Abstract
Background
The effects of amiodarone and lidocaine on the return of spontaneous circulation (ROSC) in relation to time to treatment in patients with out‐of‐hospital cardiac arrest is not known. We conducted a post hoc analysis of the ROC ALPS (Resuscitation Outcomes Consortium Amiodarone, Lidocaine, Placebo) randomized controlled trial examining the association of time to treatment (drug or placebo) with ROSC at hospital arrival.
Methods and Results
In the trial, adults with nontraumatic out‐of‐hospital cardiac arrest with initial refractory ventricular fibrillation or pulseless ventricular tachycardia after at least 1 defibrillation were randomly assigned to receive amiodarone, lidocaine, or placebo. We used logistic regression to examine the association of time to treatment (911 call to study drug administration) with ROSC. An interaction term between treatment and time to treatment was included to determine the potential effect of time on treatment effects. Overall, 1112 (36.7%) patients had ROSC at hospital arrival (350 in the amiodarone arm, 396 in the lidocaine arm, and 366 in the placebo arm). The proportion of patients who had ROSC decreased as time to drug administration increased, in patients treated with amiodarone (odds ratio, 0.92; 95% CI, 0.90–0.94 per minute increase), lidocaine (odds ratio, 0.95; 95% CI, 0.93–0.96), and placebo (odds ratio, 0.95; 95% CI, 0.93–0.96). With shorter times to drug administration, the proportion with ROSC was higher in amiodarone versus placebo recipients.
Conclusions
The probability of ROSC decreased as time to drug administration increased. The effect of amiodarone but not lidocaine to restore ROSC declined with longer times to drug administration, potentially attributable to its adverse hemodynamic effects.
Keywords: amiodarone, lidocaine, out‐of‐hospital cardiac arrest, return of spontaneous circulation
Subject Categories: Arrhythmias, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Cardiopulmonary Arrest, Mortality/Survival, Sudden Cardiac Death, Treatment
Nonstandard Abbreviations and Acronyms
- pVT
pulseless ventricular tachycardia
- ROC ALPS
Resuscitation Outcomes Consortium Amiodarone, Lidocaine, Placebo
- ROSC
return of spontaneous circulation
Clinical Perspective
What Is New?
In this secondary analysis of a randomized, placebo‐controlled trial, probability of return of spontaneous circulation was compared between amiodarone, lidocaine, and placebo on the basis of time to trial drug administration during shock‐refractory out‐of‐hospital cardiac arrest.
Overall, the proportion of patients who had return of spontaneous circulation decreased as time to drug or placebo administration increased.
Comparing amiodarone with placebo, the relative probability of return of spontaneous circulation declined with longer times to drug administration.
What Are the Clinical Implications?
Amiodarone is most effective in increasing the probability of return of spontaneous circulation when given early during shock‐refractory out‐of‐hospital cardiac arrest.
Confirming these findings in prospective studies and exploring the timing of antiarrhythmics during resuscitation could have important implications for treatment of cardiac arrest.
In the treatment of out‐of‐hospital cardiac arrest, return of spontaneous circulation (ROSC) requires 2 separate but related components: restoring organized electrical activity and restoring mechanical cardiac output. Electrophysiological outcomes such as termination of pulseless ventricular tachycardia (pVT) or ventricular fibrillation (VF) could be considered a primary outcome for studies of electrical interventions, such as defibrillation.
