Abstract
Background
Oral anticoagulation (OAC) is effective for stroke prevention in patients with atrial fibrillation. However, some patients experience stroke despite OAC therapy, and knowledge about the impact of prior treatment quality is lacking.
Methods and Results
Patients with atrial fibrillation on OAC therapy who had a first‐time ischemic stroke were identified in the Danish Stroke Registry (2005–2018). Patients treated with vitamin K antagonist (VKA) therapy were compared according to the international normalized ratio just before stroke (international normalized ratio <2 [subtherapeutic], international normalized ratio 2–3 [therapeutic], international normalized ratio >3 [supratherapeutic]), and patients on underdosed, appropriately dosed, and overdosed direct OAC (DOAC) therapy were compared. Stroke severity was determined using the Scandinavia Stroke Scale (0–58 points), and the risk of very severe stroke (0–14 points) was analyzed by multivariable logistic regression. One‐year mortality was determined using multivariable Cox regression. A total of 2319 patients with atrial fibrillation and stroke were included; 1196 were taking a VKA (subtherapeutic [46%], therapeutic [43%], supratherapeutic [11%]), and 1123 were taking DOAC (underdosed [23%], appropriately dosed [60%], and overdosed [17%]). Subtherapeutic and supratherapeutic VKA therapy (compared with therapeutic) and underdosed DOAC therapy (compared with appropriate and underdosed DOAC) patients were older, more often women, and more comorbid. Subtherapeutic VKA therapy was associated with very severe stroke (odds ratio [OR], 2.06 [95% CI, 1.28–3.31]), whereas supratherapeutic VKA therapy was not (OR, 1.24 [95% CI, 0.60–2.57]) compared with therapeutic VKA therapy. Patients on subtherapeutic and supratherapeutic VKA therapy had a higher 1‐year mortality (hazard ratio [HR], 1.66 [95% CI, 1.29–2.13]); HR, 1.55 [95% CI, 1.08–2.22], respectively) than those on therapeutic VKA therapy. Treatment with underdosed or overdosed DOAC therapy was not associated with very severe stroke (OR, 1.27 [95% CI, 0.76–2.15]; OR, 0.73 [95% CI, 0.37–1.43], respectively) and was not associated with 1‐year mortality (HR, 1.09 [95% CI, 0.83–1.44]; HR, 0.82 [95% CI, 0.57–1.18], respectively) than appropriate DOAC.
Conclusions
Half of the patients with atrial fibrillation with stroke were on inappropriate OAC therapy. Subtherapeutic VKA was associated with worse stroke severity and higher mortality rate than therapeutic VKA therapy. Neither underdosed nor overdosed DOAC was associated with worse outcomes in adjusted models compared with appropriately dosed DOAC. This study supports DOAC as a first‐line therapy over VKA.
Keywords: anticoagulation, atrial fibrillation, epidemiology, inappropriate anticoagulation, ischemic stroke
Subject Categories: Atrial Fibrillation, Ischemic Stroke, Anticoagulants, Epidemiology
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulation
- OAC
oral anticoagulation
- VKA
vitamin K antagonist
Clinical Perspective
What Is New?
Investigation of appropriateness of prior oral anticoagulation therapy in patients with atrial fibrillation with first‐time ischemic stroke with long‐term follow‐up and the first study with data on direct oral anticoagulants.
Subtherapeutic vitamin K antagonist therapy was associated with worse stroke severity, and subtherapeutic and supratherapeutic vitamin K antagonist therapies were associated with higher 1‐year mortality than therapeutic vitamin K antagonist therapy in adjusted models.
We found no difference in stroke severity and 1‐year mortality between inappropriate and appropriate direct oral anticoagulation therapy in adjusted models.
What Are the Clinical Implications?
Identification of high‐risk patients with atrial fibrillation.
The data support direct oral anticoagulants as a first‐line therapy over vitamin K antagonist.
Despite treatment with oral anticoagulation (OAC) therapy, 1% to 2% of patients with atrial fibrillation (AF) still experience stroke annually. 1 , 2 , 3 , 4 , 5 Previous studies have found an association between subtherapeutic vitamin K antagonist (VKA) therapy and worse stroke severity and higher short‐term mortality 2 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ; however, contemporary and long‐term follow‐up data are lacking. Knowledge about the treatment quality of direct OAC (DOAC) (ie, underdosed or overdosed treatment) before stroke has not been investigated and may be a potential reason for DOAC failure. 14
DOAC therapy has become the standard of care for stroke prevention in patients with AF. However, traditional VKA is still used in many patients worldwide. Appropriate treatment with DOAC is dosed according to age, renal function, body weight, and interacting drugs. In contrast, VKA is dose adjusted to maintain an international normalized ratio (INR) between 2 and 3. 1 Strokes in patients on OAC therapy have been associated with a higher risk of recurrent stroke compared with no OAC therapy 15 and often rule out the use of intravenous thrombolytics. 16 Characterization of the association between inappropriate versus appropriate dosing of OAC and outcomes may help optimize the primary stroke prophylaxis in patients with AF and reduce excess stroke risk.
Accordingly, in this Danish nationwide cohort study, we examined the characteristics, stroke severity, and long‐term outcomes of patients with AF according to OAC therapy and the appropriateness of the dosing regimen at the time of admission for stroke.
METHODS
Data Sources
In Denmark, a public health care system enables nationwide health care studies based on the personal identification number that all citizen are given at birth or immigration. 17 The diagnosis of acute stroke was obtained from the Danish Stroke Registry (2003 onward) and have a sensitivity of 97% and a positive predictive value of 90%. 18 The diagnosis of prior AF, in the absence of valvular heart diseases and prior heart valve surgery, were found in the Danish Patient Registry (1978 onward). 19 The National Prescription Registry provided information on redeemed prescriptions since 1995. 20 Vital status was drawn from the Danish Civil Registration System and date of death from The Danish Registry of Causes of Death. Data on INR and creatinine were obtained from electronic registries from laboratories of hospitals in 4 out of 5 regions in Denmark and general practitioners in the Capital Region of Denmark. The International Classification of Diseases, Eighth Revision and Tenth Revision (ICD‐8; ICD‐10) and Anatomical Therapeutic Chemical Classification System codes used are shown in Table S1. No raw data are accessible by law. The corresponding author can provide details of the analyses on request.
