Abstract
Background
Mental health conditions (MHCs) are associated with poor outcomes in patients with atrial fibrillation. However, persistence of oral anticoagulation therapy in patients with atrial fibrillation and MHCs is unknown. We aimed to evaluate the effect of MHCs on the persistence of direct oral anticoagulant (DOAC) use in patients with atrial fibrillation based on a nationwide cohort.
Methods and Results
The nationwide registry‐based FinACAF (Finnish Anticoagulation in Atrial Fibrillation) cohort included 67 503 patients with incident atrial fibrillation and indication for permanent oral anticoagulation (CHA2DS2‐VASc score >1 in men and >2 in women) starting DOAC therapy between 2011 and 2018. MHCs of interest were depression, bipolar disorder, anxiety disorder, schizophrenia, and composite of any MHC. The main outcome was nonpersistence of DOAC use, defined as the first 120‐day period without DOAC purchases after drug initiation. The mean age of the patients was 75.3±8.9 years, 53.6% were women, and the prevalence of any MHC was 17.8%. Persistence after 1 year from DOAC initiation was 79.3% in patients without MHCs and 77.2% in patients with any MHC, and after 2 years were 64.4% and 60.6%, respectively (P<0.001). Higher incidence of nonpersistence to DOACs was observed in all MHC categories: adjusted subdistribution hazard ratios, 1.16 (95% CI, 1.11–1.21) for any MHC, 1.32 (95% CI, 1.22–1.42) for depression, 1.44 (95% CI, 1.15–1.80) for bipolar disorder, 1.25 (95% CI, 1.11–1.41) for anxiety disorder, and 1.30 (95% CI, 1.02–1.64) for schizophrenia. However, patients with only anxiety disorder without other MHCs were not at higher risk of nonpersistence.
Conclusions
MHCs are associated with nonpersistence of DOAC use.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04645537.
Keywords: atrial fibrillation, depression, direct oral anticoagulants, mental health conditions, persistence
Subject Categories: Atrial Fibrillation, Mental Health, Health Equity, Anticoagulants, Compliance/Adherence
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulant
- ICPC‐2
International Classification of Primary Care, Second Edition
- MHC
mental health condition
- OAT
oral anticoagulant therapy
- VKA
vitamin K antagonist
Clinical Perspective
What Is New?
This retrospective nationwide cohort study conducted in Finland is the first to demonstrate that mental health conditions are associated with poor persistence of stroke prevention with direct oral anticoagulant therapy in patients with atrial fibrillation.
What Are the Clinical Implications?
Clinicians should be aware of the higher risk of direct oral anticoagulant therapy discontinuation in patients with atrial fibrillation suffering from mental health conditions.
Systematic monitoring of direct oral anticoagulant therapy in patients with atrial fibrillation comorbid with mental disorders is needed.
Interventions aimed at improving medication persistence in patients with atrial fibrillation with mental health conditions are warranted.
Atrial fibrillation (AF) is the most common sustained arrhythmia affecting up to 3% of the population and is a leading cause of ischemic stroke. 1 , 2 However, oral anticoagulation therapy (OAT) can reduce the risk of stroke by two thirds. 3 Currently, direct oral anticoagulants (DOACs) are recommended as the first‐line treatment over vitamin K antagonists (VKAs). 4 , 5 Mental health conditions (MHCs) are common in patients with AF, with the prevalence of depression in previous studies as high as 12%. 6 The prevalence of anxiety disorder, bipolar disorder, and schizophrenia among patients with AF have ranged between 4% to 8%, 0.2% to 0.8%, and 0.2% to 0.4%, respectively. 6 , 7 , 8 MHCs have been associated with a lower rate of OAT initiation and poorer quality of VKA therapy as well as increased risk of ischemic stroke. 7 , 9 Persistence of DOAC use, that is, continuing the medication for the prescribed duration, is crucial for effective stroke prevention, and poor persistence to DOAC therapy in patients with AF has been associated with increased stroke risk. 10 Previous studies have suggested suboptimal medication persistence among patients with MHCs, but the persistence of DOAC use in patients with AF and MHCs is currently unknown. 11 , 12 , 13 The present nationwide cohort study aimed to evaluate the effect of MHCs on the persistence of DOAC use in patients with incident AF.
Methods
Data Availability
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Finnish national register holders (Social Insurance Instituite, Finnish Institute for Health and Welfare, Population Register Centre and Tax Register).
