Abstract
Background
Assessing coronary artery calcium (CAC) is among AHA/ACC prevention guidelines for people at least 40 years old at intermediate risk for coronary heart disease (CHD). To study enhanced risk stratification, we investigated the predictive value of abdominal aorta calcium (AAC) relative to CAC for cardiovascular disease (CVD) and CHD events in Black and White early middle‐aged participants, initially free of overt CVD.
Methods and Results
In the CARDIA (Coronary Artery Risk Development in Young Adults) study, a multi‐center, community‐based, longitudinal cohort study of CVD risk, the CAC and AAC scores were assessed in 3011 participants in 2010–2011 with follow‐up until 2019 for incident CVD and CHD events. Distributions and predictions, overall and by race, were computed. During the 8‐year follow‐up, 106 incident CVD events (55 were CHD) occurred. AAC scores tended to be much higher than CAC scores. AAC scores were higher in Black women than in White women. CAC predicted CVD with HR 1.77 (1.52–2.06) and similarly for AAC, while only CAC predicted CHD. After adjustment for risk factors and calcium in the other arterial bed, the association of CAC with CVD was independent of risk factors and AAC, while the association of AAC with CVD was greatly attenuated. However, AAC predicted incident CVD when CAC was 0. Prediction did not vary by race.
Conclusions
AAC predicted CVD nearly as strongly as CAC and could be especially useful as a diagnostic tool when it is an incidental finding or when no CAC is found.
Keywords: abdominal aorta calcium, calcium score coronary artery calcium, cardiovascular disease, coronary heart disease, ethnicity, gender differences
Subject Categories: Risk Factors, Race and Ethnicity, Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- AAC
abdominal aorta calcium
- AU
Agatston units
- CARDIA
coronary artery risk development in young adults
Clinical Perspective
What Is New?
In young to middle‐aged White and Black men and women free of overt cardiovascular disease (CVD), abdominal aorta calcium (AAC) is nearly as strongly related to incident CVD as coronary artery calcium (CAC).
Higher CVD rates occur at AAC Agatston score values of over 1000, comparable to the CVD rates at CAC Agatston score values of over 300.
AAC score tends to be as high in Black women as in both Black and White men; the substantially higher AAC in Black women than in White women may help to explain their different CVD event rates.
What Are the Clinical Implications?
AAC can be used without any additional costs or harm to the patient and may aid in predicting coronary heart disease and CVD events.
Because AAC is correlated with CAC, the presence of a high AAC score raises suspicion that CAC may be present.
Because the incidental AAC finding alerts the clinician to potential CVD risk that should be further investigated, automated AAC scoring in planned abdominal computed tomography scan for a non‐CVD medical diagnostic work‐up may be used as a quick and low‐cost tool to identify patients who are at increased risk of CVD.
While coronary artery calcium (CAC) has now been incorporated in the recent AHA/ACC prevention guidelines 1 for the prediction of future cardiovascular disease (CVD) events, it is less clear whether an extra‐coronary calcification, such as abdominal aorta calcification (AAC), adds to prediction. Not utilizing information about extra‐calcium could be a missed opportunity, because information about extra‐coronary calcifications might efficiently improve upon existing risk stratification models utilizing traditional risk factors and CAC. 2 , 3 , 4 From a diagnostic perspective, extra‐coronary computed tomography is commonly performed for assessment of both cardiovascular and non‐cardiovascular conditions. Abdominal aorta calcium (AAC) may develop early in life in asymptomatic subjects. 5 AAC has been studied as a predictor of CVD in older asymptomatic persons and in chronic kidney disease. 6 , 7
Atherosclerosis starts early. 8 , 9 , 10 , 11 Carr et al. investigated the association of CAC with incident CVD in previously asymptomatic subjects aged 32 to 46 years in the CARDIA (Coronary Artery Risk Development in Young Adults) study. 12 The presence of CAC among individuals aged between 32 and 46 years was associated with increased risk of fatal and nonfatal CHD during 12.5 years of follow‐up and a CAC score of 100 or more was associated with early death. The PESA (Progression of Early Subclinical Atherosclerosis) study found that 64% of participants aged 40 to 54 years had subclinical atherosclerosis, with 25% of those sites being infrarenal aortic atherosclerosis. 13 In a meta‐analysis by Bastos Goncalves et al., the presence of AAC in an older population increased the risk for subsequent CVD even after adjustment for traditional risk factors. 14 Given the presence of early atherosclerosis and the predictiveness of CAC in early middle‐age for future CHD, we hypothesized that AAC may also prove useful in risk stratification for CVD starting in early middle‐age. If this hypothesis is true, information about AAC may improve risk determination classification of asymptomatic subjects and influence primary prevention strategies, particularly if coupled with information about CAC.