Effective resuscitation during cardiac arrest, requires restoring effective mechanical activity (ie, ROSC) in addition to restoration of organized electrical activity. However, ROSC is a more complex outcome than termination of arrhythmia and is influenced by factors such as the duration of cardiac arrest, underlying cardiac function, and the type and quality of interventions provided during cardiac arrest resuscitation. 1 , 2 These factors can affect outcomes in numerous ways. For example, antiarrhythmics or epinephrine administration may result in adverse hemodynamic effects but facilitate VF termination (or vice versa), potentially limiting their benefit during cardiac arrest. 3 , 4 Similarly, during cardiac arrest, the heart may endure some transient or permanent damage and fail to generate adequate output even in the presence of organized electrical activity. 5 A longer low‐flow or no‐flow interval can further worsen any cardiac insults or accentuate any interventions’ adverse hemodynamic effects (creating a synergistic effect). 2 , 5
In general, ROSC is the initial requirement for patient survival. Recent systematic reviews and meta‐analyses comparing amiodarone and lidocaine with placebo demonstrated that although both drugs effectively improve the probability of survival at hospital admission, only lidocaine increased the probability of ROSC. 6 , 7 This discrepancy may be explained by considering amiodarone’s principal mechanism of action, which seems to be in reducing the risks of refibrillation, 8 although it may assist in defibrillation. 8 , 9 The administration of intravenous amiodarone may have adverse effects, potentially limiting its benefit in cardiac arrest. 10 , 11 These adverse effects may be more profound when drugs are administered after prolonged ischemia. Given these findings and the established benefit of early treatment in cardiac arrest, we undertook this analysis to explore the relationship between time to antiarrhythmic treatment and ROSC, in a substudy of the ALPS (Amiodarone Lidocaine Placebo Study). A secondary objective of this study was to determine if the relationship between time to treatment and treatment effect was modified by treatment type (ie, amiodarone versus lidocaine).
Methods
The data that support the findings of this study are available from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center upon reasonable request.
The structural detail of the Resuscitation Outcome Consortium (ROC) as well as the background to the ROC ALPS (ROC Amiodarone Lidocaine Placebo Study) randomized controlled trial, methods, and results have been previously reported. 12 , 13 In brief, in the per protocol analysis, the ROC ALPS trial enrolled adult patients with out‐of‐hospital cardiac arrest (≥18 years old) who presented with pVT/VF during their first analysis and were refractory to at least 1 defibrillation. In the intention‐to‐treat analysis, patients with initial or subsequently occurring refractory VF or pVT were included. Patients received 300 mg of amiodarone (Captisol‐enabled formulation), 120 mg of lidocaine, or placebo (normal saline) via 2 identical syringes; each contained 150 mg of amiodarone or 60 mg of lidocaine (or 1 syringe if body weight was estimated to be <45 kg) in the field using a double‐blinded protocol. A supplemental dose of the same drug (150 mg for amiodarone or 60 mg for lidocaine) was administered if VF or pVT persisted after the initial dose of the trial drug, standard resuscitation measures, and additional defibrillations. The trial drug’s initial dose was administered when feasible, by rapid bolus, followed by defibrillation, and all standard resuscitation measures.
In ROC ALPS, the formulation of amiodarone (PM101, branded as Nexterone) (Prism Pharmaceuticals Inc., King of Prussia, PA) was used that has been shown to be bioequivalent to the other approved, commonly used formulation of amiodarone (polysorbate 80). 14 The diluent (Captisol, a sulfobutyl ether β‐cyclodextrin) has been demonstrated to be hemodynamically and electrophysiologically inert. This formulation of amiodarone seems likely to avoid many of the problems associated with the polysorbate 80 formulation, most importantly its effect on blood pressure and left ventricular function. 14 , 15
In the current study, we performed an individual patient‐level secondary analysis of data from the ALPS study, calculating the time interval between the 911 call and the administration of the study drug and analyzing the primary outcome of ROSC at hospital (emergency department) arrival. The primary analysis was on the “per‐protocol” population (patients whose initial cardiac arrest rhythm of VF or pVT was refractory to shock).
The ALPS trial was conducted under exception from informed consent in emergency research in compliance with all applicable regulatory requirements. The data for this study were obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center, and the study protocol was approved by the Sunnybrook Health Science Centre Research Ethics Board.
Statistical Analysis
The primary outcome was ROSC at hospital arrival. ROSC was defined as the presence of both a palpable pulse and measurable blood pressure upon hospital arrival. This definition may better represent a more accurate definition of sustained ROSC compared with ROSC “in the field” using a palpable pulse alone. A secondary objective was to determine if the relationship between time to treatment and treatment effect was modified by treatment type.
