Abstract
Background
While both renin‐dependent and renin‐independent aldosterone secretion contribute to aldosteronism, their relative associations with cardiovascular disease (CVD) risk has not been investigated.
Methods and Results
A total of 2909 participants from the FOS (Framingham Offspring Study) with baseline, serum aldosterone concentration, and plasma renin concentration who attended the sixth examination cycle and were followed up until 2014 and who were free of CVD were included. We further recruited 2612 hypertensive participants from the CONPASS (Chongqing Primary Aldosteronism Study). Captopril challenge test was performed to confirm renin‐dependent or ‐independent aldosteronism in CONPASS. Among 1433 hypertensive subjects of FOS, when compared with those with serum aldosterone concentration <10 ng dL−1 (normal aldosterone), participants who had serum aldosterone concentration ≥10 ng dL−1 and plasma renin concentration ≤15 mIU L−1 (identified as renin‐independent aldosteronism) showed a higher risk of CVD (hazard ratio, 1.40 [95% CI, 1.08–1.82]), while those who had serum aldosterone concentration ≥10 ng dL−1 and plasma renin concentration >15 mIU L−1 (identified as renin‐dependent aldosteronism) showed an unchanged CVD risk. In CONPASS, renin‐independent aldosteronism carried a significantly higher risk of CVD than normal aldosterone (odds ratio, 2.57 [95% CI, 1.13–5.86]), while the CVD risk remained unchanged in renin‐dependent aldosteronism. Elevation of the urinary potassium‐to‐sodium excretion ratio, reflective of mineralocorticoid receptor activity, was only observed in participants with renin‐independent aldosteronism.
Conclusions
Among patients with hypertension, renin‐independent aldosteronism is more closely associated with CVD risk than renin‐dependent aldosteronism.
Keywords: cardiovascular disease, hypertension, mineralocorticoid receptor activity, renin‐dependent aldosteronism, renin‐independent aldosteronism
Subject Categories: Hypertension, Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- CCT
captopril challenge test
- CONPASS
Chongqing Primary Aldosteronism Study
- FOS
Framingham Offspring Study
- PAC
plasma aldosterone concentration
- PRC
plasma renin concentration
- SAC
serum aldosterone concentration
Clinical Perspective
What Is New?
Renin‐independent aldosteronism may confer a higher risk of cardiovascular disease than renin‐dependent aldosteronism among patients with hypertension, likely because of an increase in mineralocorticoid activity.
What Are the Clinical Implications?
More attention to the renin‐independent aldosteronism may be required for interventions on the renin–angiotensin–aldosterone system and cardiovascular disease prevention.
Excess or inappropriate release of aldosterone activates the mineralocorticoid receptor (MR) to induce endothelial dysfunction and adverse cardiorenal remodeling. 1 , 2 , 3 , 4 Both renin‐dependent and renin‐independent aldosterone secretion contribute to aldosteronism. 5 , 6 , 7 It is widely known that inhibition of renin‐dependent aldosteronism, also recognized as an activated renin–angiotensin–aldosterone system, 5 confers cardiovascular benefits. 8 , 9 , 10 Subjects with renin‐independent aldosteronism also exhibited a higher risk of cardiovascular diseases (CVD) than essential hypertension. 11 However, the risk of CVD in renin‐independent compared with renin‐dependent aldosteronism has not been systematically examined.
Previous data on the relationship between aldosterone, renin, and CVD were equivocal. In the general population of the FOS (Framingham Offspring Study), neither renin nor aldosterone was found to be associated with CVD incidence during a median of 7 years follow‐up. 12 , 13 In contrast, the Jackson Heart Study showed that in a population of Black patients, either elevated baseline serum aldosterone concentration or plasma renin activity was associated with a higher risk of CVD after 12 years of follow‐up. 14 In other studies, among participants who had a high risk of CVD or cardiovascular comorbidities, the effects of circulating aldosterone and renin on the incidence of CVD were contradictory between different cohorts. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 These inconsistent results may be ascribed, at least partially, to the failure to distinguish between renin‐independent and renin‐dependent aldosteronism, as well as confounders of aldosterone and renin measurements such as medications and hypokalemia. A more recent study of 948 adults from the MESA (Multi‐Ethnic Study of Atherosclerosis) cohort who were not taking antihypertensive medications identified an association between aldosterone and risk of all‐cause mortality when plasma renin activity was suppressed, but mortality caused by CVD was not evaluated. 24
Here, using the 20‐year longitudinal data from the FOS (exploration cohort), we compared the long‐term risk of CVD in renin‐dependent and renin‐independent aldosteronism. In the cross‐sectional analysis of the CONPASS (Chongqing Primary Aldosteronism Study), we enrolled hypertensive participants who were not taking interfering medications. The captopril challenge test (CCT), together with indirect measurements of MR activity, was conducted to distinguish the types of aldosteronism. We hypothesized that renin‐independent aldosteronism would confer a higher risk of CVD compared with renin‐dependent aldosteronism.
Methods
Reproducible Research Statement
Study protocol and statistical code are available from Ying Song (e‐mail: shuiyunying@126.com) or Shumin Yang (e‐mail:443068494@qq.com).
Data Availability Statement
Data are available to approved people through written agreements with the authors and the data partner.
Study Population
The prospective cohort of the FOS has been evaluated since 1971 to 1975 with the study design previously published. 12 , 13 , 25 For the current analyses, FOS participants were included if they attended the sixth examination cycle (1995–1998, baseline) and were followed up until December 31, 2014; had serum aldosterone and plasma renin concentration measured at baseline; and had available medical records of baseline and follow‐up cardiovascular events. For the analysis of CVD outcomes, subjects with a history of CVD at baseline were excluded. The institutional review board at the Boston Medical Center approved the FOS.
