Abstract
Background
Heart failure (HF) and atrial fibrillation (AF) often coexist; yet, outcomes of ablation in patients with AF and concomitant HF are limited. This analysis assessed outcomes of cryoablation in patients with AF and HF.
Methods and Results
The Cryo AF Global Registry is a prospective, multicenter registry of patients with AF who were treated with cryoballoon ablation according to routine practice at 56 sites in 26 countries. Patients with baseline New York Heart Association class I to III (HF cohort) were compared with patients without HF. Freedom from atrial arrhythmia recurrence ≥30 seconds, safety, and health care utilization over 12‐month follow‐up were analyzed. A total of 1303 patients (318 HF) were included. Patients with HF commonly had preserved left ventricular ejection fraction (81.6%), were more often women (45.6% versus 33.6%) with persistent AF (25.8% versus 14.3%), and had a larger left atrial diameter (4.4±0.9 versus 4.0±0.7 cm). Serious procedure‐related complications occurred in 4.1% of patients with HF and 2.6% of patients without HF (P=0.188). Freedom from atrial arrhythmia recurrence was not different between cohorts with either paroxysmal AF (84.2% [95% CI, 78.6–88.4] versus 86.8% [95% CI, 84.2–89.0]) or persistent AF (69.6% [95% CI, 58.1–78.5] versus 71.8% [95% CI, 63.2–78.7]) (P=0.319). After ablation, a reduction in AF‐related symptoms and antiarrhythmic drug use was observed in both cohorts (HF and no‐HF), and freedom from repeat ablation was not different between cohorts. Persistent AF and HF predicted a post‐ablation cardiovascular rehospitalization (P=0.032 and P=0.001, respectively).
Conclusions
Cryoablation to treat patients with AF is similarly effective at 12 months in patients with and without HF.
Registration
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT02752737.
Keywords: atrial fibrillation, catheter ablation, heart failure
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Electrophysiology, Arrhythmias, Heart Failure
Nonstandard Abbreviations and Acronyms
- AAD
antiarrhythmic drug
- AE
adverse event
- AFL
atrial flutter
- AT
atrial tachycardia
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- MACE
major adverse cardiovascular events
- NYHA
New York Heart Association
- PAF
paroxysmal atrial fibrillation
- PsAF
persistent atrial fibrillation
- PV
pulmonary vein
Clinical Perspective
What Is New?
Evidence on the outcome of cryoballoon ablation in patients with atrial fibrillation (AF) who have concomitant heart failure (HF) is limited. In the Cryo AF Global Registry, 318 (24%) patients had HF with concomitant AF and were treated with cryoablation in a standard‐of‐care practice.
This analysis reconfirmed that cryoballoon ablation for the treatment of AF is similarly safe and effective in patients with AF with and without concomitant HF, despite patients with HF having higher rates of comorbidities at baseline.
The population in this analysis was primarily composed of patients who had HF with preserved ejection fraction, and 30% of patients were treated with first‐line cryoablation.
What Are the Clinical Implications?
These findings add new insights in the management of patients with AF who have HF in a standard‐of‐care practice.
Future controlled trials are needed to evaluate cryoablation as a treatment for AF in patients with concomitant HF in a relative early stage of AF disease.
Atrial fibrillation (AF) and heart failure (HF) often comanifest. 1 AF and HF independently increase the risk of hospitalization, morbidity, mortality, and symptoms that reduce quality of life (QOL) and the risk of deterioration of health is intensified by the coexistence of these conditions. 1 , 2 , 3 The reciprocal relationship between HF and AF has challenged the treatment in these patients.
Long‐term pharmacological rhythm control in patients with AF and HF has not markedly improved mortality and rehospitalization outcomes. 4 , 5 Catheter ablation to achieve pulmonary vein (PV) isolation is the cornerstone treatment for patients with AF, and it has been identified as a reasonable treatment for selected patients who have AF with concomitant HF in recently updated guidelines. 6 , 7 The randomized CASTLE‐AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation) trial identified an improvement in mortality and HF‐related rehospitalization after radiofrequency ablation for the treatment of AF in a highly selected cohort of patients with HF and reduced left ventricular ejection fraction (LVEF). 8 Subsequently, small evaluations of cryoballoon ablation for the treatment of patients with AF and HF have been reported. 9 , 10 , 11 However, clinical reports of cryoablation in a large cohort of patients with AF and HF who have either reduced or preserved LVEF (HF with reduced ejection fraction [HFrEF] and HF with preserved ejection fraction [HFpEF], respectively) are limited. The aim of this analysis was to evaluate the safety, efficacy, and health care utilization after cryoablation for the treatment of patients with AF and HF in a global, postmarket setting.
Methods
The data, analytic methods, and study materials will not be made available to other researchers. Specifically, patient data privacy within the Cryo AF Global Registry does not allow nor consent to data sharing with outside parties.
Study Design
The Cryo AF Global Registry (ClinicalTrials.gov registration: NCT02752737) is an ongoing, prospective, postmarket data collection of AF ablation procedures and outcomes conducted with Medtronic ablation products. Data for this analysis were collected from cryoballoon ablation procedures conducted at 56 centers by 139 investigators in 26 countries around the world (Table S1). Data collection milestones, data quality, clinical questions, data analysis, and potential publications for the registry are overseen by a global steering committee of international physicians, and the registry is sponsored by Medtronic, Inc. Data collection adhered to Good Clinical Practice guidelines and the principles outlined in the Declaration of Helsinki. This study was approved by each site’s institutional review board and local ethics committees, and each patient provided written informed consent for participation in the study. The objective of this analysis was to assess patient characteristics, outcomes of cryoballoon ablation, and health care utilization in patients with HF with concomitant AF.
