Abstract
Background
Tricuspid regurgitation (TR) is a common finding in adults with congenital heart disease referred for pulmonary valve replacement (PVR). However, indications for combined valve surgery remain controversial. This study aimed to evaluate early results of concomitant tricuspid valve intervention (TVI) at the time of PVR.
Methods and Results
Observational studies comparing TVI+PVR and isolated PVR were identified by a systematic search of published research. Random‐effects meta‐analysis was performed, comparing outcomes between the 2 groups. Six studies involving 749 patients (TVI+PVR, 278 patients; PVR, 471 patients) met the eligibility criteria. In the pooled analysis, both TVI+PVR and PVR reduced TR grade, pulmonary regurgitation grade, right ventricular end‐diastolic volume, and right ventricular end‐systolic volumes. TVI+PVR, but not PVR, was associated with a decrease in tricuspid valve annulus size (mean difference, −6.43 mm, 95% CI, −10.59 to −2.27; P=0.010). Furthermore, TVI+PVR was associated with a larger reduction in TR grade compared with PVR (mean difference, −0.40; 95% CI, −0.75 to −0.05; P=0.031). No evidence could be established for an effect of either treatment on right ventricular ejection fraction or echocardiographic assessment of right ventricular dilatation and dysfunction. There was no evidence for a difference in hospital mortality or reoperation for TR.
Conclusions
While both strategies are effective in reducing TR and right ventricular volumes, routine TVI+PVR can reduce TR grade to a larger extent than isolated PVR. Further studies are needed to identify the subgroups of patients who might benefit most from combined valve surgery.
Keywords: congenital heart disease, meta‐analysis, pulmonary valve insufficiency, pulmonary valve replacement, tricuspid valve
Subject Categories: Congenital Heart Disease, Cardiovascular Surgery, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- ACHD
adults with congenital heart disease
- MD
mean difference
- NYHA
New York Heart Association
- PR
pulmonary regurgitation
- PVR
pulmonary valve replacement
- RVEDV
right ventricular end‐diastolic volume
- RVESV
right ventricular end‐systolic volume
- TOF
tetralogy of Fallot
- TR
tricuspid regurgitation
- TV
tricuspid valve
- TVI
tricuspid valve intervention
Clinical Perspective
What Is New?
In this systematic review and meta‐analysis of 749 adults with congenital heart disease, we demonstrated that concomitant tricuspid valve intervention (TVI) at the time of pulmonary valve replacement (PVR) helped reduce tricuspid regurgitation (TR) grade to a larger extent than isolated PVR, while both strategies were otherwise equally effective.
What Are the Clinical Implications?
Patients with severe preoperative TR would probably derive the greatest benefit from concomitant TVI in terms of improvement in NYHA class and TR grade; however, concomitant TVI does not seem to be effective in reducing the risk of adverse events such as death, arrhythmias, and heart failure.
Current data therefore do not support the universal application of this approach for severe TR.
Further well‐designed studies focusing on specific underlying mechanisms of TR and evaluating the effect on adverse events on long‐term follow‐up may elucidate which patients stand to benefit the most from this approach.
Tricuspid regurgitation (TR) is a common finding in adults with congenital heart disease (ACHD) referred for pulmonary valve replacement (PVR), including those with tetralogy of Fallot (TOF), pulmonary stenosis, and pulmonary atresia. 1 Notably, as many as three‐quarters of these patients have at least mild TR, and one‐third present with at least moderate TR. Despite clearly demonstrated benefits of PVR on right ventricular (RV) volumes and function and the observation that isolated PVR also reduces TR, indications for combined valve surgery remain controversial. 2 , 3 Current guidelines do not suggest when concomitant tricuspid valve intervention (TVI) should be recommended. 4 , 5 Nonetheless, severe TR is strongly associated with an increased risk of adverse outcomes in ACHD. 6 Therefore, we aimed to evaluate early results of concomitant TVI at the time of PVR.
Methods
Eligibility Criteria, Databases, and Search Strategy
The data that support the findings of this study are available from the corresponding author upon reasonable request. We followed 2 internationally recognized protocols: Preferred Reporting Items for Systematic Reviews Meta‐analyses 7 and Meta‐analysis of Observational Studies in Epidemiology. 8 Using the Population, Interventions, Comparison, Outcome, and Study Design strategy, studies were included if the following criteria were fulfilled:
The population comprised ACHD (including TOF, pulmonary stenosis, and pulmonary atresia) who developed at least moderate pulmonary valve insufficiency;
The intervention group included patients who underwent combined TVI and PVR;
The control group included patients who underwent isolated PVR;
Outcomes of the studies included any of the following: tricuspid regurgitation (TR) grade, pulmonary regurgitation (PR) grade, tricuspid valve (TV) annulus size, RV dilatation, RV dysfunction, RV end‐diastolic volume (RVEDV), RV end‐systolic volume (RVESV), RV ejection fraction (RVEF), RV end‐diastolic area, RV end‐systolic area, New York Heart Association (NYHA) class, reoperation for TR, or 30‐day mortality; and
Studies were prospective or retrospective observational studies or randomized controlled trials.
Databases were searched for articles meeting our inclusion criteria and published by December 29, 2020: PubMed/MEDLINE, Embase, Scopus, and reference lists of relevant articles. The detailed search terms that were used for this search are given in Data S1. The following steps were taken: (1) identification of titles of records through database searching, (2) removal of duplicates, (3) screening and selection of abstracts, (4) assessment for eligibility through full‐text articles, and (5) final inclusion in study. Studies were selected by 2 independent reviewers (C.C. and M.L.R.). When concordance was absent, a third reviewer (J.V.D.E.) made the decision to include or exclude the study.
End Points, Risk of Bias, and Statistical Analysis
The primary end point of the study was TR grade. The secondary end points were PR grade, TV annulus size (mm), RV dilatation, RV dysfunction, RVEDV (mL), RVESV (mL), RVEF (%), RV end‐diastolic area (cm²), RV end‐systolic area (cm²), NYHA class, reoperation for TR, or 30‐day mortality. The grades of TR, PR, RV dilatation, and RV dysfunction were quantitatively assessed on echocardiography and scored on a scale from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe). Postoperative measurements were defined as the first observation within 12 months after surgery. For studies reporting interquartile ranges, the mean was estimated according to a validated formula. 9 Two independent reviewers (N.H. and A.G.) extracted the data. When concordance was absent, a third reviewer (J.V.D.E.) checked the data and made the final decision. From each study, we extracted patient characteristics, study design, and outcomes.
