Abstract
Background
Caregivers provide critical support for patients with chronic diseases, including heart disease, but often experience caregiver stress that negatively impacts their health, quality of life, and patient outcomes. We aimed to inform health care teams on an evidence‐based approach to supporting the caregivers of patients with heart disease.
Methods and Results
We conducted a systematic review and meta‐analysis of randomized controlled trials written in English that evaluated interventions to support caregivers of patients with heart disease. We identified 15,561 articles as of April 2, 2020 from 6 databases; of which 20 unique randomized controlled trials were evaluated, representing a total of 1570 patients and 1776 caregivers. Most interventions focused on improving quality of life, and reducing burden, depression, and anxiety; 85% (17 of 20) of the randomized controlled trials provided psychoeducation for caregivers. Interventions had mixed results, with moderate non‐significant effects observed for depression (Hedges’ g=−0.64; 95% CI, −1.34 to 0.06) and burden (Hedges’ g=−0.51; 95% CI, −2.71 to 1.70) at 2 to 4 months postintervention and small non‐significant effects observed for quality of life and anxiety. These results were limited by the heterogeneity of outcome measures and intervention delivery methods. A qualitative synthesis of major themes of the interventions resulted in clinical recommendations represented with the acronym “CARE” (Caregiver‐Centered, Active engagement, Reinforcement, Education).
Conclusions
This systematic review highlights the need for greater understanding of the challenges faced by caregivers and the development of guidelines to help clinicians address those challenges. More research is necessary to develop clinical interventions that consistently improve caregiver outcomes.
Keywords: anxiety, burden, cardiovascular disease, caregiver, depression, heart disease
Subject Categories: Quality and Outcomes, Statements and Guidelines, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- CARE
Caregiver‐Centered, Active Engagement, Reinforcement, Education
- NIH
National Institutes of Health
- QoL
quality of life
Clinical Perspective
What Is New?
Recently, more attention has been given to the physical and emotional challenges faced by the family members and friends that support the health care needs of patients with chronic diseases, including cardiovascular disease; these caregivers play a crucial role in the health care maintenance of our patients which can lead to stress, depression, anxiety, and decreased quality of life.
Little is known about the best ways the clinical team can support caregivers of patients with cardiovascular disease.
Our systematic review highlights and attempts to address the need for evidence‐based guidelines to help the clinical team better engage and support caregivers of patients with cardiovascular disease.
What Are the Clinical Implications?
The clinical team has a role in addressing caregiver burden by finding ways to better support the caregivers of our patients.
Based CARE (Caregiver centered, Active engagement, Reinforcement, Education) to help clinicians better support the caregivers of their patients.
Caregiver‐centered health care delivery will identify caregivers at risk of burnout, engage them as part of the team, provide educational resources to help empower them in their roles, and reinforce skills or knowledge taught.
Family and other caregivers play a vital role in the long‐term health maintenance and care of patients with chronic illnesses such as heart disease, a leading cause of hospital admission, death, and economic burden in the United States. 1 , 2 , 3 , 4 In a report published by the National Alliance on Caregiving and the American Association of Retired Persons, there are about 41.8 million Americans providing care to adults aged >50 years. 5 These caregivers perform important roles, including supporting patients in their activities of daily living and instrumental activities of daily living, providing transportation to medical appointments, and aiding in medication adherence. The overall economic value of unpaid caregivers in the United States in 2017 was estimated to be 470 billion dollars. 6
Caregiver involvement in the care of patients with heart disease has shown benefit in patient outcomes through improvement in dietary adherence, medication adherence, and patient attendance at follow‐up visits. 1 , 7 However, the caregiver role has also been associated with added stress and burden. 8 , 9 , 10 , 11 , 12 , 13 Aiding patients in adhering to a diet that is within heart disease guidelines was noted to be particularly challenging by caregivers. 14 Other common challenges that heart disease caregivers face include increased mental and physical stress, fear of the unknown, uncertainty of appropriate grocery shopping, challenges of meal preparation, and lack of acknowledgment and validation from the patient’s health care team. 14 , 15 , 16 , 17 , 18 , 19 As a result of the additional stress and less healthy lifestyles that occur from these challenges, caregivers of patients with heart disease were found to be at higher risk for CVD morbidity and mental health disorders. 15 , 20
While caregivers play a crucial role in supporting our patients, their contributions and the challenges they face have not been addressed effectively by clinicians. 21 , 22 Consequently, to address these adverse effects, there is a need for evidence‐based recommendations to guide the clinical team in supporting caregivers of patients with heart disease. In recent years, there has been increased awareness of the importance of engaging caregivers as partners in patient care; this is reflected by new policies and practices that promote caregiver support and engagement by the clinical team. 21 , 23 , 24 , 25 , 26 , 27 , 28 However, there remains a deficit in uniform evidence‐based guidelines for clinicians.
This systematic review aimed to examine the literature for interventions to inform the health care team on evidence‐based approaches to support the caregivers of patients with heart disease. We reviewed randomized controlled trials (RCTs) of caregiver interventions in populations of patients with heart disease. We performed a meta‐analysis of the results. We also conducted a qualitative synthesis of major themes of the interventions, informed by our literature review, to develop a framework to guide clinicians in recognizing, engaging, and supporting caregivers.
Methods
Study Search Strategy and Data Sources
This study did not use experimental animals, medications, biologics, or devices. This study is not human subjects research, and thus Institutional Review Board review is not required. The data that support the findings of this study are available from the corresponding author (S.S.M.) upon reasonable request. The search was developed and executed by the informationist (J. N.), in collaboration with the study team. All searches were run on June 21, 2019 and updated on April 2, 2020 in the following databases: Medline (PubMed), Embase (Embase.com), The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register), PsycINFO (EbscoHost), CINAHL (EbscoHOST), and Web of Science (Science and Social Science Citation Index). For the search strategies designed for Medline (PubMed), the Cochrane Library, PsycINFO, Embase, and CINAHL, controlled vocabulary terms for each concept were identified and combined with keyword synonyms. Web of Science was searched using keyword terms only. A Boolean search strategy was created and summarized here: (caregiver OR spouse OR family, etc) AND (cardiovascular disease OR heart disease OR myocardial infarction OR heart failure, etc) AND (intervention OR education OR support OR psychoeducation, etc). The entire search strategy can be found in Tables S1 through S6. Additional studies were identified by searching the references of review articles and included studies.
Eligibility Criteria
Studies were considered eligible for inclusion if they (1) used an RCT design, (2) were written in the English language, (3) evaluated interventions for caregivers of patients with heart disease, and (4) reported caregiver outcomes. There was no time frame restriction included in the criteria. The interventions considered targeted caregivers alone or caregivers and patients together. Outcomes of interest included caregiver burden, depression, anxiety, quality of life (QoL), and knowledge of heart disease (ie, causes, strategies for risk reduction, symptoms, and medications), though no restrictions were placed on the type of caregiver outcome evaluated. If patient outcomes were reported, these data points were also included and evaluated as an exploratory outcome. Caregivers were defined as adults (aged ≥18 years) who contributed to the health care needs and daily activities of the patient and included friends or family members. We excluded studies involving: (1) health care workers as the caregivers, since the goal of this systematic review was to understand the impact of interventions on informal caregivers (family and friends), (2) caregivers of patients with stroke, because of additional challenges of caring for patients with cognitive and motor deficits, (3) palliative care or end‐stage heart failure requiring mechanical assist devices, attributable to ethical and decision‐making roles of caregivers confounding standard care, (4) pediatric or adolescent patients, because of our focus on care for adult patients (aged ≥18 years). We also excluded conference abstracts and protocol papers.