Patient Population
All patients aged 18 years and older (or minimum age per local regulations) with a planned cryoballoon catheter ablation were eligible for inclusion. Patients were not excluded from the registry for any preexisting baseline characteristics, including medical history. For the current analysis, all consecutively enrolled patients who completed the required 12‐month follow‐up after a cryoablation procedure through January 2020 were included, except: (1) patients diagnosed with long‐standing persistent AF (continuous AF >12 months) and/or (2) patients treated with a prior cardiac ablation for the treatment of an atrial arrhythmia. During the baseline visit, each center identified patients as having HF and the New York Heart Association (NYHA) class in accordance to the 2016 European Society of Cardiology AF management guidelines (as the presence of left ventricular systolic or diastolic dysfunction). 12 Moreover, the cardiovascular history of the patient was analyzed in order to report previous cardioembolic events, HF, and cardiovascular disease. Patients with an NYHA classification of I to IV at baseline were included in the HF cohort. Patients who were reported to have no HF at baseline comprised the no‐HF group. HFrEF was defined as HF with LVEF ≤40%, HFpEF was defined as HF with LVEF ≥50%, and HF midrange LVEF was defined as HF with LVEF between 40% and 50%. Patients with AF episodes of AF <7 days in duration were classified with paroxysmal AF (PAF) and patients with episodes of ≥7 days and ≤12 months were classified with persistent AF (PsAF).
Cryoballoon Ablation Procedure
The cryoablation procedure was performed according to each local center’s standard‐of‐care. Typical procedural techniques are outlined below and have been previously described. 13 After transseptal puncture, a dedicated 15‐F outer diameter steerable sheath (FlexCath or FlexCath Advance Steerable Sheath; Medtronic) was used to guide a 23‐ or 28‐mm cryoballoon ablation catheter (Arctic Front Advance; Arctic Front Advance Pro; Medtronic, Inc.) into the left atrium. A J‐tip wire or dedicated inner‐lumen octopolar/decapolar circular mapping catheter (Achieve or Achieve Advance; Medtronic, Inc.) was used to position the sheath and cryoballoon catheter at the antrum of the targeted PVs. The number and duration of cryoapplications for each PV and any non‐PV ablation adjunctive to PV isolation were determined by physician. PV isolation was demonstrated by entrance and/or exit block following the ablation. Phrenic nerve monitoring was recommended during right‐sided cryoablation by pacing with a diagnostic catheter at the level of the right subclavian vein. The cryoapplication was terminated immediately upon detection of reduced diaphragmatic function. Adjunctive imaging, monitoring, and/or focal catheter ablation tools were used at the operator’s discretion. Intraprocedural testing of acute PV isolation, periprocedural anticoagulation, and antiarrhythmic drug (AAD) prescription was determined by physician. Patients were discharged from the hospital according to standard‐of‐care procedures.
Patient Follow‐Up
Patients were followed according to each site’s standard‐of‐care protocols by telephone visits and/or in‐person visits. Arrhythmia monitoring was not protocol required and was conducted according to the center’s standard‐of‐care practice when completed, inclusive of but not limited to the following methods: Holter monitoring, ECG, transtelephonic monitoring, implantable cardiac monitor, pacemaker, and/or implantable cardioverter‐defibrillator. Patients were asked to provide any other Holter or ECG results since the previous visit. Patients were required by protocol to have an annual follow‐up visit 12 months after the cryoablation.
End Points
The primary end point of this analysis was the 12‐month freedom from a ≥30‐second recurrence of AF/atrial flutter (AFL)/atrial tachycardia (AT) following a 90‐day blanking period during which patients were managed per standard‐of‐care. The primary safety end point was the serious procedure‐related adverse event (AE) rate. Serious procedure‐related AEs were classified by physician and included all events related to the ablation procedure that led to death or a serious deterioration of health. Arrhythmias classified by the physician as a serious adverse event related to the procedure but with arrhythmia onset postdischarge were not included in the primary safety end point for this analysis. All patients were followed until AE resolution, no further actions were planned, or the patient exited the study. QOL was measured by the 3‐level EuroQol 5‐dimensional questionnaire (EQ‐5D‐3L) (score of 1 represents maximal QOL) at baseline and 12‐month follow‐up. The following ancillary objectives were also evaluated in the HF and no‐HF cohorts: (1) baseline patient demographics; (2) procedural outcomes; (3) AAD usage at postablation discharge and 12 months; (4) change in AF‐related symptoms between baseline and 12 months; and (5) freedom from repeat ablation, all‐cause rehospitalization, and cardiovascular‐related rehospitalization at 12 months after the cryoballoon ablation procedure. Rehospitalization events were defined as nonprocedure‐related hospitalizations after the cryoablation procedure.
Statistical Analysis
Kaplan‐Meier methods were used to estimate 12‐month freedom from atrial arrhythmia recurrence, freedom from repeat ablation, and freedom from all‐cause and cardiovascular‐related rehospitalization. Standard error was calculated with the Greenwood formula. Cox regression models were utilized to assess the hazard of AF/AFL/AT recurrence, repeat ablation, all‐cause rehospitalization, and cardiovascular‐related rehospitalization between HF and no‐HF cohorts. Separate regression models were utilized for each end point. Because of the strong association of baseline AF classification with efficacy outcomes postablation, the imbalance in baseline AF classification was controlled for by including baseline AF type (PAF versus PsAF) as a covariate in all Cox regression models. Procedural complication rate was compared between cohorts with exact methods, and McNemar test was used to compare change in arrhythmia‐related symptoms from baseline to 12 months. P values of <0.050 were considered significant. Statistical analyses were completed using SAS software version 9.4 (SAS Institute Inc).
Results
Patient and Procedural Characteristics
Of the 1303 patients who completed 12‐month follow‐up during this analysis window, 985 did not have HF and 318 had HF. The HF cohort was characterized by an NYHA class of I to III and included patients with HFpEF, HF with mild reduced ejection fraction, and HFrEF (Figure 1). Baseline characteristics are presented in Table 1. Compared with patients without HF, those with HF were more often women (45.6% versus 33.6%), older (64±11 versus 60±12), with PsAF (25.8% versus 14.3%), and larger left atrial diameters (4.4±9 versus 4.0±7 cm) (all P<0.010). Patients with HF also had higher rates of hypertension (67.0% versus 49.1%), diabetes (17.0% versus 10.2%), prior myocardial infarction (3.8% versus 1.7%), and history of coronary artery disease (14.5% versus 6.1%) (all P<0.050). Procedural characteristics are detailed in Table 2. See Table S2 for baseline differences between no‐HF, HF NYHA class I, and HF NYHA class II or III groups.