We used the database (2014–2020) of CONPASS (Chongqing Primary Aldosteronism Study) to perform cross‐sectional analyses. 26 , 27 , 28 Participants of CONPASS were recruited from primary care and the largest tertiary referral center in Chongqing, China. The CONPASS recruited a wide range of patients, from those with newly diagnosed hypertension in primary care, 26 to those with resistant hypertension in the referral center with the objectives of evaluating the prevalence of primary aldosteronism and long‐term CVD outcomes in patients with primary aldosteronism and essential hypertension. 27 , 28 , 29 The CONPASS was approved by the ethical committee of Chongqing Medical University. Written informed consent was obtained from all study participants.
Physical Examination and Laboratory Measurement
Evaluations of physical examination, anthropometry, and other laboratory assessments were described in previous publications. 12 , 13 , 25 , 26 , 27 , 28 , 29 , 30 , 31 For FOS, plasma renin concentration (PRC) was measured with an immunochemiluminometric assay, and serum aldosterone concentration (SAC) was measured by radioimmunoassay. The antihypertensive medications were not withdrawn. In CONPASS, before the evaluation of renin and aldosterone concentration, all antihypertensive medications that can interfere with renin–angiotensin–aldosterone system activity were withdrawn. Hypokalemia was also corrected. Blood samples were collected in the morning. Plasma aldosterone concentration (PAC) and PRC were measured with automated chemiluminescence immunoassays. In the FOS, circulating renin and aldosterone measurement was performed in the sixth examination cycle, while MR activity was evaluated in ninth examination cycle. Detailed information is provided in Data S1.
Renin‐Independent and Renin‐Dependent Aldosteronism
In the FOS population, because no suppression test for aldosteronism was performed, renin‐independent and renin‐dependent aldosteronism was defined by SAC and PRC. Based on previous studies, circulating levels of aldosterone ≥10 ng∙dL−1 (1 ng/dL = 27 pmol/l) were deemed to be aldosteronism, 5 , 32 and PRC ≤15 mU/L was considered as low‐renin status. 7 Hence, in the FOS population, subjects with PRC ≤15 mU/L and SAC ≥10 ng∙dL−1 were considered to have renin‐independent aldosteronism, while subjects with PRC >15 mU/L and SAC ≥10 ng∙dL−1 were considered to have renin‐dependent aldosteronism. In the CONPASS population, aldosteronism was categorized by the PAC and the results of the CCT. Subjects with PAC <10 ng dL−1 before CCT were grouped as normal aldosterone. Subjects with PAC ≥10 ng dL−1 before and after the CCT were grouped as renin‐independent aldosteronism, while these who had PAC ≥10 ng dL−1 before CCT and PAC <10 ng dL−1 after CCT were grouped as renin‐dependent aldosteronism. Detailed methods on laboratory assessments are summarized in Data S2‐Data S4.
Assessment of Outcomes
In FOS, the primary outcome in this analysis is the incidence of CVD, which included coronary heart disease, congestive heart failure, stroke, or transient ischemic attack. In CONPASS, CVD was confirmed if there was a definite manifestation of coronary heart disease, stroke (including transient ischemic attack) or congestive heart failure. The diagnosis of CVD was based on evaluations by at least 2 senior physicians from the First Affiliated Hospital of Chongqing Medical University. Detailed information on the assessment of CVD is provided in Data S5.
Statistical Analysis
Normality was assessed using the Kolmogorov–Smirnov test for continuous variables. Data were presented as means and 95% CI for normal distributed variables, median (interquartile range) for skewed normal distributed variables, and percentages for categorical variables. One‐way ANOVA was conducted for the comparison of 3 groups, and least‐square difference was used for post hoc multiple comparisons. Chi‐square test was used to analyze the categorical data. All analyses were done using SPSS version 22.0 and Stata version 15.
In FOS, analyses were individually performed in the normotensive and hypertensive populations. Restricted cubic spline regression analyses were used to explore the relationship between continuous variables and CVD. The cubic spline curves were delineated on the basis of 4 equally spaced knots at 25th, 50th, 75th, and 95th percentiles. Estimated hazard ratios (HRs) with 95% CI were graphically represented. We used Cox proportional hazards regression to explore combined effects of different aldosteronism and blood pressure categories on the risk of CVD. We used multivariate models for controlling potential confounders, including age, sex, body mass index, systolic blood pressure, current smoking status, alcohol consumption, total cholesterol, presence or absence of diabetes, and sodium intake.
In CONPASS, we used restricted cubic spline regression analyses to explore the relationship between aldosterone production, MR activity, and CVD. Multivariable logistic‐regression models were used to compute the associations among different forms of aldosteronism, MR activity, and CVD, in the whole group and matched subgroup. Estimated odds ratios (OR) with 95% CI are graphically represented. We conducted multiple linear regression analysis with MR activity as the dependent variable and aldosterone and other metabolic parameters as independent variables, respectively. Potential confounders enrolled in multivariable adjustments were the same as in the FOS.
Results
Demographic and Biochemical Characteristics of Included Participants
The flow chart for the FOS and CONPASS cohorts is summarized in Figure 1. There were 1476 participants with nonhypertension and 1433 with hypertension included in FOS at baseline. The primary care and referral center cohorts from CONPASS consisted of 1020 and 1592 hypertensive participants, respectively. Characteristics of the study participants from FOS (baseline) and CONPASS are shown in Table 1. We also described baseline characteristics of FOS subjects who were classified as normal aldosterone, renin‐dependent aldosteronism, and renin‐independent aldosteronism in Table S1.
Figure 1. Study flow chart for FOS and CONPASS cohort.