Study Selection and Data Collection
The Covidence platform was used to identify and select relevant studies following Preferred Reporting Items for Systematic reviews and Meta‐Analyses guidelines. 29 Titles and abstracts, followed by full‐text articles, were screened independently by at least 2 authors (K. K., H. X., A. S., S. J., S. P., R. S., N. O., A. D.) with a third author resolving conflicts (D. W., K. K., H. X.).
Data for eligible studies were extracted by a single author (C.N.) using a pre‐made form including (1) title, (2) year, (3) journal, (4) type of journal, (5) country, (6) number of patients, (7) type of patient heart disease (8) number of caregivers, (9) type of intervention, (10) follow‐up time, (11) caregiver outcomes, and (12) patient outcomes. The extracted data were validated by a second author (A.D.) to ensure consistency, and discrepancies were resolved by a third author (K.K.). We used deductive analysis to identify repeated intervention themes that were associated with improved caregiver outcomes. Each intervention’s themes were evaluated and summarized by at least 2 independent authors (K. K., A. S., H. X.); discrepancies were resolved by discussion. Themes were then categorized and organized by discussion (K. K., A. S., H. X.).
Study Quality Assessment
Quality of the articles was assessed using an NIH (National Institutes of Health) study quality assessment tool 30 by 2 reviewers (C. N., N. O.), with resolution of conflicts by a third author (S. J.). The NIH quality assessment tool allowed answers of yes, no, or cannot determine, for 14 questions evaluating each study’s internal validity. Final assessment of study quality was determined independently by 2 reviewers (C. N., N. O.) with resolution of conflicts by a third author (S. J.) and is included in Table S7.
Statistical Analysis
A random‐effects meta‐analysis was performed for the primary outcomes of QoL, anxiety, depression, and caregiver burden. To evaluate outcomes from the heterogeneous assessment tools used across the studies, we calculated a standardized mean difference, Hedges’ g, 31 as a measure of intervention effect. It was calculated using the means and SDs of the outcome measures in the study groups at the timepoint falling within 2 to 4 months postintervention. When postintervention SDs were not available, we assumed the postintervention SD was equal to the SD at baseline. Based on the magnitude of Hedges’ g, we interpreted effects as small (0.2), medium (0.5), and large (0.8). Meta‐analysis was performed using Stata 16 (StatCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Results
Study Characteristics
A total of 15,561 articles were screened, which resulted in 25 studies that met inclusion criteria, representing 20 distinct RCTs that were included in our review (Figure 1). The additional 5 articles 32 , 33 , 34 , 35 , 36 assessed additional outcomes from 4 of the distinct RCTs. 35 , 37 , 38 , 40 Some studies defined caregivers as family caregivers. 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 Five studies focused specifically on partners who lived with and cared for patients. 32 , 38 , 48 , 49 , 50 Only 2 studies broadly specified caregivers as an unpaid person who helped the patient on a daily basis or as an individual identified as a caregiver by the patient. 51 , 52 The ratio of male to female caregivers was not reported in most studies. A summary of the included studies, which consisted of a total of 1570 patients and 1776 caregivers, is shown in Table 1. 32 , 33 , 34 , 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56
Figure 1. Preferred Reporting Items for Systematic reviews and Meta‐Analyses flow diagram.
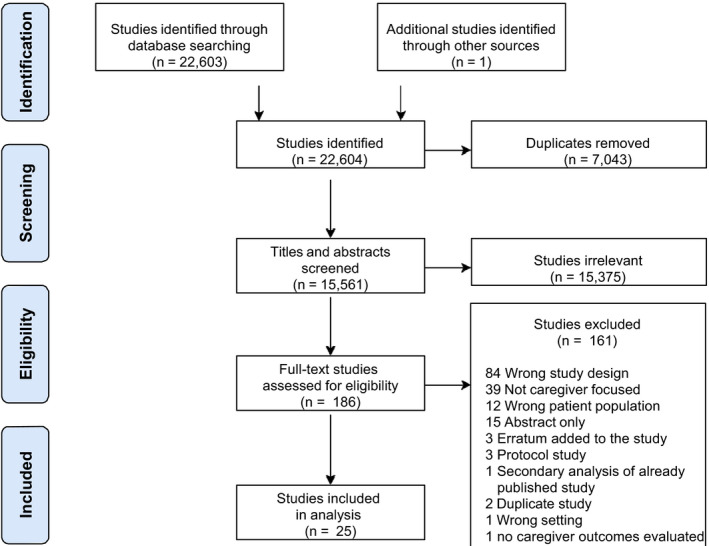
Table 1.
Summary Table of RCTs Evaluating Interventions for Caregivers of Patients With Heart Disease
| Author | Y | Heart disease | Target population | Number. of patients | Number of caregivers | Intervention summary (1. type of intervention, 2. delivery mode, 3. facilitators, 4. number of intervention sessions) | Timeline of assessment |
|---|---|---|---|---|---|---|---|
| Ågren et al, 32 Ågren et al 37 | 2015, 2015 | CHF | Caregiver and patient | 42 | 42 | (1) Psychoeducation (2) in person (3) thoracic surgeon, thoracic anesthetist and a nurse (4) 3 sessions, 30–60 mins, over 24 wk | Baseline, postintervention, 12 mo |
| Ågren et al, 38 Liljeroos et al, 33 Liljeroos et al 34 | 2012, 2015, 2017 | CHF | Caregiver and patient | 155 | 155 | (1) Psychoeducation (2) in person (3) nurse (4) 3 sessions in 12 wk | Baseline, 3 mo, 12 mo |
| Borji et al 41 | 2018 | CHF | Caregiver only | Not reported | 71 | (1) Spiritual intervention (2) in person (3) nurses (4) 6 45‐min sessions during a period of 2 wk | Baseline, 6 wk |
| Broadbent et al 39 | 2009 | MI | Caregiver and patient | 103 | 57 | (1) Psychoeducation (2) in person (3) psychologist (4) 4 sessions | 1 wk |
| Etemadifar et al, 40 Etemadifar et al 36 | 2014, 2017 | CHF | Caregiver only | 87 | 87 | (1) Psychoeducation (2) in person (3) cardiologist, a psychiatric nurse, a cardiac care nurse, and a clergyman (4) 2 h/wk for 4 wk | Baseline, postintervention, 4 mo |
| Far et al 42 | 2016 | Mixed heart disease population | Caregiver only | Not reported | 64 | Not reported | Baseline, postintervention, 1 mo |
| Fathani et al 43 | 2016 | CHF | Caregiver only | Not reported | 120 | (1) Psychoeducation, health coaching (2) in person (3) therapist (4) 1–4 sessions, 30–60 mins each | Baseline, 1 mo |
| Gary et al 44 | 2018 | CHF | Caregiver only | Not reported | 127 | (1) Psychoeducation, exercise (2) in person, telephone follow‐up (3) therapist (4) 4 sessions over 12 wk | Baseline, 6 mo |