Figure 1. Baseline characteristics of the heart failure (HF) cohort.
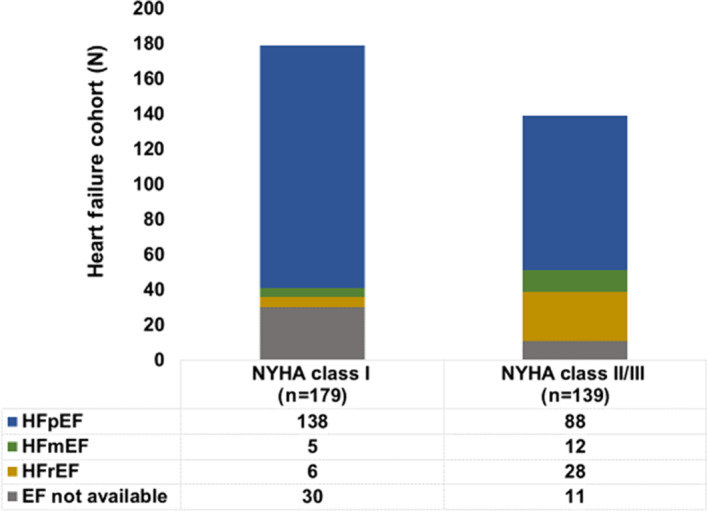
The HF cohort stratified by New York Heart Association (NYHA) class status and left ventricular ejection fraction (LVEF). Patients had an NYHA class of I to III, and most patients had preserved LVEF. EF indicates ejection fraction; HFmEF, HF with mild reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; and HFrEF, heart failure with reduced ejection fraction.
Table 1.
Baseline Patient Characteristics
| Patient characteristics |
HF (n=318) |
No HF (n=985) |
P value** |
|---|---|---|---|
| Women, n (%) | 145 (45.6) | 331 (33.6) | <0.001 |
| Age, mean±SD, y | 64±11 | 60±12 | <0.001 |
| Body mass index, mean±SD, kg/m2 * | 28±5 | 27±5 | 0.002 |
| CHA2DS2‐VASc score, mean±SD | 3.4±1.6 | 1.6±1.4 | <0.001 |
| Paroxysmal AF, n (%) | 236 (74.2) | 844 (85.7) | <0.001 |
| Years diagnosed with AF † | |||
| Mean±SD | 3.3±4.4 | 3.3±5.1 | 0.954 |
| Median (IQR) | 1.5 (0.4–4.3) | 1.2 (0.4–4.3) | |
| History of AFT, n (%) | 8 (2.5) | 50 (5.1) | 0.060 |
| History of AT, n (%) | 3 (0.9) | 12 (1.2) | 1.000 |
| Left atrial diameter, mean±SD, cm ‡ | 4.4±0.9 | 4.0±0.7 | <0.001 |
| LVEF, mean±SD, % § | 58±13 | 62±7 | <0.001 |
| Preserved LVEF ≥50%, n (%) | 226 (81.6) | 816 (97.5) | |
| Midrange LVEF 40%–50%, n (%) | 17 (6.1) | 15 (1.8) | |
| Reduced LVEF ≤40%, n (%) | 34 (12.3) | 6 (0.7) | |
| No. of failed AADs, mean±SD | 0.8±0.7 | 0.8±0.7 | 0.800 |
| First‐line cryoablation, n (%) ¶ | 89 (28.0) | 306 (31.1) | 0.326 |
| Hypertension, n (%) | 216 (67.9) | 484 (49.1) | <0.001 |
| Prior cardiac device implant, n (%) # | 29 (9.1) | 33 (3.4) | <0.001 |
| Prior myocardial infarction, n (%) | 12 (3.8) | 17 (1.7) | 0.046 |
| Prior stroke/TIA, n (%) | 24 (7.5) | 56 (5.7) | 0.229 |
| Coronary artery disease, n (%) | 46 (14.5) | 60 (6.1) | <0.001 |
| Diabetes, n (%) | 54 (17.0) | 100 (10.2) | 0.002 |
| Sleep apnea, n (%) | 14 (4.4) | 31 (3.1) | 0.291 |
AFT indicates atrial flutter; AT, atrial tachycardia, CHA2DS2‐VASc, congestive heart failure, hypertension, age (2 points); diabetes, previous stroke/transient ischemic attack (2 points), vascular disease; IQR, interquartile range; and TIA, transient ischemic attack.
A total of 1299 patients with body mass index reported; 316 with heart failure (HF) and 983 without HF.
A total of 1254 patients with atrial fibrillation (AF) diagnosis date reported; 312 with HF and 942 without HF.
A total of 862 patients with left atrial diameter reported; 277 with HF and 837 without HF.
A total of 1114 patients with left ventricular ejection fraction (LVEF) reported; 277 with HF and 837 without HF.
No prior failed antiarrhythmic drug (AAD) or not taking an AAD at enrollment.
Prior cardiac device includes implantable pulse generator, implantable cardioverter‐defibrillator, cardiac resynchronization therapy pacemaker, cardiac resynchronization therapy defibrillator, and insertable cardiac monitor.
Statistical tests comparing the HF cohort versus the no‐HF cohort. Continuous variables compared with t test and binary variables compared with exact test.
Table 2.