The Risk of Bias in Nonrandomized Studies of Interventions tool was systematically used to assess the included studies for risk of bias. 10 The articles and their characteristics were classified into A (low risk of bias), B (moderate risk of bias), C (serious risk of bias), D (critical risk of bias), or E (no information/unclear). Using the RoB 2 tool, 11 the included randomized controlled trials were assessed for biases. Two independent reviewers (C.C. and M.L.R.) assessed the risk of bias. When concordance was absent, a third reviewer (J.V.D.E.) checked the data and made the final decision.
Mean differences (MD) with 95% CI and P values were calculated for continuous variables. For binary variables, odds ratios (ORs) with 95% CI and P values were considered. Forest plots were created to represent the clinical outcomes. The chi‐square test and I 2 test were performed for assessment of statistical heterogeneity. 12 The MD and OR were combined across the studies using a random‐effects method (DerSimonian and Laird inverse variance). 13 The choice for random‐effects models was made on the basis of the assumption that the effect sizes in the individual studies represented samples from a mixing distribution. In addition, the results were reanalyzed using fixed‐effects models to explore whether this yielded differences regarding the summary inferences. The risk of publication bias could not be assessed because none of the comparisons included >10 studies. 14 , 15 All analyses were completed with R Statistical Software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria).
Institutional Review Board Approval
Institutional review board is not applicable for systematic reviews and meta‐analyses.
Results
Study Selection and Characteristics
A total of 2031 citations were identified, of which 46 studies were potentially relevant and retrieved as full text. Six publications 16 , 17 , 18 , 19 , 20 , 21 fulfilled our eligibility criteria (Figure 1). Characteristics of each study and their patients are shown in Tables 1, 2, 3. A total of 749 patients (TVI+PVR, 278 patients; PVR, 471 patients) were included from studies published from 2015 to 2020. All studies were nonrandomized observational studies. Of all patients, 60.8% were male (450/740), and 65.8% (487/740) had a transannular patch. TOF constituted 84.6% (656/775), while 15.2% (118/775) of patients had pulmonary stenosis. The pooled age at initial repair was 4.96 years (4 studies, 688 patients), and the pooled age at PVR was 34.3 years (6 studies, 775 patients). Outcomes were reported for a mean follow‐up of 10.2 months (5 studies, 721 patients). The overall internal validity was considered low risk of bias (Figure S1).
Figure 1. Flow diagram of studies included in data search.
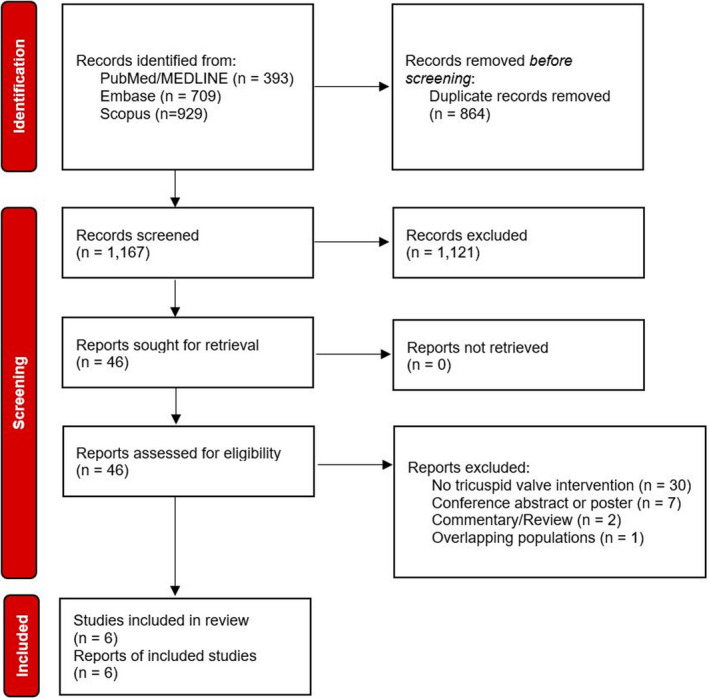
Table 1.
Study Characteristics
| First author | Year | Study period | Country | Design | Patient no. | FU time | TR grade |
|---|---|---|---|---|---|---|---|
| Deshaies 16 | 2020 | 2000–2016 | Canada | NP, NR, NM | 542 (180 TVI+PVR, 362 PVR) | 3 mo | Mild TR in 254 (19 TVI+PVR, 235 PVR), moderate TR in 192 (90 TVI+PVR, 102 PVR), severe TR in 72 (68 TVI+PVR, 4 PVR) |
| Taejung Kim 17 | 2019 | 2000–2016 | South Korea | NP, NR, NM | 67 (38 TVI+PVR, 29 PVR) | 5.5±2.7 mo | TR grade: 2.79±0.95 in TVI+PVR, 1.45±0.56 in PVR |
| Lueck 18 | 2018 | 2009–2017 | Germany | NP, NR, NM | 28 (10 TVI+PVR, 18 PVR) | ND | TR grade: 2.0±0.77 in TVI+PVR, 1.94±0.62 in PVR |
| Roubertie 19 | 2017 | 2002–2014 | France | NP, NR, NM | 41 (16 TVI+PVR, 25 PVR) | 54.6±36.6 mo | Moderate TR in 24 (8 TVI+PVR, 16 PVR), severe TR in 17 (8 TVI+PVR, 9 PVR) |
| Cramer 20 | 2015 | 1999–2012 | USA | NP, NR, NM | 36 (18 TVI+PVR, 18 PVR) | 6 mo | TR grade: 2.7±0.5 in TVI+PVR, 2.2±0.4 in PVR |
| Kogon 21 | 2015 | 2002–2008 | USA | NP, NR, NM | 35 (16 TVI+PVR, 19 PVR) | 7.0±2.8 y | TR grade: 2.63±0.43 in TVI+PVR, 2.08±0.26 in PVR |
FU indicates follow‐up; M, multicenter; ND, not determined; NM, nonmulticenter; NP, nonprospective; NR, nonrandomized; P, prospective; PVR, pulmonary valve replacement; R, randomized; TR, tricuspid regurgitation; and TVI, tricuspid valve intervention.
Table 2.