| Hartford et al 49 | 2002 | CABG | Caregiver and patient | 131 | 131 | (1) Psychoeducation (2) in person, telephone follow‐up (3) nurse (4) 6 calls in 7 wk | Baseline, week 4, week 8 |
| Hu et al 45 | 2016 | CHF | Caregiver only | 118 | 118 | (1) Psychoeducation, support group (2) in person (3) therapist (4) 30 min sessions over 3 mo | Baseline, postintervention, 6 mo |
| Johnston et al 48 | 1999 | MI | Caregiver and patient | 100 | 100 | (1) Psychoeducation (2) in person (3) nurse (4) 6 wk | Baseline, <2 wk after discharge, 2, 6, and 12 mo |
| Kim et al 46 | 2016 | Mixed heart disease population | Caregiver only | 54 | 54 | (1) Cardiopulmonary resuscitation training, psychoeducation (2) in person, telephone (3) nurses (4) a 30 min session | Baseline, postintervention, 4 wk |
| Lang et al 54 | 2018 | HFpEF | Caregiver and patient | 50 | 21 | (1) Psychoeducation (2) in person, telephone follow‐up (3) cardiac nurses (4) at least 3 sessions over 12 wk | Baseline, 3 mo, 6 mo |
| Mahler and Kulik 50 | 2002 | CABG | Caregiver and patient | 296 | 296 | (1) Psychoeducation (2) video, no follow‐up (3) cardiothoracic nurse, couples who have had CABG (4) 1 video session | Baseline, 1 mo, 3 mo, 6 mo |
| Molloy et al 53 | 2005 | CHF | Caregiver only | 60 | 42 | (1) Exercise (2) in person (3) therapist (4) 12 wk | Baseline, 3 mo, 6 mo |
| Piamjariyakul et al 51 | 2015 | CHF | Caregiver and patient | Not reported | 20 | (1) Psychoeducation and coaching (2) in person, telephone (3) nurse (4) 60–90 min session weekly for 4 wk | Baseline, 6 mo |
| Sneed et al 56 | 1997 | SCA/ICD placement | Caregiver and patient | 34 | 31 | (1) Psychoeducation, support group (2) telephone, in‐person support group (3) cardiovascular case manager (4) weekly for 8 wk with 2 in‐person support groups over 4 mo | Baseline, 5‐6 days post‐operatively, 4 mo |
| Srisuk et al 47 | 2016 | CHF | Caregiver and patient | 200 | 100 | (1) Psychoeducation (2) in person, telephone support (3) nurses (4) not reported | Baseline, 3 mo, 6 mo |
| Wingham et al 52 | 2019 | CHF | Caregiver and patient | 97 | 97 | (1) Psychoeducation (2) in person, telephone follow‐up, (3) nurses, therapists (4) 4–6 sessions over 12 wk | Baseline, 4 mo, 6 mo, 12 mo |
| Wu et al 55 | 2019 | CHF | Caregiver and patient | 43 | 43 | (1) Psychoeducation (2) in person, telephone follow‐up (3) therapist (4) bi‐weekly | Baseline, 3 mo, 6 mo |
CABG indicates coronary artery bypass graft; CHF, congestive heart failure; HFpEF, heart failure with preserved ejection fraction; ICD, implantable cardiac defibrillator; MI, myocardial infarction; and SCA, sudden cardiac arrest.
Of the 20 distinct RCTs, 5 studies were conducted in the United States, 1 in Canada, 6 in Europe, 4 in the Middle East, 3 in Asia, and 1 in New Zealand. Thirteen RCTs were conducted among patients with heart failure, 2 among patients with myocardial infarction, 2 in patients post coronary artery bypass graft surgery, 2 in mixed patient populations simply defined as having ischemic heart disease or cardiovascular disease, and 1 in patients with implantable cardioverter defibrillators for sudden cardiac arrest (Table 1). Of the 20 RCTs, 9 were published in medical journals, 6 in nursing journals, 4 in psychology journals, and 1 in a religious journal.
Intervention Characteristics
There was considerable heterogeneity among the interventions tested, but the majority (n=17, out of 20) of the RCTs provided psychoeducation for caregivers (Table 1). The goal of psychoeducation therapeutic interventions is to enhance understanding of the disease and provide support to cope with illness. With regard to intervention targets, 12 of the 20 (60%) RCTs targeted patients and caregivers together, while the remaining 8 (40%) targeted caregivers alone. Intervention duration ranged from a single 30‐minute session on the day of hospital discharge up to an entire year. The majority of interventions lasted between 4 to 12 weeks (n=11, out of 20). Intervention modalities varied and consisted of ≥1 of the following: hard copy resources such as pamphlets and books, one‐on‐one counseling telephone coaching, and both large and small group sessions led by nurses, trained research personnel, therapists/psychologists, or other medical professionals.
Timing of Interventions
Timing of the interventions and time points when teams assessed caregiver outcomes are represented in a temporal Gannt chart (Figure 2). The majority of RCTs (n=19, out of 20) assessed baseline measurements of outcomes and most (n=17) used once weekly intervention protocols. Thirteen of the RCTs assessed outcomes at time points of 6 months and beyond, and 17 of the RCTs had at least 1 assessment of outcomes within the first month. Interventions were delivered over various time points and in various intervals, so it was difficult to draw conclusions about the optimal timings of interventions for caregivers. Time points at which studies measured and assessed impact of intervention on caregiver outcomes were recorded and reported in the Gannt chart.
Figure 2. Temporal Gannt chart characterizing timing of implementation of interventions for caregivers, and points at which caregiver outcomes were assessed.
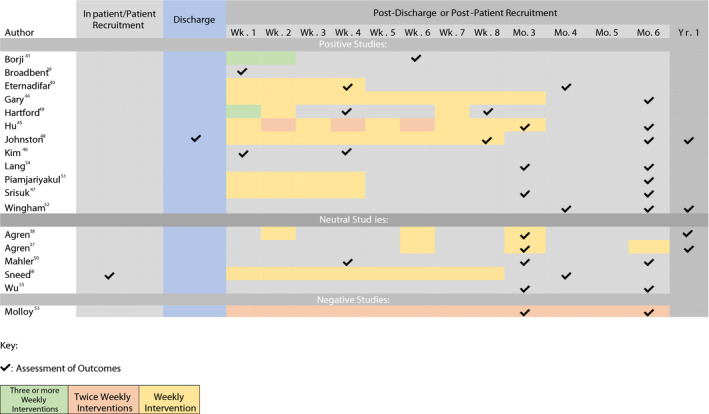
Studies are listed based on overall caregiver outcomes: positive outcomes, neutral or no change studies, and negative or adverse effect studies. Number of interventions per week (ie, once a week, twice a week, or ≥3 times a week) is color‐coded. Time points at which patients are assessed for outcomes, for instance, by phone call or electronic survey, is marked with check marks.