Procedural Characteristics
| Procedural characteristics |
HF (n=318) |
No HF (n=985) |
P value # |
|---|---|---|---|
| Total procedure time, mean±SD, m* | 85±32 | 78±34 | 0.001 |
| Left atrial dwell time, mean±SD, m † | 55±23 | 50±23 | 0.001 |
| Total fluoroscopy time, mean±SD, m ‡ | 19±18 | 16±15 | 0.002 |
| Total cryoapplication duration, mean±SD, m § | 19±6 | 18±7 | 0.001 |
| No. of applications per vein, mean±SD | 1.6±0.9 | 1.4±0.8 | <0.001** |
| Duration of cryoapplication, mean±SD, s | 189±51 | 196±52 | 0.012** |
| Cryoballoon nadir temperature, °C | −47±8 | −48±7 | 0.112** |
| Sedation method, n (%) | <0.001 | ||
| General anesthesia | 79 (24.8) | 378 (38.4) | |
| Nongeneral anesthesia | 239 (75.2) | 606 (61.5) | |
| Preprocedural imaging (CT and/or MRI) | 47 (14.8) | 187 (19.0) | 0.093 |
| PV ablation acute success, n (%) ¶ | 303 (95.3) | 930 (94.4) | 0.667 |
| PV isolation touch‐up with focal cryocatheter, n (%) | 4 (1.3) | 0 (0.0) | 0.003 |
| PV isolation touch‐up with focal radiofrequency catheter, n (%) | 6 (1.9) | 20 (2.0) | 1.000 |
| Additional ablation lesions | |||
| CTI line with focal radiofrequency catheter, n (%) | 8 (2.5) | 142 (14.4) | <0.001 |
| Other non‐PV isolation ablation, n (%) | 6 (1.9) | 27 (2.7) | 0.538 |
CT indicates computed tomography; CTI, cavotricuspid isthmus; MRI, magnetic resonance imaging; and PV, pulmonary vein.
A total of 1297 of 1303 patients reported procedure time; 318 of 318 patients with heart failure (HF) and 979 of 985 patients without HF.
A total of 1296 of 1303 patients reported left atrial dwell time; 318 of 318 patients with HF and 978 of 985 patients without HF.
A total of 1284 of 1303 patients reported fluoroscopy time; 316 of 318 patients with HF and 968 of 985 patients without HF.
A total of 1300 of 1303 patients reported total cryoablation time; 318 of 318 patients with HF and 982 of 985 patients without HF.
All targeted pulmonary veins isolated after cryoballoon ablation and focal touch‐up.
t Test for continuous variables and exact test for binary variables.
Repeated‐measures mixed model accounting for multiple veins treated within a patient.
Procedure‐Related Safety
The primary safety end point, serious cryoballoon procedure–related AEs, occurred in 13 (4.1%) patients with HF and 26 (2.6%) patients without HF (P=0.188; Table 3). Of those events, 1 (0.3%) in patients with HF and 4 (0.4%) in patients without HF were attributable to supraventricular arrhythmia recurrence onset during the index procedure hospitalization. Of the 8 serious phrenic nerve injuries observed, all but 1 asymptomatic event resolved before 12 months. A post hoc analysis completed on major adverse cardiovascular events (MACE) showed that the rate of MACE events in patients with HF was higher than the patients without HF (2.5% versus 0.8%, P=0.034). The events included 1 stroke/transient ischemic attack, which occurred in both the HF and no‐HF cohorts (0.3% versus 0.1%); myocardial infarction or ischemic cardiac events, which were observed only in the HF cohort (0.9% versus 0.0%); cardiac tamponade/pericardial effusion and postoperative hypotension, which was observed in both the HF and no‐HF cohorts (1.3% versus 0.5%, respectively); and pericarditis, which occurred only in the no‐HF cohort. In subanalysis of the HF cohort, there was no statistical difference in the safety end point between patients without HF, HF NYHA class I, and HF NYHA class II/III (P=0.265). No deaths related to the cryoablation procedure occurred during 12‐month follow‐up. The all‐cause mortality rate was 1.3% and 0.3% in the HF and no‐HF cohorts, respectively. Of the 3 deaths observed in the no‐HF group, 1 was cardiac related, occurring 104 days following the procedure. Of the 4 deaths in the HF group, 2 were cardiac related, occurring >200 days after the cryoablation.
Table 3.
Serious Procedure‐Related AEs
| Serious procedure‐related complications | No. of events (n, % of patients) | |
|---|---|---|
|
HF (n=318) |
No HF (n=985) |
|
| Total | 14 (13, 4.1) | 27 (26, 2.6) |
| Supraventricular arrhythmia recurrences* | 1 (1, 0.3) | 4 (4, 0.4) |
| Groin‐site complication † | 2 (2, 0.6) | 4 (4, 0.4) |
| Phrenic nerve injury | 1 (1, 0.3) | 7 (7, 0.7) |
| Cardiac tamponade or pericardial effusion | 3 (3, 0.9) | 4 (4, 0.4) |
| Pulmonary or bronchial complication ‡ | 1 (1, 0.3) | 3 (3, 0.3) |
| Myocardial infarction or ischemic cardiac event § | 3 (3, 0.9) | 0 (0, 0.0) |
| Pericarditis | 0 (0, 0.0) | 2 (2, 0.2) |
| Stroke or TIA ¶ | 1 (1, 0.3) | 1 (1, 0.1) |
| Postoperative hypotension | 1 (1, 0.3) | 1 (1, 0.1) |
| Face injury # | 0 (0, 0.0) | 1 (1, 0.1) |
| Sepsis | 1 (1, 0.3) | 0 (0, 0.0) |
AEs indicates adverse events; HF, heart failure; and TIA, transient ischemic attack.
Atrial fibrillation or sinus bradycardia occurring during the index procedure hospitalization.
Hematoma, vascular pseudoaneurysm, or vessel puncture site discharge.
Hematemesis, hypercapnia, pneumonia, or pleurisy.
Angina pectoris or myocardial infarction.
Cerebral infarction or cerebrovascular accident.
Caused by a postablation fall.