Procedure Characteristics
| Author | Tricuspid valve annuloplasty type | Pulmonary valve replacement type | ||||||
|---|---|---|---|---|---|---|---|---|
| Suture | Ring | Commissuroplasty | Other/combination | Bioprosthetic valve | Bioprosthetic valved conduit | Mechanical valve | Concomitant procedures other than TVI | |
| Deshaies 2020 16 | 34 | 93 | 38 | 15 replacements (1 mechanical valve, 14 bioprostheses) | ND | ND | ND | 328 (branch pulmonary arterioplasty in 109, residual VSD closure in 38, atrial ablation in 68, ventricular ablation in 70, CABG in 18, mitral valve procedure in 8, aortic valve procedure in 7, thoracic aorta±aortic valve in 5, other in 5) |
| Taejung Kim 2019 17 | 26 | 11 | 26 | 4 leaflet extension, 1 cleft repair, 2 valve replacement | ND | ND | ND | ND |
| Lueck 2018 18 | 0 | 10 | 0 | 0 | 28 | 0 | 0 | ND |
| Roubertie 2017 19 | 0 | 16 | 7 | 0 | 104 | 0 | 28 | |
| Cramer 2015 20 | 4 | 11 | 0 | 3 | 57 | 5 | 0 | 12 (Maze procedure) |
| Kogon 2015 21 | 13 | 3 | 0 | 0 | 28 | 6 | 1 | 9 (pulmonary arterioplasty in 2, VSD closure in 2, Maze procedure in 2, CABG in 1) |
CABG indicates coronary artery bypass grafting; ND, not determined; TVI, tricuspid valve intervention; and VSD, ventricular septal defect.
Table 3.
Baseline and Operative Characteristics of Patients Included in the Study
| Author | Group | Baseline characteristics | Original congenital diagnosis | Operative characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Male sex | Trans‐annular patch | Age at initial repair, y | Age at PVR, y | Interval time, y | TOF | PS | PA | Other | Cardiopulmonary bypass time, min | Aortic cross‐clamp time, min | ||
| Deshaies 2020 16 | All patients | 542 | 293 | 314 | 4.8±0.91 | 35.6±3.4 | ND | 433 | 109 | 0 | 0 | 102.0±11.0 | 60.0±6.7 |
| TVI+PVR | 180 | 89 | 89 | 6.4±1.4 | 39.8±4.1 | ND | 129 | 51 | 0 | 0 | 128.5±9.6 | 53.8±5.5 | |
| PVR | 362 | 204 | 225 | 4.2±0.78 | 34.0±3.2 | ND | 304 | 58 | 0 | 0 | 88.5±8.6 | 69.8±7.5 | |
| Taejung Kim 2019 17 | All patients | 67 | 40 | 40 | ND | ND | ND | 66 | 0 | 0 | 0 | ND | ND |
| PVR | 29 | 16 | 18 | ND | 21.7±12.3 | ND | 28 | 0 | 0 | 0 | ND | ND | |
| TVI+PVR | 38 | 24 | 22 | ND | 31.2±15.2 | ND | 38 | 0 | 0 | 0 | ND | ND | |
| Lueck 2018 18 | All patients | 28 | 17 | 21 | ND | 41.1±12.5 | 32.0±9.5 | 28 | 0 | 0 | 0 | ND | ND |
| TVI+PVR | 10 | ND | ND | ND | ND | ND | 10 | 0 | 0 | 0 | 164 | 71.0 | |
| PVR | 18 | ND | ND | ND | ND | ND | 18 | 0 | 0 | 0 | 153 | 63.5 | |
| Roubertie 2017 19 | All patients | 104 | 64 | 62 | 1.7±1.4 | 26.3±9.50 | 24.8±9.3 | 96 | 2 | 1 | 5 DORV with VSD and PS | 94.3±48.1 | 68.1±23.0 |
| TVI+PVR (moderate TR) | 8 | ND | ND | 1.7±0.6 | 24.6±12.0 | 23.0±12.0 | 8 | 0 | 0 | 0 | ND | ND | |
| TVI+PVR (severe TR) | 8 | ND | ND | 1.4±1.7 | 26.1±9.0 | 24.9±9.0 | 8 | 0 | 0 | 0 | ND | ND | |
| PVR (moderate TR) | 16 | ND | ND | 2.3±3.4 | 25.6±8.0 | 24.8±8.0 | 16 | 0 | 0 | 0 | ND | ND | |
| PVR (severe TR) | 9 | ND | ND | 1.7±0.75 | 27.8±10.0 | 26.2±9.0 | 9 | 0 | 0 | 0 | ND | ND | |
| Cramer 2015 20 | All patients | 62 | 36 | 50 | 6.9±3.6 | 35.2±8.5 | 29.5±6.2 | 62 | 0 | 0 | 0 | ND | ND |
| Kogon 2015 21 | All patients | 35 | ND | ND | 7.8±11.1 | 31.3±16.7 | 23.5±11.5 | 26 | 9 | 0 | 0 | ND | ND |
| TVI+PVR | 16 | ND | ND | 10.7±13 | 31.9±16.3 | 18.1±11.5 | 11 | 5 | 0 | 0 | ND | ND | |
| PVR | 19 | ND | ND | 6.1±9.9 | 32.3±14.6 | 26.7±10.6 | 15 | 4 | 0 | 0 | ND | ND | |
DORV indicates double outlet right ventricle; ND, not determined; PA, pulmonary atresia; PS, pulmonary stenosis; PVR, pulmonary valve replacement; TR, tricuspid regurgitation; TVI, tricuspid valve intervention; and VSD, ventricular septal defect.
Synthesis of Results
Echocardiographic Parameters
Results from the meta‐analyses of echocardiographic and magnetic resonance imaging (MRI) parameters are presented in Table 4; forest plots are given in Figures S2 through S9. Preoperative values were comparable between TVI+PVR and PVR for all parameters considered, although patients in the TVI+PVR tended to have a higher TR grade (MD, 0.64; 95% CI, −0.18 to 1.45; P=0.090; I²=85%). A decrease from preoperative to postoperative TR grade was evident in both TVI+PVR (MD, −1.53; 95% CI, −2.28 to −0.79; P=0.002; I²=94%) and PVR (MD, −0.99; 95% CI, −1.81 to −0.16; P=0.026; I²=91%). However, there was evidence for a larger decrease in TR grade in the TVI+PVR group compared with the PVR group (MD, −0.40; 95% CI, −0.75 to −0.05, P=0.031; I²=75%). As a result, postoperative TR grade was comparable between both groups (MD, 0.08; 95% CI, −0.14 to 0.29; P=0.342; I²=0%). A clinically relevant reduction in PR grade was also evident in both TVI+PVR (MD, −2.53; 95% CI, −3.98 to −1.07; P=0.029; I²=36%) and PVR (MD, −2.52; 95% CI, −3.03 to −2.02, P=0.010; I²=0%), although no evidence was found to state that TVI+PVR was associated with a larger decrease in PR (MD, 0.03; 95% CI, −0.86 to 0.92; P=0.711; I²=75%).