Unanswered questions regarding the utility of AAC in enhancing classification of risk for CVD in middle‐age led to using the CARDIA data for people aged 42 to 56 years at the time of measurement of AAC and CAC to evaluate the potential added predictive value for CVD in a younger sample. Furthermore, CAC and AAC do not occur equally in Black people as they do in White people. 7 , 15 An assessment of how CAC and AAC distributions and prediction differ by race and sex may be helpful in enhancing racial equity.
The objective of this study is to investigate in late middle age the predictive value of the AAC and CAC scores for fatal and non‐fatal CVD events and CHD events, both overall and between Black and White people. We hypothesize that AAC adds predictive value to CAC for CVD events and that the differing distributions of these subclinical disease markers influence prediction of future CVD. Moreover, we hypothesize that CAC, but not AAC, predicts future CHD events.
Methods
The data that support the findings of this study are available from the CARDIA Coordinating Center (www.cardia.dopm.uab.edu) upon reasonable request.
Study Description
CARDIA is a multi‐center, community‐based, longitudinal cohort study of the development and precursors of CVD. The CARDIA study enrolled 5115 adults initially aged 18 to 30 years in 1985 to 1986. Participants have been re‐examined approximately every 2 to 5 years. All participants have provided written informed consent, and institutional review boards approved the study at the 4 sites (Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA).
Of the 3499 participants examined at exam year 25 (2010–2011), 3117 completed a computed tomography (CT) scan of the chest and abdomen and had both CAC and AAC score. Of these, 3011 Black and White CARDIA participants had no CVD event before year 25. Missing covariate values were estimated in 25 participants by carrying forward the most recent exam value (mostly from year 20) for smoking and medication use or by averaging the year 20 and year 30 value for continuous variables (body mass index, blood lipids, estimated glomerular filtration rate [eGFR]).
Demographics and Clinical Measurements
Predictors and covariates were all assessed at exam year 25. Demographic data including age, race, and sex were self‐reported as were smoking habits. Hypertension was defined as a sitting rest blood pressure of 140/90 mm Hg or more or current use of an antihypertensive medication. Diabetes was defined as a fasting plasma glucose level of 126 mg/mL or more, a 2‐hour glucose tolerance test of 200 mg/dL or more, hemoglobin A1c of 6.5% or more, or use of an antidiabetic medication. Additional data collected following standardized protocols included body mass index (BMI), plasma total cholesterol, plasma high‐density lipoprotein cholesterol, plasma triglycerides, and medical treatment for hyperlipidemia.
Measurement of CAC and AAC by CT Scan
Agatston score was used to quantify calcification in both coronary and abdominal aortic locations. Measurements of CAC and AAC by ECG‐gated non‐contrast CT scans were performed using a standard protocol. 16 Image analysis was performed at a single center (Wake Forest University Health Sciences, Winston‐Salem, NC). The Agatston score was calculated on a workstation with FDA‐approved calcium scoring software (Aquarius Workstation, TeraRecon, Foster City, CA). Agatston scores were corrected for slice thickness and scores were calculated with a minimum calcification area of 1.87 mm2 (4 adjacent pixels) and attenuation threshold of 130 or more Hounsfield units. CAC score in Agatston units was calculated for each calcified lesion and the scores were summed across all lesions within a given coronary artery (left anterior descending, left main, circumflex, and right coronary) and across all arteries to obtain the total CAC score. In those with observed intracoronary stents, the stented area was excluded from the vessel score, 100 Agatston units (AU) were added to account for calcifications that may be obscured, and the remaining vessels were scored. 16 For participants with coronary artery bypass grafts, only native coronary arteries were scored. The accuracy, comparability, and reproducibility of the calcium score using electron beam, helical, and multidetector CT systems have been previously been published. 17 , 18 , 19 Measurement of AAC was performed in the distal aorta in a 60 mm segment centered at the aortic bifurcation. 16
Clinical Events
The incidences of all fatal‐ and non‐fatal CVD and CHD events were obtained from 2010–11 to 2019. Event finding was accomplished by annual contact with each participant; over 90% of participants were successfully contacted in the past 5 years. Two members of the CARDIA Endpoints Surveillance and Adjudication Subcommittee independently adjudicated medical records for each potential event or underlying cause of death. CVD events included CHD, heart failure, stroke, transient ischemic attack, or peripheral arterial disease. CHD events included hospitalization for myocardial infarction, acute coronary syndrome, coronary revascularization, or CHD death. Incident fatal and non‐fatal CVD and CHD events after exam year 25 (2010–2011) were analyzed through event date or censoring (last successful contact date or August 31, 2019).