Efficacy and Patient Follow‐Up
During follow‐up, 72 (5.5%) patients exited before completing a 12‐month visit; 1 patient was withdrawn by the investigator, 46 were lost to follow‐up, 21 requested withdrawal, and 2 withdrew for other reasons. Patients were followed according to local standard‐of‐care protocols. Compared with patients without HF, patients with HF were seen at clinic visits more often (3.9±2.3 and 2.9±1.8, respectively; P<0.001) and received arrhythmia monitoring (eg, 12‐lead ECG and Holter) more frequently (3.2±3.3 versus 2.5±2.3, respectively; P<0.001) on average over 12‐month follow‐up. The Kaplan‐Meier estimate of 12‐month freedom from a ≥30‐second recurrence of AF/AFL/AT after the 90‐day blanking period was 84.2% (95% CI, 78.6%–88.4%) and 86.8% (95% CI, 84.2%–89.0%) in patients with PAF and HF versus no HF, respectively. The 12‐month freedom from a ≥30‐second recurrence of AF/AFL/AT was 69.6% (95% CI, 58.1%–78.5%) and 71.8% (95% CI, 63.2%–78.7%) in patients with PsAF and HF versus no HF, respectively (Figure 2). A Cox regression model identified a significant difference in the freedom from atrial arrhythmia recurrence between patients with PAF versus PsAF (hazard ratio [HR], 0.45; 95% CI, 0.33–0.61 [P<0.001]). HF was not a predictor of AF/AT/AFL recurrence after cryoablation (HR, 0.86; 95% CI, 0.63–1.16 [P=0.319]). In subanalyses of the HF cohort, 12‐month atrial arrhythmia recurrence tended to be higher in patients with HF NYHA class II/III versus those with HF NYHA class I and no HF, although this difference was not statistically significant (P=0.079; Figure S1). Similarly, arrhythmia recurrence rate was slightly higher in patients with LVEF <50%, but, overall, the primary efficacy end point was not statistically different between patients without HF versus those with HFpEF versus HFrEF (P=0.233; Figure S2).
Figure 2. Freedom from atrial arrhythmia recurrence over 12 months.
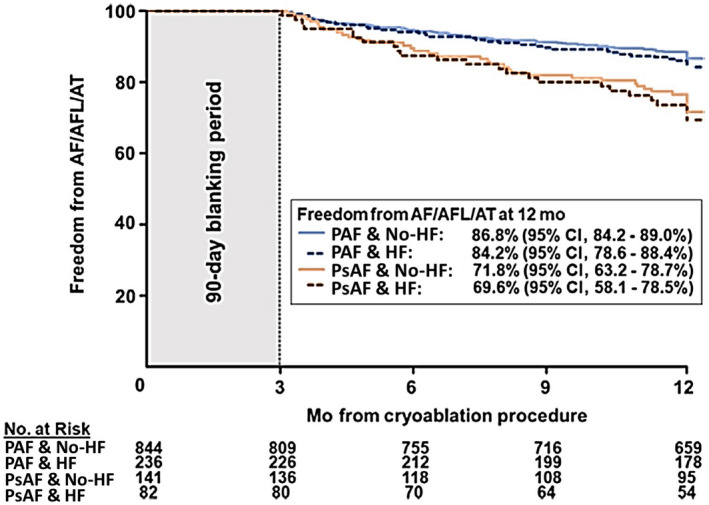
Kaplan–Maier estimate of 12‐month freedom from a ≥30‐second recurrence of atrial fibrillation (AF)/atrial flutter (AFL)/atrial tachycardia (AT) in patients with paroxysmal AF (blue) and persistent AF (red) with (dashed line) and without (solid line) heart failure (HF). Persistent AF at baseline predicted atrial arrhythmia recurrence (P<0.001), but HF status did not predict arrhythmia recurrence over the 12‐month follow‐up (P=0.319). PAF indicates paroxysmal atrial fibrillation; and PsAF, persistent atrial fibrillation.
QOL and Health Care Utilization
AF‐related symptom burden was higher at baseline in the HF versus no‐HF cohort, with 39.5% versus 16.6% of patients experiencing ≥3 symptoms, respectively. Both HF and no‐HF cohorts reported significant reductions in the number of AF‐related symptoms experienced at 12 months (P<0.001, Figure 3A). AF‐related symptoms reduced >70% from baseline to 12 months in both groups (Figure 3B). Likewise, patient‐reported QOL as measured by the EQ‐5D‐3L significantly improved from baseline to the 12‐month visit in both groups (HF cohort: P=0.001; no‐HF cohort: P<0.001 [Table 4]). The no‐HF cohort had a small but statistically greater improvement in EQ‐5D‐3L from baseline to 12 months than the HF cohort (P=0.017).
Figure 3. Change in atrial fibrillation (AF)–related symptoms after cryoballoon ablation.
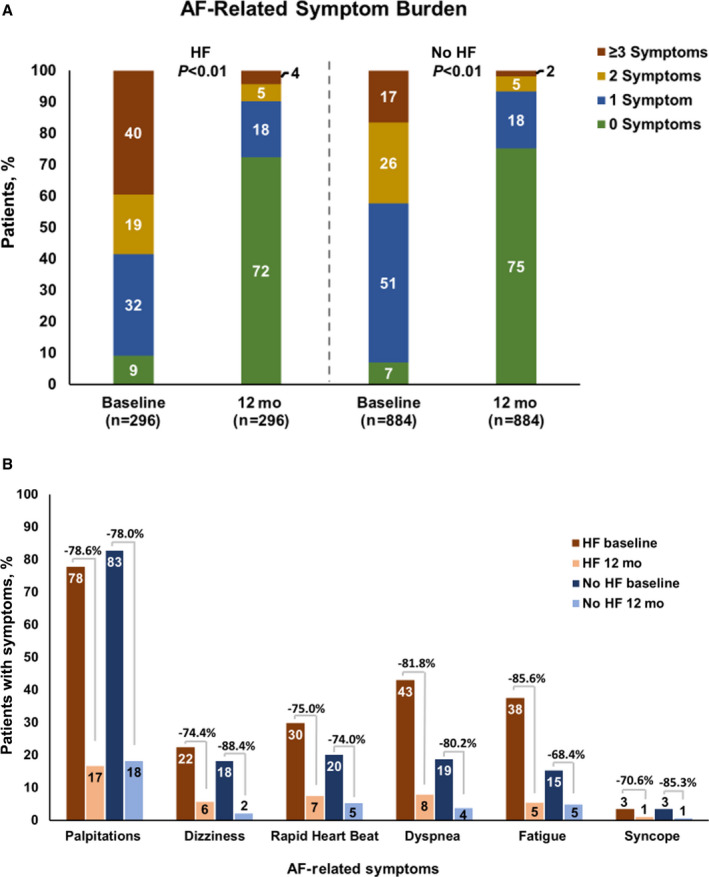
A, The percentage of patients with heart failure (HF) and patients without HF with 0 (green), 1 (blue), 2 (yellow), and ≥3 AF‐related symptoms (red) at baseline and 12‐month follow‐up is depicted. AF‐related symptom burden was higher in the HF cohort at baseline, and AF symptom burden significantly reduced from baseline to 12 months in both the HF and no‐HF cohorts (P<0.001). B, AF‐related symptoms after cryoballoon ablation were significantly reduced between baseline and the 12‐month follow‐up (P<0.001 for all except syncope in the HF group, P=0.052).
