinal adverse outcomes in patients who did and did not undergo revascularization. Future work investigating the optimal treatment strategy for this high‐risk subgroup is warranted.
Sources of Funding
None.
Disclosures
Dr Butala is funded by the John S. LaDue Memorial Fellowship at Harvard Medical School, Boston, MA and reports consulting fees and ownership interest in HiLabs, outside the submitted work. Dr Secemsky receives grants from AstraZeneca, BD Bard, Boston Scientific, Cook Medical, CSI, Medtronic, Philips, and UCSF. He consults for CSI, Medtronic, and Philips and is on the speaking bureau of BD Bard, Cook Medical and Medtronic, outside the submitted work. Dr Schermerhorn has received support from Abbott, Cook Medical, Endologix, Medtronic, and Philips, outside the submitted work. Dr Beckman has served as a consultant for AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Merck, Novo Nordisk, and Sanofi; and has served on the Data Safety and Monitoring Board of Bayer and Novartis, outside the submitted work. Dr Shishehbor has served on the Scientific Advisory Boards of Medtronic, Abbott Vascular, Phillips, Terumo, and Boston Scientific, outside the submitted work. Dr Yeh reports additional grant support from Abiomed, Astra Zeneca, and Boston Scientific and consulting fees from Abbott, Boston Scientific, Medtronic, and Teleflex, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Figures S1–S11
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023138
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, Conte MS, Murad MH. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–1651.e1643. doi: 10.1016/j.jvs.2015.07.065 [DOI] [PubMed] [Google Scholar]
- 2. Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, GVG Writing Group . Global vascular guidelines on the management of chronic limb‐threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang JC, Kim AH, Kashyap VS. Open surgical or endovascular revascularization for acute limb ischemia. J Vasc Surg. 2016;63:270–278. doi: 10.1016/j.jvs.2015.09.055 [DOI] [PubMed] [Google Scholar]
- 5. Klaphake S, de Leur K, Mulder PGH, Ho GH, de Groot HGW, Veen EJ, van der Laan L. Life expectancy and outcome of different treatment strategies for critical limb ischemia in the elderly patients. Ann Vasc Surg. 2018;46:241–248. doi: 10.1016/j.avsg.2017.06.141 [DOI] [PubMed] [Google Scholar]
- 6. Lin JH, Brunson A, Romano PS, Mell MW, Humphries MD. Endovascular‐first treatment is associated with improved amputation‐free survival in patients with critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2019;12:e005273. doi: 10.1161/CIRCOUTCOMES.118.005273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moxey PW, Brownrigg J, Kumar SS, Crate G, Holt PJ, Thompson MM, Jones KG, Hinchliffe RJ. The BASIL survival prediction model in patients with peripheral arterial disease undergoing revascularization in a university hospital setting and comparison with the FINNVASC and modified PREVENT scores. J Vasc Surg. 2013;57:1–7. doi: 10.1016/j.jvs.2012.04.074 [DOI] [PubMed] [Google Scholar]
- 8. Biancari F, Salenius JP, Heikkinen M, Luther M, Ylönen K, Lepäntalo M. Risk‐scoring method for prediction of 30‐day postoperative outcome after infrainguinal surgical revascularization for critical lower‐limb ischemia: a FINNVASC registry study. World J Surg. 2007;31:217–225; discussion 226‐217. doi: 10.1007/s00268-006-0242-y [DOI] [PubMed] [Google Scholar]
- 9. Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation‐free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48:1464–1471. doi: 10.1016/j.jvs.2008.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simons JP, Schanzer A, Flahive JM, Osborne NH, Mills JL Sr, Bradbury AW, Conte MS. Survival prediction in patients with chronic limb‐threatening ischemia who undergo infrainguinal revascularization. J Vasc Surg. 2019;69:137S–151S.e133. doi: 10.1016/j.jvs.2018.08.169 [DOI] [PubMed] [Google Scholar]
- 11. McAlister F, van Walraven C. External validation of the Hospital Frailty Risk Score and comparison with the Hospital‐patient One‐year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf. 2019;28:284–288. doi: 10.1136/bmjqs-2018-008661 [DOI] [PubMed] [Google Scholar]
- 12. Smith RJ, Reid DA, Santamaria JD. Frailty is associated with reduced prospect of discharge home after in‐hospital cardiac arrest. Intern Med J. 2019;49:978–985. doi: 10.1111/imj.14159 [DOI] [PubMed] [Google Scholar]
- 13. Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. Association of frailty with 30‐day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 2019;4:1084–1091. doi: 10.1001/jamacardio.2019.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morisaki K, Yamaoka T, Iwasa K, Ohmine T. Influence of frailty on treatment outcomes after revascularization in patients with critical limb ischemia. J Vasc Surg. 2017;66:1758–1764. doi: 10.1016/j.jvs.2017.04.048 [DOI] [PubMed] [Google Scholar]
- 15. Takeji Y, Yamaji K, Tomoi Y, Okazaki J, Tanaka K, Nagae A, Jinnouchi H, Hiramori S, Soga Y, Ando K. Impact of frailty on clinical outcomes in patients with critical limb ischemia. Circ Cardiovasc Interv. 2018;11:e006778. doi: 10.1161/CIRCINTERVENTIONS.118.006778 [DOI] [PubMed] [Google Scholar]
- 16. Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, Jaff MR. Determinants of long‐term outcomes and costs in the management of critical limb ischemia: a population‐based cohort study. J Am Heart Assoc. 2018;7:e009724. doi: 10.1161/JAHA.118.009724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims‐based frailty indicator anchored to a well‐established frailty phenotype. Med Care. 2017;55:716–722. doi: 10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims‐based frailty index in the national health and aging trends study cohort. Am J Epidemiol. 2017;186:745–747. doi: 10.1093/aje/kwx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 20. Secemsky EA, Schermerhorn M, Carroll BJ, Kennedy KF, Shen C, Valsdottir LR, Landon B, Yeh RW. Readmissions after revascularization procedures for peripheral arterial disease: a nationwide cohort study. Ann Intern Med. 2018;168:93–99. doi: 10.7326/M17-1058 [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 22. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23. Rodríguez‐Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko‐Zajko W, Gonzalez‐Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition‐consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shishehbor MH, Li J. Frailty for critical limb ischemia: fruitful or futile? Circ Cardiovasc Interv. 2018;11:e007009. doi: 10.1161/CIRCINTERVENTIONS.118.007009 [DOI] [PubMed] [Google Scholar]
- 25. van Aalst FM, Verwijmeren L, van Dongen EPA, de Vries JPM, de Groot E, Noordzij PG. Frailty and functional outcomes after open and endovascular procedures for patients with peripheral arterial disease: a systematic review. J Vasc Surg. 2020;71:297–306.e291. doi: 10.1016/j.jvs.2018.12.060 [DOI] [PubMed] [Google Scholar]
- 26. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. doi: 10.1002/pds.3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S11