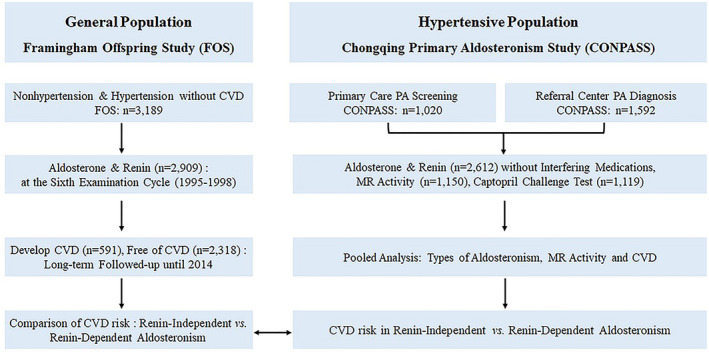
The current study includes a prospective analysis of Framingham Offspring Study FOS (Framingham Offspring Study) and post hoc analyses of CONPASS (Chongqing Primary Aldosteronism Study). In FOS, participants who measured aldosterone and renin did not withdraw interfering medications, and no confirmatory test was performed to explore the cause of aldosteronism. In CONPASS, participants from primary care and referral center were included, and all participants were asked to withdraw interfering medications and correct the electrolyte imbalance (if comorbid) for screening. Participants with positive screening received confirmatory tests to demonstrate the cause of aldosteronism. CVD indicates cardiovascular diseases; PA, primary aldosteronism; and MR, mineralocorticoid receptor.
Table 1.
Demographic, Clinical, and Biochemical Characteristics of Participants from FOS and CONPASS
| Participants from FOS | Participants from CONPASS | P value for hypertension ‡ | ||
|---|---|---|---|---|
| Nonhypertension | Hypertension | Hypertension | ||
| Total number | 1476 | 1433 | 2612 | … |
| Median age, y | 54 (48, 61) | 61 (54, 68) | 50 (40, 59) | <0.001 |
| Women, % | 894 (61) | 712 (50) | 1295 (50) | 0.974 |
| Body mass index, kg/m2 | 26 (23, 29) | 28 (25, 32) | 25 (23, 27) | <0.001 |
| Average SBP, mm Hg | 117 (109, 125) | 138 (127, 149) | 151 (140, 163) | <0.001 |
| Average DBP, mm Hg | 72 (67, 78) | 80 (72, 85) | 93 (84, 101) | <0.001 |
| History of diabetes, % | 39 (3) | 208 (15) | 313 (12) | 0.024 |
| Current smoker, no., % | 252 (17) | 180 (13) | 634 (24) | <0.001 |
| Alcohol user, no., % | 1032 (70) | 704 (49) | 783 (30) | <0.001 |
| Triglyceride, mg/dL | 101 (71, 148.75) | 129 (93, 184) | 130 (89, 193) | 0.032 |
| HDL‐c, mg/dL | 52 (42, 63) | 47 (39, 59) | 48 (39, 57) | 0.078 |
| LDL‐c, mg/dL | 126 (102, 148) | 126 (107, 148) | 111 (89, 134) | <0.001 |
| FPG, mg/dL | 94 (88, 101) | 100 (93, 111) | 97 (88, 110) | <0.001 |
| Aldosterone concentration, ng/dL* | 10 (7, 14) | 10 (7, 15) | 12 (8, 20) | <0.001 |
| Plasma renin concentration, mIU/L † | 13 (8, 20) | 12 (6, 24) | 9 (3, 22) | <0.001 |
Data are expressed as median (interquartile range) and number (%). CONPASS indicates Chongqing Primary Aldosteronism Study; DBP, diastolic blood pressure; FOS, the Framingham Offspring Study; FPG, fasting plasma glucose; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
Serum aldosterone concentration was measured by radioimmunoassay in FOS; plasma aldosterone concentration was measured with automated chemiluminescence immunoassays in CONPASS.
Plasma renin concentration was measured with an immunochemiluminometric assay in FOS, and with automated chemiluminescence immunoassays in CONPASS. For circulating aldosterone concentration, 1 ng/dL = 27 pmol/L.
P value for hypertension: comparison between hypertensive participants from FOS and CONPASS.
Aldosterone, Renin, and CVD Risk
Among 2909 participants from FOS who were free of CVD at baseline, 591 subjects developed CVD during a median of 18 years of follow‐up. In the nonhypertension group, neither PRC nor SAC was associated with the risk of CVD. Among participants with hypertension, the risk of CVD was significantly increased with an increasing SAC (HR, 1.11 [95% CI, 1.02–1.21]), but not with an increasing PRC (HR, 0.97 [95% CI, 0.83–1.13]) (Table 2). Relationships between circulating concentrations of aldosterone and renin at baseline and long‐term risk of CVD are further delineated in Figure S1. Among 2612 hypertensive participants from CONPASS, 160 participants (6.1%) had a history of CVD. The risk of CVD was similarly increased with increasing aldosterone (Figure S2A), but only in those with PRC <15 mU/L (Figure S2C) (Table 2).
Table 2.