Table 4.
Changes in QOL as Measured by EQ‐5D‐3L
| (n=1101*) |
No HF (n=815) |
HF (n=286) |
P value † |
|---|---|---|---|
| Baseline | 0.90±0.14 | 0.88±0.14 | 0.017 |
| 12 mo | 0.93±0.12 | 0.90±0.13 | |
| Absolute difference | 0.034±0.15 | 0.026±0.14 |
QOL indicates quality of life.
A total of 1101 of 1303 patients completed a 12‐month visit and the 3‐level EuroQol 5‐dimensional questionnaire (EQ‐5D‐3L) at baseline and 12 months.
Linear regression model: outcome=EQ‐5D‐3L change, covariates=baseline EQ‐5D‐3L, baseline atrial fibrillation type, and heart failure (HF).
Health care utilization after the cryoablation was evaluated by AAD usage, repeat ablations, and rehospitalizations after the cryoablation (Figure 4). AAD prescription was higher in patients with PsAF (66.2% HF and 63.2% no HF) than patients with PAF (46.1% HF and 47.6% no HF) at procedure discharge irrespective of HF diagnosis and decreased between discharge and 12‐months in all groups (Figure 4A). Freedom from repeat ablation was 94.3% (95% CI, 92.4%–95.7%) and 92.4% (95% CI, 88.1%–95.2%) in the PAF no‐HF versus the PAF HF groups, respectively. Freedom from repeat ablation in patients with PsAF was 87.1% (95% CI, 80.1%–91.8%) and 87.2% (95% CI, 77.5%–92.9%) for those with no HF and HF, respectively (Figure 4B). Patients with PsAF had a significantly higher risk of a reablation than patients with PAF (P=0.001), but HF diagnosis at baseline did not predict reablation over 12 months (P=0.439). Freedom from all‐cause and cardiovascular‐related rehospitalization after the cryoablation are displayed in Figure 4C through 4D. Freedom from cardiovascular‐related rehospitalization in patients with PAF was 87.1% (95% CI, 81.9%–90.8%) and 92.4% (95% CI, 90.4%–94.1%) in the HF and no‐HF cohorts, respectively. Freedom from cardiovascular rehospitalization in patients with PsAF was 78.7% (95% CI, 68.0–86.2) and 89.5% (95% CI, 83.0–93.7) in the HF and no‐HF cohorts, respectively. Baseline AF type did not predict all‐cause rehospitalization (P=0.179), but HF status was a significant predictor of all‐cause rehospitalization (P<0.001). Similarly, HF status most strongly predicted a cardiovascular‐related rehospitalization event after the cryoablation (P<0.001), and AF baseline classification also increased the risk of a cardiovascular‐related hospitalization after cryoablation (P=0.032). In total, 83 cardiovascular‐related rehospitalizations were reported in 74 patients without HF, and 61 cardiovascular‐related rehospitalizations were observed in 46 patients with HF. AF/AFL/AT recurrence was the predominant cause of rehospitalization, and, in total, 57 (5.8%) patients without HF and 37 (11.6%) patients with HF were rehospitalized for AF/AFL/AT recurrences over 12‐month follow‐up.
Figure 4. Health care utilization after cryoballoon ablation.
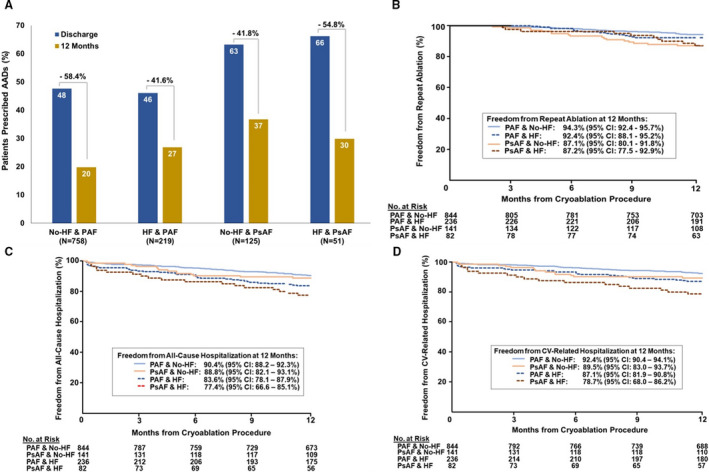
A, Antiarrhythmic drug utilization decreased between discharge (blue) and 12 months (yellow) among patient subgroups. B, Kaplan‐Meier estimate of freedom from reablation over 12 months is displayed. Persistent atrial fibrillation (AF), but not heart failure (HF) status (P=0.439), predicted reablation (P=0.001) over follow‐up. Kaplan–Meier estimates of (C) freedom from all‐cause and (D) cardiovascular‐related rehospitalization over 12‐month follow‐up are presented. HF predicted both all‐cause (P<0.001) and cardiovascular‐related (P<0.001) rehospitalization. Persistent AF did not predict all‐cause hospitalization (P=0.179) but did predict cardiovascular‐related rehospitalization (P=0.032) over follow‐up. AAD indicates antiarrhythmic drug; PAF, paroxysmal atrial fibrillation; and PsAF, persistent atrial fibrillation.