Study Population
The FinACAF (Finnish Anticoagulation in Atrial Fibrillation) study (Clinical Trials identifier: NCT04645537; European Network of Centres for Pharmacoepidemiology and Pharmacovigilance identifier: EUPAS29845) is a nationwide, retrospective registry‐based cohort study including all patients with an AF diagnosis in Finland from 2004 to 2018. Patients were identified from 3 national health care registers (hospitalizations and outpatient specialist visits register; primary health care register; and National Reimbursement Register upheld by Social Insurance Institute). The inclusion criterion for the cohort was an International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis code I48 (including AF and atrial flutter, together referred as AF) recorded between 2004 and 2018 and cohort entry occurred at the date of the first recorded AF diagnosis. The exclusion criteria were age <18 years at AF diagnosis and permanent migration abroad before December 31, 2018.
The present substudy focused on patients with incident AF recommended to receive permanent OAT and starting DOAC therapy from 2011 to 2018, when DOACs were approved for stroke prevention in patients with AF in Finland. Patients with a recorded AF diagnosis from 2004 to 2006 were excluded because 2 years of medical history was considered too short to exclude the presence of an AF diagnosis before cohort entry. In addition, patients who had fulfilled an OAT prescription from 2004 to 2006 or in the year preceding the first AF diagnosis were excluded because most of them likely had a previous diagnosis of AF. To include only patients recommended to receive permanent OAT across the observation period according to the contemporary guidelines, women with CHA2DS2‐VASc scores ≤2 and men with CHA2DS2‐VASc scores ≤1 were excluded. 14 , 15 Finally, patients not receiving DOAC therapy from 2011 to 2018 were excluded. The patient selection process is presented in Figure S1.
Exposure to Oral Anticoagulation
In the current substudy, the date of first DOAC (Anatomical Therapeutic Chemical codes B01AE08, dabigatran; B01AF01, rivaroxaban; B01AF02, apixaban; and B01AF03, edoxaban) purchase was the index date, and follow‐up continued until death or December 31, 2018, whichever occurred first. Nonpersistence of DOAC use, the main outcome in our study, was defined as the first >120‐day period without DOAC purchase. In Finland, it is possible to purchase drugs with reimbursement for a maximum of 90 days. Therefore, an additional grace period of 30 days was allowed in our study. The outcome was considered to occur at the end of the 120‐day period. The impact of varying the grace period to 15 or 45 days was additionally examined. Individuals switching to VKAs during the 120‐day period were censored. Patients switching from 1 DOAC to another during the 120‐day period were considered persistent.
Mental Health Conditions
MHCs of interest were depression, bipolar disorder, anxiety disorder, schizophrenia, and any MHC. These specific MHCs were chosen because of their high prevalence and burden in the aging population of patients with AF. 16 Patients were classified into diagnostic groups if they were recorded with the ICD‐10 diagnosis code or International Classification of Primary Care, Second Edition (ICPC‐2) entry of the condition before the index date as follows: depression (ICD‐10: F32, F33, F34.1; ICPC‐2: P76), anxiety disorder (ICD‐10: F40‐F42, F43.1; ICPC‐2: P74), bipolar disorder (ICD‐10: F31; ICPC‐2: P73), and schizophrenia (ICD‐10: F20; ICPC‐2: P72). Patients with >1 of these conditions were classified into each diagnostic category separately. This double counting was allowed because of the high prevalence of co‐occurring MHCs. Patients were classified to have any MHC if they had any of these 4 MHCs or had filled a prescription for an antidepressant, antipsychotic, or mood‐stabilizing medication within the year before the index date (Anatomical Therapeutic Chemical codes N05A, N05BE01, N06A). Medication data were not used to further classify patients to specific conditions. In addition, considering the possible bias resulting from double counting patients with >1 MHC to multiple categories and that these psychotropic medications are also prescribed for indications other than MHCs, sensitivity analyses were performed on patients with only a single diagnosed MHC, patients with >1 diagnosed MHC, and patients with any diagnosed MHC.
Study Ethics
The study protocol was approved by the Ethics Committee of the Medical Faculty of Helsinki University, Helsinki, Finland (number 15/2017) and granted research permission from the Helsinki University Hospital (HUS/46/2018). Respective permissions were obtained from the Finnish register holders (Social Insurance Institute 138/522/2018, Finnish Institute for Health and Welfare 2101/5.05.00/2018, Population Register Centre VRK/1291/2019‐3, and Tax Register VH/874/07.01.03/2019). Informed consent was waived because of the retrospective registry nature of the study. The study conforms to the Declaration of Helsinki as revised in 2002.