Statistical Analysis
Participant characteristics and traditional CVD risk factors were obtained at exam year 25. We presented the separate distributions of CAC and AAC both in clinically useful categories and in percentiles, as well as their joint distribution in clinical categories, overall and stratified by race and sex. We presented unadjusted cumulative incidence of all fatal and non‐fatal CVD events for participants in clinical categories overall and stratified by race and by sex. We performed proportional hazards regression with dependent variable CVD events and CAC and AAC as continuous variables on the natural log scale. Adjustment was for demographics (age, race, and sex) as well as for smoking habits, systolic and diastolic blood pressures, antihypertensive medication, body mass index, total cholesterol, HDL‐cholesterol, triglycerides, lipid lowering medication, diabetes, and estimated glomerular filtration rate. We also report a model that included both CAC and AAC as predictor variables, to evaluate whether they predict disease independently of each other. This analysis was repeated for fatal and non‐fatal CHD event incidence as the dependent variable. Because many people had no CAC, we also examined risk according to continuous AAC when CAC=0. To further examine race and sex, we added interaction terms to the proportional hazards models. We also examined the extent to which the race and sex differences in CVD event rates were explained by adjustment for CAC, AAC, and risk factors.
Results
The 3011 participants at CARDIA Y25 were average age 50.1±3.6 years, range 42 to 56 years (Table 1). Nearly half were male and nearly half were Black. The sample had mean BMI over 30 kg/m2, 17% were cigarette smokers, 26% were using antihypertensive medication, 14% were using lipid lowering medication, and 13% had type 2 diabetes. Black participants had attained fewer years of education, were more likely to be cigarette smokers, had higher blood pressure, higher BMI, and higher prevalence of diabetes. White men had the highest plasma triglycerides. White women had the most favorable risk factor profile. CVD events between Y25 and the end of follow‐up in Y33 occurred in 5.7% of Black men, 4% of White men, 3.6% of Black women, and 1.6% of White women. Although about half of the events were CHD, CHD incidence difference by race and sex. CHD constituted 23 of 29 events in White men, but was much less common in other race‐sex groups. Stroke was most common in women. All but 2 of the 19 heart failure events were in Black participants.
Table 1.
Participant Characteristics Overall and By Race and Sex Year 25, 2010 to 2011, n=3011
| All | Black men | Black women | White men | White women | |
|---|---|---|---|---|---|
| Sample size | 3011 | 562 | 852 | 735 | 862 |
| Age, y | 50.1±3.6 | 49.3±3.8 | 49.5±3.8 | 50.6±3.4 | 50.7±3.4 |
| Education attained, y | 15.4±2.5 | 14.2±2.2 | 14.7±2.2 | 16.2±2.6 | 15.4±2.5 |
| Current cigarette smoking | 517 (17.2) | 155 (27.6) | 166 (19.5) | 91 (12.4) | 105 (12.2) |
| Systolic blood pressure, mm Hg | 119.6±15.9 | 125.8±14.6 | 123±17.3 | 119.5±13.4 | 112.3±14.5 |
| Diastolic blood pressure, mm Hg | 74.9±11.1 | 78.6±10.9 | 78.1±11 | 74.3±10 | 70±10.4 |
| Antihypertensive medication | 773 (25.7) | 176 (31.3) | 334 (39.2) | 141 (19.2) | 122 (14.2) |
| Body mass index, (kg/m2) | 30.2±7.1 | 30.1±6.3 | 33.3±7.9 | 28.9±5 | 28.3±7.2 |
| Total cholesterol, mg/dL | 193.3±36.6 | 187.6±37 | 191.9±38.5 | 193±35.9 | 198.8±34.2 |
| HDL cholesterol, mg/dL | 58.3±18.1 | 53.1±16.3 | 61.4±17.3 | 49.6±14.3 | 65.9±18.8 |
| Triglycerides, mg/dL | 114.1±85.8 | 113.3±72.7 | 97.7±72 | 144.4±118.7 | 104.9±63.5 |
| Cholesterol‐lowering medication | 431 (14.3) | 82 (14.6) | 138 (16.2) | 129 (17.6) | 82 (9.5) |
| Diabetes | 392 (13) | 101 (18) | 151 (17.7) | 75 (10.2) | 65 (7.5) |
| eGFR, mL/min/1.73 m2 | 96.3±16.1 | 98.1±18.4 | 102.8±17.8 | 92.1±12.5 | 92.3±12.6 |
| Incident CVD | 106 (3.5) | 32 (5.7) | 31 (3.6) | 29 (4.0) | 14 (1.6) |
| Incident CHD | 55 (1.8) | 16 (2.9) | 11 (1.3) | 23 (3.1) | 5 (0.6) |
| Incident stroke | 39 (1.3) | 11 (2.0) | 15 (1.8) | 4 (0.5) | 9 (1.0) |
| Incident heart failure | 19 (0.6) | 10 (1.8) | 7 (0.8) | 2 (0.3) | 0 (0.0) |
Cells show Mean±SD or n (%).
Abbreviations: eGFR indicates estimated glomerular filtration rate; CVD, cardiovascular disease; and CHD, coronary heart disease.
Note that each individual may qualify for more than one CVD subtype (CHD, stroke, and heart failure). Incident disease in the bottom 4 rows occurred Year 25 in 2010–11 and end of follow‐up, August 31, 2019.
