Abstract
Background
Influenza infection may increase the risk of stroke and acute myocardial infarction (AMI). Whether influenza vaccination may reduce mortality in patients with hypertension is currently unknown.
Methods and Results
We performed a nationwide cohort study including all patients with hypertension in Denmark during 9 consecutive influenza seasons in the period 2007 to 2016 who were prescribed at least 2 different classes of antihypertensive medication (renin‐angiotensin system inhibitors, diuretics, calcium antagonists, or beta‐blockers). We excluded patients who were aged <18 years, >100 years, had ischemic heart disease, heart failure, chronic obstructive lung disease, cancer, or cerebrovascular disease. The exposure to influenza vaccination was assessed before each influenza season. The end points were defined as death from all‐causes, from cardiovascular causes, or from stroke or AMI. For each influenza season, patients were followed from December 1 until April 1 the next year. We included a total of 608 452 patients. The median follow‐up was 5 seasons (interquartile range, 2–8 seasons) resulting in a total follow‐up time of 975 902 person‐years. Vaccine coverage ranged from 26% to 36% during the study seasons. During follow‐up 21 571 patients died of all‐causes (3.5%), 12 270 patients died of cardiovascular causes (2.0%), and 3846 patients died of AMI/stroke (0.6%). After adjusting for confounders, vaccination was significantly associated with reduced risks of all‐cause death (HR, 0.82; P<0.001), cardiovascular death (HR, 0.84; P<0.001), and death from AMI/stroke (HR, 0.90; P=0.017).
Conclusions
Influenza vaccination was significantly associated with reduced risks of death from all‐causes, cardiovascular causes, and AMI/stroke in patients with hypertension. Influenza vaccination might improve outcome in hypertension.
Keywords: acute myocardial infarction, all‐cause death, hypertension, influenza, influenza vaccination, stroke, vaccination
Subject Categories: Hypertension, High Blood Pressure, Epidemiology, Ischemic Stroke, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- PIN
personal identification number
Clinical Perspective
What Is New?
Our study shows that influenza vaccination may reduce the risk of all‐cause death, cardiovascular death, and death from acute myocardial infarction or stroke in patients with hypertension.
What Are the Clinical Implications?
Our results emphasize the benefit of influenza vaccination in patients with hypertension and our findings suggest that the benefit of vaccination may stretch beyond reducing the likelihood of influenza and respiratory infections to include a reduced risk of cardiovascular death.
Influenza infection has been suggested as a major trigger of acute myocardial infarction (AMI) 1 , 2 , 3 and stroke. 2 Yet, little is known about the effect of influenza vaccination on mortality in individuals with hypertension. It is known that the risk of AMI and stroke is elevated during the acute phase of influenza infection. 2 , 4 Accordingly, influenza vaccination has been shown to reduce the risk of AMI and cardiovascular death in small‐scale randomized clinical trials of patients with previous AMI or stable coronary artery disease. 5 , 6 In a meta‐analysis of several smaller randomized clinical trials of patients with high‐risk cardiovascular disease, influenza vaccination was shown to significantly reduce the risk of major adverse cardiovascular outcome. 7 Consequently, influenza vaccination is recommended by the American Heart Association in patients with coronary artery disease as a secondary preventative measure (Class of recommendation: I, level of evidence B). 8 However, no studies have investigated the effect of influenza vaccination on mortality in individuals with hypertension without prevalent significant cardiovascular disease. Since individuals with hypertension are at a greatly increased risk of dying from cardiovascular causes, particularly from stroke or AMI, 9 it is possible that annual influenza vaccination may reduce mortality and improve outcome in hypertension. If this is the case, the cost‐effectiveness, safety, and feasibility of annual influenza vaccination make it an ideal preventive measure for improving outcome in hypertension. Therefore, this study sought to investigate the effect of influenza vaccination on mortality and outcome in a large nationwide cohort free of serious cardiovascular disease.
Methods
Data Availability Statement
For this study, the authors were granted full access to raw data in nationwide administrative registers following central encryption of personal identification numbers (PIN) by Statistics Denmark (Central Authority on Danish Statistics). According to Danish law, informed consent and approval by a local ethics committee is not required for register‐based studies. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Since this study uses data from human subjects, the data and everything pertaining to it are governed by the Danish Data Protection Agency and can only be made available to any additional researchers if a formal request is filed with the Danish Authorities.
Data Sources
All Danish citizens are assigned a unique PIN at birth. 10 This PIN is used all throughout the Danish public sector for data registration and general administrative purposes including the healthcare system. With this PIN, health and administrative data at the individual level may be linked throughout registers ensuring complete follow‐up. 11 The single‐payer healthcare system in Denmark provides equally available health care to all Danish citizens free of charge irrespective of social status and financial means. In this study data we collected data from several nationwide registers. More detail and an overview of the registers used in this study are available in Table S1.