Table 4.
Summary of Outcomes
| Variable (unit) | Comparison | No. of (sub)studies (No. of patients) | MD (95% CI) | P value | I² (%) | P value |
|---|---|---|---|---|---|---|
| TR grade (0–3) | Preoperative TVI+PVR vs PVR | 4 (82 TVI+PVR/84 PVR) | 0.64 (−0.18 to 1.45) | 0.090 | 85 | <0.001 |
| Postoperative TVI+PVR vs PVR | 4 (82 TVI+PVR/84 PVR) | 0.08 (−0.14 to 0.29) | 0.342 | 0 | 0.670 | |
| Change from preoperative to postoperative in TVI+PVR | 7 (249 TVI+PVR) | −1.53 (−2.28 to −0.79) | 0.002 | 94 | <0.001 | |
| Change from preoperative to postoperative in PVR | 7 (415 PVR) | −0.99 (−1.81 to −0.16) | 0.026 | 91 | <0.001 | |
| Difference in change with TVI+PVR vs PVR* | 7 (249 TVI+PVR/415 PVR) | −0.40 (−0.75 to −0.05) | 0.031 | 75 | <0.001 | |
| PR grade (0–3) | Preoperative TVI+PVR vs PVR | 2 (34 TVI+PVR/37 PVR) | −0.03 (−0.59 to 0.53) | 0.657 | 30 | 0.234 |
| Postoperative TVI+PVR vs PVR | 2 (34 TVI+PVR/37 PVR) | −0.01 (−0.25 to 0.23) | 0.603 | 0 | 0.889 | |
| Change from preoperative to postoperative in TVI+PVR | 2 (34 TVI+PVR) | −2.53 (−3.98 to −1.07) | 0.029 | 36 | 0.210 | |
| Change from preoperative to postoperative in PVR | 2 (37 PVR) | −2.52 (−3.03 to −2.02) | 0.010 | 0 | 0.701 | |
| Difference in change with TVI+PVR vs PVR* | 2 (34 TVI+PVR/37 PVR) | 0.03 (−0.85;0.0.92) | 0.711 | 75 | 0.045 | |
| TV annulus (mm) | Preoperative TVI+PVR vs PVR | 2 (56 TVI+PVR/47 PVR) | 1.10 mm (−7.44 to 9.09) | 0.350 | 0 | 0.425 |
| Postoperative TVI+PVR vs PVR | 2 (56 TVI+PVR/47 PVR) | −1.50 mm (−21.18 to 18.19) | 0.511 | 82 | 0.020 | |
| Change from preoperative to postoperative in TVI+PVR | 2 (56 TVI+PVR) | −6.43 mm (−10.59 to −2.27) | 0.032 | 0 | 0.550 | |
| Change from preoperative to postoperative in PVR | 2 (47 PVR) | −4.20 mm (−10.42 to 2.02) | 0.074 | 0 | 0.592 | |
| Difference in change with TVI+PVR vs PVR* | 2 (56 TVI+PVR/47 PVR) | −2.45 mm (−13.25 to 8.35) | 0.212 | 93 | <0.001 | |
| RV dilatation (0–3) | Preoperative TVI+PVR vs PVR | 2 (26 TVI+PVR/37 PVR) | 0.08 (−0.90 to 1.06) | 0.490 | 0 | 0.713 |
| Postoperative TVI+PVR vs PVR | 2 (26 TVI+PVR/37 PVR) | 0.22 (−0.73 to 1.18) | 0.207 | 0 | 0.732 | |
| Change from preoperative to postoperative in TVI+PVR | 2 (26 TVI+PVR) | −0.14 (−6.32 to 6.04) | 0.823 | 71 | 0.065 | |
| Change from preoperative to postoperative in PVR | 2 (37 PVR) | −0.24 (−6.52 to 6.04) | 0.714 | 85 | 0.011 | |
| Difference in change with TVI+PVR vs PVR* | 2 (26 TVI+PVR//37 PVR) | 0.14 (0.08 to 0.19) | 0.020 | 0 | 0.956 | |
| RV dysfunction (0–3) | Preoperative TVI+PVR vs PVR | 2 (26 TVI+PVR/37 PVR) | 0.39 (−1.32 to 2.10) | 0.212 | 0 | 0.574 |
| Postoperative TVI+PVR vs PVR | 2 (26 TVI+PVR/37 PVR) | 0.71 (−2.21 to 3.63) | 0.199 | 0 | 0.334 | |
| Change from preoperative to postoperative in TVI+PVR | 2 (26 TVI+PVR) | 0.25 (−3.94 to 4.43) | 0.592 | 33 | 0.222 | |
| Change from preoperative to postoperative in PVR | 2 (37 PVR) | 0.04 (−5.61 to 5.69) | 0.948 | 76 | 0.040 | |
| Difference in change with TVI+PVR vs PVR* | 2 (26 TVI+PVR/37 PVR) | 0.28 (−1.05 to 1.62) | 0.277 | 25 | 0.247 | |
| RVEDV (mL) | Preoperative TVI+PVR vs PVR | 3 (34 TVI+PVR/43 PVR) | 1.07 mL (−32.04 to 34.18) | 0.902 | 0 | 0.416 |
| Postoperative TVI+PVR vs PVR | 3 (34 TVI+PVR/43 PVR) | −2.87 mL (−23.83 to 18.09) | 0.615 | 0 | 0.502 | |
| Change from preoperative to postoperative in TVI+PVR | 3 (34 TVI+PVR) | −84.46 mL (−107.36 to −61.57) | 0.004 | 0 | 0.405 | |
| Change from preoperative to postoperative in PVR | 3 (43 PVR) | −76.66 mL (−114.22 to −39.11) | 0.013 | 25 | 0.264 | |
| Difference in change with TVI+PVR vs PVR* | 3 (34 TVI+PVR/43 PVR) | −0.74 mL (−24.90 to 23.43) | 0.908 | 62 | 0.072 | |
| RVESV (mL) | Preoperative TVI+PVR vs PVR | 3 (34 TVI+PVR/43 PVR) | 1.32 mL (−26.18 to 28.82) | 0.855 | 0 | 0.388 |
| Postoperative TVI+PVR vs PVR | 3 (34 TVI+PVR/43 PVR) | −0.39 mL (−18.28 to 17.51) | 0.934 | 0 | 0.934 | |
| Change from preoperative to postoperative in TVI+PVR | 3 (34 TVI+PVR) | −28.45 mL (−37.65 to −19.25) | 0.006 | 0 | 0.863 | |
| Change from preoperative to postoperative in PVR | 3 (43 PVR) | −25.83 mL (−39.20 to −12.46) | 0.014 | 0 | 0.704 | |
| Difference in change with TVI+PVR vs PVR* | 3 (34 TVI+PVR/43 PVR) | −0.37 mL (−11.84 to 11.09) | 0.901 | 12 | 0.320 | |