Baseline characteristics were summarized using mean (SD) and median (interquartile range) for continuous data and number of patients (with percentages) for categorical data. Logistic regression models were fitted for the outcome of ROSC at hospital arrival. In our analyses, time to administration of the trial drug as a continuous variable and the allocated treatment were modeled as explanatory factors. The interaction of these variables was considered in the model to account for the possible effect of time on the estimated treatment effects. A potential nonlinear effect in the continuous variable of time to administration of the trial drug was assessed using a restricted cubic spline. To compare the models with linear and nonlinear effects, we used the likelihood ratio test. We kept the interaction term in the models if it was statistically significant (P<0.05). We also repeated the models with additional adjustment for prehospital arrest characteristics. In a post hoc analysis, the models were replicated separately for a witnessed cardiac arrest subgroup. All results were summarized using odds ratio (OR) and corresponding 95% CI. R, version 3.6.1 (R Core Team, 2019) was used for all statistical analyses in the study and figures were produced using the package ggplot 2. 16 , 17
Results
In the ROC ALPS, 3026 patients were enrolled in the 3 arms of the trial. Of those, 2994 patients with known drug administration times were included in our analysis (1046 in the placebo arm, 962 in the amiodarone arm, and 986 in the lidocaine arm). Baseline characteristics are shown in Table. A comprehensive description of all baseline patient characteristics and outcomes in the per‐protocol population has been reported in the study’s primary analysis. 12 The distribution of time intervals between 911 emergency call (or time of cardiac arrest for paramedic‐witnessed cases) and trial drug administration were similar in all groups. As was expected in a large randomized controlled trial, the baseline characteristics were well balanced between trial groups. Also, patients in the 3 study arms were similar with regard to the time of drug administration (Table S1). Overall, 1091 (36.6%) patients had ROSC at hospital arrival (342 in the amiodarone arm, 390 in the lidocaine arm, and 359 in the placebo arm). The probability of ROSC at hospital arrival based on time to the first dose of the trial drug is shown in Figure 1. The odds of ROSC at hospital arrival decreased as time to drug administration increased, in patients treated with amiodarone (OR, 0.92; 95% CI, 0.90–0.94 per minute increase in time interval), lidocaine (OR, 0.95; 95% CI, 0.93–0.96), and placebo (OR, 0.95; 95% CI, 0.93–0.96). The change in odds of ROSC at hospital arrival based on time to the first dose of amiodarone (compared with placebo) is shown in Figure 2A. With shorter times to drug administration, the odds of ROSC at hospital arrival were higher in amiodarone‐ versus placebo‐treated patients, whereas the odds of ROSC were higher with placebo than amiodarone at later times after drug administration. Figure 2B shows the change in odds of ROSC at hospital arrival based on time to the first dose of amiodarone (compared with lidocaine). With shorter times to drug administration, the odds of ROSC at hospital arrival were not significantly different after amiodarone versus lidocaine, whereas ROSC was significantly more likely with lidocaine than amiodarone at later times after drug administration. Comparing lidocaine with placebo (Figure 2C), there was an increased likelihood of ROSC at hospital arrival for all times of drug administration (OR, 1.29; 95% CI, 1.07–1.59). There was a significant time by treatment interaction for the comparison of amiodarone versus placebo (P=0.007) and lidocaine versus placebo (P=0.004). For each additional minute of wait time from 911 call to drug, the odds of ROSC were lower in amiodarone versus placebo (OR, 0.96; 95% CI, 0.93–0.99) and amiodarone versus lidocaine (OR, 0.96; 95% CI, 0.94–0.98). Time was not significantly associated with ROSC when comparing lidocaine to placebo (1.00; 95% CI, 0.97–1.03; P=0.87). Comparing amiodarone with placebo (Figure 2A), there was a higher probability of ROSC after amiodarone when drug or placebo was administered at <19.5 minutes after dispatch (red dotted line; ie, OR, 19.5=1.00); beyond this time point, the relative efficacy of amiodarone versus placebo had an OR <1, suggesting a lower odds of ROSC at hospital arrival (compared with placebo) if amiodarone is administered later than 19.5 minutes after dispatch. Similarly, comparing amiodarone with lidocaine (Figure 2B), amiodarone was associated with a higher probability of ROSC if administered <13.5 minutes after dispatch (red dotted line); beyond this time point, the relative efficacy of amiodarone versus lidocaine was reversed, with an OR <1. The study’s findings remained consistent after adjustment for prehospital arrest characteristics (Data S1).
Table 1.