Outcomes Measurement
Outcome assessment tools were heterogeneous across the studies. For instance, 2 out of 5 studies that measured QoL in caregivers and patients used Short form‐36, 43 , 45 and 1 study used the comparable shortened version, Short form‐12. 47 Depression and/or anxiety of caregivers were assessed as outcomes in 12 studies: 4 studies used the Hospital Anxiety and Depression Scale 48 , 52 , 53 , 54 2 studies used the Center for Epidemiologic Studies Depression Scale, 45 , 51 2 studies used the Beck Anxiety Inventory, 41 , 49 and the remaining studies used various other metrics. Caregiver burden was evaluated in 9 studies using various metrics including Zarit Burden Inventory, 36 , 45 Caregiver Burden Scale, 32 , 38 and Caregiver Burden Questionnaire—Heart Failure. 52 , 54 Caregiver knowledge was evaluated in only 2 studies using different metrics. 48 , 55
Effects of Interventions on Caregiver Health and Behavioral Outcomes
Across studies, we estimate small increases in physical QoL (Hedges’ g=0.178; 95% CI, −0.09 to 0.45) and mental QoL (Hedges’ g=0.22; 95% CI, −0.04 to 0.48), and small reductions in anxiety (Hedges’ g=−0.48; 95% CI, −1.08 to 0.12) (Table 2). For each of the small effect sizes on QoL and anxiety, the CI included the null value which suggests that the intervention may not have had an effect on these outcomes. We noted moderate reductions in caregiver depression (Hedges’ g=−0.64; 95% CI, −1.34 to 0.06) and caregiver burden (Hedges’ g=−0.51; 95% CI, −2.71 to 1.70) (Table 2). Similarly, given the wide CIs for these effect sizes that crossed null, there is a possibility that the interventions had no effect on these outcomes. Forest plots of the individual study effects show heterogeneity across studies for each caregiver outcome (Figure 3). Both caregiver depression and caregiver burden showed considerable heterogeneity across studies (I2=91.54% and I2=98.72%, respectively), suggesting the combined effect size for these outcomes should not be interpreted as an average intervention effect.
Table 2.
Hedges’ g Effect Size of Psychoeducational Interventions and CIs
| Caregiver outcome | No. of studies | Hedges’ g (95% CI) |
|---|---|---|
| Physical quality of life | 5 | 0.18 (−0.09 to 0.45) |
| Mental quality of life | 5 | 0.22 (−0.04 to 0.48) |
| Depression | 5 | −0.64 (−1.34 to 0.06) |
| Anxiety | 3 | −0.48 (−1.08 to 0.12) |
| Burden | 4 | −0.51 (−2.71 to 1.70) |
Figure 3. Forest plots representing effect size (Hedges’ g) distribution for the outcomes.
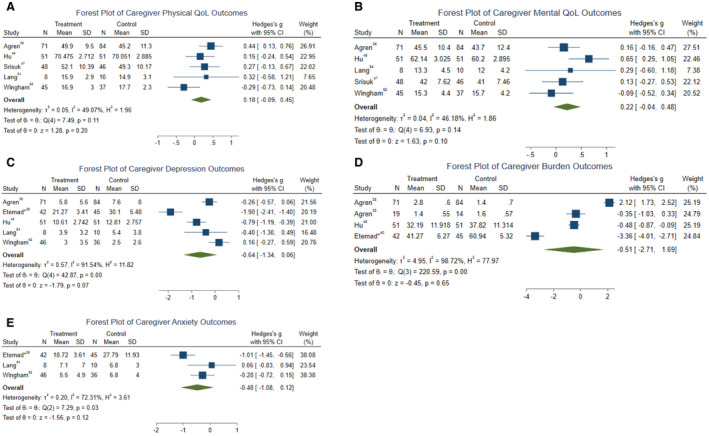
A, Physical quality of life (Hedges’ g=0.178; 95% CI, −0.09 to 0.45). B, Mental quality of life (Hedges’ g=0.22; 95% CI, −0.04 to 0.48). C, Depression (Hedges’ g=−0.64; 95% CI, −1.34 to 0.06). D, Burden (Hedges’ g=−0.51; 95% CI, −2.71 to 1.70). E, Anxiety (Hedges’ g=−0.48; 95% CI, −1.08 to 0.12).
Effects of Interventions on Patient Health and Behavioral Outcomes
As an exploratory aim, we evaluated the patient outcomes of medication adherence and hospital readmission in the RCTs that targeted caregivers and patients together. Patient medication adherence was only studied by Wu and colleagues 55 ; they reported a statistically significant improvement in adherence. Three studies measured rehospitalization with mixed outcomes. 50 , 51 , 54 Because of the limited number of reports, no additional statistical analysis was performed.
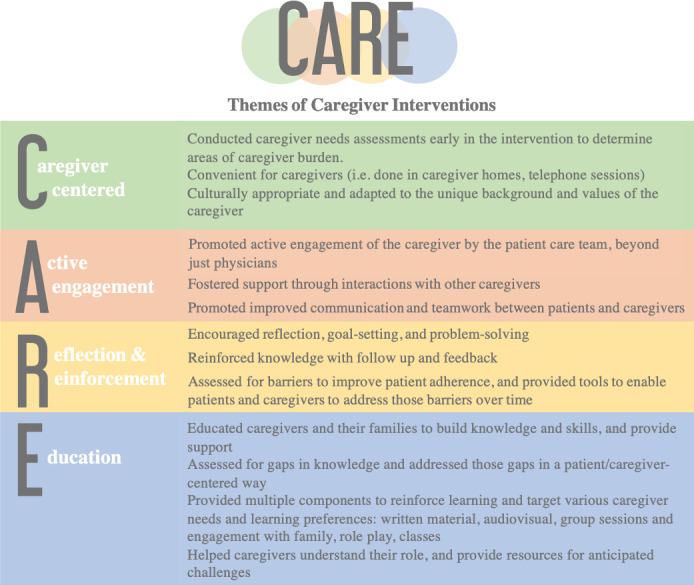
CARE Model and Themes of Interventions
We summarized and evaluated key themes from the psychoeducational interventions. When themes were organized, 4 central ones arose, which were subsequently organized under a framework, represented by Caregiver‐Centered, Active Engagement, Reinforcement, and Education (CARE) (Figure 4). The 4 thematic categories of “CARE” are: (1) Caregiver‐centered approaches that assess the unique needs of caregivers, and promote convenient and culturally appropriate responses to their needs, (2) Active engagement of the caregiver by the patient care team using a variety of resources and tools, (3) Reinforcement of knowledge and shared goals, and (4) Continued education of caregivers after assessing for gaps in knowledge to empower them to confidently provide care to patients.
Figure 4. Evidence‐based themes and framework to aid clinical team support of caregivers.
Quality of Evidence
Our quality review (Table S7) found 13 studies (65%) of good quality, 6 studies (30%) of fair quality, and 1 study (5%) of poor quality. The details of the interventions, such as the frequency and length, total duration of the intervention period, and material content were reported in most studies. Of note, 1 study by Far and colleagues 42 did not report details of the intervention, and 2 studies, 32 , 51 were pilot studies that were mainly hypothesis‐generating.
There was also a high degree of heterogeneity in the tools used for outcome assessment and in the outcomes that were measured for patients and caregivers. Similarly, we found a lack of standardization in defining the inclusion criteria for the caregiver as aforementioned.