Study Population
Patients were included if they were admitted with first‐time ischemic stroke or unspecified stroke and a history of AF at any time before admission for stroke (ie, index date). We included patients from January 1, 2005 to ensure higher data completeness in the Danish Stroke Registry. Hereafter, stroke refers to a group of both ischemic and unspecified strokes. Previously, Krarup et al found that two‐thirds of unspecified strokes registered in the Danish National Patient Registry were ischemic strokes and were therefore included. 21 Patients without a filled prescription for either a VKA (warfarin or marcoumar) or DOAC (dabigatran, rivaroxaban, apixaban, or edoxaban) up to 90 days before the stroke were excluded. Patients on VKA therapy without an INR value measured ≤30 days before the stroke or on the day of hospitalization were also excluded (Figure 1).
Figure 1. Selection of the study population.
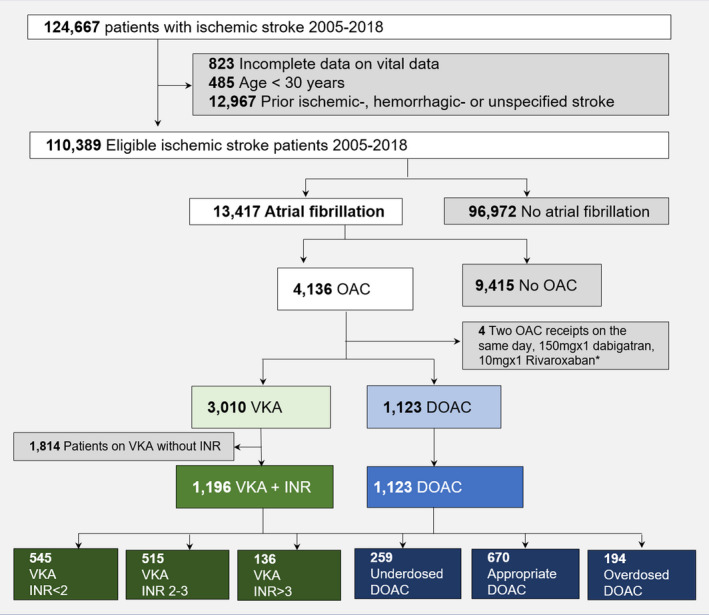
DOAC indicates direct oral anticoagulation, INR, international normalized ratio; OAC, oral anticoagulation; and VKA, vitamin K antagonist. *Patients prescribed dabigatran 150 mg or rivaroxaban 10 mg were excluded because these doses are typically given after orthopedic procedures.
Covariates
Baseline concomitant pharmacotherapy, apart from anticoagulation therapy, was defined as redeemed prescriptions ≤180 days before the index date. Information on comorbidities was obtained any time before the index admission from the Danish National Patient Registry. Information on smoking status, type of residence, civil status, thrombolysis, and thrombectomy were obtained from the Danish Stroke Registry. Modified CHA2DS2–VASc (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, history of stroke/transient ischemic attack/systemic arterial thromboembolism [doubled], vascular disease, age 65–74 years, and sex category [women]), and HAS‐BLED (hypertension, abnormal renal or liver function, stroke, bleeding history, labile INR, elderly [age >65 years], drug consumption, or alcohol abuse) scores were calculated as described previously. 22 , 23 The estimated glomerular filtration rate was calculated using the chronic kidney disease–epidemiology collaboration equation on an individual basis using creatinine values measured within 3 years before the stroke.
Exposure—Appropriateness of Oral Anticoagulation Therapy
Patients on VKA therapy were categorized according to the latest INR value within 30 days before stroke or on the day of admission: INR <2 (subtherapeutic), INR 2 to 3 (therapeutic), INR >3 (supratherapeutic). The dose reduction criteria applied in this study were based on recommendations from the European Society of Cardiology, Danish national guidelines, and data availability (ie, we did not have data on body weight and creatinine values for all patients). 1 The criteria are displayed in Table S2. Underdosed DOAC therapy was defined as low‐dose DOAC without guideline recommendations justifying this. Overdosed DOAC therapy was defined as high‐dose DOAC therapy despite fulfilling the guideline criteria for low‐dose DOAC. Inappropriate DOAC was defined as either underdosed or overdosed DOAC.
Outcomes
The primary outcome was stroke severity acquired from the Danish Stroke Registry determined on admission and graded according to the Scandinavian Stroke Scale based on 9 different parameters: speech (10–0 points), orientation (6–0 points), facial palsy (2–0 points), consciousness (6–0 points), eye movement (4–0 points), gait (12–0 points) and hand, arm, and leg motor power (6–0 points each). The Scandinavian Stroke Scale can be categorized into very severe stroke (0–14 points), severe stroke (15–29 points), moderate stroke (30–44 points), and mild stroke (45–58 points). 24 The Scandinavian Stroke Scale is equally as good as the National Institutes of Health Stroke Scale in predicting mortality and disability. 25 , 26 , 27 The secondary outcome was 1‐year all‐cause mortality. Patients were followed from the date of stroke admission until the occurrence of death or the end of the study (December 31, 2019), whichever came first.
Statistical Analysis
Baseline characteristics were described as frequencies and percentages and medians with 25th to 75th percentiles. Covariates were compared using the χ2 test for categorical variables and the Kruskal‐Wallis test for continuous variables. Differences within groups were tested using the Cochran‐Mantel‐Haenszel test.