Patient Characteristics and Outcomes by Treatment Arm (N=2994)
| Patient characteristics |
Placebo (n=1046*) |
Amiodarone (n=962*) |
Lidocaine (n=986*) |
|---|---|---|---|
| Mean age, y (SD) | 62.7 (14.6) | 63.7 (14.1) | 63.2 (14.6) |
| Median time to drug, min (IQR) | 17.6 (7.7) | 17.6 (8.4) | 17.6 (7.8) |
| Male, n (%) | 835 (79.8) | 762 (78.5) | 816 (82.0) |
| Witnessed, n (%) | 730 (71.0) | 669 (69.5) | 676 (70.1) |
| Public location, n (%) | 312 (29.5) | 301 (31.1) | 308 (31.0) |
| Bystander CPR, n (%) | 588 (60.3) | 550 (61.5) | 546 (59.3) |
| Outcomes | |||
| ROSC at hospital arrival, n (%) | 359 (34.3) | 342 (35.6) | 390 (39.6) |
| Number of EMS shocks after first dose of the trial drug, median (IQR) | 3 (5) | 2 (3) | 2 (2) |
| Total dose of epinephrine (mg), median (IQR) | 4 (3) | 4 (2) | 4 (3) |
CPR indicates cardiopulmonary resuscitation; EMS, emergency medical services; IQR, interquartile range; and ROSC, return of spontaneous circulation.
May vary depending on missing values.
Figure 1. Probability (shaded area shows 95% CI) of ROSC at hospital arrival based on time to the first dose of the trial drug.
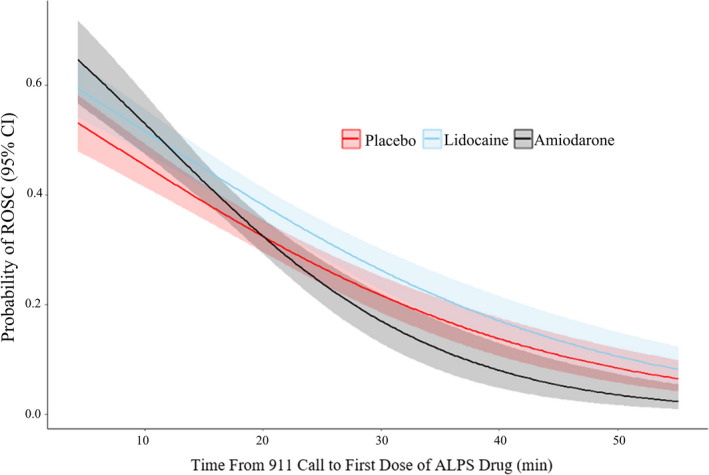
ALPS indicates Amiodarone Lidocaine Placebo Study; and ROSC, return of spontaneous circulation.
Figure 2. Change in odds ratio (OR) of return of spontaneous circulation (ROSC) at hospital arrival (solid black line) based on time to the first dose of (A) amiodarone (vs placebo).
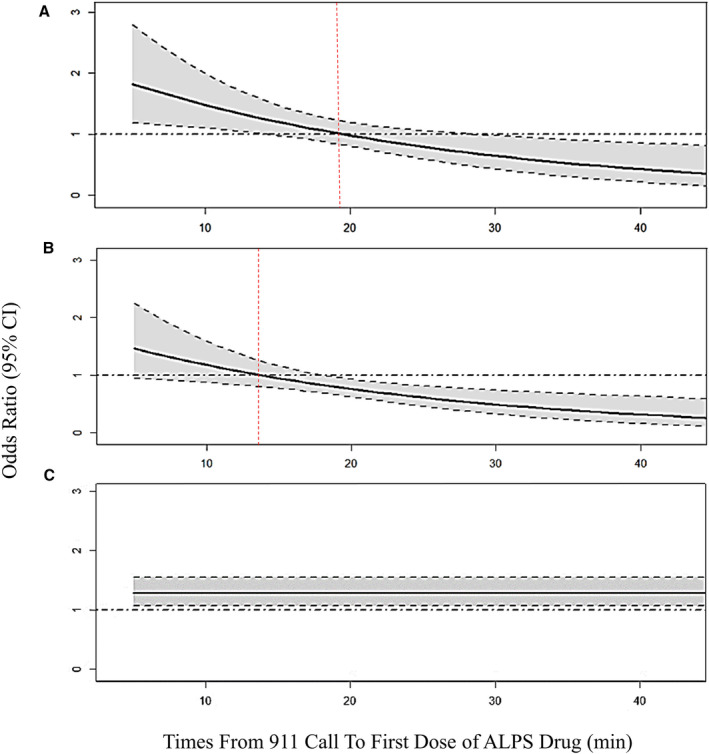
(B) Amiodarone (vs lidocaine). (C) Lidocaine (vs placebo). Black dashed line indicates OR=1; Red dashed line indicates time when OR of ROSC crosses 1; Shaded area shows 95% CI. ALPS indicates Amiodarone Lidocaine Placebo Study; OR, odds ratio; and ROSC, return of spontaneous circulation.