Discussion
HF and AF often coexist and together can worsen patient QOL and increase the risk of morbidity and mortality. 1 , 2 , 3 In this evaluation of standard‐of‐care practice, 24% of patients treated with cryoablation had HF with concomitant AF. Although patients with HF had more comorbidities, there was no statistical difference in the rate of the predefined study safety end point, serious procedure‐related AEs, between patients with HF and those without HF (4.1% versus 2.6%, respectively; P=0.188). A post hoc analysis was completed on a subset of the primary safety end point events, MACE. A statistically different rate in MACE events was observed between patients with HF and those without HF (2.5% versus 0.8%, respectively). However, both the rate of MACE and overall periprocedural events in our HF population was similar, and even slightly lower, to those recorded in previous studies on AF catheter ablation of patients with both preserved and reduced ejection fraction. 14 , 15 HF status did not increase the risk of arrhythmia recurrence at 12 months (P=0.319) despite a higher frequency of clinic visits reported during follow‐up. A significant reduction in AF‐related symptoms and AAD use after the cryoablation was observed in both groups, and freedom from repeat ablation at 12 months was not different between the HF and no‐HF cohorts. Atrial arrhythmia recurrence was the predominant cause of cardiovascular‐related rehospitalization events and reinforces the importance of AF management in the AF and HF population. An increased mean number of follow‐up visits (over a 1‐year period) was reported in the HF cohort as compared with the no‐HF cohort (3.9 versus 2.9, respectively), and this difference could be explained, in part, by the observation that patients with HF were considered at higher risk and were seen more often in routine clinical follow‐up care.
Previous studies have demonstrated that catheter ablation is more effective than medical therapy in reducing major clinical events in patients with HF who have concomitant AF. 16 , 17 Moreover, restoration of sinus rhythm by catheter ablation in patients with HF leads to significant and sustained improvements in LVEF. 18 , 19 Published studies have focused on outcomes of radiofrequency AF ablation for the treatment of patients with HFrEF, but AF may incur even greater morbidity and mortality in patients with HFpEF. The population in this analysis was primarily composed of patients with HFpEF, and 30% of patients were treated with first‐line cryoablation. Therefore, the findings add new insights in the management of these patients. This HF cohort had a higher rate of preexisting baseline cardiovascular comorbidities than patients without HF, and a statistically significant but small increase in procedure time was used to treat patients with HF. Despite these baseline comorbidities in patients with HF, cryoablation was similarly effective in treating AF in patients with and without HF. Cryoablation was recently found to have a superior efficacy/safety profile than radiofrequency as a first‐option ablation technique, 20 and future controlled trials should evaluate cryoablation as a treatment for AF in patients with concomitant HF in a relative early stage of AF disease.
A recent pooled analysis of randomized data on patients with AF and HF showed that catheter ablation (as compared with medical therapy) significantly reduced rates of mortality and rehospitalization. 21 Accordingly, catheter ablation has been advocated as cost‐effective for the management of patients with AF and HF. 22 Our study showed that rates of reablation and both all‐cause and cardiovascular‐related rehospitalization were relatively low in both cohorts, and recurrence of atrial arrhythmias was the primary cause of rehospitalization for patients both with and without HF. Overall, AF/AFL/AT recurrence rates were similar between patients with HF and those without HF. Therefore, the higher rate of cardiovascular rehospitalization in the HF group may be attributable to: (1) more serious effects of an arrhythmia recurrence in patients with HF, and/or (2) the additional cardiovascular comorbidity burden in patients with HF. Additionally, the use of AADs significantly decreased at 12 months after ablation similarly in patients with and without HF. Although hospitalization rates before ablation were unavailable, these data indicate a positive effect of catheter ablation on health care resource utilization in patients with AF and HF. In agreement with previous studies, 21 these data demonstrated a significant improvement in AF‐related symptoms and QOL in patients treated with cryoablation irrespective of HF status. These consistent findings reinforce the importance of a rhythm control strategy by means of catheter ablation to improve QOL in patients with AF and HF.
Study Limitations
This is an observational study of cryoablation; therefore, no comparisons to other therapies were possible. Nevertheless, these data are representative of global clinical practice and are useful to confirm the safety and efficacy of cryoablation in specific populations. The rate of hospitalization before catheter ablation was not collected and prevented assessment of change in rates after ablation. Although all serious AEs were required by protocol to be reported, it is possible that delayed AEs were not identified, and there may have been insufficient statistical power to detect a difference in the risk of an AE after cryoablation between the HF and no‐HF cohorts. While our subanalysis did not show a difference in outcomes, the sample size was not large enough to draw definite conclusions on the safety and effectiveness of cryoablation in different subgroups of patients with HF (eg, HFrEF, HFpEF, and NYHA class). Similarly, multiple end points and subgroups were assessed but no adjustment for multiple testing was used.
Moreover, HF status was defined by the enrolling physicians based on patients’ symptoms and clinical history, but diagnostic testing (ie, biomarkers and imaging) was not required to confirm the HF diagnosis. Last, as per the observational nature of this registry, no protocol was provided to the participating centers in terms of follow‐up and intensive monitoring of AF recurrence. Thus, some asymptomatic AF episodes could be missed. However, for comparative data assessment, it is important to recognize that the HF cohort was (on average) more often seen in routine follow‐up care, and, hence, these patients had more opportunity to record an AF episode compared with the cohort without HF in this study.
Conclusions
In this large, multicenter, international registry, cryoablation of AF was found to be safe and effective in the treatment of patients with AF and HF. Cryoablation for patients in an early stage of AF with a concomitant diagnosis of HF resulted in high rates of freedom from arrhythmia recurrence and low rates of hospital utilization after treatment.
Sources of Funding
The registry was sponsored by Medtronic, Inc., Minneapolis, MN.