Different Phenotypes of Aldosterone and Renin at Baseline and Long‐Term Risk of Cardiovascular Diseases, Among Participants from FOS and Hypertensive Participants from CONPASS
| Nonhypertensive participants | Hypertensive participants | |||
|---|---|---|---|---|
| Incident/total | HR (95% CI ) | Incident/total | HR/OR (95% CI) † | |
| Continuous increment of aldosterone and renin in FOS | ||||
| 1‐SD increment of PRC | 193/1476 | 1.00 (0.90, 1.12) | 398/1433 | 0.97 (0.83, 1.13) |
| 1‐SD increment of SAC | 0.90 (0.76, 1.07) | 1.11 (1.02, 1.21) ‡ | ||
| Continuous increment of aldosterone, by renin phenotype in FOS | ||||
| 1‐SD increment of SAC when PRC >15 mIU/L | 61/544 | 0.84 (0.61, 1.17) | 141/562 | 1.09 (0.98, 1.23) |
| 1‐SD increment of SAC when PRC ≤15 mIU/L | 132/932 | 0.97 (0.74, 1.26) | 257/871 | 1.19 (1.02, 1.39) ‡ |
| Categories of aldosterone and renin phenotype in FOS* | ||||
| SAC <10 ng/dL | 105/715 | Reference | 161/644 | Reference |
| SAC ≥10 ng/dL and PRC >15 mIU/L | 32/357 | 0.86 (0.61, 1.23) | 93/357 | 1.19 (0.89, 1.60) |
| SAC≥10 ng/dL and PRC ≤15 mIU/L | 56/404 | 1.16 (0.86, 1.56) | 144/432 | 1.40 (1.08, 1.82) ‡ |
| Continuous increment of aldosterone and renin in CONPASS | ||||
| 1‐SD increment of PRC | … | … | 160/2612 | 1.01 (0.88, 1.18) |
| 1‐SD increment of PAC | … | 1.12 (1.01, 1.27) ‡ | ||
| Continuous increment of aldosterone, by renin phenotype in CONPASS | ||||
| 1‐SD increment of SAC when PRC >15 mIU/L | … | … | 51/1002 | 1.04 (0.78, 1.54) |
| 1‐SD increment of SAC when PRC ≤15 mIU/L | … | … | 109/1610 | 1.28 (1.10, 1.46) ‡ |
| Categories of aldosterone and renin phenotype in CONPASS* | ||||
| PAC<10 ng/dL | … | … | 58/1080 | Reference |
| PAC ≥10 ng/dL and PRC >15 mIU/L | … | … | 28/568 | 1.43 (0.88, 2.32) |
| PAC ≥10 ng/dL and PRC ≤15 mIU/L | … | … | 74/964 | 1.59 (1.10, 2.31) ‡ |
| Categories of aldosteronism in CONPASS § | ||||
| Normal aldosterone: PAC <10 ng/dL | … | … | 58/1080 | Reference |
| Confirmed renin‐dependent aldosteronism by CCT | … | … | 24/575 | 1.78 (0.72, 4.41) |
| Confirmed renin‐independent aldosteronism by CCT | … | … | 78/957 | 2.57 (1.13, 5.86) ‡ |
All of these effects are based on the multivariate model, which adjusted for age, sex, body mass index, systolic blood pressure, current smoking status, alcohol consumption, total cholesterol, presence or absence of diabetes, antihypertensive medication use, and sodium status. CCT indicates captopril challenge test; CONPASS, Chongqing Primary Aldosteronism Study; FOS, Framingham Offspring Study; HR, hazard ratio; OR, odds ratio; PAC, plasma aldosterone concentration (ng/dL); PRC, plasma renin concentration (mIU/L); and SAC, serum aldosterone concentration (ng/dL). For SAC, 1 ng/dL = 27 pmol/L.
SAC ≥10 ng dL−1 was suspected as aldosteronism. PRC ≤15 mU/L was considered as low‐renin status. Subjects with PRC ≤15 mU/L and SAC ≥10 ng dL−1 were considered as renin‐independent aldosteronism, and subjects with PRC >15 mU/L and SAC ≥10 ng dL−1 were considered as renin‐dependent aldosteronism.
HR/OR (95% CI): HR (95% CI) for FOS, OR (95% CI) for CONPASS.
P value was <0.05.
PAC <10 ng dL−1 was considered as normal aldosterone. Subjects with PAC ≥10 ng dL−1 at screening and unsuppressed aldosterone secretion in the captopril challenge test (defined as PAC ≥10 ng dL−1 after the test) were confirmed as renin‐independent aldosteronism, while subjects with PAC ≥10 ng dL−1 and suppressed aldosterone secretion in CCT (defined as PAC <10 ng dL−1 after the test) were confirmed as renin‐dependent aldosteronism.
Risk of CVD in Renin‐Independent and Renin‐Dependent Aldosteronism
In hypertensive participants of FOS, the risk of CVD was significantly increased with an increasing SAC in the setting of PRC ≤15 mIU/L (HR, 1.19 [95% CI, 1.02–1.39]), but not in the subset with PRC >15 mIU/L (HR, 1.09 [95% CI, 0.98–1.23]) (Table 2, Figure S3). Hypertensive participants classified as having renin‐independent aldosteronism showed a higher risk of CVD when compared with these with normal‐aldosterone levels, namely, SAC <10 ng dL−1 (HR, 1.40 [95% CI, 1.08–1.82]), while participants who were classified as renin‐dependent aldosteronism did not show any increased risk of CVD (HR, 1.19 [95% CI, 0.89–1.60]) (Figure 2, Table 2).
Figure 2. Categories of aldosterone and renin phenotype at baseline and long‐term risk of cardiovascular diseases, according to classifications of blood pressure in the whole group of FOS (Framingham Offspring Study).
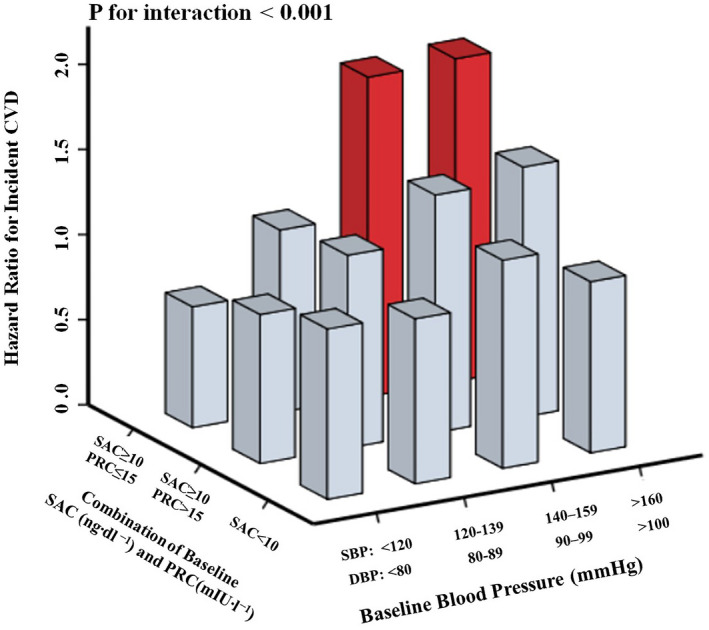
Serum aldosterone concentration (SAC) ≥10 ng dL−1 was suspected as aldosteronism; plasma renin concentration (PRC) ≤15 mU/L was considered as low‐renin status; subjects with PRC ≤15 mU/L and SAC ≥10 ng dL−1 were classified as renin‐independent aldosteronism, and subjects with PRC >15 mU/L and SAC ≥10 ng dL−1 were classified as renin‐dependent aldosteronism. Baseline blood pressure was stratified on the basis of definitions for normotension, prehypertension, and stages 1–3 of hypertension. For SAC, 1 ng/dL = 27 pmol/L. All of these effects are based on the multivariate model, which adjusted for age, sex, body mass index, current smoking status, alcohol consumption, total cholesterol, presence or absence of diabetes, antihypertensive medication use, and sodium status. Columns colored in red indicate that the false discovery rate is <0.05. CVD indicates cardiovascular disease; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
In CONPASS, when compared with participants with normal aldosterone (PAC <10 ng dL−1), the increased risk of CVD was observed in participants who had PRC ≤15 mU/L and PAC ≥10 ng dL−1 (OR, 1.59 [95% CI, 1.10–2.31]) or CCT‐confirmed renin‐independent aldosteronism (OR, 2.57 [95% CI, 1.13–5.86]), but not among participants who had PRC >15 mU/L and PAC≥10 ng dL−1 (OR, 1.43 [95% CI, 0.88–2.32]) or CCT‐confirmed renin‐dependent aldosteronism (OR, 1.78 [95% CI, 0.72–4.41]) (Table 2, Figure 3A through 3B).