The odds ratio (OR) of very severe stroke (0–14 points) versus the composite of mild, moderate, and severe stroke (15–58 points) was analyzed for patients on VKA therapy (VKA with INR 2–3 [reference], VKA INR <2, VKA INR >3) and DOAC therapy (appropriate DOAC [reference], underdosed DOAC, overdosed DOAC) in 2 separate multivariable logistic regression models. The models were adjusted for age, sex, calendar year, diabetes, chronic kidney disease, transient ischemic attack/thromboembolism, ischemic heart disease, chronic obstructive lung disease, cancer, peripheral artery disease, heart failure, prior bleeding, liver disease, hypertension, dementia, alcohol abuse, and prior use of statins, aspirin, nonsteroidal anti‐inflammatory drugs, and adenosine diphosphate receptor inhibitors. Patients with missing Scandinavian Stroke Scale values were excluded from the analysis. Interaction with age, sex, and calendar year were tested, and no clinically meaningful interactions were found. Continuous variables were tested for linearity.
The cumulative incidence of 1‐year mortality (including in‐hospital mortality) was assessed using the Kaplan‐Meier method, and differences were tested using the log‐rank test. The relative rate of mortality associated with OAC treatment quality was determined using a multivariable Cox proportional hazards model and adjusted for the same factors as the multivariable logistic regression models. The Cox models were tested for the proportional hazards assumption and linearity of continuous variables. The interaction between exposure and age, sex, and calendar year on the outcome were tested, and no clinically meaningful interactions were found. A 2‐sided P value of <0.05 was considered statistically significant. Numbers ≤3 are not reported because of local data protection regulations. All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC).
Sensitivity Analysis
We performed several sensitivity analyses to test the robustness of our findings.
Categorization of patients on DOAC into underdosed, overdosed, and appropriately dosed were analyzed in a sensitivity analysis with the original guideline criteria (Table S2). As such, we included all patients on DOAC with an available creatinine value within the past 3 years before the stroke, and for patients on apixaban and edoxaban, a body weight estimate (from index stroke admission) was also required.
In a sensitivity analysis of patients on apixaban, the effect of applying 1 of 2 criteria for dose reduction was assessed compared with applying 2 of 2 criteria.
The analyses were repeated on a population, excluding all patients with unspecified strokes.
We performed a sensitivity analysis excluding all patients on VKA before the first DOAC was approved in Denmark (August 22, 2011).
Ethics
In Denmark, no ethical approval is needed for registry‐based research, because individuals cannot be identified in the data. The Danish Data Protection Agency approved this study (approval number: P‐2019‐191).
RESULTS
Baseline Characteristics
There were 13 417 patients with AF admitted with stroke (2005–2018), of whom 4136 (30.8%) were treated with OAC. After the last exclusion criteria were applied, 2319 patients were included: 1196 patients on VKA and 1123 on DOAC. Data on the duration of OAC therapy and switching of OAC therapy before stroke are shown in Table S3. Of all strokes included, 2205 patients had an ischemic stroke (97.9% and 92.5% in the VKA and DOAC groups, respectively), and 114 had an unspecified stroke (2.1% and 7.5% in the VKA and DOAC groups, respectively). The baseline characteristics of the patients on VKA and DOAC overall are shown in Table S4.
Baseline Characteristics of Patients on VKA
In total, 545 (45.6%) patients on VKA had an INR <2, 515 (43.1%) had an INR between 2 and 3, and 136 (11.4%) had an INR >3. The median INR of all patients on VKA was 2.0 (25th–75th percentile, 1.5–2.6), and the majority of INR values were either measured at the index date for stroke (52.3%) or the day before (38.1%).
Overall, patients on sub‐ or supratherapeutic VKA therapy were more often women, slightly older, more comorbid, and more often on concomitant therapy with aspirin than those on therapeutic VKA therapy (Table). Among all patients on VKA, 245 (20.7%) patients were included before 2010, when recommendations of VKA to patients with AF were included in the European Society of Cardiology guidelines. 28
Table 1.
Baseline Patient Characteristics of Patients on VKA Stratified Upon INR and DOAC Stratified Upon Underdosed, Appropriate, and Overdosed Treatment
| Variable |
VKA INR <2 |
VKA INR 2–3 |
VKA INR >3 |
Underdosed DOAC | Appropriate DOAC | Overdosed DOAC |
|---|---|---|---|---|---|---|