Discussion
Caregivers play a vital role in supporting patients with heart disease, but experience significant burden while performing this role, emphasizing the need for greater support from the clinical team. However, it is still not always clear to the clinical teams who a patient’s caregivers are and how to best support them. Prior systematic reviews have focused on evaluating and understanding the caregiver experience and caregiver burden. 20 , 22 , 57 We build upon this knowledge by investigating RCTs of interventions designed to educate and support caregivers of patients with heart disease. The goal was to identify interventions with statistically significant outcomes to help provide guidelines for clinical teams to better engage the caregivers of their patients.
Our systematic review found that most interventions were designed to focus on psychoeducation, were delivered over multiple weeks, and had at least 1 in‐person meeting. In summary, meta‐analysis across studies showed no change compared with control in caregiver burden, anxiety, depression, physical quality of life or mental quality of life. However, it is important to note that some individual RCTs did demonstrate statistically significant improvement in at least 1 caregiver health or behavioral outcome. It may be reasonable to assume that these specific interventions have scalable potential, but the limited number of studies and heterogeneity of interventions and outcomes measured in this meta‐analysis possibly resulted in a type II error.
As an exploratory aim we also evaluated the medication adherence and rehospitalization of patients in the RCTs that targeted both caregivers and their patients. It is interesting to note that none of the patient health and behavioral outcomes evaluated in these studies was negatively impacted. Two studies showed significant improvements in patient medication adherence 55 and rehospitalization. 51 Similarly, while the meta‐analysis was limited by the number of studies, these optimistic findings warrant additional investigation utilizing standardized methodologies.
Given the variation in study intervention design, implementation, and outcome measures, direct comparison between studies was challenging. However, during our review, we noted multiple themes that were key for effective caregiver interventions. Using a qualitative approach, we organized the recurring themes and developed the CARE model (Figure 4) to highlight strategies and tools for the clinical team to better engage and support caregivers and to provide a foundation for the design of future interventions. Using the CARE model, we developed scripts and strategies that health care professionals can use to better engage and navigate the caregiver relationship (Figure 5).
Figure 5. Building dialogue with caregivers.

We use the Caregiver‐Centered, Active Engagement, Reinforcement, Education (CARE) model to develop scripts for health care professionals to build dialogue with caregivers. We provide statements that recognize the important role caregivers play, engage the caregiver in conversation, and follow‐up questions to navigate caregiver interactions.
While our review focused on caregivers of patients with heart disease because of its prevalence and lack of representation in the caregiver literature, we believe that the guidelines presented are generalizable to the patient‐caregiver dyad in other chronic diseases. The aspects of the CARE model highlighted by the interventions in this review are also well supported in the existing caregiver literature.
Caregiver‐Centered Approach
Wolff and colleagues, and others have highlighted the spectrum of caregiver needs, arguing for a more tailored approach to care that has the caregiver in focus. 21 , 34 , 42 , 43 At baseline some caregivers are at higher risk of incurring the negative health effects of being a caregiver and require more immediate intervention to prevent harm. 21 , 58 Some of these risk factors include poor health, lack of choice in being a caregiver, and low‐wage jobs without flexibility. 21 Several of the studies in the review included caregiver needs assessment in the form of questionnaires or interviews, allowing the interventions to be more aligned with the identified caregiver needs instead of a one size fits all approach. 40 , 42 , 43 , 52
Active Engagement
Caregivers support patients in various ways, including assisting with activities of daily living, instrumental activities of daily living, and health care‐related activities such as medication administration, organizing appointments, and providing transportation. Buck and colleagues and others have underscored the importance of caregiver engagement by the medical team as co‐providers of care. 21 , 22 , 27 , 57 , 59 , 60 Caregivers want to be treated as part of the care team and are often frustrated by their health care teams’ lack of acknowledgment and support. 27 , 59 As supported by our review, successful interventions used various methods to actively engage caregivers. These included the use of multimedia resources, 40 , 44 , 46 , 47 role play, 55 teach back, 44 , 47 , 51 , 55 peer support groups, 45 small group activities, 45 and workbooks/manuals. 46 , 47 , 51 , 52 , 54 Engagement by the health care team should be multidisciplinary, extending to physicians, pharmacists, nurses, dieticians, physical therapists, social workers, and more.
Reinforcement
Most studies in this review, apart from a select few, 35 , 53 provided ongoing training, follow‐up, or booster sessions to reinforce and build on skills or knowledge gained. Because of knowledge attrition and ongoing needs, follow‐up and reinforcement is likely important for improved outcomes. What is less clear in the literature is intervals at which reinforcement is required, and frequency, though it will likely be driven by the unique needs of the caregiver and patient.
Education
Caregivers often feel ill‐prepared to deliver care, and lack understanding and knowledge about the patient’s disease process and their role in supporting patients, which results in increased uncertainty and distress. 22 , 27 , 35 , 61 , 62 , 63 , 64 This highlights the value placed by caregivers in understanding their loved ones disease and how they can effectively support them. In a study of an intervention for caregivers of patients who had a heart attack, caregivers had increased understanding, less anxiety, and more positive expectations when they attended 1 half‐hour patient‐and‐spouse session with a psychologist, focused on illness perception, in addition to standard of care. 35 All interventions in our review offered some form of education covering illness perception, clinical knowledge base, or psychosocial components of care. Topics for education are diverse and should be guided by the disease process and by caregiver and patient needs.
We acknowledge that while these best practices may be identified, the level of support that can be offered to caregivers of patients may be limited, because of time constraints in busy practices and the patient‐centered focus of our reimbursement system. Consequently, initial caregiver interventions should be designed to be cost and resource effective. For example, in their study, Lang and colleagues, discuss the societal, health care utilization, and intervention costs associated with their positive caregiver and patient outcomes. 54 In a recent article, Wolff and colleagues outlined strategies at a clinical and policy level to begin addressing this inequity of care. 21 They argue that the clinical team has a responsibility for identification of at‐risk caregivers, assessment of caregiver burden, and provision of tailored support to caregivers. 21 However, Wolff and colleagues also acknowledged that the clinical team needs to be better educated to engage and support caregivers, and reimbursement strategies must change to hold the entire clinical team accountable in the support of caregivers. 21 As we await continued policy shifts, we propose the CARE model as a way to enable clinicians to identify and engage caregivers most at risk and to provide a framework for continued research. The CARE model provides themes in which clinicians may engage, build dialogue with, and identify needs of caregivers. While it is our hope that this model provides strategies that will reduce caregiver stress and improve their quality of life, we must acknowledge that there are inherent qualities of the caregiver such as age, comorbidities, resilience, and existing social support that are not as easily impacted and can also have an important effect on caregiver outcomes. Consequently, further translational studies are necessary to identify effective interventions for caregivers that are resource‐efficient, and adaptable to the workflow of members of the health care team.
Strengths and Limitations
To build upon prior caregiver intervention literature, we categorized the themes and outcomes of the included studies and created the CARE model. The model highlights specific areas for future caregiver interventions to target. We also analyzed and reported outcomes among both patients and caregivers, highlighting the dynamic and complex interactions that exist in chronic disease management. The studies also included a wide spectrum of demographics and countries.