| RVEF (%) | Preoperative TVI+PVR vs PVR | 3 (34 TVI+PVR/43 PVR) | 12.77% (−41.75 to 67.30) | 0.420 | 97 | <0.001 |
| Postoperative TVI+PVR vs PVR | 3 (34 TVI+PVR/43 PVR) | 6.96% (−18.98 to 32.89) | 0.368 | 90 | <0.001 | |
| Change from preoperative to postoperative in TVI+PVR | 3 (34 TVI+PVR) | 8.38% (−9.77 to 26.54) | 0.185 | 79 | 0.008 | |
| Change from preoperative to postoperative in PVR | 3 (43 PVR) | 14.35% (−31.49 to 60.19) | 0.310 | 97 | <0.001 | |
| Difference in change with TVI+PVR vs PVR* | 3 (34 TVI+PVR/43 PVR) | −6.00% (−34.44 to 22.45) | 0.460 | 99 | <0.001 |
MD indicates mean difference; PR, pulmonary regurgitation; PVR, pulmonary valve regurgitation; RV, right ventricular; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end‐systolic volume; TR, tricuspid regurgitation; TV, tricuspid valve; and TVI, tricuspid valve intervention.
(Difference in change with TVI+PVR vs PVR)=(Change from preoperative to postoperative in TVI+PVR)−(Change from preoperative to postoperative in PVR).
With regard to TV annulus size, a clear decrease from preoperative to postoperative was observed in TVI+PVR (MD, −6.43 mm; 95% CI, −10.59 to −2.27; P=0.032), whereas it was not evident whether a similar effect was present in the PVR group (MD, −4.20; 95% CI, −10.42 to 2.02; P=0.074; I²=0%) (Table 4). Although no evidence was found for an effect of either TVI+PVR or PVR on qualitative score for RV dilatation, TVI+PVR tended to be associated with a greater increase in qualitative score for RV dilatation compared with PVR (MD, 0.14; 95% CI, 0.08 to 0.19; P=0.020; I²=0%); however, this result should be interpreted cautiously given that Lueck et al 18 reported a tendency toward an increase in RV dilatation, whereas Kogon et al 21 reported a decrease in RV dilatation with both procedures. No evidence of effects of either treatment or differences between the effects could be observed with regard to RV dysfunction as qualitatively assessed by echocardiography (Table 4).
RV end‐diastolic area and RV end‐systolic area were reported by only one study. Cramer et al 20 reported a decrease from preoperative to postoperative RV end‐diastolic area in both TVI+PVR (39.6±12.0 cm² to 28.6±5.7 cm²; P=0.001) and PVR (36.2±12.0 cm² to 28.7±8.8 cm²; P=0.040). In contrast, they found no evidence of an effect of RV end‐systolic area with either TVI+PVR (28.4±8.5 cm² to 23.1±13.1 cm², P=0.16) or PVR (25.1±8.6 cm² to 20.1±7.2 cm², P=0.07).
MRI Parameters
A clinically relevant decrease from preoperative to postoperative RVEDV was observed in both TVI+PVR (MD, −84.5 mL; 95% CI, −107 to −61.6; P=0.004) and PVR (MD, −76.7 mL; 95% CI, −114 to −39.1; P=0.013). Similarly, a clinically relevant decrease was observed for RVESV in both TVI+PVR (MD, −28.5 mL; 95% CI, −37.7 to −19.3; P=0.006) and PVR (MD, −25.8 mL; 95% CI, −39.2 to −12.5; P=0.014). However, no evidence could be found for any differences between both treatments with regard to the decreases in RVEDV (MD, −0.74; 95% CI, −24.90 to 23.43; P=0.908; I²=62%) and RVESV (MD, −0.37; 95% CI, −11.84 to 11.09; P=0.901; I²=12%). No evidence of effects of either treatment nor differences between the effects could be observed with regard to RVEF (Table 4).
NYHA Class
NYHA class was only reported by a single study. Roubertie et al 19 demonstrated that postoperative NYHA class was better in TVI+PVR compared with PVR in patients who had preoperative severe TR (postoperative NYHA class I in 8/8 [100%] with TVI+PVR versus 2/9 [22.2%] with PVR, respectively; P=0.004), whereas they could find no evidence for a benefit of concomitant TVI in patients with preoperative moderate TR (7/8 [87.5%] versus 16/16 [100%], respectively; P=0.333).
Short‐Term Outcomes
The overall OR for 30‐day mortality showed no evidence of a difference between TVI+PVR and PVR (OR, 1.86; 95% CI, 0.24 to 14.61; P=0.324) (Figure S10). Reoperation for TR was only reported by Roubertie et al 19 and they could establish no evidence for a different between both groups. In this study, 2 of 9 (22%) of patients with severe TR who had undergone isolated PVR required reoperations, compared with 0 of 8 (0%) in the TVI+PVR arm (P=0.47).