The distribution of the Agatston score for AAC was substantially more spread out than for CAC (Table 2). Whereas 73% had no detectable CAC, 48% had no detectable AAC. At the high end of the distributions, 0.5% of participants had CAC 1000+, compared with 5.3% with AAC 1000+. CAC score was highest in White men, followed by Black men, and all women. AAC score was high and similar among Black men, followed by White men, then Black women, and least in White women (Tables 1, 2, and 3). The 90th percentile of CAC ranged from 187 in White men and 125 in Black men to 22 and 26 in Black and White women, while the 90th percentile of AAC was 192 in White women, but ranged from 600 to 649 in the 2 groups of men and was nearly the same, namely 579 in Black Women. CAC and AAC had a moderate correlation coefficient, 0.39, whether computed on the natural scale or after ln transformation. This correlation manifested as 56% having AAC 0 of those with CAC 0 and 60% having AAC 1000+ of the 15 people with CAC 1000+ (Table 3). The correlation between CAC and AAC was similar across race and sex groups (not shown).
Table 2.
Univariate Distributions of Coronary Artery Calcification and Abdominal Aortic Artery Calcification in Clinically Relevant Categories
| Agatston Score, n (row %) | Percentile, Agatston Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–99 | 100–299 | 300–999 | 1000+ | P25 | P50 | P75 | P90 | P95 | |
| CAC | ||||||||||
| All | 2199 (73.0) | 569 (18.9) | 147 (4.9) | 81 (2.7) | 15 (0.5) | 0 | 0 | 3 | 67 | 200 |
| Black men | 374 (66.6) | 123 (21.9) | 36 (6.4) | 22 (3.9) | 7 (1.3) | 0 | 0 | 10 | 125 | 307 |
| Black women | 708 (83.1) | 106 (12.4) | 24 (2.8) | 12 (1.4) | 2 (0.2) | 0 | 0 | 0 | 22 | 87 |
| White men | 396 (53.9) | 233 (31.7) | 62 (8.4) | 38 (5.2) | 6 (0.8) | 0 | 0 | 31 | 187 | 332 |
| White women | 721 (83.6) | 107 (12.4) | 25 (2.9) | 9 (1.0) | 0 (0.0) | 0 | 0 | 0 | 26 | 69 |
| AAC | ||||||||||
| All | 1438 (47.8) | 876 (29.1) | 296 (9.8) | 243 (8.1) | 158 (5.3) | 0 | 1 | 82 | 451 | 1107 |
| Black men | 224 (39.9) | 172 (30.6) | 78 (13.9) | 49 (8.7) | 39 (6.9) | 0 | 10 | 156 | 649 | 1395 |
| Black women | 420 (49.3) | 242 (28.4) | 64 (7.5) | 74 (8.7) | 52 (6.1) | 0 | 1 | 73 | 579 | 1466 |
| White men | 308 (41.9) | 223 (30.3) | 84 (11.4) | 76 (10.3) | 44 (6.0) | 0 | 4 | 130 | 600 | 1211 |
| White women | 486 (56.4) | 239 (27.7) | 70 (8.1) | 44 (5.1) | 0 (0) | 0 | 0 | 30 | 192 | 518 |
AAC indicates aortic artery calcification; and CAC coronary artery calcification.
Table 3.
Distribution of Abdominal Aortic Artery Calcification Within Coronary Artery Calcification Clinically Relevant Categories, n (row %)
| AAC 0 | AAC 1–99 | AAC 100–299 | AAC 300–999 | AAC 1000+ | |
|---|---|---|---|---|---|
| CAC 0 | 1237 (56.3) | 631 (28.7) | 164 (7.5) | 121 (5.5) | 46 (2.1) |
| CAC 1–99 | 170 (29.9) | 177 (31.1) | 90 (15.8) | 77 (13.5) | 55 (9.7) |
| CAC 100–299 | 24 (16.3) | 45 (30.6) | 30 (20.4) | 24 (16.3) | 24 (16.3) |
| CAC 300–999 | 7 (8.6) | 20 (24.7) | 11 (13.6) | 19 (23.5) | 24 (29.6) |
| CAC 1000+ | 0 (0.0) | 3 (20.0) | 1 (6.7) | 2 (13.3) | 9 (60.0) |
AAC indicates aortic artery calcification; and CAC coronary artery calcification.
CAC was strongly predictive of incident CHD (HR: 2.09), with little effect on estimates of adjustment for demographics, risk factors or AAC (Table 4). The association of CAC with incident CVD was somewhat weaker (HR: 1.77) and more attenuated with full adjustment (HR: 1.42). AAC was also a strong predictor of incident CVD (HR: 1.77), but was largely attenuated with full adjustment (HR: 1.25). Further adjustment for years of education attained did not substantially affect the estimated hazard ratios. It correlated with CHD but was attenuated to nonsignificance with adjustment for CAC. In the common situation in which CAC was 0, AAC was predictive of the 49 CVD events in the fully adjusted model among 2199 people (HR: 1.52 [1.12–2.06]). Only 17 of these events were CHD, which were not predicted by AAC. The overall gradient for incident CVD was only slightly shallower for AAC than for CAC. No gradient in risk was seen between AAC categories 0 and 1–99 (Figure).