Comparison of models with linear and nonlinear time covariates identified that linear models offered the best fit for all cases. In addition, subgroup analyses of patients with witnessed cardiac arrest (Data S2, Figure S1) and in the intention‐to‐treat population, which included patients with initial shockable rhythm or subsequently occurring shockable rhythm, yielded similar results (Data S3, Table S2, Figures S2 and S3).
Discussion
In our post hoc analysis of ROC ALPS data, the probability of ROSC at hospital arrival decreased as the time interval from cardiac arrest to the administration of amiodarone, lidocaine, or placebo increased. The highest ROSC rates were seen in patients treated early after the onset of cardiac arrest. When an interaction between time to drug administration and treatment type was included in the models, our results showed a pattern, which suggests the relative effects of amiodarone but not lidocaine (compared with placebo) on the probability of ROSC might change with different times to drug administration. With shorter times to drug administration, the probability of ROSC was higher after amiodarone compared with placebo, while the probability of ROSC was higher with both lidocaine and placebo compared with amiodarone at the later times. Comparing lidocaine with placebo, the likelihood of ROSC remained higher in those treated with lidocaine; this relation was independent of time to drug administration.
In our study, comparison of a drug versus placebo and the interaction between drug versus placebo and time made it possible to explore any potential time effect that is independent of any drug effect, as well as the time influence on drug effect (versus placebo). While the probability of ROSC at the time of hospital arrival decreased over time for amiodarone, lidocaine, and placebo, the rate of decrease was faster with amiodarone relative to placebo and lidocaine. The decrease in ROSC rate with longer time to placebo is likely attributable to early placebo administration correlating with shorter times for any intervention. In the majority of cardiovascular emergencies, the effectiveness of time‐sensitive interventions is dependent on the timing of administration and can affect clinical outcomes. In prolonged VF, ischemic injury may suppress myocardial function and prevent ROSC even after VF termination. The global myocardial ischemia precipitated by VF and antiarrhythmic drugs’ adverse effects can lead to hypotension and bradycardia after VF termination. At shorter times after VF onset, the potential adverse effects of amiodarone (negative inotropic effect) may be blunted by its effectiveness in improving the probability of successful, durable defibrillation. Conversely, at later times, treatment with amiodarone might decrease the likelihood of ROSC potentially because of its adverse effects on hemodynamics. However, in the primary analysis of the ROC ALPS trial, there was no evidence of any overall decrease in survival at hospital discharge in the amiodarone group compared with placebo or lidocaine. 12 This may be related to the efficacy of amiodarone in preventing ventricular tachyarrhythmias in the immediate postresuscitation period (refibrillation) or reversal of these transient adverse events. 18 Similarly, the benefit of lidocaine in cardiac arrest most likely relates to its efficacy in minimizing arrhythmia recurrences, once an organized rhythm is restored by other means such as defibrillation. 19
Negative time‐dependent hemodynamic effects of amiodarone in the early postresuscitation period have been previously observed and described in experimental studies. In an experiment by Ji et al, 20 administering amiodarone versus placebo 4 minutes after the onset of VF (untreated VF) increased ROSC in a swine model of VF arrest. However, Karlis et al 21 observed that the administration of amiodarone after 8 minutes of untreated VF did not improve ROSC rates and was associated with worse hemodynamic parameters during cardiopulmonary resuscitation and immediately after resuscitation. In our study, we saw similar results with early administration of amiodarone. The newer Captisol‐enabled formulation of amiodarone, devoid of diluent‐related adverse hemodynamic effects, was used during this study. Even with this formulation, amiodarone did not improve the probability of ROSC compared with placebo with longer times to treatment. Amiodarone’s adverse effects on hemodynamics could be attributed to amiodarone’s α‐blocking action, antagonizing the α‐stimulation of adrenaline. 21 It may be that amiodarone itself may be partly responsible for the reported hypotension and bradycardia after ROSC (the parent drug is also a negative inotrope; however, less than the diluent). 4 Given the transient adverse effect of antiarrhythmics on hemodynamic status, using the absence of a palpable pulse as a sole predictor of ROSC or futility may not be reliable and could result in misclassifying a small number of potential survivors. 22 Improved methods for detecting ROSC for prehospital health care providers, especially for those who continue to provide on‐scene resuscitation (versus early transport), are needed. 23