Statistical Analysis
Statistical analyses were performed with the IBM SPSS Statistics software (version 27.0; IBM, Inc., Armonk, NY) and R (version 4.0.5; https://www.R‐project.org). The χ2 test was used to compare differences between proportions, and the independent‐samples t test was used to analyze continuous variables. Poisson regression was used to determine the unadjusted and adjusted incidence rate ratios of nonpersistence separately for each MHC category. Incidence of nonpersistence event might be hindered by mortality occurring during the study period. Therefore, competing risk analyses with the Fine‐Gray subdistribution hazard model were performed to estimate the incidence of nonpersistence considering all‐cause mortality as a competing event. Unadjusted and adjusted subdistribution hazard ratios for the incidence of nonpersistence in patients with MHCs were calculated. In the Fine‐Gray subdistribution hazard and Poisson regression models, adjustments were made for age, sex, and calendar year of DOAC initiation and additionally for stroke and bleeding risk factors (heart failure, hypertension, diabetes, prior stroke, vascular disease, prior bleeding, alcohol abuse, renal failure, and liver cirrhosis or failure), dementia, income (highest annual income during follow‐up divided in quintiles), dosage of the first purchased DOAC (once or twice a day), and polypharmacy (>5 different medications during the year preceding DOAC initiation) because these have been shown to associate with DOAC persistence and adherence in previous studies. 17 , 18 , 19 , 20 The definitions of the comorbidities are presented in Table S1.
Results
Altogether, 67 503 patients (53.6% women) with incident AF initiating DOAC therapy were identified. The mean age was 77.3 years (SD, 8.3) in women and 73.1 years (SD, 9.0) in men. The overall prevalence of any MHC at index date was 17.8%. Patients with any MHC were more often women and had lower income and a higher prevalence of cardiovascular risk factors, dementia, and alcohol abuse than patients with no history of MHCs. The mean CHA2DS2‐VASc score was as high as 3.9 (SD, 1.4) in patients without MHCs and 4.2 (SD, 1.5) in patients with any MHC (P<0.001; Table 1).
Table 1.
Descriptive Characteristics of the Cohort According to the Presence of MHCs
| No MHC, n=55 454 | Any MHC, n=12 049 | Depression, n=3230 | Bipolar disorder, n=302 | Anxiety disorder, n=1341 | Schizophrenia, n=343 | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y, mean (SD) | 75.4 (8.7) | 75.0 (9.7)* | 73.3 (10.1)* | 69.0 (9.3)* | 73.1 (10.3)* | 70.4 (9.0)* |
| Female sex | 28 461 (51.3) | 7966 (63.9)* | 2089 (64.7)* | 159 (52.6) | 939 (70.0)* | 193 (56.3) |
| Income quintiles | * | * | * | * | * | |
| First (lowest) | 9743 (17.6) | 2744 (22.8) | 682 (21.1) | 85 (28.1) | 302 (22.5) | 166 (48.4) |
| Second | 10 843 (19.6) | 2698 (22.4) | 742 (23.5) | 74 (24.5) | 326 (24.3) | 95 (27.7) |
| Third | 11 335 (20.4) | 2588 (21.5) | 721 (22.3) | 55 (18.2) | 279 (20.8) | 52 (15.2) |
| Fourth | 11 759 (21.2) | 2222 (18.4) | 656 (20.3) | 48 (15.9) | 265 (19.8) | 17 (5.0) |
| Fifth (highest) | 11 774 (21.2) | 1797 (14.9) | 429 (13.3) | 40 (13.2) | 169 (12.6) | 13 (3.8) |
| Comorbidities and medications | ||||||
| Alcohol abuse | 1045 (1.9) | 926 (7.7)* | 410 (12.7)* | 72 (23.8)* | 159 (11.9)* | 33 (9.6)* |
| Dementia | 2929 (5.3) | 1531 (12.7)* | 416 (12.9)* | 27 (8.9)* | 130 (9.7)* | 34 (9.9)* |