Table 4.
Hazard Ratios (95% CI) Predicting Cardiovascular Disease and Coronary Heart Disease from Continuous CAC or AAC
| Cardiovascular disease (n=106 events) | HR (CI) | P value | Coronary heart disease (n=55 events) | HR (CI) | P value |
|---|---|---|---|---|---|
| Independent variable CAC, per 1 SD of ln(CAC)=1.84 | |||||
| ARS | 1.77 (1.52‐2.06) | <0.0001 | ARS | 2.09 (1.69‐2.57) | <0.0001 |
| ARS and AAC | 1.54 (1.30‐1.82) | <0.0001 | ARS and AAC | 1.91 (1.51‐2.40) | <0.0001 |
| ARS and RF | 1.52 (1.29‐1.79) | <0.0001 | ARS and RF | 1.96 (1.57‐2.46) | <0.0001 |
| ARS, RF and AAC | 1.42 (1.19‐1.69) | <0.0001 | ARS, RF and AAC | 1.89 (1.48‐2.42) | <0.0001 |
| Independent variable AAC, per 1 SD of ln(AAC)=2.56 | |||||
| ARS | 1.77 (1.47‐2.12) | <0.0001 | ARS | 1.74 (1.35‐2.25) | <0.0001 |
| ARS and CAC | 1.45 (1.18‐1.77) | 0.0003 | ARS and CAC | 1.27 (0.97‐1.68) | 0.09 |
| ARS and RF | 1.45 (1.18‐1.77) | 0.0003 | ARS and RF | 1.49 (1.13‐1.98) | 0.005 |
| ARS, RF and CAC | 1.25 (1.01‐1.54) | 0.04 | ARS, RF and CAC | 1.12 (0.83‐1.50) | 0.46 |
For each of CVD and CHD, the table presents results from 6 regression models. Other than 4 models that included only CAC or only AAC, the fifth model provided HR for CAC adjusted for ARS and AAC and for AAC adjusted for ARS and CAC. The sixth model provided HR for CAC adjusted for ARS, RF and AAC and for AAC adjusted for ARS, RF and CAC. Both CAC and AAC are expressed as ln(Agatston score); this table includes 3011 participants followed from Y25 (2010–2011) through August 31, 2019.
ARS: Model adjusted for age, race, and sex. RF: Model adjusted for cigarette smoking, systolic blood pressure, diastolic blood pressure, antihypertensive medication use, body mass index, total cholesterol, high‐density lipoprotein cholesterol, triglycerides, cholesterol‐lowering medication, diabetes mellitus, and eGFR.
AAC indicates abdominal aorta calcification; CAC,coronary artery calcification; and HR, hazard ratio.
Figure 1. Incident CVD by CAC Score Category (left) and by AAC Score Category (right), for Black vs White race (upper) and for men vs women (lower), unadjusted % events.
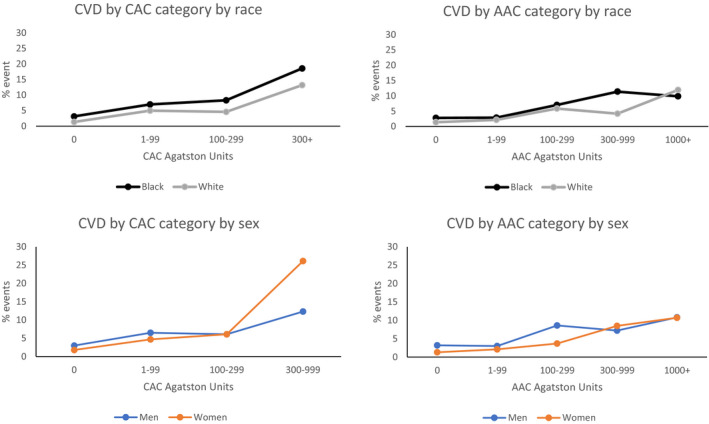
Abbreviations: CAC indicates coronary artery calcification; AAC, abdominal aorta calcification; and CVD, cardiovascular disease.
Consistent with the findings for all participants in Table 4, the risk across clinical Agatston score categories showed a strong gradation (Table 5, Figure). This gradation was similarly seen within Black and within White people. The age and sex adjusted hazard ratio predicting CVD per SD of ln(CAC) was 1.83 (1.45–2.31) in White people, and nonsignificantly lower in Black people (1.73 [1.43‐2.09], P for interaction 0.69). Similarly the age and sex adjusted hazard ratio predicting CVD per SD of ln(AAC) was 1.92 (1.44–2.56), again nonsignificantly lower in Black people (1.67 [1.32–2.11], P for interaction 0.45). Further adjustment for education had little effect on these estimates. The age and sex adjusted hazard ratio was 1.80 (1.21–2.67) for Black versus White people. This hazard ratio for race became non‐significant in the risk factor adjusted model (designated ARS+RF in Table 4): adjusting for CAC, the hazard ratio for race was 1.41 (0.95–2.22); adjusting for AAC, the hazard ratio for race 1.25 (0.85–1.96).