| No. (%) | 545 (45.6) | 515 (43.1) | 136 (11.4) | 259 (23.1) | 670 (59.7) | 194 (17.3) |
| Sex (men) | 283 (51.9) | 322 (62.5) | 74 (54.4) | 95 (36.7) | 374 (55.8) | 111 (57.2) |
| Median age, y, 25th–75th percentile | 80 (73–86) | 79 (73–84) | 81 (75–85) | 85 (79–89) | 77 (71–83) | 76 (70–82) |
| Comorbidities, n (%) | ||||||
| Diabetes | 114 (20.9) | 90 (17.5) | 29 (21.3) | 46 (17.8) | 137 (20.4) | 39 (20.1) |
| Hypertension | 410 (75.2) | 362 (70.3) | 97 (71.3) | 156 (60.2) | 445 (66.4) | 150 (77.3) |
| Alcohol abuse | 20 (3.7) | 10 (1.9) | 7 (5.1) | 17 (6.6) | 39 (5.8) | 19 (9.8) |
| Prior bleeding | 156 (28.6) | 138 (26.8) | 35 (25.7) | 82 (31.7) | 163 (24.3) | 51 (26.3) |
| Cancer | 145 (26.6) | 113 (21.9) | 29 (21.3) | 57 (22.0) | 169 (25.2) | 36 (18.6) |
| Congestive heart failure | 190 (34.9) | 157 (30.5) | 53 (39.0) | 90 (34.7) | 184 (27.5) | 55 (28.4) |
| Chronic kidney disease | 61 (11.2) | 47 (9.1) | 13 (9.6) | 5 (1.9) | 52 (7.8) | 27 (13.9) |
| TIA/TE | 89 (16.3) | 80 (15.5) | 33 (24.3) | 39 (15.1) | 81 (12.1) | 14 (7.2) |
| COPD | 88 (16.1) | 81 (15.7) | 26 (19.1) | 47 (18.1) | 108 (16.1) | 28 (14.4) |
| PAD | 46 (8.4) | 47 (9.1) | 21 (15.4) | 25 (9.7) | 52 (7.8) | 18 (9.3) |
| Ischemic heart disease | 203 (37.2) | 198 (38.4) | 58 (42.6) | 101 (39.0) | 220 (32.8) | 71 (36.6) |
| Concomitant medication, n (%) | ||||||
| Acetylsalicylic acid | 98 (18.0) | 61 (11.8) | 24 (17.6) | 32 (12.4) | 75 (11.2) | 36 (18.6) |
| ADP inhibitors | 11 (2.0) | 8 (1.6) | ≤3 | 20 (7.7) | 25 (3.7) | 7 (3.6) |
| NSAID | 49 (9.0) | 41 (8.0) | 10 (7.4) | 18 (6.9) | 64 (9.6) | 28 (14.4) |
| Diuretics* | 356 (65.3) | 312 (60.6) | 81 (59.6) | 145 (56.0) | 355 (53.0) | 117 (60.3) |
| Digoxin | 188 (34.5) | 201 (39.0) | 50 (36.8) | 97 (37.5) | 208 (31.0) | 61 (31.4) |
| Verapamil | 71 (13.0) | 55 (10.7) | 13 (9.6) | 9 (3.5) | 33 (4.9) | 6 (3.1) |
| Statins | 208 (38.2) | 201 (39.0) | 46 (33.8) | 95 (36.7) | 248 (37.0) | 81 (41.8) |
| HAS‐BLED score | ||||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ≤3 | 0 (0.0) |
| 1 | 12 (2.2) | 12 (2.3) | 4 (2.9) | 4 (1.5) | 19 (2.8) | 0 (0.0) |
| 2 | 80 (14.7) | 99 (19.2) | 30 (22.1) | 64 (24.7) | 165 (24.6) | 10 (5.2) |
| ≥3 | 453 (83.1) | 404 (78.4) | 102 (75.0) | 191 (73.7) | 485 (72.4) | 184 (94.8) |
| CHA2DS2‐VASc score | ||||||
| 0 | 4 (0.7) | 4 (0.8) | ≤3 | ≤3 | 13 (1.9) | 0 (0.0) |
| 1 | 20 (3.7) | 28 (5.4) | ≤3 | 6 (2.3) | 52 (7.8) | 6 (3.1) |
| ≥2 | 521 (95.6) | 483 (93.8) | 133 (97.8) | 252 (97.3) | 605 (90.3) | 188 (96.9) |
| Smoking | ||||||
| Smoker | 60 (14.1) | 73 (17.2) | 25 (21.0) | 31 (15.4) | 103 (17.9) | 45 (26.5) |
| Former smoker, >0.5 y | 161 (37.8) | 164 (38.7) | 51 (42.9) | 80 (39.8) | 253 (43.9) | 63 (37.1) |
| Never | 205 (48.1) | 187 (44.1) | 43 (36.1) | 90 (44.8) | 220 (38.2) | 62 (36.5) |
| Civil status | ||||||
| Cohabiting | 275 (51.3) | 315 (62.6) | 67 (52.3) | 81 (31.8) | 359 (54.6) | 107 (55.4) |
| Alone | 246 (45.9) | 177 (35.2) | 58 (45.3) | 163 (63.9) | 274 (41.6) | 80 (41.5) |
| Other | 15 (2.8) | 11 (2.2) | ≤3 | 11 (4.3) | 25 (3.8) | 6 (3.1) |
| Type of residence | ||||||
| Own residence | 491 (92.1) | 472 (93.5) | 123 (95.3) | 212 (83.1) | 595 (90.7) | 180 (93.8) |
| Care home | 34 (6.4) | 27 (5.3) | 6 (4.7) | 43 (16.9) | 58 (8.8) | 11 (5.7) |
| Other | 8 (1.5) | 6 (1.2) | 0 (0.0) | 0 (0.0) | ≤3 | ≤3 |
| Thrombolysis † | ||||||
| Yes | 36 (7.6) | ≤3 | 0 (0.0) | 7 (2.7) | 23 (3.5) | 6 (3.2) |
| No | 40 (8.4) | 44 (9.4) | 17 (13.8) | ≤3 | 6 (0.9) | ≤3 |
| Contraindicated | 400 (84.0) | 420 (90.1) | 106 (86.2) | 245 (96.1) | 636 (95.6) | 181 (95.8) |
| Thrombectomy † | ||||||
| Yes | 18 (4.9) | 10 (2.6) | ≤3 | 10 (3.9) | 31 (4.7) | 11 (5.8) |
| No | 17 (4.6) | 16 (4.2) | 6 (7.1) | 4 (1.6) | 8 (1.2) | ≤3 |
| Not indicated | 331 (90.4) | 354 (93.2) | 76 (89.4) | 241 (94.5) | 625 (94.1) | 175 (92.6) |
| eGFR, mL/min per 1.73 m2 | ||||||
| ≥90 | 43 (11.3) | 48 (14.2) | 9 (8.3) | ≤3 | 52 (17.6) | 22 (25.6) |
| 60–89 | 156 (40.8) | 162 (47.8) | 43 (39.8) | 44 (35.5) | 147 (49.7) | 33 (38.4) |
| 30–59 | 157 (41.1) | 118 (34.8) | 50 (46.3) | 72 (58.1) | 90 (30.4) | 30 (34.9) |
| 15–29 | 23 (6.0) | 9 (2.7) | 5 (4.6) | 5 (4.0) | 6 (2.0) | ≤3 |
| <15 | ≤3 | ≤3 | ≤3 | 0 | ≤3 | 0 |
ADP indicates adenosine diphosphate; COPD, chronic obstructive lung disease; DOAC, direct oral anticoagulation; eGFR, estimated glomerular filtration rate; HAS‐BLED, hypertension, abnormal renal or liver function, stroke, bleeding history, labile international normalized ratio, elderly (age >65 years), drug consumption or alcohol abuse; INR, international normalized ratio; NSAID, nonsteroidal anti‐inflammatory drugs; PAD, peripheral artery disease; TIA/TE, transient ischemic attack/thromboembolism; and VKA, vitamin K antagonist.