| Diabetes | 15 275 (27.5) | 3906 (32.4)* | 1183 (36.6)* | 156 (51.7)* | 442 (33.0)* | 166 (48.4)* |
| Dyslipidemia | 34 045 (61.4) | 8024 (66.6)* | 2207 (68.3)* | 221 (73.2) | 906 (67.6)* | 185 (53.9)* |
| Heart failure | 7736 (14.0) | 2117 (17.6)* | 591 (18.3)* | 60 (19.9)* | 236 (17.6)* | 101 (29.4)* |
| Hypertension | 49 426 (89.1) | 12 049 (91.9)* | 2992 (92.6)* | 282 (93.4)* | 1238 (92.3)* | 298 (86.9) |
| Liver cirrhosis or failure | 185 (0.3) | 58 (0.5)* | 13 (0.4) | 3 (1.0) | 4 (0.3) | 3 (0.9) |
| CHA2DS2‐VASc score, mean (SD) | 3.9 (1.4) | 4.2 (1.5)* | 4.2 (1.5)* | 3.9 (1.4) | 4.2 (1.5)* | 3.8 (1.4) |
| Modified HAS‐BLED score, maximum score 7, mean (SD) | 2.2 (0.8) | 2.3 (0.9)* | 2.4 (0.9)* | 2.4 (1.0)* | 2.4 (0.9)* | 2.2 (0.9) |
| Prior bleeding | 6629 (12.0) | 1919 (15.9)* | 562 (17.4)* | 56 (18.5)* | 232 (17.3)* | 58 (16.9)* |
| Prior stroke | 9418 (17.0) | 2567 (21.3)* | 710 (22.0)* | 66 (21.9)* | 308 (23.0)* | 61 (17.8) |
| Polypharmacy (>5 drugs) | 39 835 (71.8) | 10 804 (89.7)* | 2880 (89.2)* | 282 (93.4)* | 1197 (89.3)* | 304 (88.6)* |
| Renal failure or dialysis | 928 (1.7) | 253 (2.1)* | 77 (2.4)* | 4 (1.3) | 35 (2.6)* | 6 (1.7) |
| Vascular disease | 15 937 (28.7) | 3806 (31.6)* | 1053 (32.6)* | 91 (30.1) | 396 (29.5) | 62 (18.1)* |
| VKA therapy before DOAC | 17 846 (32.2) | 4105 (34.1)* | 897 (27.8)* | 94 (31.1) | 384 (28.6)* | 86 (25.1)* |
Values are provided as number (percentage) unless otherwise noted. CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥75 years, diabetes, history of stroke or transient ischemic attack, vascular disease, age 65–74 years, sex category (female sex); DOAC, direct oral anticoagulant; HAS‐BLED, hypertension, abnormal renal or liver function, prior stroke, bleeding history, age >65 years, alcohol abuse (no labile international normalized ratio or concomitant antiplatelet/nonsteroidal anti‐inflammatory drugs use); MHC, mental health condition; and VKA, vitamin K antagonist.
P<0.05 when compared with patients without MHC.
Overall, 14 912 (22.1%) patients were nonpersistent to DOAC therapy during the mean follow‐up of 1.3 years (SD, 1.6). Persistence of DOAC use reduced substantially over time, particularly among patients with MHCs (Figures 1 and 2). Persistence at 1 and 2 years after DOAC initiation were significantly lower in patients with any MHC (77.2% and 60.6%, both P<0.001), depression (75.8% and 57.1%, both P<0.001), and bipolar disorder (72.6% and 53.5%, both P<0.05), but not in patients with anxiety disorder (76.5%, P=0.07; and 60.1%, P=0.08) and schizophrenia (76.2%, P=0.33; and 61.4%, P=0.60), when compared with patients without MHCs (79.3% and 64.4%). Of the nonpersistent patients, 10 357 (69.5%) restarted DOAC therapy later during follow‐up and 975 (5.4%) switched to VKAs after the 120‐day period without OAT purchases. Restarting OAT with either DOAC or VKA was less common in patients with any MHC than in those without MHCs (73.1% versus 76.2%, P<0.001).
Figure 1. Cumulative incidence function of direct oral anticoagulant therapy nonpersistence in patients with and without any mental health condition (MHC).
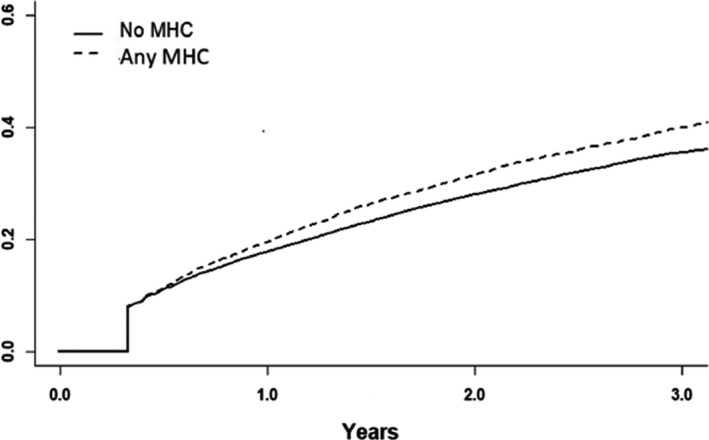
Figure 2. Cumulative incidence function of direct oral anticoagulant therapy nonpersistence in patients with depression, bipolar disorder, anxiety disorder, or schizophrenia vs patients without a mental health condition (MHC).