Table 5.
Unadjusted Cardiovascular Disease and Coronary Heart Disease Event Rates by Race
| Cardiovascular disease | Agatston score categories | ||||
|---|---|---|---|---|---|
| 0 | 1–99 | 100–299 | 300+ | 1000+ | |
| CAC: All | 2.2 (49/2199) | 5.8 (33/569) | 6.1 (9/147) | 15.6 (15/96) | |
| AAC: All | 2 (29/1438) | 2.5 (22/876) | 6.4 (19/296) | 7.8 (19/243) | 10.8 (17/158) |
| CAC: Black race | 3.1 (34/1082) | 7.0 (16/229) | 8.3 (5/60) | 18.6 (8/43) | |
| CAC: White race | 1.3 (15/1117) | 5.0 (17/340) | 4.6 (4/87) | 13.2 (7/53) | |
| AAC: Black race | 2.8 (18/644) | 2.9 (12/414) | 7 (10/142) | 11.4 (14/123) | 9.9 (9/91) |
| AAC: White race | 1.4 (11/794) | 2.2 (10/462) | 5.8 (9/154) | 4.2 (5/120) | 11.9 (8/67) |
| CAC: Men | 3.0 (23/770) | 6.5 (23/356) | 6.1 (6/98) | 12.3 (9/73) | |
| CAC: Women | 1.8 (26/1429) | 4.7 (10/213) | 6.1 (3/49) | 26.1 (6/23) | |
| AAC: Men | 3.2 (17/532) | 3.0 (12/395) | 8.6 (14/162) | 7.2 (9/125) | 10.8 (9/83) |
| AAC: Women | 1.3 (12/906) | 2.1 (10/481) | 3.7 (5/134) | 8.5 (10/118) | 10.7 (8/75) |
Cells contain % events (n/N); because of small sample size for CAC 1000+, the CAC categories 300‐999 and 1000+ were combined for event analyses.
AAC indicates abdominal aorta calcification; and CAC, coronary artery calcification.
In the corresponding analysis by sex, the age and sex adjusted hazard ratio predicting CVD per SD of ln(CAC) was 1.57 (1.30–1.90) in men, and larger in women (2.13 [1.7–2.68], P for interaction 0.04, influenced by a high CVD event rate in the 23 women with CAC 300+ Agatston units). Similarly the age and sex adjusted hazard ratio predicting CVD per SD of ln(AAC) was 1.59 (1.25–2.02), again substantially larger in women, although the interaction was not significant (2.02 [1.54–2.66], p for interaction 0.18). Further adjustment for education had little effect on these estimates of the sex specific associations of CAC or AAC with incident CVD. The age and race adjusted hazard ratio was 0.53 (0.36–0.78) for women versus men. The difference between women and men was reduced in the model adjusting for risk factors: adjusting for CAC, the hazard ratio for sex was 0.75 (0.50–1.14); adjusting for AAC, the hazard ratio for sex was only slightly attenuated 0.60 (0.40–0.89).
Discussion
Coronary atherosclerosis is known to be present in young adulthood 5 and the presence of CAC in individuals in the CARDIA cohort aged 32 to 46 years was associated with increased risk of fatal and non‐fatal CHD events and death. 12 Using the same cohort 10 years later, we demonstrated in this paper that knowledge of AAC scores in middle‐aged individuals also has predictive value for incident CVD events. Calcification amounts in the abdominal aorta may have less immediate disease implications compared with the same Agatston score value in the coronary arteries. AAC was nearly as strongly related to incident CVD as CAC is, but higher CVD rates occurred at AAC Agatston score values of over 1000, comparable to the CVD rates at CAC Agatston score values of over 300. For example, the unadjusted incident CVD event rate was 2.2% both for CAC 0 and for AAC 0 combined with 1 to 99, while it was 15.6% for CAC 300 or more, compared with about 10.8% for AAC 1000 or more. However, CAC is specific to CHD. The unadjusted incident CHD event rates were about 0.8% for CAC 0 and 1.1% for AAC 0 combined with 1–99, compared with 10.4% for CAC 300 or more, but only 4.4% for AAC 1000 or more. Of note, AAC did predict CVD among the 73% of the sample with CAC score 0.