Figure 3. Different types of aldosteronism and risk of cardiovascular diseases (CVD) or mineralocorticoid receptor (MR) activity, among hypertensive participants from CONPASS.
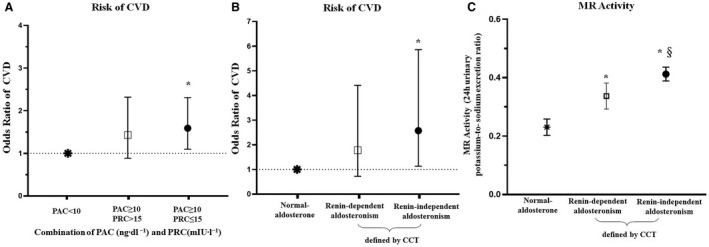
For PAC, 1 ng/dL = 27 pmol/L. A, PAC <10 ng dL−1 was classified as normal aldosterone; PAC ≥10 ng dL−1 was classified as aldosteronism; PRC ≤15 mU/L was classified as low‐renin status. Subjects with PRC ≤15 mU/L and PAC ≥10 ng dL−1 were classified as renin‐independent aldosteronism, and subjects with PRC >15 mU/L and PAC ≥10 ng dL−1 were classified as renin‐dependent aldosteronism. B, Shows the odd ratios and 95% CIs of CVD for renin‐dependent or renin‐independent aldosteronism as determined by the captopril challenge test (CCT). PAC <10 ng dL−1 was considered as normal aldosterone. Subjects with PAC ≥10 ng dL−1 at screening and unsuppressed aldosterone secretion in the CCT (defined as PAC ≥10 ng dL−1 after the test) were confirmed as renin‐independent aldosteronism, while subjects with PAC ≥10 ng dL−1 and suppressed aldosterone secretion in CCT (defined as PAC <10 ng dL−1 after the test) were confirmed as renin‐dependent aldosteronism. All of these effects were based on the multivariate model, which adjusted for age, sex, body mass index, systolic blood pressure, current smoking status, alcohol consumption, total cholesterol, presence or absence of diabetes, antihypertensive medication use, and sodium status. C, Further comparison of the MR activity across 3 groups in the CONPASS (Chongqing Primary Aldosteronism Study). PAC indicates plasma aldosterone concentration; and PRC, plasma renin concentration. *P value <0.05 when compared with subjects with normal aldosterone, §P value <0.05 when compared with subjects with renin‐dependent aldosteronism. P value <0.05 was considered as significantly different.
Estimated MR Activity in Renin‐Independent and Renin‐Dependent Aldosteronism
Relationships among estimated MR activity (24‐h urinary potassium‐to‐sodium excretion ratio), different subtypes of aldosteronism, and CVD risk are provided in Figure S4. In the CONPASS cohort, participants who had renin‐independent aldosteronism showed a higher 24‐hour urinary potassium‐to‐sodium excretion ratio than participants with renin‐dependent aldosteronism (0.41±0.28 versus 0.33±0.31, P=0.001) or normal aldosterone (0.41±0.28 versus 0.23±0.11, P<0.001) (Figure 3C).
Discussion
Using longitudinal data from FOS and cross‐sectional data from CONPASS, we demonstrated that a higher level of aldosterone was associated with a higher incidence of CVD in hypertensive subjects but not in normotensive subjects. Importantly, hypertensive patients with renin‐independent aldosteronism carried a higher risk of CVD than those with renin‐dependent aldosteronism or normal‐aldosterone levels. Moreover, a persistently increased estimated MR activity, as reflected by the 24‐hour urinary potassium‐to‐sodium excretion ratio, may account for the increased CVD risk.
Our results extend the earlier observations on the relationship between aldosterone and CVD by differentiating the population with hypertension from those with normal blood pressure at baseline. Several previous studies demonstrated an association between aldosterone and CVD. The Jackson Heart Study, 14 Chronic Renal Insufficiency Cohort (CRIC), 21 and Ludwigshafen Risk and Cardiovascular Health (LURIC) 18 confirmed the potential detrimental effects of aldosterone on major adverse cardiovascular events. However, these studies did not evaluate the risk separately in normotensive and hypertensive patients. Our study identified that the risk of CVD was significantly higher with increasing aldosterone only in the group of patients with hypertension at baseline.