Study Design and Definition of Hypertension
We used a modified cohort design for this study. This design uses a season‐specific approach to assess the association between influenza vaccination and outcome. It is reasonable to assume that a causal association between vaccination and reduced mortality, if present, would result from either a reduced probability of influenza infection or a reduced severity of infection. In Denmark, epidemiological studies have found that the majority all influenza activity occurs in months December, January, February, and March. 12 Consequently, a potential association between influenza vaccination and a reduced risk of death is assumed to be strongest during these months. Therefore, we chose to confine our study to consider these 4 months. For the remainder of this report, the period December 1 to April 1 the following year (a time period spanning the 4 months of high influenza activity) will be referred to as an influenza “season”. For the present study, we considered all influenza seasons in the period 2007 to 2016, resulting in a total of 9 seasons and 9 distinct periods of observation. In each of these seasons, all patients living with hypertension in Denmark on December 1 were identified using nationwide registers and followed until April 1 the following year (Figure 1). In Denmark, hypertension is mainly diagnosed and managed by primary care physicians. Thus, the in‐hospital International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis code for essential hypertension (ICD‐10: I10) does not accurately reflect the burden of hypertension in the population. Therefore, we defined patients with hypertension as patients treated with at least 2 antihypertensive drugs in the 6 months leading up to each season (ie, in the 6 months leading up to December 1), including beta‐blockers, diuretics, calcium antagonists, and renin‐angiotensin system inhibitors, as previously described. 13 , 14 Defining hypertension through use of 2 antihypertensive drugs has a high positive predictive value of 80% and a specificity of 94%. 13 Since 1995, all prescriptions filled in Danish pharmacies have been recorded in the National Prescription register. The register is used for drug cost reimbursement purposes and has been shown to be accurate. 15 Thus, using the National Prescription register, we identified all prescriptions filled in Denmark in the 6 months before the start of each season (December 1) for the 4 common classes of antihypertensive medications using Anatomical Therapeutic Chemical Classification System codes (renin‐angiotensin system inhibitors‐inhibitors [C09], calcium antagonists [C08], beta‐blockers [C07], and diuretics [C03]) (Figure 1). A patient was classified as hypertensive if the patient had filled ≥1 prescriptions for at least 2 of these 4 classes of medications (Figure 1). To ensure that antihypertensive drugs were prescribed for hypertension, and to include primarily individuals with hypertension without significant comorbidity, we excluded patients with known ischemic heart disease, heart failure, chronic obstructive pulmonary disease, cancer, and prior or prevalent cerebrovascular disease (Figure 1). Furthermore, we only considered patients aged >18 years and <100 years of age. Figure 1 displays the inclusion process for the 2007 to 2008 season. For the remaining 8 seasons, an inclusion procedure identical to the process displayed in Figure 1 was used.
Figure 1. Flowchart depicting the inclusion procedure.
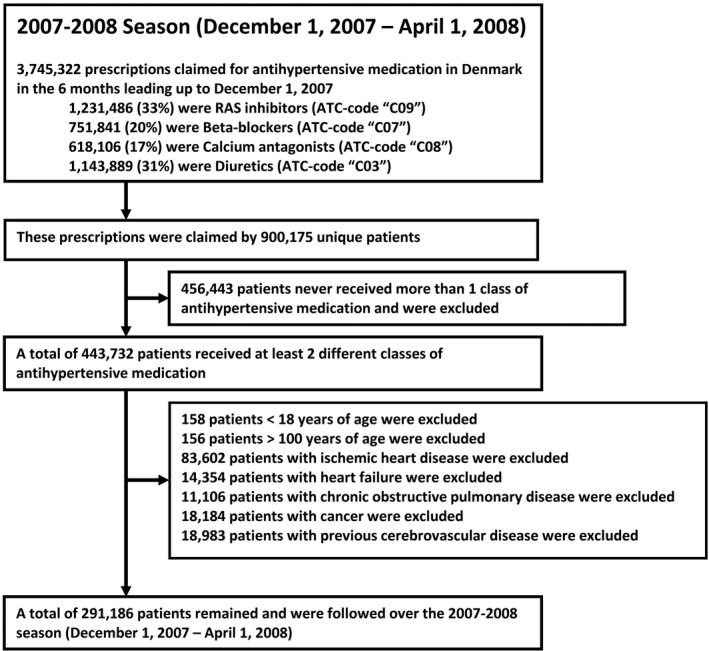
In this example, the inclusion process for the 2007 to 2008 season is shown. An identical process was used for including patients in the remaining 8 seasons from 2008 to 2016. ATC indicates Anatomical Therapeutic Chemical Classification System; and RAS, renin‐angiotensin system.
Patient Characteristics
We assessed patient characteristics at the beginning of each season (December 1). Information on comorbidities, medications, household income, and education level were obtained from nationwide administrative registers. Please refer to Data S1 and tables S2 and S3 for further details.
Influenza Vaccination Status
Influenza vaccine administration in general practice and the PIN of the patient receiving the vaccine is recorded in the General Practitioners Reimbursement register. In Denmark, general practitioners receive compensation from the government for services provided to Danish patients through a fee‐for‐service payment model. The general practitioners use the General Practitioners Reimbursement register to document services provided to patients and thus rely on the accuracy of this register for reimbursement purposes. We used this register to assess the exposure of patients to influenza vaccination. We assessed whether patients had been vaccinated in the 4 months before the start of each season (before December 1). This period was chosen because almost all influenza vaccines administered in Denmark are dispensed in months September, October, and November. If a patient had received a vaccination in the 4 months before the beginning of a given season, we considered the patient vaccinated for that season.