Our other major finding is that in this early middle age sample AAC score tended to be higher in Black people than in White people, especially among Black women. The incident CVD risk associated with a given Agatston score, whether for CAC or AAC, was similar for Black as for White people. The substantially higher AAC in Black women than in White women may help to explain their different CVD event rates, 3.6% in Black women versus 1.6% in White women. The finding that Black men and women have similar AAC scores is in agreement with the Jackson Heart Study 15 ; however, Black people were reported to have less AAC than White people in MESA. 3 It is interesting to note that a substantial part of the Black race excess CVD rate was explained by adjustment for risk factors and the subclinical disease represented by CAC and AAC. Much less of the male excess CVD risk was explained by these factors.
Extra‐coronary calcium has been identified for decades in vascular beds including carotid, aortic, and femoral arteries. 5 , 6 , 20 , 21 The differential calcification may be related to hemodynamic effects in various vascular beds. Differences in shear stress between vascular beds may account for the variable expression of calcium and atherosclerotic disease given that arterial vascular bifurcations are associated with accelerated atherosclerosis as a result of increased shear stress. 22 , 23 Of most relevance to differential prediction of incident disease between AAC and CAC is that the diameter of the aorta is about 10 times as great as that of the coronary arteries.
Even when AAC is the only available vascular information, as recently meta‐analyzed, 6 AAC has been shown in many studies to predict CVD, both in the general population and even more strongly in people with chronic kidney disease. AAC could be used without any additional costs or harm to the patient and may aid in prediction for CHD and CVD events. Because AAC is correlated with CAC, the presence of AAC, especially a high score, raises suspicion that CAC may be present. Also, in our unadjusted or minimally adjusted models, AAC was nearly as a good a predictor of CVD as CAC. The incidental AAC finding alerts the clinician to potential CVD risk that should be further investigated. Automated AAC scoring in planned abdominal CT scan for a non‐CVD medical diagnostic work‐up may be used as a quick and low‐cost tool to identify patients who are at increased risk of CVD. This provides an opportunity to consider higher intensity CVD prevention strategies.
If risk factor levels are known to the clinician and CAC is present, our data suggest that AAC adds only a little to prediction of CVD or CHD. However, AAC score is an earlier indicator of subclinical disease: it predicts CVD risk when CAC is not found. Another advantage of AAC as a risk marker is that it can be detected with other radiographic techniques that might be used, as noted by Loew et al. 6 For example, AAC was identified in lumbar radiographs done starting 1967 in the Framingham Study. 24 There were 1049 men and 1466 women with a mean age of 61 years old, followed for 22 years for CHD, CVD, and CVD death. AAC measured this way was predictive.
The utility of informing clinicians and patients of the CAC score was demonstrated in the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) study in middle‐aged subjects without a history of CVD events. 25 In EISNER, the risk profile improved over 4 years in participants who were randomly assigned to have CAC assessed at baseline compared with those randomized to have no scan. No comparable study for clinical use of AAC has been performed.
Limitations of this study include a relatively small number of CVD and CHD events, balanced by the value of providing information in middle age. The small number of events limits the power for fine‐tuned judgments about predictiveness of AAC and CAC; the power of this study for the general predictive associations is in the continuous regression models in Table 4. The raw counts shown in Figure and Table 5 are less precise than the continuous regression models, but provide information to the reader that is both confirmatory of Table 4 findings and also expands those findings concerning shape of relationship and lack of race and sex differences in the associations. Findings in this study using CARDIA data may not be applicable to individuals of races other than White and Black. All observational studies are unable to completely rule out residual confounding.
Conclusions
Knowledge of the presence of AAC in middle‐age may be useful in strengthening risk stratification for risk of CVD events and CHD events and may inform clinical decision making surrounding primary prevention. Presence of AAC in Black people may help to explain the pathology by which they have increased CVD compared with White people, particularly among women. Additional prospective investigations to further evaluate this approach within clinical practice would be desirable.
Sources of Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI) and grant R01‐HL098445 from the National Heart, Lung, and Blood Institute (NHLBI) to Vanderbilt University and Wake Forest University.
Disclosures
None.
Acknowledgments
The authors thank the investigators, the staff and the participants of the CARDIA study for their dedication and highly valued contributions. This article has been reviewed prior to submission by CARDIA for scientific content.