Currently, the optimal sequence of Advanced Cardiac Life Support interventions for VF/pVT cardiac arrest, including administration of epinephrine or antiarrhythmic drug, at the optimal dose and the timing of medication administration in relation to other interventions, is not known. 24 In this study, we saw a higher ROSC rate with early amiodarone administration (compared with placebo); a secondary analysis of the PARAMEDIC‐2 (Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration in Cardiac Arrest) trial showed an increased chance of ROSC with a longer time to epinephrine administration (compared with placebo). 3 Exploring the timing of antiarrhythmics during resuscitation and the order of antiarrhythmics versus epinephrine (ie, amiodarone before epinephrine versus after epinephrine) may result in improved overall rate of ROSC. Resuscitation from cardiac arrest is complex, with hemodynamic, autonomic, and electrophysiologic factors playing a significant role. Each phase of resuscitation may require different, tailored, antiarrhythmic therapy. 24 Improved understanding of these phases is important to the identification of novel antiarrhythmic targets for resuscitation and the optimal bundle of care for shock‐refractory VF/pVT. The translation of optimized basic life support and advanced life support interventions into the best possible outcome is contingent on the optimal transition between intra‐arrest and post–cardiac arrest care. This requires effective implementation of techniques to identify ROSC reliably and timely implementation of therapeutic strategies to give patients resuscitated from cardiac arrest the best chance for survival with favorable neurological function.
This study has several limitations. During the ROC ALPS trial, patients were randomized on the basis of either incessant or recurrent VF. Thus, the time to drug administration may be longer in patients who had ROSC early on following 1 shock, maintained a pulse for a few minutes, and then had a recurrence. For this study, we used the time that a 911 call was received as the cardiac arrest time. This estimate may not reflect the cardiac arrest’s exact time (especially in those with unwitnessed cardiac arrest), yet it is a reasonable estimate considering the 2 events’ proximity and has been used in published studies. In many prior studies, ROSC has been defined inconsistently. For example, the exact duration required for return of pulse or blood pressure was not specified in the initial Utstein definitions, 25 whereas the 2004 Utstein definitions specified 20 minutes of pulse as a criterion for the new term sustained ROSC. 26 In this study, ROSC was defined as the presence of both a palpable pulse and measurable blood pressure, whereas several previous studies defined ROSC as having a palpable pulse alone. As with all retrospective analyses, the results of our study are subject to the influence of unmeasured confounders and should be regarded as hypothesis generating. It is possible that there are systematic differences between patients treated earlier versus later, which could account for some of the observed drug‐by‐time interactions. Similarly, the time points at which our data suggest relative drug effects are directionally reversed are post hoc estimates and should be regarded as hypothesis generating. Finally, the results of our analyses at both ends of the curves are limited by low‐frequency counts and, therefore, should be interpreted with caution.
Conclusions
In this study, the probability of ROSC decreased as time to drug or placebo treatment increased. Comparing lidocaine with placebo, the relative probability of ROSC did not change with time to drug administration. The effect of amiodarone to restore ROSC declined with longer times to drug administration, potentially because of its adverse hemodynamic effects. Exploring the timing of antiarrhythmics during resuscitation may improve the overall rate of ROSC.
Sources of Funding
None.
Disclosures
No authors have any relevant conflicts of interest to declare.