Diuretics: loop diuretics, non‐loop diuretics, thiazide, spironolactone, diuretics in combination (Anatomical Therapeutic Chemical Classification System codes C07C, C08G, C03B, C03X).
Registered from September 2011 onward. Missing (n): smoking (403), civil (46), type of residence (49), thrombolysis (145), thrombectomy (379), eGFR (984).
Baseline Characteristics of Patients on DOAC
The majority of patients on DOAC were appropriately dosed (670 patients [59.7%]), whereas 259 (23.1%) were underdosed, and 194 (17.3%) were overdosed. The distribution of different specific DOAC drugs is shown in Table S5. Patients on underdosed DOAC were more likely to be women and more comorbid, were a median of 8 to 9 years older, were more often living alone, and more often at a care home than patients who were on appropriately dosed DOAC and overdosed DOAC (Table).
Outcome
Stroke Severity Among Patients on VKA
The proportion of patients with very severe stroke was higher for patients on subtherapeutic VKA therapy (68 [13.5%]) and supratherapeutic VKA therapy (12 [9.6%]) than therapeutic VKA therapy (30 [6.2%]) (Figure 2A). In the adjusted models, subtherapeutic VKA therapy was associated with a higher likelihood of very severe stroke (OR, 2.06 [95% CI, 1.28–3.31]), but for supratherapeutic VKA therapy, the association was nonsignificant (OR, 1.24 [95% CI, 0.60–2.57]) (Figure 3A).
Figure 2. Stroke severity among patients on vitamin K antagonist (VKA) and direct oral anticoagulants (DOAC).

A, Median Scandinavian Stroke Scale (SSS): VKA with international normalize ratio (INR) <2 (46.0 [25th–75th percentile, 26.0–54.0]), INR 2 to 3 (51.0 [25th–75th percentile, 42.0–56.0]), INR >3 (51.0 [25th–75th percentile, 42.0–56.0]). B, Median SSS: Underdosed DOAC (43.0 [25th–75th percentile, 26.0–52.0]), appropriate DOAC (48.0 [25th–75th percentile, 36.0–55.0]), overdosed DOAC (51.0 [25th–75th percentile, 41.0–55.0]). Missing on SSS: VKA (86 [7.2%]) and DOAC (28 [2.5%]); p indicates points. *Cochran‐Mantel‐Haenszel test.
Figure 3. Multivariable logistic regression model.

Odds ratio (OR) of very severe stroke among patients on vitamin K antagonist (VKA) (A) and patients on direct oral anticoagulation (DOAC) (B). Scandinavian Stroke Scale: Very severe stroke (0–14 points), mild‐severe stroke (15–58 points). INR indicates international normalized ratio.
Stroke Severity Among Patients on DOAC
Patients on underdosed DOAC were more likely to have very severe stroke than those on appropriate and overdosed DOAC therapy (32 [12.8%], 54 [8.3%], 13 [6.8%], respectively) (Figure 2B). After adjustment, the estimates became nonsignificant for patients on underdosed DOAC (OR, 1.27 [95% CI, 0.76–2.15]) and overdosed DOAC (OR, 0.73 [95% CI, 0.37–1.43]) (Figure 3B).
Mortality Among Patients on DOAC
The absolute risk and relative mortality rates were higher for patients on subtherapeutic VKA than for therapeutic VKA (absolute risk difference 15.3% [P<0.01]; hazard ratio [HR], 1.66 [95% CI, 1.29–2.13]). The absolute risk and relative mortality rates were also higher for patients on supratherapeutic VKA than those on therapeutic VKA (absolute risk difference 13.0% [P<0.01]; HR, 1.55 [95% CI, 1.08–2.22]) (Figure 4A).
Figure 4. Cumulative incidence of 1‐year mortality and adjusted hazard ratio (HR) among patients on vitamin K antagonist (VKA) and direct oral anticoagulation (DOAC).

A, VKA: VKA INR <2: (34.7% [95% CI, 30.7–38.7]),* adjusted HR, 1.66 (95% CI, 1.29–2.13); VKA INR 2 to 3: (19.4% [95% CI, 16.1–22.9]), reference; VKA INR >3: (32.4% [95% CI, 24.6–40.3]),† adjusted HR, 1.55 (95% CI, 1.08–2.22). *P<0.01. † P<0.01. B, DOAC: Underdosed DOAC: (36.7% [95% CI, 30.8–42.5]),‡ adjusted HR, 1.09 (95% CI, 0.83–1.44); Appropriate DOAC: (25.8% [95% CI, 22.6–29.2]), reference; Overdosed DOAC: (20.1% ([95% CI, 14.8–26.0]),{} adjusted HR 0.82 (95% CI, 0.57–1.18). ‡ P <0.01. {} P=0.09. INR indicates international normalized ratio.
Mortality Among Patients on DOAC
The absolute 1‐year mortality was significantly higher for patients on underdosed DOAC compared with appropriate DOAC (risk difference of 10.9% [P<0.01]) but became nonsignificant in adjusted models (HR, 1.09 [95% CI, 0.83–1.44]). The absolute and relative 1‐year mortality rates were lower for patients on overdosed DOAC compared with appropriate DOAC, but statistically nonsignificant (risk difference of 5.7% [P = 0.09]; HR, 0.82 [95% CI, 0.57–1.18]) (Figure 4B).
Sensitivity analysis
First, we included all patients on DOAC with an available creatinine value, and for apixaban and edoxaban also an available body weight estimate, and analyzed the appropriateness of the DOAC dose using the original dose‐reduction criteria (Table S2). In total, 53 (15.1%) were underdosed, 211 (59.9%) were appropriately dosed, and 88 (25.0%) were overdosed according to the guideline‐specific criteria. The baseline characteristics were similar; however, outcome analyses were limited by power (Table S6 and Figure S1).
Second, we tested the categorization of apixaban patients according to dose reduction criteria requiring 1 of 2 criteria instead of 2 of 2 criteria for dose reduction. Applying 1 of 2 criteria for dose reduction categorized 21 (5.5%) patients as underdosed, 270 (70.5%) patients as appropriately dosed, and 92 (24.0%) as overdosed.