Outcomes
The main end points of this study were all‐cause death, cardiovascular death, and death from AMI or stroke. Both AMI and stroke were included in the cardiovascular death end point. AMI was defined as ICD‐10 codes I21‐I22. Stroke was defined as ICD‐10 codes I61‐I64. In each season, we followed patients from December 1 until their death or until April 1 the following year, whichever occurred first. For the purposes of sensitivity analysis, we also examined the incidence of cancer during follow‐up (ICD‐10 codes: C00‐C97).
Statistical Analysis
The statistical analysis was performed with Stata 16 (StataCorp, College Station, TX). Because of the season‐specific cohort approach, patients were allowed to contribute with follow‐up in multiple seasons when considering all seasons included in the study. For example, a patient diagnosed with hypertension fulfilling inclusion criteria and treated with at least 2 antihypertensive drugs before December 1, 2007 would be included in the 2007 to 2008 season. Then, should the patient not die in the 2007 to 2008 season, and if the patient did not develop any of the exclusion criteria conditions (ischemic heart disease, heart failure, chronic obstructive pulmonary disease, cancer, or cerebrovascular disease) and continued to receive at least 2‐drug antihypertensive therapy in the 6 months before the index of the next season (December 1, 2008), the patient would also be included in the 2008 to 2009 season. Thus, in the Table, patients were classified per whether they received at least 1 influenza vaccination in at least 1 season during the study period. Furthermore, the characteristics listed in the Table correspond to patient characteristics at the time of their first inclusion into the study. A figure illustrating the modified cohort design of the study may be found in Figure S3. We used survival analysis implemented through Stata stcox command to assess the association between vaccination and outcome. When considering all seasons, we used multivariable Cox regression with multiple follow‐up intervals per patient. The multivariable model (referred to as “fully adjusted results” for the remainder of this report) was adjusted for all variables in the Table. The follow‐up interval for each season began on December 1 and ended at the time of death or April 1 of the following year, whichever occurred first. This allowed for a contribution of up to 120 days per patient per season. To account for multiple observation periods per patient, the analyses were stratified by year and a clustered variance estimator treating observation periods from the same patient as clusters was used. Patient characteristics were reassessed and updated on the index date of each season to account for any changes between seasons. For sensitivity purposes, the association between vaccination and mortality was assessed in the “off‐season” months (April 1–December 1 the following year). In this analysis, we extended follow‐up from the 4 months “in‐season” period (December 1–April 1 the following year) to 1 full year (December 1–December 1 the following year). To address the robustness of our results, we performed multiple sensitivity analyses which are described in Data S1 and S2. We used survival probabilities and hazard ratio estimates from the fully adjusted Cox regression estimates to derive adjusted numbers needed to treat to prevent 1 death over 1 season associated with vaccination when considering all seasons included in the study. 16 To investigate whether vaccination may be more beneficial for patients with hypertension than patients with normotension, we matched patients with hypertension 1:1 on birth year and sex with patients with normotension sourced from the Danish general background population in each season (defined as no prescriptions for any antihypertensive medications in the 6 months leading up to each season and otherwise identical inclusion criteria) and assessed the fully adjusted association between vaccination and outcome in both groups. Finally, we assessed the association between vaccination and death in each separate season included in the study. In influenza seasons 2007 to 2008 and 2015 to 2016 known partial mismatches between vaccine influenza strain and circulating influenza strains occurred. In these 2 seasons a moderate amount of influenza B activity was recorded in Denmark (2007–2008: 36% influenza B, 64% influenza A)(2015–2016: 44% influenza B, 56% influenza A), and epidemiological surveillance data from Statens Serum Institut indicate that the influenza B vaccine component in these 2 seasons did not match the circulating influenza B strains. These analyses are described in Data S1, S2 and Figure S2.
Table .