There are a few limitations of this study. Given the scope of our topic and a lack of clearly established search terms in the literature to encompass for example, family and other unpaid caregivers, we used broad search terms that resulted in thousands of returned articles. The low specificity led to many unrelated search returns, which had to be manually filtered. We also discovered a lack of consistency in who was considered a caregiver across studies. Since, for example, the needs of spousal caregivers may differ from the needs of caregivers who live outside the home, making direct comparisons between studies is more challenging. The heterogeneity of caregiver interventions and reported outcomes and small sample sizes may have resulted in an error of omission, potentially masking significant findings. The considerable heterogeneity across studies means that the combined effect sizes may not be meaningful as an average intervention effect. Further, the small number of studies do not allow for exploration of the causes of such heterogeneity. Future work should focus on developing standardized approaches with common metrics to reduce the potential for a type II error.
We recognize that another limitation of our systematic review is that it does not capture unpublished, institutionalized support for caregivers. For example, Called to Care at Johns Hopkins Bayview Medical Center (Baltimore, MD) provides supportive services, education, and access to community resources for caregivers of patients at our institution, that is separate from assistance by the clinical team. Additionally, organizations like the American Heart Association and American College of Cardiology provide resources to support caregivers of patients with CVD. There are a myriad of other national and smaller local programs that exist to support caregivers. Our findings and evidence‐based recommendations must be contextualized within these existing infrastructures to collaboratively promote improved caregiver support and engagement.
Future Directions
There is a need for better designed clinical trials to help establish evidence‐based clinical practices for supporting caregivers. Additionally, establishing a clear relationship between caregiver support and patient outcomes may be valuable to help promote changes in policy and clinical practice. Using heart disease as an index case, our model’s reliability and validity should be further studied. Thereafter, it can serve as a model for other chronic diseases that require extensive caregiver involvement.
We note that in our systematic review, there was also a high degree of heterogeneity in what outcomes were measured, and how they were assessed for both caregivers and patients. This reflects a need for more standardization of caregiver interventions and outcomes assessment. Similarly, we found a lack of standardization in defining “caregiver”, such as “who is a caregiver?” and “what roles do they play?”. This is important to note because moving forward, we must agree on a standard definition of “caregiver”, in order to better represent the caregiver population, recognize them and their role in the health of the patient, and develop practices that help them maintain their health and quality of life. We also acknowledge that in practice, the terminology “caregiver” may not be universally accepted. For example, while a spouse or adult child may support a loved one with their health care decisions and needs, they may not consider themselves to be a caregiver.
In our review, we also noted a high degree of heterogeneity in the timing of interventions and the intervals over which they were delivered. This made drawing conclusions about optimal timing challenging. We hypothesize that the timing of the intervention may be an important factor. For example, during transitions from hospital to home or after a new or worsening diagnosis, an intervention may have a greater impact on caregiver outcomes. Furthermore, in our study, evaluation of outcomes occurred periodically throughout and immediately following interventions. We hypothesize that timing of follow‐up impacts the magnitude of detected outcomes. Consequently, the timing of interventions is a potentially important factor that warrants further investigation.
Conclusions
The vital role that caregivers play in supporting the care of patients with heart disease and the resulting burden experienced by performing this role highlights the need for greater support from the clinical team. Many of the caregivers in the intervention groups did not exhibit a significant improvement from control groups. However, statistically significant improvements in some outcomes were demonstrated and rarely did the interventions worsen caregiver outcomes. Our systematic review highlighted themes on engaging and supporting caregivers (CARE) based on the RCTs. There remains a need for evidence‐based recommendations to guide the health care team in best practices for engaging and aiding caregivers of patients with heart disease.
Sources of Funding
This study did not receive specific funding. Martin has received research support from the American Heart Association (20SFRN35380046 and COVID19‐811000), Patient‐Centered Outcomes Research Institute (ME‐2019C1‐15328), NIH (P01 HL108800), the Aetna Foundation, the David and June Trone Family Foundation, the Pollin Digital Innovation Fund, the PJ Schafer Cardiovascular Research Fund, Sandra and Larry Small, CASCADE Familial Hypercholesterolemia, Apple, Google, and Amgen. Spaulding has received the following financial support for the research, authorship, and publication of this article: NIH/National Heart, Lung, and Blood Institute T32 HL007024 Post‐Doctoral Fellowship in Cardiovascular Epidemiology Institutional Training, NIH/National Institute of Nursing Research F31 NR017328 Ruth L. Kirschstein National Research Service Award, and NIH/National Institute of Nursing Research T32 NR012704 Pre‐Doctoral Fellowship in Interdisciplinary Cardiovascular Health Research. Wongvibulsin is supported by the Johns Hopkins School of Medicine Medical Scientist Training Program (NIH: Institutional Predoctoral Training Grant—T32, 5T32GM007309) and the NIH: Ruth L. Kirschstein Individual Predoctoral National Service Research Award for MD/PhD: F30 Training Grant (F30HL142131). This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research which is funded in part by Grant Number UL1 TR003098 from the National Center for Advancing Translational Sciences a component of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins Institute for Clinical and Translational Research, National Center for Advancing Translational Sciences, or NIH.
Disclosures
Under a license agreement between Corrie Health and the Johns Hopkins University, the University owns equity in Corrie Health and the University and Drs Marvel, Lee, and Martin are entitled to royalty distributions related to technology described in the study discussed in this publication. Additionally, Drs Marvel, Lee, and Martin are cofounders of and hold equity in Corrie Health. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Dr Martin has served as a consultant to Akcea, Amgen, AstraZeneca, Esperion, Kaneka, Novo Nordisk, Quest Diagnostics, Regeneron, REGENXBIO, Sanofi, and 89bio. He is a coinventor on a system to estimate low‐density lipoprotein cholesterol levels, patent application pending. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Acknowledgments
The authors would like to acknowledge Dr W. Daniel Hale of Called to Care for his invaluable insights which helped provide guidance for this project. We would like to thank Leah Jager, PhD, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health for her expertise in biostatistics.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019706
For Sources of Funding and Disclosures, see page 12.
References
- 1. Mosca L, Mochari‐Greenberger H, Aggarwal B, Liao M, Suero‐Tejeda N, Comellas M, Rehm L, Umann TM, Mehran R. Patterns of caregiving among patients hospitalized with cardiovascular disease. J Cardiovasc Nurs. 2011;26:305–311. doi: 10.1097/JCN.0b013e3181f34bb3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood). 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38 [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 4. National Heart, Lung, and Blood Institute . Fact book fiscal year 2011. NIH; 2011. [Google Scholar]
- 5. AARP and National Alliance for Caregiving . Caregiving in the United States 2020. AARP; 2020. [Google Scholar]
- 6. Reinhard SC, Feinberg LF, Houser A, Choula R & Evans M Valuing the Invaluable: 2019 Update Charting a Path Forward. AARP Public Policy Institute; 2019.