Sensitivity Analysis
The treatment effect estimates from fixed‐effects models were largely comparable to those from random‐effects models (Figures S2–S10). In contrast to the random‐effects models, the fixed‐effects models suggested some evidence for a greater decrease in TV annulus size (MD, −2.47; 95% CI, −2.91 to −2.03; P<0.001), a greater increase in RV dysfunction as qualitatively assessed by echocardiography (MD, 0.29; 95% CI, 0.12 to 0.46; P<0.001), and a smaller increase in RVEF (MD, −6.41; 95% CI, −7.80 to −5.02; P<0.001) with TVI+PVR compared with PVR; however, all of these results should be interpreted with caution given the important statistical heterogeneity in these analyses (I² of 93%, 25%, and 99%, respectively). Furthermore, the greater increase in qualitative score for RV dilatation with TVI+PVR compared with PVR was no longer evident in fixed‐effects analyses (OR, 0.14; 95% CI, −0.01 to 0.29; P=0.077); no evidence for heterogeneity was evident in this analysis (I²=0%).
Discussion
Summary of Evidence
This meta‐analysis investigated the effect of concomitant TVI at the time of PVR in ACHD. The key findings are summarized in Figure 2. Our results demonstrated that both TVI+PVR and PVR reduced TR grade, PR grade, RVEDV, and RVESV. TVI+PVR, but not PVR alone, was associated with a decrease in TV annulus size after the procedure. Furthermore, TVI+PVR was associated with a larger decrease in TR grade compared with PVR. No evidence could be established for an effect of either treatment on RVEF or echocardiographic assessment of RV dilatation and dysfunction. There was no evidence for a difference in hospital mortality or reoperation for TR. These results suggest that TVI might have a favorable effect on TR grade, although specific indications for combined valve surgery remain unclear.
Figure 2. Summary of the key findings of the meta‐analysis.
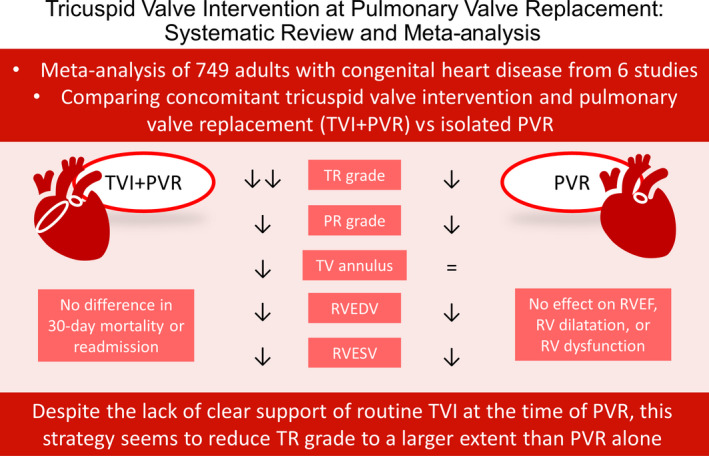
Both TVI+PVR and PVR reduced TR grade, PR grade, RVEDV, and RVESV. TVI+PVR, but not PVR, was associated with a decrease in TV annulus. Furthermore, TVI+PVR was associated with a larger decrease in TR grade compared with PVR. No evidence could be established for an effect of either treatment on RVEF or RV dilatation and RV dysfunction as qualitatively assessed by echocardiography of either treatment. There was no evidence for a difference in hospital mortality or reoperation for TR. PR indicates pulmonary regurgitation; PVR, pulmonary valve replacement; RV, right ventricular; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end‐systolic volume; TR, tricuspid regurgitation; TV, tricuspid valve; and TVI, tricuspid valve intervention.
Comments
Dilatation of the RV is a common complication following repair of TOF, pulmonary stenosis, and pulmonary atresia, primarily attributable to chronic PR. 1 This, in turn, leads to dilatation of the TV annulus, resulting in varying degrees of TR and further RV dilatation. Although the transannular patch repair approach causes PR, many additional factors can contribute to TR in these patients. 22 These include damage to the TV leaflets or chordae tendineae during initial surgery, as well as the presence of additional valve abnormalities. Regardless of the causative mechanism, moderate to severe preoperative TR is a well‐described risk factor for adverse outcomes in ACHD, leading to heart failure, arrhythmia, and death. 6 Although concomitant TVI has been shown to reduce TR in these patients, there has been considerable debate regarding this approach.
Several studies have recommended PVR alone to address both PR and TR following TOF repair, arguing that the reduction in RV volume overload resulting from PVR is enough to ameliorate the observed TR. In a comparison between patients undergoing PVR alone versus those with TVI+PVR, Kogon et al 21 found that patients in the latter group experienced a greater increase in TR at medium follow‐up (7.0±2.8 years). These results led them to recommend PVR alone in patients with moderate or greater TR. Similarly, Kurkluoglu et al 23 found that dilatation of the TV annulus improved after PVR alone, suggesting that additional parameters should be taken into account when evaluating patients for TVI+PVR. Results from a single‐center study by Lueck et al 18 found longer intensive care unit stays for the TVI+PVR group, as well as greater rates of arrhythmia, renal insufficiency, sternal wound infection, and delirium. Notably, all of these findings were drawn from single‐center studies composed of relatively small populations. Conversely, results from a multicenter study performed by Deshaies et al 16 found that TVI+PVR results in a greater reduction in TR. With the exception of a slightly higher incidence of major infections, there was no evidence for differences in adverse outcomes between TVI+PVR and PVR alone.
Another area of debate that our study could not address is the optimal treatment strategy for patients who undergo TVI. With the exception of Lueck et al, 18 where the TV was replaced in all 10 of their patients with TVI+PVR, TV repair was the most common TVI in the studies we analyzed. This is similar to other studies of ACHD patients undergoing TVI. A recent single‐center study from Australia analyzing TVI in adults with Ebstein anomaly and other ACHD found that TV repair was performed in 61% (22/36) of their cohort, while the remaining 39% (14/36) underwent TV replacement. 24 In this cohort, 4 patients required reintervention (with 1 death 9 days after reintervention), of which 2 had initial TV replacement and 2 underwent TV repair. Of the 30 patients with available echocardiographic data, all 5 with moderate or greater TR underwent TV repair. 24 In an analysis of 109 TV repairs and 19 replacements in 128 patients with ACHD other than Ebstein anomaly, Lo Rito et al 25 found that those who underwent suture annuloplasty had a higher rate of moderate or greater TR at latest follow‐up (4.95 years; 7.7 interquartile range) compared with those with ring annuloplasty. The only patient who required TV reintervention had an initial biological valve replacement. Importantly, both studies describe a high incidence of atrial arrhythmias following TVI, regardless of surgical approach. 25 , 26
Currently, there are not enough data to identify which patients may benefit the most from concomitant TVI. Our study, however, highlights several salient features that warrant further exploration. In the only included study to report NYHA class, Roubertie et al 19 found that patients with severe preoperative TR experienced an improvement in NYHA class and TR grade following TVI+PVR. This study similarly found no patients with residual moderate or greater TR in the TVI+PVR group, compared with 78% (7/9) of those with PVR alone when analyzing patients with severe TR before surgery. In accordance with this, Deshaies et al 16 found that severe preoperative TR was associated with a higher risk of residual postoperative TR (OR, 9.43; 95% CI, 4.20–21.33; P<0.001), while TVI+PVR reduced this risk (OR, 0.44; 95% CI, 0.25–0.77; P=0.004). Importantly, only 5.6% (4/72) of patients with severe preoperative TR underwent isolated PVR in this study. In the Cramer et al 20 series, 75% (12/16) of patients with severe TR had TVI+PVR, with both approaches resulting in mild residual TR at 6‐month follow‐up.