Third, we conducted a sensitivity analysis excluding 114 patients (4.9%) with unspecified stroke and found similar results to the main analysis. Among patients on VKA, 502 (45.4%) had an INR <2, 479 (43.3%) had an INR between 2 and 3, and 125 (11.3%) had an INR >3. Among patients on DOAC, 253 (23.0%) were underdosed, 654 (59.5%) were appropriately dosed, and 192 (17.5%) were overdosed.
Fourth, we excluded all patients on VKA before August 22, 2011. There were 799 patients who experienced a stroke treated with VKA, and 348 (43.6%) had an INR <2, 370 (46.3%) had an INR between 2 and 3, and 81 (10.1%) had an INR >3. Outcomes on stroke severity (Table S7) and mortality (Figure S2) were comparable to the main analysis.
DISCUSSION
This study examined the characteristics of patients with ischemic stroke and prior AF on inappropriate versus appropriate OAC therapy in a real‐world setting and yielded 3 major findings. First, patients on sub‐ or supratherapeutic VKA therapy were slightly older, were more likely to be women, and had a higher comorbidity burden than patients on therapeutic VKA. Patients on underdosed DOAC were older, more likely to be women, and were more comorbid than patients on appropriate or overdosed DOAC. Second, subtherapeutic VKA therapy was associated with worse stroke severity in absolute and relative numbers than therapeutic VKA therapy. This difference was not found between inappropriate and appropriate DOAC therapy after adjustment for baseline covariates. Third, subtherapeutic and supratherapeutic VKA therapy was associated with higher 1‐year mortality compared with therapeutic VKA therapy. This difference in mortality was not found among patients receiving inappropriate versus appropriate DOAC therapy.
To our knowledge, this is the first study to describe the appropriateness of prior VKA and DOAC treatment in patients with first‐time ischemic stroke with long‐term follow‐up. Previous observational studies and clinical trials 2 , 6 , 7 , 8 , 9 , 13 , 29 , 30 , 31 , 32 included patients with a history of prior stroke/transient ischemic attack, yet different mechanisms and risk factors exist in this patient group. 33 Furthermore, this heterogeneous study population is not optimal for the characterization of stroke patients on OAC therapy. This lack of knowledge is supported by a systematic review of the literature by Meinel et al. 14 Many studies have examined the risk of stroke according to OAC therapy. However, by looking at prior OAC therapy in patients who present with stroke potential, preventable strokes might be identified. Of all patients with first‐time ischemic stroke, 30.8% were on prior OAC treatment, which is comparable to what was found by Gundlund et al and Gadsbøll et al. 34 , 35 Of the patients on VKA, 57% had an INR out of range before stroke, and 40.4% of the patients on DOAC were inappropriately dosed.
Patient Characteristics
In line with prior studies, we found that patients on therapeutic VKA were only slightly younger than patients on subtherapeutic and supratherapeutic VKA. 7 , 9 , 11 , 13 Additionally, we found that stroke in patients on underdosed DOAC were 8 to 9 years older than patients on appropriate and overdosed DOAC. This has only been described in a much younger Asian study population by Jung et al. 36 As in previous studies, 2 , 7 we also found a higher proportion of women among subtherapeutic and supratherapeutic patients on VKA. However, Sakamoto et al found fewer women among patients on subtherapeutic VKA therapy. 6 In this study, we also found a higher proportion of women among patients on underdosed DOAC, but not among patients on overdosed DOAC, which was not found by Jung et al. 36 Patients on subtherapeutic and supratherapeutic VKA were more comorbid compared with patients on therapeutic VKA in the present study. However, other studies did not find this clear contrast in comorbidity burden among patients on VKA. 2 , 7 , 8 , 9 , 13 , 29 , 37 This study additionally describes that patients on underdosed DOAC were older and more often comorbid women than patients on appropriate and overdosed DOAC. Patients on overdosed DOAC were more like patients on appropriate DOAC but were slightly more comorbid.
Stroke Severity
Subtherapeutic VKA therapy, rather than therapeutic VKA therapy, was associated with worse stroke severity in the adjusted models. Prior studies found that patients on therapeutic VKA therapy were associated with lower stroke severity, measured on the National Institutes of Health Stroke Scale compared with subtherapeutic VKA therapy. 2 , 6 , 8 , 9 , 10 , 11 , 12 , 13 However, Indredavik et al found no significant difference in stroke severity among patients on VKA with an INR of <2 and ≥2. 38
In the present study, underdosed or overdosed DOAC was not significantly associated with very severe stroke in the adjusted models. Jung et al found, in a Korean population, lower stroke severity among patients on underdosed DOAC compared with standard dose DOAC, yet they were limited by power. 36
Mortality
In our study, we found that inappropriate versus appropriate VKA therapy at the time of stroke was associated with higher 1‐year mortality. However, among patients on DOAC, this difference did not hold true in adjusted models, because it was largely explained by patients on inappropriate therapy being older and/or with more comorbidities.
Most of the existing literature only reports in‐hospital mortality or mortality as a part of the modified Rankin Scale, and most studies are limited by power. 2 , 7 , 8 , 9 , 13 Xian et al found higher in‐hospital mortality, and Schwammenthal et al found a higher risk of 1‐year mortality (nonsignificant in adjusted models) for patients on VKA, with INR <2, compared with VKA, with INR ≥2. 2 , 11 Patients on underdosed or overdosed DOAC compared with appropriately dosed DOAC were not associated with a higher rate of 1‐year mortality in adjusted models.
The reasons for inappropriate VKA therapy are probably not the same as those for inappropriate DOAC therapy; this will also affect characteristics and outcomes. Nevertheless, this study supports the clinical guidelines in recommending DOAC as first‐line therapy over VKA.
Limitations
This was an observational study using nationwide real‐world data enabling the characterization of patients outside a clinical trial setting. However, this type of study only describes associations; residual bias cannot be precluded, and there might be risk of confounding by indication in a population who already have experienced a stroke. The different DOAC groups and VKA groups have different distribution of age and sex, and we cannot fully elucidate the effect of age and sex on the association with the outcome. This is an inherent limitation of the study design.