The excess secretion of aldosterone, also known as aldosteronism, is often classified into renin‐independent and renin‐dependent. 5 , 33 Physiologically, renin‐dependent aldosterone secretion is activated in response to intravascular volume depletion such as hypovolemia and renal hypoperfusion, while renin‐independent aldosterone secretion is secondary to extracellular hyperkalemia. 5 Pathologically, renin‐dependent aldosteronism (also recognized as renin–angiotensin–aldosterone system activation), characterized by high levels of renin and aldosterone, was associated with metabolic disorders and vascular injuries. 5 , 34 In contrast, renin‐independent aldosteronism, defined as increased aldosterone production with a suppressed renin level, ranges from subtle autonomous aldosterone secretion to overt aldosteronism caused by bilateral adrenal hyperplasia or aldosterone‐producing adenomas. 5
It is widely explored that renin‐independent aldosteronism is associated with an increased risk for hypertension, 3 , 25 , 35 which is an important risk factor of CVD. However, we are unaware of previous studies that directly compare the associations of renin‐dependent and renin‐independent aldosteronism with the risk of CVD, although they were individually evaluated in observational and interventional studies among varied populations. In the general population of 883 Japanese, an increased risk of stroke incidence was observed with each 1‐SD increase in the aldosterone‐to‐renin ratio, which is typically elevated in renin‐independent aldosteronism. 36 In another cohort of 125 patients with hypertension, a high level of aldosterone‐to‐renin ratio carried a 2.7‐fold higher risk of CVD than a low aldosterone‐to‐renin ratio. 20 However, these studies had relatively limited sample size and did not segregate by aldosterone or renin level to compare the effects of renin‐dependent and renin‐independent aldosteronism on CVD risk. Beyond these observational studies, results from double‐blind randomized clinical trials offer clues to the cardiovascular effects of renin‐dependent and renin‐independent aldosteronism: aliskiren, a direct renin inhibitor, was not associated with improved cardiovascular outcomes 37 , 38 , 39 , 40 ; in contrast, MR antagonists, including eplerenone and finerenone, have been shown to be associated with lower risk of CVD. 41 , 42 , 43 Although these randomized clinical trials were not performed in patients with hypertension, the observational evidence and indirect comparisons of renin inhibitor versus MR antagonists suggest that renin‐independent aldosteronism exerts more detrimental cardiovascular effects than renin‐dependent aldosteronism. In the current analyses of FOS, among hypertensive subjects, renin‐independent aldosteronism was associated with a 1.4‐fold higher CVD risk compared with normal aldosterone, while renin‐dependent aldosteronism was not associated with an increased risk of incident CVD. Moreover, these findings were consistent with the cross‐sectional data from CONPASS, in which renin‐independent aldosteronism was confirmed using the CCT without the interference of medications and hypokalemia.
One explanation for renin‐independent aldosteronism promoting CVD is the enhanced aldosterone‐induced MR activity. Among normotensive persons from the MESA study, higher aldosterone concentrations were associated with increased estimated MR activity only when plasma renin activity was suppressed to ≤0.50 μg/L per hour. 3 Our cross‐sectional analysis of CONPASS, when adjusted for confounding factors in a multiple regression analysis, indicated that a 1‐unit increment in PAC was associated with a significantly higher level of MR activity in renin‐independent aldosteronism, but not renin‐dependent aldosteronism. Moreover, in CONPASS, we observed an increased OR of CVD with a higher MR activity (Figure S4A), and only hypertensive subjects with combined renin‐independent aldosteronism and high MR activity exhibited significantly higher risk of CVD (Figure S4B). Another explanation for the relationship between renin‐independent aldosteronism and CVD is that low renin is a sensitive marker of MR activity. In patients with primary aldosteronism treated with MR antagonists, those who had a plasma renin activity ≥1 μg/L per hour did not exhibit an increased CVD risk, while patients with a suppressed plasma renin activity (<1 μg/L per hour) showed a 2.8‐fold higher CVD risk than essential hypertension. 44 A low renin in the context of an inappropriate or elevated aldosterone is therefore reflective of the magnitude of MR activity and predictive of incident cardiovascular events over time.
The main strengths of the current study included the large sample size, long‐term follow‐up data from the FOS cohort, detailed evaluation of renin–angiotensin–aldosterone system activity without interfering factors in the CONPASS cohort, and confirmation of the study outcomes in 2 independent populations. There are several limitations of the current study. First, confirmatory tests for renin‐independent aldosteronism were not performed in FOS and confounders such as interfering medications and electrolyte imbalance could not be eliminated. However, in CONPASS, these confounders were controlled and the final outcomes were the same. Secondly, presence or absence of renin‐independent aldosteronism in the CONPASS cohort was determined by the CCT, which is not considered the criterion standard for the diagnosis of primary aldosteronism. However, it has previously been shown to be a robust confirmatory test in the Chinese population, 27 and recommended by the European Society of Hypertension. 45 Thirdly, MR activity was not measured at baseline in FOS, and to evaluate the short‐term impact of aldosterone and renin on MR activity, cross‐sectional data from CONPASS was used. Fourthly, the FOS and CONPASS cohorts are inherently different because of their ethnicity, geographic location, time of data collection, and the different measurement methods of renin and aldosterone. To obtain the same outcomes in both cohorts is therefore significant, although the findings should be tested prospectively in other populations where multiple measurements of aldosterone and renin are performed and potential confounders can be fully controlled. Finally, we did not observe a relationship between renin‐independent aldosteronism and CVD incidence among nonhypertensive participants. Given that nonhypertensive participants with low renin are more likely to develop hypertension over time which then predisposes them to CVD, 3 , 35 a longer follow‐up duration and larger sample size may be needed to demonstrate cardiovascular events in these participants.