Author contributions: P.T.J, D.A.D, D.R.J designed the study, D.R.J, J.J.C, J.G.T analyzed data, P.T.J, D.R.J, D.A.D drafted the manuscript. P.T.J, J.J.C, J.G.T, J.S.R, D.R.J, D.A.D interpreted the results, critically reviewed and edited the manuscript. P.T.J, J.J.C, J.T.G, D.R.J had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors were involved in preparing this manuscript and agreed upon its content.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140–146. doi: 10.1161/ATVBAHA.111.235234 [DOI] [PubMed] [Google Scholar]
- 3. Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, Cohoon KP, Srikanthan P, Watson KE, Wong ND. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1574–1579. doi: 10.1161/ATVBAHA.114.303268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong ND, Lopez VA, Allison M, Detrano RC, Blumenthal RS, Folsom AR, Ouyang P, Criqui MH. Abdominal aortic calcium and multi‐site atherosclerosis: the Multiethnic Study of Atherosclerosis. Atherosclerosis. 2011;214:436–441. doi: 10.1016/j.atherosclerosis.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solberg LA, Strong JP. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis. 1983;3:187–198. doi: 10.1161/01.ATV.3.3.187 [DOI] [PubMed] [Google Scholar]
- 6. Leow K, Szulc P, Schousboe JT, Kiel DP, Teixeira‐Pinto A, Shaikh H, Sawang M, Sim M, Bondonno N, Hodgson JM, et al. Prognostic value of abdominal aortic calcification: a systematic review and meta‐analysis of observational studies. J Am Heart Assoc. 2021;10:e017205. 10.1161/JAHA.120.017205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann U, Massaro JM, D'Agostino RB Sr, Kathiresan S, Fox CS, O'Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5:e003144. 10.1161/JAHA.115.003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gidding SS, Rana JS, Prendergast C, McGill H, Carr JJ, Liu K, Colangelo LA, Loria CM, Lima J, Terry JG, et al. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score in young adults predicts coronary artery and abdominal aorta calcium in middle age: the CARDIA study. Circulation. 2016;133:139–146. doi: 10.1161/CIRCULATIONAHA.115.018042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miedema MD, Dardari ZA, Nasir K, Blankstein R, Knickelbine T, Oberembt S, Shaw L, Rumberger J, Michos ED, Rozanski A, et al. Association of coronary artery calcium with long‐term, cause‐specific mortality among young adults. JAMA Netw Open. 2019;2:e197440. doi: 10.1001/jamanetworkopen.2019.7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tota‐Maharaj R, Blaha MJ, Blankstein R, Silverman MG, Eng J, Shaw LJ, Blumenthal RS, Budoff MJ, Nasir K. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the Multi‐Ethnic Study of Atherosclerosis: a secondary analysis of a prospective, population‐based cohort. Mayo Clin Proc. 2014;89:1350–1359. doi: 10.1016/j.mayocp.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tota‐Maharaj R, Blaha MJ, McEvoy JW, Blumenthal RS, Muse ED, Budoff MJ, Shaw LJ, Berman DS, Rana JS, Rumberger J, et al. Coronary artery calcium for the prediction of mortality in young adults < 45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–2962. doi: 10.1093/eurheartj/ehs230 [DOI] [PubMed] [Google Scholar]
- 12. Carr JJ, Jacobs DR, Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernández‐Friera L, Peñalvo JL, Fernández‐Ortiz A, Ibañez B, López‐Melgar B, Laclaustra M, Oliva B, Mocoroa A, Mendiguren J, Martínez de Vega V, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle‐aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131:2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310 [DOI] [PubMed] [Google Scholar]
- 14. Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta‐analysis. Heart. 2012;98:988–994. doi: 10.1136/heartjnl-2011-301464 [DOI] [PubMed] [Google Scholar]
- 15. Tullos BW, Sung JH, Lee JE, Criqui MH, Mitchell ME, Taylor HA. Ankle‐Brachial Index (ABI), Abdominal Aortic Calcification (AAC), and Coronary Artery Calcification (CAC): the Jackson heart study. Int J Cardiovasc Imaging. 2013;29:891–897. doi: 10.1007/s10554-012-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 17. Carr JJ, Crouse JR 3rd, Goff DC Jr, D'Agostino RB Jr, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174:915–921. doi: 10.2214/ajr.174.4.1740915 [DOI] [PubMed] [Google Scholar]
- 18. Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt‐Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility–MESA study. Radiol. 2005;236:477–484. doi: 10.1148/radiol.2362040513 [DOI] [PubMed] [Google Scholar]
- 19. Budoff MJ, McClelland RL, Chung H, Wong ND, Carr JJ, McNitt‐Gray M, Blumenthal RS, Detrano RC. Reproducibility of coronary artery calcified plaque with cardiac 64‐MDCT: the multi‐ethnic study of atherosclerosis. AJR Am J Roentgenol. 2009;192:613–617. doi: 10.2214/AJR.08.1242 [DOI] [PubMed] [Google Scholar]
- 20. Barasch E, Gottdiener JS, Marino Larsen EK, Chaves PH, Newman AB. Cardiovascular morbidity and mortality in community‐dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study). Am J Cardiol. 2006;97:1281–1286. doi: 10.1016/j.amjcard.2005.11.065 [DOI] [PubMed] [Google Scholar]
- 21. Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, Wong ND, Blumenthal RS, Budoff MJ, Nasir K. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all‐cause mortality: the multi‐ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9:406–414. doi: 10.1016/j.jcct.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215 [DOI] [PubMed] [Google Scholar]
- 23. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.CIR.103.11.1529 [DOI] [PubMed] [Google Scholar]
- 25. Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda‐Peats L, Wong ND, Rana JS, Orakzai R, Hayes SW, Friedman JD, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]