Although TR grade and measurements of cardiac volumes and function are valuable indices of the efficacy of TVI, the actual goal of such intervention in ACHD should be the prevention of adverse events such as arrhythmias and heart failure. In this regard, the results of a study by Bokma et al 6 are concerning. In their cohort of 129 patients with TOF undergoing isolated PVR, those with severe preoperative TR remained at increased risk for adverse events (including death, sustained ventricular tachycardia, heart failure, or supraventricular tachycardia), regardless of their postoperative TR grade. The authors suggested that both long‐standing volume overload attributable to PR and long‐standing right atrial volume and pressure overload attributable to TR might contribute to this risk, leading to RV dysfunction and arrhythmias, respectively. While our findings suggest that patients with severe preoperative TR benefit most from TVI+PVR in terms of improvement of TR grade, a benefit in terms of “hard” outcomes can thus not be directly inferred. These data therefore do not support the universal application of this approach for severe TR. Further well‐designed studies focusing on specific underlying mechanisms of TR and evaluating the effect on adverse events on long‐term follow‐up may elucidate which patients stand to benefit the most from this approach.
Sources of Heterogeneity
Given the nonrandomized nature of the existing studies comparing TVI+PVR against PVR, underlying center‐ and surgeon‐specific bias with regard to treatment allocation was likely. Kogon et al 21 intervened on 46% (16/35) of patients with moderate or greater TR, stating bias toward a conservative approach based on their prior work 26 showing improvement in TV function without concomitant TVI, a view shared by Cramer et al. 20 In contrast, Taejung Kim et al 17 performed concomitant TVI in 56.7% (38/67) of patients in their cohort, with no signficant difference in baseline TV annulus diameter but larger RV volumes in their TVI+PVR group, reflecting a more aggressive approach to TR at their center. In Deshaies et al, 16 almost 59.8% (158/264) of patients with moderate or greater TR had TVI+PVR, as opposed to only 7.9% (22/278) of those with mild TR. Taken together, these data suggest that considerable heterogeneity may have been present with regard to indications for concomitant TVI. Such indication bias would be expected to result in a greater prevalence of higher‐risk patients in the TVI+PVR group, as observed in the studies by Taejung Kim et al, 17 Cramer et al, 20 and Kogon et al. 21 In every study reviewed for this meta‐analysis, the addition of TVI was performed on the basis of surgeon and cardiologist preference, which further adds patient‐specific heterogeneity regardless of the degree of preoperative TR.
The use of echocardiography and/or MRI also varied among studies. While the use of cardiac MRI has evolved in recent years, only Roubertie et al 19 and Taejung Kim et al 17 incorporated MRI data into their analyses out of the 6 included studies. Expanded use of cardiac MRI can further quantify TV function and help better understand the role of concomitant TVI in patients with TOF and PR.
Limitations
While the use of meta‐analysis enabled us to pool studies and increase our sample size, we were ultimately limited to 6 studies that met the inclusion criteria of comparing PVR with and without concomitant TVI. Accordingly, some of the analyses were based on a low number of subjects. As described earlier, our results may have been susceptible to selection bias. Another limitation is the lack of data regarding patient anatomy and underlying causes of TR, which can be critical in determining when TVI+PVR offers the greatest benefit. Since all included studies focused on adults with childhood TOF repair, the operative technique and age at repair reflect treatment strategies from earlier decades, which have since evolved. 27 , 28 Furthermore, long‐term follow‐up studies of patients with TVI+PVR remains scarce, which precludes the ability to draw definitive conclusions on durability of the results.
Conclusions
While both TVI+PVR and PVR alone are effective in the reduction of TR and RV volumes, routine TVI at the time of PVR can reduce TR grade to a larger extent than isolated PVR. Further studies are needed to identify the subgroups of patients who might benefit most from combined valve surgery, as current data do not support the universal application of this approach.
Sources of Funding
None.
Disclosures
J. Van den Eynde was supported by the Belgian American Educational Foundation. Dr Budts is proctor for Abbott and Occlutech. Dr Gewillig is proctor for Edwards and Medtronic. Dr Kutty is consultant for GE Healthcare. The remaining authors have no disclosures to report.
Supporting information
Data S1
Figures S1–S10
Acknowledgments
J.V.D.E.: concept/design, data collection, data interpretation, drafting article, critical revision of article, approval of article, C.C.: data collection, data interpretation, critical revision of article, approval of article; M.L.R.: data collection, data interpretation, critical revision of article, approval of article, N.H.: data collection, data interpretation, critical revision of article, approval of article; H.C.: data interpretation, critical revision of article, approval of article; A.G.: data collection, data interpretation, critical revision of article, approval of article; A.R.: data interpretation, critical revision of article, approval of article; A.W.: data interpretation, critical revision of article, approval of article; W.B.: data interpretation, critical revision of article, approval of article; M.G.: data interpretation, critical revision of article, approval of article; M.P.S.: concept/design, data analysis/interpretation, statistics, drafting article, critical revision of article, approval of article; S.K.: data interpretation, critical revision of article, approval of article.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022909
For Sources of Funding and Disclosures, see page 12.