In conclusion, data from FOS and CONPASS jointly suggested that among patients with hypertension, renin‐independent aldosteronism is more closely associated with CVD risk than renin‐dependent aldosteronism, likely because of a sustained increase in MR activity. Given the wide availability of MR antagonists, the timely detection of renin‐independent aldosteronism would lead to active intervention. Given the concordance of the results from FOS and CONPASS, our study emphasizes the need for the timely evaluation of renin and aldosterone in all hypertensive patients so as to offer a more precise measure of cardiovascular risk and enable selection of targeted therapy to achieve optimal cardiovascular outcomes.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (81670785, 81800701, 81870567, 81800731, and 81970720). Joint Medical Research Project of Chongqing Science and Technology Commission & Chongqing Health and Family Planning Commission (Youth Project, 2018QNXM001). Outstanding Talents of the First Affiliated Hospital of Chongqing Medical University 2019 (2019‐4‐22). Chongqing Outstanding Youth Funds (cstc2019jcyjjq0006). Bethune Merck Diabetes Research Foundation (G2018030).
Disclosures
None.
Supporting information
Data S1–S5
Table S1
Figures S1–S4
Acknowledgments
We thank the participants and staff of the Framingham Offspring Study. We also thank Laboratory of Endocrine and Laboratory of Lipid and Glucose Metabolism, as well as the First Affiliated Hospital of Chongqing Medical University. Membership of the Chongqing Primary Aldosteronism Study (CONPASS) Group: Qifu Li, MD, PhD, Ying Song, MD, Shumin Yang, MD, PhD, Wenwen He, MD, Mei Mei, MD, PhD, Jinbo Hu, PhD, Suxin Luo, MD, PhD, Kangla Liao, MD, Yao Zhang, MD, PhD, Yunfeng He, MD, PhD, Yihong He, MD, Ming Xiao, PhD, and Bin Peng, PhD. Author Contributions: Conception and design: Q.F. Li, S.M. Yang, Y. Song, J. Cai, and J.B. Hu; Analysis and interpretation of the data: J.B. Hu, H. Shen, and P.Q. Huo; Drafting of the article: J.B. Hu, H. Shen, and P.Q. Huo, P. Fuller, Yang, J.B. Hu, S.M. Yang and J. Cai; Critical revision of the article for important intellectual content: P. Fuller, J. Yang, J. Cai, Q.F. Li, and S.M. Yang; Statistical expertise: J.B. Hu, K.R. Wang, and Y. Yang; Obtaining of funding: Q.F. Li, S.M. Yang, Y. Song, and J.B. Hu; Administrative, technical, or logistic support: R. Luo, Y. Song, T. Luo, and W.W. He; Collection and assembly of data: W.W. He, Z.P. Feng, Q.F. Cheng, Z.P. Du, M. Mei, and L.Q. Ma. All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023082
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Ying Song, Email: shuiyunying@126.com.
Shumin Yang, Email: 443068494@qq.com.
References
- 1. Buonafine M, Bonnard B, Jaisser F. Mineralocorticoid receptor and cardiovascular disease. Am J Hypertens. 2018;31:1165–1174. [DOI] [PubMed] [Google Scholar]
- 2. Ferrario CM, Schiffrin EL. Role of mineralocorticoid receptor antagonists in cardiovascular disease. Circ Res. 2015;116:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JM, Robinson‐Cohen C, Luque‐Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baudrand R, Guarda FJ, Fardella C, Hundemer G, Brown J, Williams G, Vaidya A. Continuum of renin‐independent aldosteronism in normotension. Hypertension. 2017;69:950–956. doi: 10.1161/HYPERTENSIONAHA.116.08952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39:1057–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96:1327–1384. [DOI] [PubMed] [Google Scholar]
- 7. Buffolo F, Monticone S, Pecori A, Pieroni J, Losano I, Cavaglia G, Tetti M, Veglio F, Mulatero P. The spectrum of low‐renin hypertension. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101399. doi: 10.1016/j.beem.2020.101399 [DOI] [PubMed] [Google Scholar]
- 8. Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin‐angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6 [DOI] [PubMed] [Google Scholar]
- 10. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Investigators C, et al. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the charm‐preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 11. Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. [DOI] [PubMed] [Google Scholar]
- 12. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 13. Parikh NI, Gona P, Larson MG, Wang TJ, Newton‐Cheh C, Levy D, Benjamin EJ, Kannel WB, Vasan RS. Plasma renin and risk of cardiovascular disease and mortality: the Framingham Heart Study. Eur Heart J. 2007;28:2644–2652. doi: 10.1093/eurheartj/ehm399 [DOI] [PubMed] [Google Scholar]
- 14. Joseph JJ, Echouffo‐Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, Casanova R, Sims M, Wu WC, Wand GS, et al. Aldosterone, renin, cardiovascular events, and all‐cause mortality among African Americans: the Jackson heart study. JACC. Heart Failure. 2017;5:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, Yusuf S, Lonn EM. Plasma renin activity predicts cardiovascular mortality in the heart outcomes prevention evaluation (hope) study. Eur Heart J. 2011;32:2135–2142. doi: 10.1093/eurheartj/ehr066 [DOI] [PubMed] [Google Scholar]
- 16. Tomaschitz A, Pilz S, Ritz E, Morganti A, Grammer T, Amrein K, Boehm BO, März W. Associations of plasma renin with 10‐year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J. 2011;32:2642–2649. doi: 10.1093/eurheartj/ehr150 [DOI] [PubMed] [Google Scholar]
- 17. Yuyun MF, Jutla SK, Quinn PA, Ng LL. Aldosterone predicts major adverse cardiovascular events in patients with acute myocardial infarction. Heart Asia. 2012;4:102–107. doi: 10.1136/heartasia-2012-010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the ludwigshafen risk and cardiovascular health (luric) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019 [DOI] [PubMed] [Google Scholar]
- 19. Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated gfr: the ludwigshafen risk and cardiovascular health (luric) study. Am J Kidney Dis. 2011;57:403–414. doi: 10.1053/j.ajkd.2010.10.047 [DOI] [PubMed] [Google Scholar]