Of the entire study population, 4.9% had an unspecified stroke, and based on Krarup et al, around one‐third of these may be hemorrhagic strokes. 21 Because we did not have information on body weight and creatinine values for all patients, we had to modify the guideline‐specific dose‐reduction criteria for DOAC. However, we conducted a sensitivity analysis of 25% of the patients on DOAC who had a creatinine value within 3 years before stroke and body weight estimates and yielded similar results. We used creatinine values taken within 3 years before stroke to avoid too much missing data. In the main analysis for apixaban, we choose to apply 2 of 2 criteria for dose reduction, instead of 1 of 2 criteria, as is recommended by Alexander et al. 39
CONCLUSIONS
Approximately half of the included patients with AF, who experienced an ischemic stroke on OAC therapy, were on inappropriately dosed therapy. Patients who were on subtherapeutic VKA treatment just before the stroke had a higher associated stroke severity and higher rate of 1‐year mortality compared with patients on therapeutic VKA. Among patients treated with underdosed or overdosed DOAC than appropriately dosed DOAC, no association with more severe strokes and higher 1‐year mortality was found after adjustment for baseline characteristics. This study supports DOACs as a first‐line therapy over VKA.
Sources of Funding
This work was supported by an internal grant from the Department of Cardiology, Copenhagen University Hospital, Copenhagen, Denmark. The funding source did not have influence on any part of the submitted work including the study design; in the collection, analysis, and interpretation of data; in the writing of the report, and in the decision to submit the article for publication.
Disclosures
Drs Vinding, Butt, Rørth, Bonde, Gundlund, Yafasova, and Weeke have nothing to disclose. Dr Olesen received speaker’s honoraria or consultancy fees from Bristol‐Myers Squibb and Pfizer outside of the submitted work. Dr Xian received a research grant to the Duke Clinical Research Institute from Janssen and Daiichi Sankyo. Dr Kristensen received a speaker’s fee from Astra Zeneca outside of the submitted work. Dr Gislason received research grants from Boerhinger Ingelheim, Bristol‐Myers Squibb, and Pfizer. Dr Torp‐Pedersen received grants for studies from Bayer and Novo Nordisk. Dr Køber received speaker’s honoraria from Novo, Novartis, AstraZeneca, and Boehringer, unrelated to this article. Dr Fosbøl received an independent research grant from the Novo Nordisk Foundation.
Supporting information
Tables S1–S7
Figures S1–S2
Acknowledgments
The National Quality Improvement Program made it possible to use the Danish Stroke Registry in this study.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2. Xian Y, O’Brien EC, Liang LI, Xu H, Schwamm LH, Fonarow GC, Bhatt DL, Smith EE, Olson DM, Maisch L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in‐hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317:1057–1067. doi: 10.1001/jama.2017.1371 [DOI] [PubMed] [Google Scholar]
- 3. Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913 [DOI] [PubMed] [Google Scholar]
- 4. Almutairi AR, Zhou L, Gellad WF, Lee JK, Slack MK, Martin JR, Lo‐Ciganic WH. Effectiveness and safety of non–vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta‐analyses. Clin Ther. 2017;39:1456–1478. doi: 10.1016/j.clinthera.2017.05.358 [DOI] [PubMed] [Google Scholar]
- 5. Ding WY. Residual stroke risk in atrial fibrillation. Arrhythm Electrophysiol Rev. 2021;10:147–153. doi: 10.15420/aer.2021.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakamoto Y, Okubo S, Nito C, Suda S, Matsumoto N, Abe A, Aoki J, Shimoyama T, Takayama Y, Suzuki K, et al. The relationship between stroke severity and prior direct oral anticoagulant therapy in patients with acute ischaemic stroke and non‐valvular atrial fibrillation. Eur J Neurol. 2017;24:1399–1406. doi: 10.1111/ene.13405 [DOI] [PubMed] [Google Scholar]
- 7. Tomita H, Hagii J, Metoki N, Saito S, Shiroto H, Hitomi H, Kamada T, Seino S, Takahashi K, Sasaki S, et al. Severity and functional outcome of patients with cardioembolic stroke occurring during non‐vitamin K antagonist oral anticoagulant treatment. J Stroke Cerebrovasc Dis. 2015;24:1430–1437. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 8. Hellwig S, Grittner U, Audebert H, Endres M, Haeusler KG. Non‐vitamin K‐dependent oral anticoagulants have a positive impact on ischaemic stroke severity in patients with atrial fibrillation. Europace. 2018;20:569–574. doi: 10.1093/europace/eux087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tokunaga K, Koga M, Itabashi R, Yamagami H, Todo K, Yoshimura S, Kimura K, Sato S, Terasaki T, Inoue M, et al. Prior anticoagulation and short‐ or long‐term clinical outcomes in ischemic stroke or transient ischemic attack patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2019;8:e010593. doi: 10.1161/JAHA.118.010593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auer E, Frey S, Kaesmacher J, Hakim A, Seiffge DJ, Goeldlin M, Arnold M, Fischer U, Jung S, Meinel TR. Stroke severity in patients with preceding direct oral anticoagulant therapy as compared to vitamin K antagonists. J Neurol. 2019;266:2263–2272. doi: 10.1007/s00415-019-09412-y [DOI] [PubMed] [Google Scholar]
- 11. Schwammenthal Y, Bornstein N, Schwammenthal E, Schwartz R, Goldbourt U, Tsabari R, Koton S, Grossman E, Tanne D. Relation of effective anticoagulation in patients with atrial fibrillation to stroke severity and survival (from the National Acute Stroke Israeli Survey [NASIS]). Am J Cardiol. 2010;105:411–416. doi: 10.1016/j.amjcard.2009.09.050 [DOI] [PubMed] [Google Scholar]
- 12. Haeusler KG, Konieczny M, Endres M, Villringer A, Heuschmann PU. Impact of anticoagulation before stroke on stroke severity and long‐term survival. Int J Stroke. 2012;7:544–550. doi: 10.1111/j.1747-4949.2011.00672.x [DOI] [PubMed] [Google Scholar]