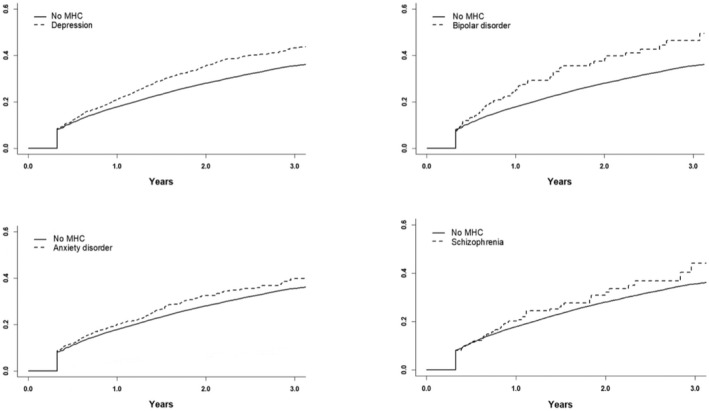
In the Poisson regression models, when compared with patients without MHCs, all MHC diagnostic groups were individually associated with higher incidences of nonpersistence before and after adjustment for covariates, although the difference in the unadjusted incidence did not reach statistical significance in patients with schizophrenia (Table 2). In the sensitivity analyses, wherein 105‐day and 135‐day periods without a DOAC purchase were used to define nonpersistence, similar results were observed (Table S2). Furthermore, when considering all‐cause death as a competing event in the Fine‐Gray subdistribution hazard model, all MHC groups presented with higher adjusted incidences of nonpersistence than patients without MHCs (Table 3).
Table 2.
Incidence of DOAC Nonpersistence According to the Presence of MHC
| Clinical condition | Events, n | Proportion of patients with events, % | Patient‐y | Incidence, per patient‐y (95% CI) | Unadjusted IRR (95% CI) | Adjusted IRR (95% CI) |
|---|---|---|---|---|---|---|
| No MHC | 12 039 | 21.7 | 68 872 | 0.18 (0.17–0.18) | Reference | Reference |
| Any MHC | 2 873 | 23.8 | 14 101 | 0.20 (0.20–0.21) | 1.17 (1.12–1.21) | 1.19 (1.14–1.24) |
| Depression | 804 | 24.9 | 3 620 | 0.22 (0.21–0.24) | 1.27 (1.18–1.37) | 1.35 (1.25–1.45) |
| Bipolar disorder | 84 | 27.8 | 325 | 0.26 (0.21–0.32) | 1.48 (1.20–1.84) | 1.54 (1.24–1.92) |
| Anxiety disorder | 305 | 22.7 | 1 541 | 0.20 (0.18–0.22) | 1.13 (1.01–1.27) | 1.26 (1.13–1.35) |
| Schizophrenia | 72 | 21.0 | 331 | 0.22 (0.17–0.27) | 1.25 (0.99–1.57) | 1.42 (1.12–1.79) |
Adjusted IRRs estimated by Poisson regression and adjusted for age, sex, calendar year, heart failure, hypertension, diabetes, prior stroke, vascular disease, prior bleeding, alcohol abuse, renal failure, liver cirrhosis or failure, dementia, income, DOAC dosage, and polypharmacy. DOAC indicates direct oral anticoagulant; IRR, incidence rate ratio; and MHC, mental health condition.
Table 3.
SHRs of Nonpersistence According to the Presence of MHC
| Clinical condition | Unadjusted SHR (95% CI) | Adjusted SHR (95% CI) |
|---|---|---|
| Any MHC | 1.13 (1.09–1.18) | 1.16 (1.11–1.21) |
| Depression | 1.24 (1.16–1.33) | 1.32 (1.22–1.42) |
| Bipolar disorder | 1.42 (1.15–1.75) | 1.44 (1.15–1.80) |
| Anxiety disorder | 1.13 (1.01–1.26) | 1.25 (1.11–1.41) |
| Schizophrenia | 1.10 (0.88–1.38) | 1.30 (1.02–1.64) |
SHRs estimated by Fine‐Gray subdistribution hazard regression with all‐cause death as competing event and adjusted for age, sex, calendar year, heart failure, hypertension, diabetes, prior stroke, vascular disease, prior bleeding, alcohol abuse, renal failure, liver cirrhosis or failure, dementia, income, direct oral anticoagulant dosage, and polypharmacy. MHC indicates mental health condition; and SHR, subdistribution hazard ratio.
Although the sensitivity analysis on patients with only a single specific MHC yielded otherwise similar results as the main analysis including also patients with >1 MHC, no significant difference in the incidence of nonpersistence was observed between patients with only anxiety disorder and those without MHCs. The rate of nonpersistence was higher in patients with >1 MHC and in those with any diagnosed MHC (excluding patients without diagnosed MHC) when compared with patients without MHCs (Table S3).
Discussion
In this nationwide cohort study, patients with AF and MHCs had lower persistence of DOAC use than patients without MHC. A composite of any MHC as well as depression, bipolar disorder, and schizophrenia individually were associated with 19% to 54% higher adjusted incidences of nonpersistence to DOACs, with the highest risk estimates emerging in patients with bipolar disorder. The proportion of persistent patients in the cohort reduced substantially over time, especially among patients with MHCs, and considering the high stroke risk scores of the cohort population, DOAC persistence appears alarmingly poor.