- 20. Kisaka T, Ozono R, Ishida T, Higashi Y, Oshima T, Kihara Y. Association of elevated plasma aldosterone‐to‐renin ratio with future cardiovascular events in patients with essential hypertension. J Hypertens. 2012;30:2322–2330. doi: 10.1097/HJH.0b013e328359862d [DOI] [PubMed] [Google Scholar]
- 21. Deo R, Yang W, Khan AM, Bansal N, Zhang X, Leonard MB, Keane MG, Soliman EZ, Steigerwalt S, Townsend RR, et al. Serum aldosterone and death, end‐stage renal disease, and cardiovascular events in blacks and whites: findings from the chronic renal insufficiency cohort (cric) study. Hypertension. 2014;64:103–110. doi: 10.1161/HYPERTENSIONAHA.114.03311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meade TW, Cooper JA, Peart WS. Plasma renin activity and ischemic heart disease. N Engl J Med. 1993;329:616–619. [DOI] [PubMed] [Google Scholar]
- 23. Doyle AE, Jerums G, Johnston CI, Louis WJ. Plasma renin levels and vascular complications in hypertension. Br Med J. 1973;2:206–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi‐ethnic study of atherosclerosis. Hypertension. 2020;76:113–120. doi: 10.1161/HYPERTENSIONAHA.120.15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. [DOI] [PubMed] [Google Scholar]
- 26. Xu Z, Yang J, Hu J, Song Y, He W, Luo T, Cheng Q, Ma L, Luo R, Fuller PJ, et al. Chongqing primary aldosteronism study G. primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. 2020;75:1913–1922. [DOI] [PubMed] [Google Scholar]
- 27. Song Y, Yang S, He W, Hu J, Cheng Q, Wang Y, Luo T, Ma L, Zhen Q, Zhang S, et al. Chongqing primary aldosteronism study G. Confirmatory tests for the diagnosis of primary aldosteronism: a prospective diagnostic accuracy study. Hypertension. 2018;71:118–124. doi: 10.1161/HYPERTENSIONAHA.117.10197 [DOI] [PubMed] [Google Scholar]
- 28. Wang K, Hu J, Yang J, Song Y, Fuller PJ, Hashimura H, He W, Feng Z, Cheng Q, Du Z, et al. Development and validation of criteria for sparing confirmatory tests in diagnosing primary aldosteronism. J Clin Endocrinol Metab. 2020;105(7):e2449–e2456. doi: 10.1210/clinem/dgaa282 [DOI] [PubMed] [Google Scholar]
- 29. Yang YI, Williams TA, Song Y, Yang S, He W, Wang K, Cheng Q, Ma L, Luo T, Yang J, et al. Nomogram‐based preoperative score for predicting clinical outcome in unilateral primary aldosteronism. J Clin Endocrinol Metab. 2020;105:e4382–e4392. doi: 10.1210/clinem/dgaa634 [DOI] [PubMed] [Google Scholar]
- 30. Lin C, Yang J, Fuller PJ, Jing H, Song Y, He W, Du Z, Luo T, Cheng Q, Yang S, et al. Chongqing primary aldosteronism study G. A combination of captopril challenge test after saline infusion test improves diagnostic accuracy for primary aldosteronism. Clin Endocrinol (Oxf). 2020;92:131–137. [DOI] [PubMed] [Google Scholar]
- 31. Ma L, Song Y, Mei M, He W, Hu J, Cheng Q, Tang Z, Luo T, Wang Y, Zhen Q, et al. The chongqing primary aldosteronism study G. Age‐related cutoffs of plasma aldosterone/renin concentration for primary aldosteronism screening. Int. J. Endocrinol. 2018;2018:8647026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126–148. [DOI] [PubMed] [Google Scholar]
- 33. Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism. Circulation. 2018;138:823–835. doi: 10.1161/CIRCULATIONAHA.118.033597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jia G, Aroor AR, Hill MA, Sowers JR. Role of renin‐angiotensin‐aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension. 2018;72:537–548. doi: 10.1161/HYPERTENSIONAHA.118.11065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Newton‐Cheh C, Guo C‐Y, Gona P, Larson MG, Benjamin EJ, Wang TJ, Kathiresan S, O’Donnell CJ, Musone SL, Camargo AL, et al. Clinical and genetic correlates of aldosterone‐to‐renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49:846–856. doi: 10.1161/01.HYP.0000258554.87444.91 [DOI] [PubMed] [Google Scholar]
- 36. Satoh M, Kikuya M, Ohkubo T, Mori T, Metoki H, Hara A, Utsugi MT, Hashimoto T, Hirose T, Obara T, et al. Aldosterone‐to‐renin ratio as a predictor of stroke under conditions of high sodium intake: the ohasama study. Am J Hypertens. 2012;25:777–783. [DOI] [PubMed] [Google Scholar]
- 37. Solomon SD, Hee Shin S, Shah A, Skali H, Desai A, Kober L, Maggioni AP, Rouleau JL, Kelly RY, Hester A, et al. Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur Heart J. 2011;32:1227–1234. doi: 10.1093/eurheartj/ehq522 [DOI] [PubMed] [Google Scholar]
- 38. McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016;374:1521–1532. [DOI] [PubMed] [Google Scholar]
- 39. Nicholls SJ, Bakris GL, Kastelein JJP, Menon V, Williams B, Armbrecht J, Brunel P, Nicolaides M, Hsu A, Hu BO, et al. Effect of aliskiren on progression of coronary disease in patients with prehypertension: the aquarius randomized clinical trial. JAMA. 2013;310:1135–1144. doi: 10.1001/jama.2013.277169 [DOI] [PubMed] [Google Scholar]
- 40. Gheorghiade M, Böhm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the astronaut randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954 [DOI] [PubMed] [Google Scholar]
- 41. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 42. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.21073363 [Google Scholar]
- 43. Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Schloemer P, Tornus I, Joseph A, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540–552. doi: 10.1161/CIRCULATIONAHA.120.051898. doi: 10.1161/CIRCULATIONAHA.120.051898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro M‐C, Beuschlein F, Rossi GP, Nishikawa T, Morganti A, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38:1919–1928. doi: 10.1097/HJH.0000000000002510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S5
Table S1
Figures S1–S4
Data Availability Statement
Data are available to approved people through written agreements with the authors and the data partner.