Patient Characteristics Assessed at the Time of Inclusion Stratified by Vaccination Status
| Demographics | All patients | No vaccine | Ever vaccinated in study | P Value |
|---|---|---|---|---|
| n | 608 452 | 321 623 (52.9%) | 286 829 (47.1%) | |
| Age, y | 65.2 (13.1) | 60.1 (12.8) | 70.8 (11.0) | <0.001 |
| Men | 266 683 (43.8%) | 146 803 (45.6%) | 119 880 (41.8%) | <0.001 |
| Household income quartile | <0.001 | |||
| 1st quartile | NA | 58 785 (8.4%) | 92 407 (32.3%) | |
| 2nd quartile | NA | 65 189 (20.5%) | 86 003 (30.1%) | |
| 3rd quartile | NA | 85 513 (26.8%) | 65 679 (23.0%) | |
| 4th quartile | NA | 109 256 (34.3%) | 41 936 (14.7%) | |
| Highest education level | <0.001 | |||
| Basic school <10 y | 237 382 (39.0%) | 110 439 (34.3%) | 126 943 (44.3%) | |
| High school, +3 y | 13 879 (2.3%) | 9457 (2.9%) | 4422 (1.5%) | |
| Vocational education | 215 765 (35.5%) | 123 505 (38.4%) | 92 260 (32.2%) | |
| Short/medium higher education, +2–4 y | 84 933 (14.0%) | 50 201 (15.6%) | 34 732 (12.1%) | |
| Long higher education, +5 y or more | 22 180 (3.7%) | 13 145 (4.1%) | 9035 (3.2%) | |
| Unknown | 34 313 (5.6%) | 14 876 (4.6%) | 19 437 (6.8%) | |
| Vaccination in previous season | 131 602 (21.6%) | 7172 (2.2%) | 124 430 (43.4%) | <0.001 |
| No. of seasons included | 5 (2–8) | 4 (2–7) | 6 (3–9) | <0.001 |
| Comorbidities | ||||
| Valvular disease | 10 132 (1.7%) | 3844 (1.2%) | 6288 (2.2%) | <0.001 |
| Systemic embolus | 3160 (0.5%) | 1358 (0.4%) | 1802 (0.6%) | <0.001 |
| Atrial fibrillation or flutter | 33 291 (5.5%) | 12 784 (4.0%) | 20 507 (7.2%) | <0.001 |
| Chronic renal failure | 8080 (1.3%) | 4129 (1.3%) | 3951 (1.4%) | 0.001 |
| Anemia | 10 477 (1.7%) | 4370 (1.4%) | 6107 (2.1%) | <0.001 |
| Diabetes | 79 822 (13.1%) | 31 816 (10.0%) | 48 006 (16.7%) | <0.001 |
| Peripheral vascular disease | 9199 (1.5%) | 3531 (1.1%) | 5668 (2.0%) | <0.001 |
| Liver disease | 5703 (0.9%) | 3271 (1.0%) | 2432 (0.9%) | <0.001 |
| Rheumatic disease | 9739 (1.6%) | 3822 (1.2%) | 5917 (2.1%) | <0.001 |
| Peptic ulcer | 13 619 (2.2%) | 5865 (1.8%) | 7763 (2.7%) | <0.001 |
| Medications | ||||
| No. of antihypertensive drugs | <0.001 | |||
| 2 drugs | 482 440 (79.3%) | 265 250 (82.4%) | 217 190 (75.2%) | |
| 3 drugs | 108 986 (17.9%) | 49 339 (15.3%) | 59 647 (20.8%) | |
| 4 drugs | 17 026 (2.8%) | 7034 (2.2%) | 9992 (3.5%) | |
| Renin‐angiotensin system inhibitor | 458 579 (75.4%) | 247 094 (76.8%) | 211 485 (73.3%) | <0.001 |
| Beta blocker | 222 531 (36.6%) | 110 623 (34.4%) | 111 908 (39.0%) | <0.001 |
| Diuretic | 357 311 (58.7%) | 176 397 (54.9%) | 180 914 (63.0%) | <0.001 |
| Calcium antagonist | 321 521 (52.8%) | 172 539 (53.7%) | 148 982 (51.9%) | <0.001 |
| Statin | 192 417 (31.6%) | 84 642 (26.3%) | 107 775 (37.6%) | <0.001 |
| Lipid‐lowering | 194 548 (32.0%) | 132 535 (29.7%) | 62 013 (38.4%) | <0.001 |
| Glucose‐lowering | 79 822 (13.1%) | 31 816 (10.0%) | 48 006 (16.7%) | <0.001 |
| Antithrombotic | 180 473 (29.7%) | 65 989 (20.5%) | 114 484 (39.9%) | <0.001 |
| Spironolactone | 16 964 (2.8%) | 7565 (2.4%) | 9399 (3.3%) | <0.001 |
| Digoxin | 17 284 (2.8%) | 5298 (1.7%) | 11 986 (4.2%) | <0.001 |
| Aspirin | 146 065 (24.0%) | 53 349 (16.7%) | 92 716 (32.2%) | <0.001 |
| Opioid | 70 265 (11.6%) | 29 953 (9.3%) | 40 312 (14.1%) | <0.001 |
| Antipsychotic | 17 203 (2.8%) | 7824 (2.4%) | 9379 (3.3%) | <0.001 |
| Antidepressant | 72 719 (12.0%) | 33 442 (10.4%) | 39 277 (13.7%) | <0.001 |
| Antiepileptic | 18 158 (3.0%) | 8450 (2.6%) | 9708 (3.4%) | <0.001 |
| Systemic glucocorticoid | 23 606 (3.9%) | 9340 (2.9%) | 14 266 (5.0%) | <0.001 |
| Proton‐pump inhibitor | 85 310 (14.0%) | 37 799 (11.8%) | 47 511 (16.6%) | <0.00116 |
A patient was considered “ever vaccinated” if the patient was vaccinated at least once in at least 1 season.
Percentages may not sum to 100% because of rounding.
Results
Study Population
We included 608 452 unique patients with hypertension over 9 consecutive influenza seasons during the time period 2007 to 2016 (Figure 1). The majority of patients were included before the first season (2007–2008). The median follow‐up was 5 seasons (interquartile range, 2–8). The vaccination coverage varied by season and ranged from 26% in the 2007 to 2008 season to 35% in the 2015 to 2016 season with a peak of 36% in the 2013 to 2014 season (Figure S1). During the study period, 24.9% of vaccines were administered during November, 68.2% were administered in October, and 5.5% in September. A total of 16637 patients (3.7%) received a vaccine during the follow‐up period in at least 1 season and were thus categorized as not vaccinated in those seasons. The Table displays the characteristics of patients who were vaccinated at least once during the study period and patients who were never vaccinated during the study at the time of their first inclusion into the study. Generally, vaccinated patients were older, less likely to be men, had lower household income, lower education level, displayed a higher prevalence of almost all comorbidities and used more medications (Table).