Disclosures
F.J. Kueffer and K.M. Braegelmann are employees of Medtronic. Dr Földesi has received compensation for teaching and proctoring from Medtronic, Johnson & Johnson, Abbott Laboratories, and Biotronik SE & Co. Dr Rordorf has received modest speaking fees from Abbott and Boston Scientific. Dr Okumura has received compensation from Medtronic and compensation from Johnson & Johnson outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S2
Figures S1–S2
Acknowledgments
The authors extend their gratitude to the Cryo AF Global Registry sites and staff for their contributions to the trial. The authors thank Bob Hokanson, Hae Lim, Scott A. Sarazin, and Valentine Obidigbo from Medtronic for their support of the trial and oversight of the article development.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021323
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Santhanakrishnan R, Wang NA, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4:740–746. doi: 10.1161/CIRCHEARTFAILURE.111.962688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherian TS, Shrader P, Fonarow GC, Allen LA, Piccini JP, Peterson ED, Thomas L, Kowey PR, Gersh BJ, Mahaffey KW. Effect of atrial fibrillation on mortality, stroke risk, and quality‐of‐life scores in patients with heart failure (from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation [ORBIT‐AF]). Am J Cardiol. 2017;119:1763–1769. doi: 10.1016/j.amjcard.2017.02.050 [DOI] [PubMed] [Google Scholar]
- 4. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JMO, Buxton AE, Camm AJ, et al.; Atrial Fibrillation and Congestive Heart Failure Investigators . Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789 [DOI] [PubMed] [Google Scholar]
- 5. Zhao Y, Krupadev V, Dagher L, Mahnkopf C, Sohns C, Sehner S, Suling A, Sanders P, Boersma L, Schunkert H, et al. Pharmacological rhythm versus rate control in patients with atrial fibrillation and heart failure: the CASTLE‐AF trial. J Interv Card Electrophysiol. 2021;61:609–615. doi: 10.1007/s10840-020-00856-1 [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 7. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al; ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 8. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379:492. doi: 10.1056/NEJMc1806519. PMID: 30067924 [DOI] [PubMed] [Google Scholar]
- 9. Rattka M, Pott A, Kühberger A, Weinmann K, Scharnbeck D, Stephan T, Baumhardt M, Bothner C, Iturbe Orbe M, Rottbauer W, et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace. 2020;22:1328–1336. doi: 10.1093/europace/euaa101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pott A, Jäck S, Schweizer C, Baumhardt M, Stephan T, Rattka M, Weinmann K, Bothner C, Scharnbeck D, Keßler M, et al. Atrial fibrillation ablation in heart failure patients: improved systolic function after cryoballoon pulmonary vein isolation. ESC Heart Fail. 2020;7:2258–2267. doi: 10.1002/ehf2.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heeger CH, Abdin A, Mathew S, Reissmann B, Yalin K, Liosis S, Fink T, Proietti R, Eitel C, Vogler J, et al. Efficacy and safety of cryoballoon ablation in patients with heart failure and reduced left ventricular ejection fraction‐ a multicenter study. Circ J. 2019;83:1653–1659. doi: 10.1253/circj.CJ-19-0151 [DOI] [PubMed] [Google Scholar]
- 12. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295 [DOI] [PubMed] [Google Scholar]
- 13. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ, Elvan A, Arentz T, Bestehorn K, Pocock SJ, et al; FIRE AND ICE Investigators . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245: doi: 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
- 14. Ruzieh M, Foy AJ, Aboujamous NM, Moroi MK, Naccarelli GV, Ghahramani M, Kanjwal S, Marine JE, Kanjwal K, et al. Meta‐analysis of atrial fibrillation ablation in patients with systolic heart failure. Cardiovasc Ther. 2019;8181657. doi: 10.1155/2019/8181657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aldaas OM, Lupercio F, Darden D, Mylavarapu PS, Malladi CL, Han FT, Hoffmayer KS, Krummen D, Ho G, Raissi F, et al. Meta‐analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021;142:66–73. doi: 10.1016/j.amjcard.2020.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S, et al. Catheter ablation of atrial fibrillation in patients with heart failure: a meta‐analysis of randomized controlled trials. Ann Intern Med. 2019;170:41–45. doi: 10.7326/M18-0992 [DOI] [PubMed] [Google Scholar]
- 17. Packer DL, Piccini JP, Monahan KH, Al‐Khalidi HR, Silverstein AP, Noseworthy PA, Poole JE, Bahnson TD, Lee KL, Mark DB. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sohns C, Zintl K, Zhao Y, Dagher L, Andresen D, Siebels J, Wegscheider K, Sehner S, Boersma L, Merkely B, et al. Impact of left ventricular function and heart failure symptoms on outcomes post ablation of atrial fibrillation in heart failure: CASTLE‐AF trial. Circ Arrhythm Electrophysiol. 2020;13:e008461. doi: 10.1161/CIRCEP.120.008461 [DOI] [PubMed] [Google Scholar]
- 19. Sugumar H, Prabhu S, Costello B, Chieng D, Azzopardi S, Voskoboinik A, Parameswaran R, Wong GR, Anderson R, Al‐Kaisey AM, et al. Catheter ablation versus medication in atrial fibrillation and systolic dysfunction: late outcomes of CAMERA‐MRI study. JACC Clin Electrophysiol. 2020;6:1721–1731. doi: 10.1016/j.jacep.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 20. Fortuni F, Casula M, Sanzo A, Angelini F, Cornara S, Somaschini A, Mugnai G, Rordorf R, De Ferrari GM. Meta‐analysis comparing cryoballoon versus radiofrequency as first ablation procedure for atrial fibrillation. Am J Cardiol. 2020;125:1170–1179. doi: 10.1016/j.amjcard.2020.01.016 [DOI] [PubMed] [Google Scholar]
- 21. Chen S, Pürerfellner H, Meyer C, Acou WJ, Schratter A, Ling Z, Liu S, Yin Y, Martinek M, Kiuchi MG, et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J. 2020;41:2863–2873. doi: 10.1093/eurheartj/ehz443 [DOI] [PubMed] [Google Scholar]
- 22. Chew DS, Loring Z, Anand J, Fudim M, Lowenstern A, Rymer JA, Weimer KED, Atwater BD, DeVore AD, Exner DV, et al. Economic evaluation of catheter ablation of atrial fibrillation in patients with heart failure with reduced ejection fraction. Circ Cardiovasc Qual Outcomes. 2020;13:e007094. doi: 10.1161/CIRCOUTCOMES.120.007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S2
Figures S1–S2