- 13. O'Donnell M, Oczkowski W, Fang J, Kearon C, Silva J, Bradley C, Guyatt G, Gould L, D'Uva C, Kapral M, et al. Preadmission antithrombotic treatment and stroke severity in patients with atrial fibrillation and acute ischaemic stroke: an observational study. Lancet Neurol. 2006;5:749–754. doi: 10.1016/S1474-4422(06)70536-1 [DOI] [PubMed] [Google Scholar]
- 14. Meinel TR, Frey S, Arnold M, Kendroud S, Fischer U, Kaesmacher J, Heldner MR, Jung S. Clinical presentation, diagnostic findings and management of cerebral ischemic events in patients on treatment with non‐vitamin K antagonist oral anticoagulants – a systematic review. PLoS One. 2019;14:e0213379. doi: 10.1371/journal.pone.0213379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, Macha, MD K, Tsivgoulis G, Ambler G, Arihiro S, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87:677–687. doi: 10.1002/ana.25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Purrucker JC, Haas K, Rizos T, Khan S, Poli S, Kraft P, Kleinschnitz C, Dziewas R, Binder A, Palm F, et al. Coagulation testing in acute ischemic stroke patients taking non‐vitamin K antagonist oral anticoagulants. Stroke. 2017;48:152–158. doi: 10.1161/STROKEAHA.116.014963 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, Sørensen HT. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, Johnsen SP. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2013;6:27–36. doi: 10.2147/CLEP.S50449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Toft SH. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pottegård A, Schmidt SJA, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krarup L‐H, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28:150–154. doi: 10.1159/000102143 [DOI] [PubMed] [Google Scholar]
- 22. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen A‐M S, Gislason GH, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olesen JB, Lip GYH, Hansen PR, Lindhardsen J, Ahlehoff O, Andersson C, Weeke P, Hansen ML, Gislason GH, Torp‐Pedersen C. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost. 2011;9:1460–1467. doi: 10.1111/j.1538-7836.2011.04378.x [DOI] [PubMed] [Google Scholar]
- 24. Jørgensen H, Nakayama H, Raaschou H, Vive‐Larsen J, Støier M, Olsen T. Outcome and time course of recovery in stroke. Part I: outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2 [DOI] [PubMed] [Google Scholar]
- 25. Askim T, Bernhardt J, Churilov L, Indredavik B. The Scandinavian Stroke Scale is equally as good as the National Institutes of Health Stroke Scale in identifying 3‐month outcome. J Rehabil Med. 2016;48:909–912. doi: 10.2340/16501977-2155 [DOI] [PubMed] [Google Scholar]
- 26. Lindenstrøm E, Boysen G, Waage Christiansen L, à Rogvi Hansen B, Würtzen Nielsen P. Reliability of Scandinavian neurological stroke scale. Cerebrovasc Dis. 1991;1:103–107. doi: 10.1159/000108825 [DOI] [Google Scholar]
- 27. Barber M, Fail M, Shields M, Stott DJ, Langhorne P. Validity and reliability of estimating the Scandinavian Stroke Scale Score from medical records. Cerebrovasc Dis. 2004;17:224–227. doi: 10.1159/000075795 [DOI] [PubMed] [Google Scholar]
- 28. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, et al. Guidelines for the management of atrial fibrillation. Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350 [DOI] [PubMed] [Google Scholar]
- 29. Jung YH, Kim YD, Kim J, Han SW, Oh MS, Lee JS, Lee KY. Initial stroke severity in patients with atrial fibrillation according to antithrombotic therapy before ischemic stroke. Stroke. 2020;51:2733–2741. doi: 10.1161/STROKEAHA.120.030138 [DOI] [PubMed] [Google Scholar]
- 30. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 31. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 32. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 33. Edwards JD, Kapral MK, Fang J, Swartz RH. Long‐term morbidity and mortality in patients without early complications after stroke or transient ischemic attack. CMAJ. 2017;189:E954–E961. doi: 10.1503/cmaj.161142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gundlund A, Xian Y, Peterson ED, Butt JH, Gadsbøll K, Bjerring Olesen J, Køber L, Torp‐Pedersen C, Gislason GH, Loldrup EF. Prestroke and poststroke antithrombotic therapy in patients with atrial fibrillation: Results From a Nationwide Cohort. JAMA Netw Open. 2018;1:e180171. doi: 10.1001/jamanetworkopen.2018.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gadsbøll K, Staerk L, Fosbøl EL, Sindet‐Pedersen C, Gundlund A, Lip GYH, Gislason GH, Olesen JB. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;38:899–906. doi: 10.1093/eurheartj/ehw658 [DOI] [PubMed] [Google Scholar]
- 36. Jung YO, Choi H‐Y, Lee K‐Y, Cheon K, Han SW, Park JH, Cho H‐J, Park HJ, Nam HS, Heo JI, et al. Stroke severity in patients on non‐vitamin K antagonist oral anticoagulants with a standard or insufficient dose. Thromb Haemost. 2018;118:2145–2151. doi: 10.1055/s-0038-1675602 [DOI] [PubMed] [Google Scholar]
- 37. Merbach D, Lawrence E, Mallick D, Marsh EB. A therapeutic international normalized ratio results in smaller infarcts and better outcomes for patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:104278. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.036 [DOI] [PubMed] [Google Scholar]
- 38. Indredavik B, Rohweder G, Lydersen S. Frequency and effect of optimal anticoagulation before onset of ischaemic stroke in patients with known atrial fibrillation. J Intern Med. 2005;258:133–144. doi: 10.1111/j.1365-2796.2005.01512.x [DOI] [PubMed] [Google Scholar]
- 39. Alexander J, Andersson U, Lopes R, Hijazi Z, Hohnloser S, Ezekowitz J, Halvorsen S, Hanna M, Commerford P, Ruzyllo W, et al. Apixaban 5 mg twice daily and clinical outcomes in patients with atrial fibrillation and advanced age, body weight, or high creatinine. a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:673–681. doi: 10.1001/jamacardio.2016.1829 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S2