Follow‐Up
Patients were followed for a total of 975 902 person‐years. During this follow‐up, 21 571 patients died of all‐causes (3.5%), 12 270 patients died of cardiovascular causes (2.0%) and 3846 patients died of AMI or stroke (0.6%). In unadjusted analysis considering all seasons, influenza vaccination was significantly associated with a higher risk of all‐cause death, cardiovascular death and death from AMI or stroke (all‐cause death: hazard ratio [HR], 1.84; 95% CI, 1.79–1.89; P<0.001; cardiovascular death: HR, 1.89; 95% CI, 1.82–1.96; P<0.001; death from stroke or AMI: HR, 2.06; 95% CI, 1.93–2.20; P<0.001). Following adjustment for age, vaccination was significantly associated with a reduced risk of all‐cause death, cardiovascular death and death from AMI or stroke (all‐cause death: HR, 0.84; 95% CI, 0.81–0.86; P<0.001; cardiovascular death: HR, 0.85; 95% CI, 0.82–0.88; P<0.001; death from stroke or AMI: HR, 0.93; 95% CI, 0.88–0.99; P=0.042). In fully adjusted analysis, vaccination remained significantly associated with a reduced risk of all‐cause death, cardiovascular death, and death from AMI or stroke (all‐cause death: HR, 0.82; 95% CI, 0.79–0.85; P<0.001; cardiovascular death: HR, 0.84; 95% CI, 0.80–0.89; P<0.001; death from stroke or AMI: HR, 0.90; 95% CI, 0.82–0.98; P=0.017) (Figure 2). The adjusted number needed to treat (NNT) to prevent 1 death over 1 season associated with vaccination was 977 (95% CI, 837–1172). When we extended follow‐up to 1 full year, we found that the fully adjusted association between vaccination and reduced mortality appeared strongest in months December to January and February to March (Figure 3), although the association between vaccination and a reduced risk of death in the “off‐season” months April to November remained statistically significant (Figure 3). When patients with hypertension were matched 1:1 to patients with normotension in each season, in fully adjusted analysis, vaccination was associated with a reduced risk of all‐cause death in both the hypertensive and normotensive group (hypertensive: HR, 0.82; 95% CI, 0.79–0.85; P<0.001; normotensive: HR, 0.81; 95% CI, 0.77–0.86; P<0.001). However, the NNT to prevent 1 death over a single season associated with vaccination was statistically significantly lower in the hypertensive group (NNT: 977; 95% CI, 837–1172) as compared with the normotensive group (NNT: 2026; 95% CI, 1674–2750; P value for difference: P<0.001). The association between vaccination and all‐cause death and between vaccination and cardiovascular death was significantly modified by age (P for interaction: P<0.001 and P=0.004, respectively). However, when considering the outcome death from AMI or stroke no interaction between vaccination and age was present (P=0.21). In fully adjusted analysis considering only patients aged <65 years, no statistically significant association between vaccination and all‐cause death (HR, 0.95; 95% CI, 0.82–1.09; P=0.47) or cardiovascular death (HR, 0.92; 95% CI, 0.75–1.12; P=0.41) was present. In fully adjusted analysis considering only patients aged ≥65 years, vaccination was significantly associated with a reduced risk of both all‐cause (HR, 0.81; 95% CI, 0.78–0.85; P<0.001) and cardiovascular death (HR, 0.84; 95% CI, 0.80–0.89; P<0.001).
Figure 2. Association between influenza vaccination and the risk of death when considering all seasons included in the study with 95% CIs depicted as error bars.
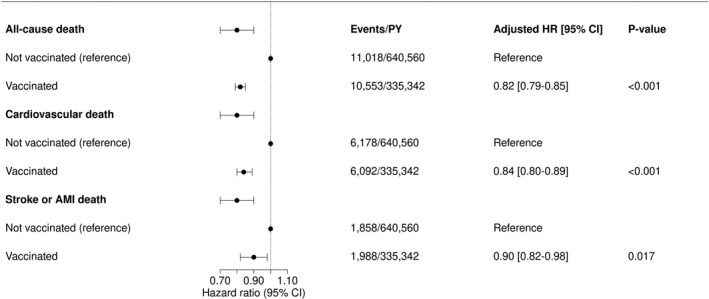
Hazard ratios were produced with multivariable Cox regression models stratified on season year with patient‐level cluster variances. The models were adjusted for all variables from the Table. AMI indicates acute myocardial infarction; HR, hazard ratio; and PY, person‐years.
Figure 3. Association between vaccination and all‐cause mortality was assessed for each 2‐month period of follow‐up as landmark analyses.