References
- 1. Mahle WT, Parks WJ, Fyfe DA, Sallee D. Tricuspid regurgitation in patients with repaired tetralogy of Fallot and its relation to right ventricular dilatation. Am J Cardiol. 2003;92:643–645. doi: 10.1016/S0002-9149(03)00746-X [DOI] [PubMed] [Google Scholar]
- 2. Van den Eynde J, Sá MPBO, Vervoort D, Roever L, Meyns B, Budts W, Gewillig M, Ruhparwar A, Zhigalov K, Weymann A. Pulmonary valve replacement in tetralogy of Fallot: an updated meta‐analysis. Ann Thorac Surg. 2020;S0003‐4975(20)32173‐1. doi: 10.1016/j.athoracsur.2020.11.040 [DOI] [PubMed] [Google Scholar]
- 3. Jones TK, Rome JJ, Armstrong AK, Berger F, Hellenbrand WE, Cabalka AK, Benson LN, Balzer DT, Cheatham JP, Eicken A, et al. Transcatheter pulmonary valve replacement reduces tricuspid regurgitation in patients with right ventricular volume/pressure overload. J Am Coll Cardiol. 2016;68:1525–1535. doi: 10.1016/j.jacc.2016.07.734 [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H, De Backer J, Babu‐Narayan SV, Budts W, Chessa M, Diller G‐P, lung B, Kluin J, Lang IM, Meijboom F, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554 [DOI] [PubMed] [Google Scholar]
- 5. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 6. Bokma JP, Winter MM, Oosterhof T, Vliegen HW, van Dijk AP, Hazekamp MG, Koolbergen DR, Groenink M, Mulder BJ, Bouma BJ. Severe tricuspid regurgitation is predictive for adverse events in tetralogy of Fallot. Heart. 2015;101:794–799. doi: 10.1136/heartjnl-2014-306919 [DOI] [PubMed] [Google Scholar]
- 7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 9. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H‐Y, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 15. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deshaies C, Trottier H, Khairy P, Al‐Aklabi M, Beauchesne L, Bernier PL, Dhillon S, Gandhi SK, Haller C, Hancock Friesen CL, et al. Tricuspid intervention following pulmonary valve replacement in adults with congenital heart disease. J Am Coll Cardiol. 2020;75:1033–1043. doi: 10.1016/j.jacc.2019.12.053 [DOI] [PubMed] [Google Scholar]
- 17. Taejung Kim S, Song J, Kim YS, Huh J, Kang IS, Yang JH, Jun TG. Repair of tricuspid valve with pulmonary valve replacement in repaired tetralogy of Fallot. Scand Cardiovasc J. 2019;53:148–152. doi: 10.1080/14017431.2019.1610572 [DOI] [PubMed] [Google Scholar]
- 18. Lueck S, Bormann E, Rellensmann K, Martens S, Rukosujew A. Impact of additional tricuspid valve annuloplasty in TOF patients undergoing pulmonary valve replacement. J Cardiovasc Surg. 2019;60:268–273. doi: 10.23736/S0021-9509.18.10385-5 [DOI] [PubMed] [Google Scholar]
- 19. Roubertie F, Séguéla PEP‐E, Jalal Z, Iriart X, Roques X, Kreitmann B, Al‐Yamani M, Pillois X, Thambo JB. Tricuspid valve repair and pulmonary valve replacement in adults with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg. 2017;154:214–223. doi: 10.1016/j.jtcvs.2016.12.062 [DOI] [PubMed] [Google Scholar]
- 20. Cramer JW, Ginde S, Hill GD, Cohen SB, Bartz PJ, Tweddell JS, Earing MG. Tricuspid repair at pulmonary valve replacement does not alter outcomes in tetralogy of Fallot. Ann Thorac Surg. 2015;99:899–904. doi: 10.1016/j.athoracsur.2014.09.086 [DOI] [PubMed] [Google Scholar]
- 21. Kogon B, Mori M, Alsoufi B, Kanter K, Oster M. Leaving moderate tricuspid valve regurgitation alone at the time of pulmonary valve replacement: a worthwhile approach. Ann Thorac Surg. 2015;99:2117–2123. doi: 10.1016/j.athoracsur.2015.01.062 [DOI] [PubMed] [Google Scholar]
- 22. Cheng JW, Russell H, Stewart RD, Thomas J, Backer CL, Mavroudis C. The role of tricuspid valve surgery in the late management of tetralogy of Fallot: collective review. World J Pediatr Congenit Hear Surg. 2012;3:492–498. doi: 10.1177/2150135112450037 [DOI] [PubMed] [Google Scholar]
- 23. Kurkluoglu M, John AS, Cross R, Chung D, Yerebakan C, Zurakowski D, Jonas RA, Sinha P. Should tricuspid annuloplasty be performed with pulmonary valve replacement for pulmonary regurgitation in repaired tetralogy of Fallot? Semin Thorac Cardiovasc Surg. 2015;27:159–165. doi: 10.1053/j.semtcvs.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 24. Offen S, Cham J, Tan C, Chard RB, Cordina R, Celermajer DS. Tricuspid valve surgery in adults with congenital heart disease: indications, techniques and outcomes. Int J Cardiol Congenit Heart Dis. 2021;4:100159. doi: 10.1016/j.ijcchd.2021.100159 [DOI] [Google Scholar]
- 25. Lo Rito M, Grandinetti M, Muzio G, Varrica A, Frigiola A, Micheletti A, Chessa M, Giamberti A. Results for tricuspid valve surgery in adults with congenital heart disease other than Ebstein’s anomaly. Eur J Cardiothorac Surg. 2019;56:706–713. doi: 10.1093/ejcts/ezz093 [DOI] [PubMed] [Google Scholar]
- 26. Kogon B, Patel M, Leong T, McConnell M, Book W. Management of moderate functional tricuspid valve regurgitation at the time of pulmonary valve replacement: is concomitant tricuspid valve repair necessary? Pediatr Cardiol. 2010;31:843–848. doi: 10.1007/s00246-010-9717-6 [DOI] [PubMed] [Google Scholar]
- 27. Starr JP. Tetralogy of Fallot: yesterday and today. World J Surg. 2010;34:658–668. doi: 10.1007/s00268-009-0296-8 [DOI] [PubMed] [Google Scholar]
- 28. Cunningham ME, Donofrio MT, Peer SM, Zurakowski D, Jonas RA, Sinha P. Optimal timing for elective early primary repair of tetralogy of Fallot: analysis of intermediate term outcomes. Ann Thorac Surg. 2017;103:845–852. doi: 10.1016/j.athoracsur.2016.07.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figures S1–S10