When analyzing all patients with AF with anxiety disorder, a 26% higher adjusted incidence of DOAC discontinuation was observed. However, interestingly, in the sensitivity analysis, patients suffering from only anxiety disorder without other MHCs were not at higher risk of therapy discontinuation than patients without MHCs. Therefore, other co‐occurring MHCs appear to explain largely the observed higher rate of DOAC nonpersistence in patients with AF suffering from anxiety disorder.
There are no prior studies investigating the association of MHCs and persistence of DOAC therapy in patients with AF. However, our results indicating a higher risk of discontinuation of DOAC therapy in patients with AF with MHCs are in accordance with previous findings of suboptimal drug adherence and persistence in treatment of other chronic conditions among patients with MHCs. 11 , 13 , 21 In addition, the observed 1‐year persistence rates of 77% to 79% in our study are concordant with a recent multinational study reporting an average 1‐year DOAC persistence rate of 82%. 22
Previous studies have indicated that patients with AF with MHCs have a higher risk of ischemic stroke than patients without MHCs. 9 Nonpersistence to DOACs has been shown to increase stroke risk in patients with AF, and our finding of higher nonpersistence to DOACs in patients with MHCs, in addition to the previously reported lower OAT initiation rate in this patient group, is likely 1 mechanism underlying the higher stroke risk. 9 , 10 Another concerning finding in our study—further decreasing the total OAT coverage among patients with MHCs—is that patients with MHCs are less likely to resume OAT, either with VKAs or DOACs, after discontinuing DOAC therapy.
The observed lower DOAC persistence in patients with MHCs is most likely multifactorial. First, poor socioeconomic conditions prevalent in this patient group may affect the use of DOAC therapy, which is relatively expensive compared with VKAs. 23 However, in our study, MHCs remained clearly associated with DOAC nonpersistence even after adjusting for income level. The social and cognitive difficulties sometimes associated with MHCs may influence communication between patients and health care professionals, possibly impairing patients’ understanding of the purpose and importance of OAT as well as its lifelong nature. 24 , 25 In addition, deficits in self‐care resources likely impair the commitment to continuous lifelong therapies in patients with MHCs. 23 Excessive alcohol consumption, which is more common among patients with MHCs than in those without MHCs (Table 1), has been associated with poor persistence to prescribed therapies. 23 Furthermore, fragmented care as a result of the separation of psychiatric and somatic health care services may impair OAT follow‐up, and medication discontinuation may therefore go unnoticed.
The frequent nonpersistence of DOAC therapy in patients with AF with MHCs highlights the need to improve the care of these challenging patients and explore the factors underlying the poor persistence. The EHRA (European Heart Rhythm Association) advocates regular follow‐ups to review persistence in DOAC users, and the importance of systematic monitoring of DOAC therapy is highlighted among patients suffering from MHCs. In addition, the EHRA recommends strategies, including adequate patient education and use of technical aids, to improve DOAC persistence, which could also help to tackle nonpersistence in patients with AF and MHCs. 26 Furthermore, improving collaboration between somatic and mental health services and increasing social support to this vulnerable patient group may be helpful in optimizing DOAC therapy.
The main strength of our study is the large sample size and comprehensive nationwide nature of the data. The FinACAF cohort includes all patients with AF in Finland gathered from all available national registries from all levels of care, including uniquely also primary care. These well‐validated registries have high diagnostic accuracy. 27 , 28 , 29 Use of DOACs is based on complete nationwide data of redeemed prescriptions and includes all DOAC purchases because DOACs are not sold over the counter without prescription.
The challenges inherent to real‐world retrospective registry studies are the main limitations of our study. In addition, a gold standard to define persistence is lacking, and there are numerous methods to measure persistence, which may influence the results considerably. 30 However, our aim was to evaluate the association of MHCs and persistence rather than to define the absolute persistence rates, and therefore our results are likely not critically influenced by the chosen methodology. Furthermore, the difference in DOAC persistence between patients with and without MHCs remained similar in the sensitivity analysis using 105‐day and 135‐day gaps as the nonpersistence outcome event. Information bias may be caused by inaccurate recording of psychiatric diagnoses as well as by the diagnostic accuracy of the ICD‐10 and ICPC‐2 codes. However, we attemped to reduce this bias by using the any MHC variable, which included also patients with purchases of drugs used to treat MHCs, albeit these medications are also marginally used for other indications. We lacked data on lifestyle‐related factors, except for diagnosed alcohol abuse disorders. In addition, except for the dementia diagnoses, we lacked data on the cognitive function status of the patients. Although we were able to adjust our findings for multiple covariates, residual confounding cannot be excluded. Finally, we lacked data on the actual reasons for therapy discontinuation.