- 7. Aggarwal B, Liao M, Mosca L. Medication adherence is associated with having a caregiver among cardiac patients. Ann Behav Med. 2013;46:237–242. doi: 10.1007/s12160-013-9492-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hwang B, Fleischmann KE, Howie‐Esquivel J, Stotts NA, Dracup K. Caregiving for patients with heart failure: impact on patients’ families. Am J Crit Care. 2011;20:431–441. doi: 10.4037/ajcc2011472 [DOI] [PubMed] [Google Scholar]
- 9. Gérain P, Zech E. Informal caregiver burnout? Development of a theoretical framework to understand the impact of caregiving. Front Psychol. 2019;10:1748. doi: 10.3389/fpsyg.2019.01748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108:23–27. doi: 10.1097/01.NAJ.0000336406.45248.4c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burton LC, Zdaniuk B, Schulz R, Jackson S, Hirsch C. Transitions in spousal caregiving. Gerontologist. 2003;43:230–241. doi: 10.1093/geront/43.2.230 [DOI] [PubMed] [Google Scholar]
- 12. Hirst M. Carer distress: a prospective, population‐based study. Soc Sci Med. 2005;61:697–708. doi: 10.1016/j.socscimed.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 13. Ramsay CE, Walker ER, Ramsay R, Compton MT, Thompson N. An Exploration of perceptions of possible depression prevention services for caregivers of elderly or chronically ill adults in rural Georgia. Community Ment Health J. 2012;48:167–178. doi: 10.1007/s10597-010-9361-x [DOI] [PubMed] [Google Scholar]
- 14. Bakas T, Pressler SJ, Johnson EA, Nauser JA, Shaneyfelt T. Family caregiving in heart failure. Nurs Res. 2006;55:180–188. doi: 10.1097/00006199-200605000-00004 [DOI] [PubMed] [Google Scholar]
- 15. Robinson R, Barnett T. Health related quality of life and the support needs of carers of cardiac surgical patients: an exploratory study. Int J Nurs Pract. 2012;18:205–209. doi: 10.1111/j.1440-172X.2012.02020.x [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal B, Liao M, Christian A, Mosca L. Influence of caregiving on lifestyle and psychosocial risk factors among family members of patients hospitalized with cardiovascular disease. J Gen Intern Med. 2009;24:93–98. doi: 10.1007/s11606-008-0852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29:29–37. doi: 10.1097/JCN.0b013e3182784123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andreu ET, Asencio JMM, Sanchez JCC, Sanchez JS, Rico RM, Ruiz FR. Effects and consequences of caring for persons with heart failure: (ECCUPENIC study) a nested case‐control study. J Adv Nurs. 2015;2987–2997. doi: 10.1111/jan.12732 [DOI] [PubMed] [Google Scholar]
- 19. Wingham J, Frost J, Britten N. Behind the smile: qualitative study of caregivers’ anguish and management responses while caring for someone living with heart failure. BMJ Open. 2017;7:e014126. doi: 10.1136/bmjopen-2016-014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strömberg A, Luttik ML. Burden of caring: risks and consequences imposed on caregivers of those living and dying with advanced heart failure. Curr Opin Support Palliat Care. 2015;9:26–30. doi: 10.1097/SPC.0000000000000111 [DOI] [PubMed] [Google Scholar]
- 21. Wolff JL, Feder J, Schulz R. Supporting family caregivers of older Americans. N Engl J Med. 2016;375:2513–2515. doi: 10.1056/NEJMp1612351 [DOI] [PubMed] [Google Scholar]
- 22. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311:1052–1060. doi: 10.1001/jama.2014.304 [DOI] [PubMed] [Google Scholar]
- 23. Wolff JL. Family matters in health care delivery. JAMA. 2012;308:1529–1530. doi: 10.1001/jama.2012.13366 [DOI] [PubMed] [Google Scholar]
- 24. Gillick MR. The critical role of caregivers in achieving patient‐centered care. JAMA. 2013;310:575–576. doi: 10.1001/jama.2013.7310 [DOI] [PubMed] [Google Scholar]
- 25. Lynn J. Strategies to ease the burden of family caregivers. JAMA. 2014;311:1021–1022. doi: 10.1001/jama.2014.1769 [DOI] [PubMed] [Google Scholar]
- 26. Saunders MM. Family caregivers need support with heart failure patients. Holist Nurs Pract. 2003;17:136–142. doi: 10.1097/00004650-200305000-00004 [DOI] [PubMed] [Google Scholar]
- 27. Reinhard SC, Given B, Petlick NH, Bemis A. Supporting family caregivers in providing care. In: Hughes RG, ed. Patient Safety and Quality: An Evidence‐Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2011:341–363. [PubMed] [Google Scholar]
- 28. Ornstein KA, Wolff JL, Bollens‐Lund E, Rahman O‐K, Kelley AS. Spousal caregivers are caregiving alone in the last years of life. Health Aff. 2019;38:964–972. doi: 10.1377/hlthaff.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 30.Quality Assessment of Controlled Intervention Studies. In: National Heart, Blood, and Lung Institute [Internet]. Available at: https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. Accessed October 14, 2019.
- 31. Higgins JPT, Li T, Deeks JJ, eds. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021:143–176. Available at: www.training.cochrane.org/handbook Accessed April 15, 2021. [Google Scholar]
- 32. Ågren S, Strömberg A, Jaarsma T, Luttik MLA. Caregiving tasks and caregiver burden; effects of a psycho‐educational intervention in partners of patients with post‐operative heart failure. Heart Lung. 2015;44:270–275. doi: 10.1016/j.hrtlng.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 33. Liljeroos M, Ågren S, Jaarsma T, Årestedt K, Strömberg A. Long term follow‐up after a randomized integrated educational and psychosocial intervention in patient‐partner dyads affected by heart failure. PLoS One. 2015;10:e0138058. doi: 10.1371/journal.pone.0138058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liljeroos M, Ågren S, Jaarsma T, Årestedt K, Strömberg A. Long‐term effects of a dyadic psycho‐educational intervention on caregiver burden and morbidity in partners of patients with heart failure: a randomized controlled trial. Qual Life Res. 2017;26:367–379. doi: 10.1007/s11136-016-1400-9 [DOI] [PubMed] [Google Scholar]
- 35. Broadbent E, Ellis CJ, Thomas J, Gamble G, Petrie KJ. Further development of an illness perception intervention for myocardial infarction patients: A randomized controlled trial. J Psychosom Res. 2009;67:17–23. doi: 10.1016/j.jpsychores.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 36. Etemadifar S, Bahrami M, Shahriari M. The family centered empowerment program can relieve stress, anxiety and depression of heart failure patients’ family caregivers. World Fam Med. 2017;15:99–104. doi: 10.5742/MEWFM.2017.93145 [DOI] [Google Scholar]
- 37. Ågren S, Berg S, Svedjeholm R, Strömberg A. Psychoeducational support to post cardiac surgery heart failure patients and their partners—a randomised pilot study. Intensive Crit Care Nurs. 2015;31:10–18. doi: 10.1016/j.iccn.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 38. Ågren S, Evangelista LS, Hjelm C, Strömberg A. Dyads affected by chronic heart failure: a randomized study evaluating effects of education and psychosocial support to patients with heart failure and their partners. J Card Fail. 2012;18:359–366. doi: 10.1016/j.cardfail.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Broadbent E, Ellis CJ, Thomas J, Gamble G, Petrie KJ Can an illness perception intervention reduce illness anxiety in spouses of myocardial infarction patients? A randomized controlled trial. J Psychosom Res. 2009;67:11–15. doi: 10.1016/j.jpsychores.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 40. Etemadifar S, Bahrami M, Shahriari M, Farsani AK. The effectiveness of a supportive educative group intervention on family caregiver burden of patients with heart failure. Iran J Nurs Midwifery Res. 2014;19:217–223. [PMC free article] [PubMed] [Google Scholar]