Supporting information
Data S1–S3
Tables S1–S2
Figures S1–S3
Acknowledgments
The authors acknowledge the help of the National Heart, Lung, and Blood Institute by providing the ROC ALPS trial data. This research would not have been possible without their support.
For Sources of Funding and Disclosures, see page 7.
References
- 1. Deyell MW, AbdelWahab A, Angaran P, Essebag V, Glover B, Gula LJ, Khoo C, Lane C, Nault I, Nery PB, et al. 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Position Statement on the management of ventricular tachycardia and fibrillation in patients with structural heart disease. Can J Cardiol. 2020;36:822–836. doi: 10.1016/j.cjca.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 2. Yao Y, Johnson NJ, Perman SM, Ramjee V, Grossestreuer AV, Gaieski DF. Myocardial dysfunction after out‐of‐hospital cardiac arrest: predictors and prognostic implications. Intern Emerg Med. 2018;13:765–772. doi: 10.1007/s11739-017-1756-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perkins GD, Kenna C, Ji C, Deakin CD, Nolan JP, Quinn T, Scomparin C, Fothergill R, Gunson I, Pocock H, et al. The influence of time to adrenaline administration in the Paramedic 2 randomised controlled trial. Intensive Care Med. 2020;46:426–436. doi: 10.1007/s00134-019-05836-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kudenchuk PJ, Cobb LA, Copass MK, Cummins RO, Doherty AM, Fahrenbruch CE, Hallstrom AP, Murray WA, Olsufka M, Walsh T. Amiodarone for resuscitation after out‐of‐hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341:871–878. doi: 10.1056/NEJM199909163411203 [DOI] [PubMed] [Google Scholar]
- 5. Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097:00130-1 [DOI] [PubMed] [Google Scholar]
- 6. Ali MU, Fitzpatrick‐Lewis D, Kenny M, Raina P, Atkins DL, Soar J, Nolan J, Ristagno G, Sherifali D. Effectiveness of antiarrhythmic drugs for shockable cardiac arrest: a systematic review. Resuscitation. 2018;132:63–72. doi: 10.1016/j.resuscitation.2018.08.025 [DOI] [PubMed] [Google Scholar]
- 7. McLeod SL, Brignardello‐Petersen R, Worster A, You J, Iansavichene A, Guyatt G, Cheskes S. Comparative effectiveness of antiarrhythmics for out‐of‐hospital cardiac arrest: a systematic review and network meta‐analysis. Resuscitation. 2017;121:90–97. doi: 10.1016/j.resuscitation.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 8. Fain ES, Lee JT, Winkle RA. Effects of acute intravenous and chronic oral amiodarone on defibrillation energy requirements. Am Heart J. 1987;114:8–17. doi: 10.1016/0002-8703;90300-0 [DOI] [PubMed] [Google Scholar]
- 9. Frame LH. The effect of chronic oral and acute intravenous amiodarone administration on ventricular defibrillation threshold using implanted electrodes in dogs. Pacing Clin Electrophysiol. 1989;12:339–346. doi: 10.1111/j.1540-8159.1989.tb02667.x [DOI] [PubMed] [Google Scholar]
- 10. Levine JH, Massumi A, Scheinman MM, Winkle RA, Platia EV, Chilson DA, Gomes A, Woosley RL. Intravenous amiodarone for recurrent sustained hypotensive ventricular tachyarrhythmias. Intravenous Amiodarone Multicenter Trial Group. J Am Coll Cardiol. 1996;27:67–75. doi: 10.1016/0735-1097:00427-0 [DOI] [PubMed] [Google Scholar]
- 11. Kowey PR, Levine JH, Herre JM, Pacifico A, Lindsay BD, Plumb VJ, Janosik DL, Kopelman HA, Scheinman MM. Randomized, double‐blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. The Intravenous Amiodarone Multicenter Investigators Group. Circulation. 1995;92:3255–3263. doi: 10.1161/01.cir.92.11.3255 [DOI] [PubMed] [Google Scholar]
- 12. Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, et al. Amiodarone, lidocaine, or placebo in out‐of‐hospital cardiac arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204 [DOI] [PubMed] [Google Scholar]
- 13. Davis DP, Garberson LA, Andrusiek DL, Hostler D, Daya M, Pirrallo R, Craig A, Stephens S, Larsen J, Drum AF, et al. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11:369–382. doi: 10.1080/10903120701537147 [DOI] [PubMed] [Google Scholar]
- 14. Kudenchuk PJ, Brown SP, Daya M, Morrison LJ, Grunau BE, Rea T, Aufderheide T, Powell J, Leroux B, Vaillancourt C, et al. Resuscitation Outcomes Consortium‐Amiodarone, Lidocaine or Placebo Study (ROC‐ALPS): rationale and methodology behind an out‐of‐hospital cardiac arrest antiarrhythmic drug trial. Am Heart J. 2014;167:653–659.e4. doi: 10.1016/j.ahj.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torres‐Arraut E, Singh S, Pickoff AS. Electrophysiologic effects of Tween 80 in the myocardium and specialized conduction system of the canine heart. J Electrocardiol. 1984;17:145–151. doi: 10.1016/s0022-0736(84)81088-2 [DOI] [PubMed] [Google Scholar]
- 16. R Core Team . R: A Language and Environment for Statistical Computing. Published online 2019. https://www.r‐project.org. Accessed August 15, 2021.