In conclusion, the present nationwide cohort study is the first to demonstrate that MHCs are associated with poor persistence of DOAC therapy in patients with AF. However, patients suffering only from anxiety disorder without other MHCs were not at higher risk of therapy discontinuation. Our findings indicate a need for additional monitoring of DOAC therapy and interventions aimed at improving medication persistence in patients with AF and MHCs.
Sources of Funding
This work was supported by the Aarne Koskelo Foundation, The Finnish Foundation for Cardiovascular Research, and Helsinki and Uusimaa Hospital District research fund. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosures
Dr Putaala reports personal fees from Boehringer‐Ingelheim, Portola, Herantis Pharma, Terve Media, and Abbott; personal fees and other from Bayer; grants and personal fees from Bristol Myers Squibb‐Pfizer; and other from Amgen and Vital Signum outside the submitted work. Dr Mustonen reports consulting for Roche, Bristol Myers Squibb‐Pfizer Alliance, Novartis Finland, Boehringer Ingelheim, and Merck Sharp and Dohme Finland. Dr Haukka reports consulting for Research Janssen Research and Development and speaking at Bayer Finland. Dr Linna reports speaking at Bristol Myers Squibb‐Pfizer Alliance, Bayer, and Boehringer‐Ingelheim. Hartikainen reports research grants from The Finnish Foundation for Cardiovascular Research, European Union Horizon 2020, and European Union FP7; is an advisory board member of Bristol Myers Squibb‐Pfizer Alliance, Novo Nordisk, and Amgen; and speaking at Cardiome and Bayer. Dr Airaksinen reports research grants from The Finnish Foundation for Cardiovascular Research; speaking at Bayer, Pfizer, and Boehringer‐Ingelheim; and is a member of the Bayer, Pfizer, and AstraZeneca advisory boards. Lehto reports consulting for Bristol Myers Squibb‐Pfizer Alliance, Bayer, Boehringer‐Ingelheim, and Merck Sharp and Dohme; speaking at Bristol Myers Squibb‐Pfizer Alliance, Bayer, Boehringer Ingelheim, Merck Sharp and Dohme, Terve Media, and Orion Pharma; a nd received research grants from the Aarne Koskelo Foundation, The Finnish Foundation for Cardiovascular Research, and Helsinki and Uusimaa Hospital District research fund, and Boehringer‐Ingelheim. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
Author Contributions: Dr. Teppo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Teppo, Jaakkola, Putaala, Mustonen, Haukka, Airaksinen, and Lehto contributed to the concept and design. Teppo, Jaakkola, Airaksinen, Putaala, Mustonen, Haukka, Hartikainen, Luojus, Niemi, Linna, and Lehto contributed to the acquisition, analysis, or interpretation of data and the critical revision of the manuscript for important intellectual content. Teppo drafted the manuscript. Teppo and Jaakkola contributed to the statistical analysis. Lehto obtained funding. Jaakkola, Halminen, and Haukka contributed administrative, technical, or material support. Jaakkola, Putaala, Mustonen, Haukka, Airaksinen, and Lehto provided supervision.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024119
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population‐based study. Stroke. 2013;44:3103–3108. doi: 10.1161/STROKEAHA.113.002329 [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Ze F, Li J, Mi L, Han B, Niu H, Zhao N. Trends of global burden of atrial fibrillation/flutter from Global Burden of Disease Study 2017. Heart. 2021;107:881–887. doi: 10.1136/heartjnl-2020-317656 [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 4. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. The Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, ESC Scientific Document Group , et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 6. Fenger‐Grøn M, Vestergaard CH, Frost L, Davydow DS, Parner ET, Christensen B, Ribe AR. Depression and uptake of oral anticoagulation therapy in patients with atrial fibrillation: a Danish nationwide cohort study. Med Care. 2020;58:216–224. doi: 10.1097/MLR.0000000000001268 [DOI] [PubMed] [Google Scholar]
- 7. Fenger‐Grøn M, Vestergaard CH, Ribe AR, Johnsen SP, Frost L, Sandbæk A, Davydow DS. Association between bipolar disorder or schizophrenia and oral anticoagulation use in Danish adults with incident or prevalent atrial fibrillation. JAMA Network Open. 2021;4:e2110096. doi: 10.1001/jamanetworkopen.2021.10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Søgaard M, Skjøth F, Kjældgaard JN, Larsen TB, Hjortshøj SP, Riahi S. Atrial fibrillation in patients with severe mental disorders and the risk of stroke, fatal thromboembolic events and bleeding: a nationwide cohort study. BMJ Open. 2017;7. doi: 10.1136/bmjopen-2017-018209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teppo K, Jaakkola J, Lehto M, Biancari F, Airaksinen KEJ. The impact of mental health conditions on oral anticoagulation therapy and outcomes in patients with atrial fibrillation: a systematic review and meta‐analysis. Am J Prev Cardiol. 2021;7:100221. doi: 10.1016/j.ajpc.2021.100221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komen JJ, Heerdink ER, Klungel OH, Mantel‐Teeuwisse AK, Forslund T, Wettermark B, Hjemdahl P. Long‐term persistence and adherence with non‐vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur Heart J–Cardiovasc Pharmacother. 2021;7:f72–f80. doi: 10.1093/ehjcvp/pvaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holvast F, Wouters H, Hek K, Schellevis F, Oude Voshaar R, van Dijk L, Burger H, Verhaak P. Non‐adherence to cardiovascular drugs in older patients with depression: a population‐based cohort study. Int J Cardiol. 2019;274. doi: 10.1016/j.ijcard.2018.08.100 [DOI] [PubMed] [Google Scholar]