- 41. Borji M, Mousavimoghadam SR, Salimi E, Otaghi M, Azizi Y. The impact of spiritual care education on anxiety in family caregivers of patients with heart failure. J Relig Health. 2019;58:1961–1969. doi: 10.1007/s10943-018-0689-9 [DOI] [PubMed] [Google Scholar]
- 42. Far FS, Moeini M, Kheirabadi GR. Analysis of the effects of a support program based on psychosocial needs of families on care burdens of family caregivers of patients with ischemic heart disease hospitalized in Chamran heart ward of Isfahan University of Medical Sciences. Int J Med Res Health Sci. 2016;5:152–157. [Google Scholar]
- 43. Fathani M, Afzal Aghaee M, Tadayonfar M. Evaluation of the effect of designated educational intervention on the improvement of quality of life in caregivers of patients with chronic heart failure. J Babol Univ Med Sci. 2016;18:20–25. [Google Scholar]
- 44. Gary R, Dunbar SB, Higgins M, Butts B, Corwin E, Hepburn K, Butler J, Miller AH. An intervention to improve physical function and caregiver perceptions in family caregivers of persons with heart failure. J Appl Gerontol. 2020;39:181–191. doi: 10.1177/0733464817746757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu X, Dolansky MA, Su Y, Hu X, Qu M, Zhou L. Effect of a multidisciplinary supportive program for family caregivers of patients with heart failure on caregiver burden, quality of life, and depression: a randomized controlled study. Int J Nurs Stud. 2016;62:11–21. doi: 10.1016/j.ijnurstu.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 46. Kim HS, Kim HJ, Suh EE. The effect of patient‐centered CPR education for family caregivers of patients with cardiovascular diseases. J Korean Acad Nurs. 2016;46:463–474. doi: 10.4040/jkan.2016.46.3.463 [DOI] [PubMed] [Google Scholar]
- 47. Srisuk N, Cameron J, Ski CF, Thompson DR. Randomized controlled trial of family‐based education for patients with heart failure and their carers. J Adv Nurs. 2017;73:857–870. doi: 10.1111/jan.13192 [DOI] [PubMed] [Google Scholar]
- 48. Johnston M, Foulkes J, Johnston DW, Pollard B, Gudmundsdottir H. Impact on patients and partners of inpatient and extended cardiac counseling and rehabilitation: a controlled trial. Psychosom Med. 1999;61:225–233. doi: 10.1097/00006842-199903000-00015 [DOI] [PubMed] [Google Scholar]
- 49. Hartford K, Wong C, Zakaria D. Randomized controlled trial of a telephone intervention by nurses to provide information and support to patients and their partners after elective coronary artery bypass graft surgery: effects of anxiety. Heart Lung. 2002;31:199–206. doi: 10.1067/mhl.2002.122942 [DOI] [PubMed] [Google Scholar]
- 50. Mahler HI, Kulik JA. Effects of a videotape information intervention for spouses on spouse distress and patient recovery from surgery. Health Psychol. 2002;21:427–437. doi: 10.1037/0278-6133.21.5.427 [DOI] [PubMed] [Google Scholar]
- 51. Piamjariyakul U, Werkowitch M, Wick J, Russell C, Vacek JL, Smith CE. Caregiver coaching program effect: reducing heart failure patient rehospitalizations and improving caregiver outcomes among African Americans. Heart Lung. 2015;44:466–473. doi: 10.1016/j.hrtlng.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 52. Wingham J, Frost J, Britten N, Greaves C, Abraham C, Warren FC, Jolly K, Miles J, Paul K, Doherty PJ, et al. Caregiver outcomes of the REACH‐HF multicentre randomized controlled trial of home‐based rehabilitation for heart failure with reduced ejection fraction. Eur J Cardiovasc Nurs. 2019;18:611–620. doi: 10.1177/1474515119850011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Molloy GJ, Johnston DW, Gao C, Witham MD, Gray JM, Argo IS, Struthers AD, McMurdo MET. Effects of an exercise intervention for older heart failure patients on caregiver burden and emotional distress. Eur J Cardiovasc Prev Rehabil. 2006;13:381–387. doi: 10.1097/01.hjr.0000198916.60363.85 [DOI] [PubMed] [Google Scholar]
- 54. Lang CC, Smith K, Wingham J, Eyre V, Greaves CJ, Warren FC, Green C, Jolly K, Davis RC, Doherty PJ, et al. A randomised controlled trial of a facilitated home‐based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH‐HFpEF Pilot Study. BMJ Open. 2018;8:e019649. doi: 10.1136/bmjopen-2017-019649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu J‐R, Mark B, Knafl GJ, Dunbar SB, Chang PP, DeWalt DA. A multi‐component, family‐focused and literacy‐sensitive intervention to improve medication adherence in patients with heart failure‐a randomized controlled trial. Heart Lung. 2019;48:507–514. doi: 10.1016/j.hrtlng.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sneed NV, Finch NJ, Michel Y. The effect of psychosocial nursing intervention on the mood state of patients with ICDs and their caregivers. Prog Cardiovasc Nurs. 1997;12:4–14. [PubMed] [Google Scholar]
- 57. Buck HG, Harkness K, Wion R, Carroll SL, Cosman T, Kaasalainen S, Kryworuchko J, McGillion M, O’Keefe‐McCarthy S, Sherifali D, et al. Caregivers' contributions to heart failure self‐care: a systematic review. Eur J Cardiovasc Nurs. 2015;14:79–89. doi: 10.1177/1474515113518434 [DOI] [PubMed] [Google Scholar]
- 58. Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA. 2012;307:398–403. doi: 10.1001/jama.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heinrich M, Neufeld A, Harrison MJ. Seeking support: caregiver strategies for interacting with health personnel. Can J Nurs Res. 2003;35:38–56. [PubMed] [Google Scholar]
- 60. Wolff JL, Spillman BC, Freedman VA, Kasper JD. A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Intern Med. 2016;176:372–379. doi: 10.1001/jamainternmed.2015.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Walden JA, Dracup K, Westlake C, Erickson V, Hamilton MA, Fonarow GC. Educational needs of patients with advanced heart failure and their caregivers. J Heart Lung Transplant. 2001;20:766–769. doi: 10.1016/S1053-2498(00)00239-4 [DOI] [PubMed] [Google Scholar]
- 62. Bucher JA, Loscalzo M, Zabora J, Houts PS, Hooker C, BrintzenhofeSzoc K. Problem‐solving cancer care education for patients and caregivers. Cancer Pract. 2001;9:66–70. doi: 10.1046/j.1523-5394.2001.009002066.x [DOI] [PubMed] [Google Scholar]
- 63. Scherbring M. Effect of caregiver perception of preparedness on burden in an oncology population. Oncol Nurs Forum. 2002;29:E70–E76. doi: 10.1188/02.ONF.E70-E76 [DOI] [PubMed] [Google Scholar]
- 64. Schumacher KL, Stewart BJ, Archbold PG, Dodd MJ, Dibble SL. Family caregiving skill: development of the concept. Res Nurs Health. 2000;23:191–203. doi: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