- 17. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. Springer‐Verlag New York; 2016. https://ggplot2.tidyverse.org. Accessed August 15, 2021. [Google Scholar]
- 18. Karlis G, Iacovidou N, Lelovas P, Niforopoulou P, Papalois A, Siafaka I, Mentzelopoulos S, Xanthos T. Nifekalant versus amiodarone in the treatment of cardiac arrest: an experimental study in a swine model of prolonged ventricular fibrillation. Cardiovasc Drugs Ther. 2015;29:425–431. doi: 10.1007/s10557-015-6604-7 [DOI] [PubMed] [Google Scholar]
- 19. Kudenchuk PJ, Newell C, White L, Fahrenbruch C, Rea T, Eisenberg M. Prophylactic lidocaine for post resuscitation care of patients with out‐of‐hospital ventricular fibrillation cardiac arrest. Resuscitation. 2013;84:1512–1518. doi: 10.1016/j.resuscitation.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 20. Ji XF, Li CS, Wang S, Yang L, Cong LH. Comparison of the efficacy of nifekalant and amiodarone in a porcine model of cardiac arrest. Resuscitation. 2010;81:1031–1036. doi: 10.1016/j.resuscitation.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 21. Karlis G, Iacovidou N, Lelovas P, Niforopoulou P, Zacharioudaki A, Papalois A, Sunde K, Steen PA, Xanthos T. Effects of early amiodarone administration during and immediately after cardiopulmonary resuscitation in a swine model. Acta Anaesthesiol Scand. 2014;58:114–122. doi: 10.1111/aas.12226 [DOI] [PubMed] [Google Scholar]
- 22. Drennan IR, Case E, Verbeek PR, Reynolds JC, Goldberger ZD, Jasti J, Charleston M, Herren H, Idris AH, Leslie PR, et al. A comparison of the universal TOR Guideline to the absence of prehospital ROSC and duration of resuscitation in predicting futility from out‐of‐hospital cardiac arrest. Resuscitation. 2017;111:96–102. doi: 10.1016/j.resuscitation.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheskes S, Vaillancourt C. Multiple shocks or early transfer for shock refractory ventricular fibrillation? Can J Emerg Med. 2019;21:315–317. doi: 10.1017/cem.2019.23 [DOI] [PubMed] [Google Scholar]
- 24. Panchal AR, Berg KM, Kudenchuk PJ, Del Rios M, Hirsch KG, Link MS, Kurz MC, Chan PS, Cabañas JG, Morley PT, et al. 2018 American Heart Association focused update on advanced cardiovascular life support use of antiarrhythmic drugs during and immediately after cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2018;138:e740–e749. doi: 10.1161/CIR.0000000000000613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett PJ, Becker L, Bossaert L, Delooz HH, Dick WF, Eisenberg MS. Recommended guidelines for uniform reporting of data from out‐of‐hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke. Circulation. 1991;84:960–975. doi: 10.1161/01.cir.84.2.960 [DOI] [PubMed] [Google Scholar]
- 26. Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation. Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1–S3
Tables S1–S2
Figures S1–S3