- 12. Springer SA, Dushaj A, Azar MM. The impact of DSM‐IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16. doi: 10.1007/s10461-012-0212-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panish J, Karve S, Candrilli SD, Dirani R. Association between adherence to and persistence with atypical antipsychotics and psychiatric relapse among US Medicaid‐enrolled patients with schizophrenia. J Pharm Health Serv Res. 2013;4:29–39. doi: 10.1111/jphs.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;2016:37. doi: 10.5603/KP.2016.0172 [DOI] [Google Scholar]
- 15. John Camm A, Lip GYH, de Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, et al. 2012 Focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33. doi: 10.1016/j.rec.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 16. GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowres N, Giskes K, Hespe C, Freedman B. Reducing stroke risk in atrial fibrillation: adherence to guidelines has improved, but patient persistence with anticoagulant therapy remains suboptimal. Korean Circ J. 2019;49:883. doi: 10.4070/kcj.2019.0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez‐Bernal CL, Peiró S, Hurtado I, García‐Sempere A, Sanfélix‐Gimeno G. Primary nonadherence to oral anticoagulants in patients with atrial fibrillation: real‐world data from a population‐based cohort. J Manag Care Spec Pharm. 2018;24. doi: 10.18553/jmcp.2018.24.5.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banerjee A, Benedetto V, Gichuru P, Burnell J, Antoniou S, Schilling RJ, Strain WD, Ryan R, Watkins C, Marshall T, et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population‐based study. Heart. 2020;106:119–126. doi: 10.1136/heartjnl-2019-315307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoehr GP, Lu S‐Y, Lavery L, Bilt JV, Saxton JA, Chang C‐C, Ganguli M. Factors associated with adherence to medication regimens in older primary care patients: the steel valley seniors survey. Am J Geriatr Pharmacother. 2008;6:255–263. doi: 10.1016/j.amjopharm.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gajria K, Lu M, Sikirica V, Greven P, Zhong Y, Qin P, Xie J. Adherence, persistence, and medication discontinuation in patients with attention‐deficit/hyperactivity disorder—a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543–1569. doi: 10.2147/NDT.S65721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komen JJ, Pottegård A, Mantel‐Teeuwisse AK, Forslund T, Hjemdahl P, Wettermark B, Hellfritzsch M, Hallas J, Olesen M, Bennie M, et al. Persistence and adherence to non‐vitamin K antagonist oral anticoagulant treatment in patients with atrial fibrillation across five Western European countries. EP Europace. 2021;23:1722–1730. doi: 10.1093/europace/euab091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22:313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- 24. Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta‐analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15. doi: 10.1007/s11065-005-6254-9 [DOI] [PubMed] [Google Scholar]
- 25. Gillis JC, Chang SC, Devore EE, Rosner BA, Grodstein F, Okereke OI. Patterns of late‐life depressive symptoms and subsequent declines in cognitive domains. Int J Geriatr Psychiatry. 2017;32. doi: 10.1002/gps.4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, Rowell N, et al. 2021 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Europace. 2021;23:1612–1676. doi: 10.1093/europace/euab065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wikström K, Toivakka M, Rautiainen P, Tirkkonen H, Repo T, Laatikainen T. Electronic health records as valuable data sources in the health care quality improvement process. Health Serv Res Manag Epidemiol. 2019;6. doi: 10.1177/2333392819852879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rapola JM, Virtamo J, Korhonen P, Haapakoski J, Hartman AM, Edwards BK, Heinonen OP. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol. 1997;13:133–138. doi: 10.1023/a:1007380408729 [DOI] [PubMed] [Google Scholar]
- 29. Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40:505–515. doi: 10.1177/1403494812456637 [DOI] [PubMed] [Google Scholar]
- 30. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Finnish national register holders (Social Insurance Instituite, Finnish Institute for Health and Welfare, Population Register Centre and Tax Register).