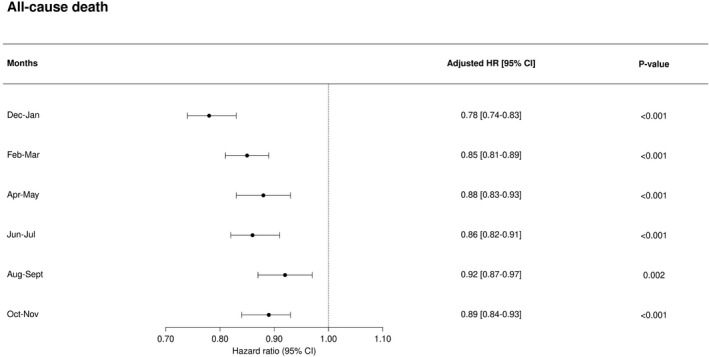
This analysis considers all seasons with follow‐up extended from the 4 months “in‐season” period (December 1–April 1 the following year) to a full year (December 1–December 1 the following year). The reference is no vaccination in the given season and the Cox regression models were adjusted for all variables displayed in the Table.
Sensitivity Analyses
We found that vaccination was significantly associated with an increased risk of incident cancer (HR, 1.59; 95% CI, 1.55–1.63; P<0.001) in unadjusted analysis considering all seasons. In fully adjusted analysis, vaccination remained significantly associated with an increased risk of incident cancer although the strength of association was markedly reduced (HR, 1.06; 95% CI, 1.02–1.11; P=0.002). In fully adjusted analysis, vaccination in the second season was associated with a reduced risk of all‐cause death (HR, 0.79; 95% CI, 0.73–0.87; P<0.001) and cardiovascular death (HR, 0.82; 95% CI, 0.73–0.92; P=0.001) during the second season, while vaccination in the previous season was not associated with either a reduced or increased risk of death in the second season (all‐cause death: HR, 0.97; 95% CI, 0.89–1.06; P=0.54; cardiovascular death: HR, 0.94; 95% CI, 0.84–1.006; P=0.31). In fully adjusted analysis, when excluding patients receiving loop diuretics (n=75 388), vaccination was significantly associated with a reduced risk of all‐cause death and cardiovascular death (all‐cause death: HR, 0.83; 95% CI 0.79–0.87; P<0.001; cardiovascular death: HR, 0.86; 95% CI, 0.81–0.92; P<0.001).
Discussion
In a Danish nationwide study considering 9 consecutive influenza seasons from 2007 to 2016, including >600 000 unique patients with hypertension, we found that influenza vaccination was significantly associated with a reduced risk death from all‐causes, cardiovascular causes, and from AMI or stroke. Finally, we found statistically significant interactions between vaccination and age with respect to outcomes all‐cause death and cardiovascular death, with results suggesting that vaccination may be more beneficial for elderly patients (over 65 years of age) with hypertension. To our knowledge, this study is the first to specifically assess the association between influenza vaccination and outcome in patients with hypertension without significant cardiovascular comorbidity.
Hypertension and Influenza Vaccination
No prior studies have assessed the effect of influenza vaccination in patients with hypertension without significant cardiovascular disease. Influenza vaccination is not mentioned in the 2017 hypertension guidelines from the American Heart Association 17 or the 2013 guidelines from the European Society of Cardiology. 18 Yet, influenza infection has been identified as a trigger of AMI, 1 and it has been shown in several small‐scale randomized clinical trials that influenza vaccination reduces the risk of AMI in patients with coronary artery disease. 5 , 7 , 19 Thus, it is becoming increasingly apparent that infections may increase the risk of acute ischemic events. 3 Accordingly, influenza vaccination is now recommended in the guidelines on secondary prevention and risk reduction in patients with coronary artery disease and other atherosclerotic vascular disease by the American Heart Association, as a secondary preventative measure in patients with coronary artery disease. 8 However, no recommendation on the role of influenza vaccination for the primary prevention of adverse cardiovascular outcomes in patients with hypertension exists, 17 , 18 and a systematic Cochrane review of randomized trials recently concluded that more evidence is needed before such recommendation can be made. 20 Hypertension is a major risk factor for stroke and coronary artery disease, and together these conditions are responsible for a large part of the deaths caused by hypertension. 9 Furthermore, influenza‐like illness has been shown to increase the risk of stroke, 4 and this risk is attenuated by influenza vaccination. 19 , 21 Therefore, it is likely that influenza vaccination may also reduce the risk of AMI and stroke in patients with hypertension without prevalent coronary artery disease or previous stroke and lead to improved outcome and reduced mortality. This notion is supported by our results, since we found that influenza vaccination was associated with significant reductions in the risk of all‐cause, cardiovascular, and AMI or stroke death. Also, since the NNT to prevent 1 death over 1 season associated with vaccination was significantly lower in patients with hypertension (NNT 977) as compared to patients without hypertension (NNT 2026), our results support the notion that vaccination may be particularly beneficial for improving outcome in patients with hypertension.
Causality, Confounding, and Limitations
In this study, we used a sensitive method based on drug use to identify a widely representative cohort of patients with hypertension free from significant cardiovascular or chronic disease. Even though we excluded significant cardiovascular disease, we cannot guarantee that the antihypertensive medications we used to identify patients were prescribed solely for hypertension, as the indication for treatment was not available in the registers. However, this method has been proven effective for identifying patients with hypertension in the general population using the same Danish registers as used in this study. 13 , 14 Because of this limitation, our results should be replicated in a population of patients with an adjudicated diagnosis of hypertension. Furthermore, since our study was observational, we cannot prove causation. However, several aspects of our findings provide support for a potential causal association between vaccination and improved outcome in hypertension: (1) In unadjusted analysis, influenza vaccination was associated with a markedly higher risk of death. Patients who underwent vaccination were older, had lower household income, lower education attainment, more comorbidity, and higher medication use. After adjustment for these confounders, vaccination was significantly associated with a substantially reduced risk of death. This change in association with adjustment suggests that our analysis effectively controlled for confounding by indication and speaks to the validity of our results. 22 Also, that the association changed from hazardous to protective with adjustment suggests that our data were confounded by selection bias, possibly because patients who were vaccinated were older and sicker. 22 This is supported by our results, since we found that vaccination was significantly associated with an increased risk of incident cancer in both unadjusted and fully adjusted analysis. It is highly unlikely that vaccination would increase cancer rates, and thus an increased risk of cancer with vaccination suggests sicker patients with more comorbidities were more likely to receive vaccination. (2) When follow‐up was extended from the 4 months “in‐season” period (December 1—April 1 the following year) to 1 full year (December 1—December 1 the following year), we found that the association between vaccination and reduced mortality was strongest in months December 1 to April 1 and declined throughout months April 1 to December 1. Thus, the association appeared strongest in months with high influenza activity but was not absent in the “off‐season” months following the influenza season. This pattern is consistent with recent findings suggesting the risk of ischemic events associated with acute infection is highest shortly following infection and declines but remains elevated for months after the infection before returning to baseline. 3 , 23 However, it is also possible that the association between vaccination and outcome found in the “off‐season” months is the result of residual confounding perhaps because of increased propensity for health‐seeking behavior among vaccinated patients. (3) When considering only the outcome of patients during their second season in the study, in fully adjusted analysis, vaccination in the second season was associated with a reduced risk of death, while vaccination in the prior season was not significantly related to survival during the second season. If vaccination was a marker of a healthier patient, we would expect vaccination in the prior season to be associated with improved outcome in the second season. (4) We found that in the 2007 to 2008 season (1 of the 2 seasons where known mismatches between vaccine strains and circulating strains occurred) no association between vaccination and a reduced risk of cardiovascular death was present. This adds further support for a potential causal relationship between vaccination and a reduced risk of death. However, another known partial mismatch occurred in the 2015 to 2016 season, and in this season vaccination was associated with a reduced risk of both all‐cause and cardiovascular death, which we did not expect. Since the known mismatch occurred only between the influenza B vaccine component and the circulating B strain, it is possible that the 2 different influenza A strains contained in the vaccine offered adequate protection from influenza infection in that season. As only 44% of recorded influenza activity in the 2015 to 2016 season were of type B, and the remaining 56% were of type A, this might explain why an association between vaccination and a reduced risk of death was present in this season. Ideally, more information on the degree of match between influenza vaccine strains and circulating viruses would have allowed for more detailed comparisons. However, although unlikely, it must be acknowledged that our study was observational, and therefore another potential explanation is also that our findings may be explained by residual confounding not addressed by our analyses. Therefore, our results must be replicated in future randomized controlled trials. Finally, we only had information on vaccines administered by general practitioners. Hence, if some patients received vaccines from a different provider, for instance from a job‐related healthcare program, this would not be detected. However, these patients would have been classified as unvaccinated, and this would only serve to weaken any potential association between influenza vaccination and outcome. Also, since this was a register‐based study, we did not have access to important hypertension‐related parameters such as the presence of obesity or the level of blood pressure control achieved by each individual patient.
Conclusions
In this nationwide study which included 9 consecutive influenza seasons and >600 000 patients with hypertension free from significant cardiovascular disease identified through medication use, we found that influenza vaccination was significantly associated with a reduced risk of death from all‐causes, cardiovascular causes, and AMI or stroke. The association between vaccination and improved outcome was strongest for elderly patients aged >65 years. Influenza vaccination might improve patient outcome in hypertension, and the potential effect on outcome may be strongest in elderly patients >65 years.
Sources of Funding
Daniel Modin was supported by the Herlev & Gentofte University Hospital Internal Research Fund and by the Novo Nordisk Foundation (grant number: NNF18OC0052966) during the preparation of this manuscript. Dr Biering‐Sørensen was supported by the Fondsbørsvekselerer Henry Hansen og Hustrus Hovedlegat 2016. The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the article.
Disclosures
Dr Vardeny reports grants from Sanofi‐Pasteur, grants from National Institutes of Health National Heart Lung and Blood Institute, outside the submitted work; Dr Solomon reports grants from Sanofi, during the conduct of the study; grants and personal fees from Alnylam, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants from Bellerophon, grants and personal fees from BMS, grants from Celladon, grants and personal fees from Gilead, grants and personal fees from GSK, grants from Ionis, grants from Lone Star Heart, grants from Mesoblast, grants and personal fees from MyoKardia, grants from National Institutes of Health/National Heart, Lung and Blood Institute, grants and personal fees from Novartis, grants from Sanofi Pasteur, grants and personal fees from Theracos, personal fees from Akros, grants and personal fees from Bayer, personal fees from Corvia, personal fees from Ironwood, personal fees from Merck, personal fees from Roche, personal fees from Takeda, personal fees from Quantum Genomics, personal fees from AoBiome, personal fees from Janssen, personal fees from Cardiac Dimensions, grants from Eidos, grants and personal fees from Cytokinetics, personal fees from Tenaya, outside the submitted work.
Supporting information
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021715
For Sources of Funding and Disclosures, see page 10.
References
- 1. Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–610. doi: 10.1016/S1473-3099(09)70233-6 [DOI] [PubMed] [Google Scholar]
- 2. Warren‐Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory‐confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self‐controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51:1701794. doi: 10.1183/13993003.01794-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musher DM, Abers MS, Corrales‐Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137 [DOI] [PubMed] [Google Scholar]
- 4. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747 [DOI] [PubMed] [Google Scholar]
- 5. Ciszewski A, Bilinska ZT, Brydak LB, Kepka C, Kruk M, Romanowska M, Ksiezycka E, Przyluski J, Piotrowski W, Maczynska R, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–1358. doi: 10.1093/eurheartj/ehm581 [DOI] [PubMed] [Google Scholar]
- 6. Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J. 2004;25:25–31. [DOI] [PubMed] [Google Scholar]
- 7. Udell JA, Zawi R, Bhatt DL, Keshtkar‐Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, et al. Association between influenza vaccination and cardiovascular outcomes in high‐risk patients: a meta‐analysis. JAMA. 2013;310:1711–1720. doi: 10.1001/jama.2013.279206 [DOI] [PubMed] [Google Scholar]
- 8. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d [DOI] [PubMed] [Google Scholar]
- 9. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet Lond Engl. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 10. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 11. Thygesen LC, Daasnes C, Thaulow I, Brønnum‐Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39:12–16. doi: 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 12. Statens Serum Institut, Influnza, ugens opgørelse . Available at: http://www.ssi.dk/sygdomme‐beredskab‐og‐forskning/sygdomsovervaagning/i/influenza‐ugens‐opgoerelse. Accessed January 1, 2019.
- 13. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen A‐M S, Gislason GH, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jørgensen ME, Hlatky MA, Køber L, Sanders RD, Torp‐Pedersen C, Gislason GH, Jensen PF, Andersson C. β‐Blocker–associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med. 2015;175:1923–1931. doi: 10.1001/jamainternmed.2015.5346 [DOI] [PubMed] [Google Scholar]
- 15. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription register. Scand J Public Health. 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 16. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 18. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;2013:34. [DOI] [PubMed] [Google Scholar]
- 19. Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32:1730–1735. doi: 10.1093/eurheartj/ehr004 [DOI] [PubMed] [Google Scholar]
- 20. Clar C, Oseni Z, Flowers N, Keshtkar‐Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015:2015:CD005050. doi: 10.1002/14651858.CD005050.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36:1501–1506. doi: 10.1161/01.STR.0000170674.45136.80 [DOI] [PubMed] [Google Scholar]
- 22. Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316:1818–1819. doi: 10.1001/jama.2016.16435 [DOI] [PubMed] [Google Scholar]
- 23. Corrales‐Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C‐C, Newman A, Loehr L, Folsom AR, Elkind MS, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 27. Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027 [DOI] [PubMed] [Google Scholar]
- 28. Weinberg GA, Szilagyi PG. Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J Infect Dis. 2010;201:1607–1610. doi: 10.1086/652404 [DOI] [PubMed] [Google Scholar]
- 29. Modin D, Jørgensen ME, Gislason G, Jensen JS, Køber L, Claggett B, Hegde SM, Solomon SD, Torp‐Pedersen C, Biering‐Sørensen T. Influenza vaccine in heart failure. Circulation. 2019;139:575–586. doi: 10.1161/CIRCULATIONAHA.118.036788 [DOI] [PubMed] [Google Scholar]
- 30. Kümler T, Gislason GH, Kirk V, Bay M, Nielsen OW, Køber L, Torp‐Pedersen C. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10:658–660. doi: 10.1016/j.ejheart.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 31. Andersson C, Norgaard ML, Hansen PR, Fosbøl EL, Schmiegelow M, Weeke P, Olesen JB, Raunsø J, Jørgensen CH, Vaag A, et al. Heart failure severity, as determined by loop diuretic dosages, predicts the risk of developing diabetes after myocardial infarction: a nationwide cohort study. Eur J Heart Fail. 2010;12:1333–1338. doi: 10.1093/eurjhf/hfq160 [DOI] [PubMed] [Google Scholar]
- 32. Andersson C, Lyngbæk S, Nguyen CD, Nielsen M, Gislason GH, Køber L, Torp‐Pedersen C. Association of clopidogrel treatment with risk of mortality and cardiovascular events following myocardial infarction in patients with and without diabetes. JAMA. 2012;308:882–889. doi: 10.1001/2012.jama.10779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For this study, the authors were granted full access to raw data in nationwide administrative registers following central encryption of personal identification numbers (PIN) by Statistics Denmark (Central Authority on Danish Statistics). According to Danish law, informed consent and approval by a local ethics committee is not required for register‐based studies. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Since this study uses data from human subjects, the data and everything pertaining to it are governed by the Danish Data Protection Agency and can only be made available to any additional researchers if a formal request is filed with the Danish Authorities.


