Abstract
Background
This study examined the associations between quantitative optical coherence tomography angiography (OCTA) parameters and myocardial abnormalities as documented on cardiovascular magnetic resonance imaging in patients with systemic hypertension.
Methods and Results
We conducted a cross‐sectional study of 118 adults with hypertension (197 eyes). Patients underwent cardiovascular magnetic resonance imaging and OCTA (PLEX Elite 9000, Carl Zeiss Meditec). Associations between OCTA parameters (superficial and deep retinal capillary density) and adverse cardiac remodeling (left ventricular mass, remodeling index, interstitial fibrosis, global longitudinal strain, and presence of left ventricular hypertrophy) were studied using multivariable linear regression analysis with generalized estimating equations. Of the 118 patients with hypertension enrolled (65% men; median [interquartile range] age, 59 [13] years), 29% had left ventricular hypertrophy. After adjusting for age, sex, systolic blood pressure, diabetes, and signal strength of OCTA scans, patients with lower superficial capillary density had significantly higher left ventricular mass (β=−0.150; 95% CI, −0.290 to −0.010), higher interstitial volume (β=−0.270; 95% CI, −0.535 to −0.0015), and worse global longitudinal strain (β=−0.109; 95% CI, −0.187 to −0.032). Lower superficial capillary density was found in patients with hypertension with replacement fibrosis versus no replacement fibrosis (16.53±0.64 mm‐1 versus 16.96±0.64 mm‐1; P=0.003).
Conclusions
We showed significant correlations between retinal capillary density and adverse cardiac remodeling markers in patients with hypertension, supporting the notion that the OCTA could provide a non‐invasive index of microcirculation alteration for vascular risk stratification in people with hypertension.
Keywords: cardiovascular magnetic resonance, optical coherence tomography angiography, systemic hypertension
Subject Categories: High Blood Pressure, Hypertension, Imaging, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- ECV
extracellular volume fraction
- FAZ
foveal avascular zone
- GLS
global longitudinal strain
- OCTA
optical coherence tomography angiography
- RI
remodeling index
Clinical Perspective
What Is New?
To our knowledge, this study is the first to demonstrate a significant relationship between retinal capillary density and adverse cardiac remodeling markers in patients with systemic hypertension.
What Are the Clinical Implications?
This study supports the viewpoint that the optical coherence tomography angiography may be a promising tool to provide a noninvasive measure of the microcirculation in the field of hypertensive cardiovascular disease.
Hypertensive heart disease is a life‐threatening disease that affects multiple organ systems with significant impact on mortality and morbidity. 1 In the heart, hypertension results in the alterations of cardiomyocyte, interstitium, and coronary microvasculature. 2 , 3 , 4 Early detection of these adverse cardiac manifestations would help identify patients at risk of future development of heart failure for more intensive treatment. Left ventricular (LV) geometrical (eg, hypertrophy), myocardial structural changes (eg, fibrosis) and cardiomyocyte dysfunction (eg, global longitudinal strain) can be evaluated accurately by non‐invasive non‐radiating cardiovascular magnetic resonance (CMR) imaging as markers of subclinical LV dysfunction. 5 , 6 , 7 , 8 There is increasing research that suggests microvascular disease plays a key role in cardiac remodeling and dysfunction. 9 The coronary microvasculature, however, cannot be directly visualized using in vivo imaging techniques. 10 Non‐invasive evaluation of cardiac microcirculation has relied on functional tests using echocardiography, positron emission tomography, or CMR imaging, 11 , 12 , 13 which are often performed in specialized centers for research purposes.
The eye is the only organ that allows direct visualization of the microcirculation non‐invasively and retinal microcirculation abnormalities have been associated with an increased risk of cardiovascular diseases. Technologies such as retinal fundus imaging 14 have shown that some retinal microvascular measures (eg, retinopathy, retinal vessel caliber) may predict cardiovascular diseases. 15 , 16 , 17 , 18 , 19 , 20 Increasingly, however, newer technologies such as optical coherence tomography angiography (OCTA), 21 , 22 offer highly accurate, reproducible, 23 , 24 and relatively inexpensive quantification of the retinal capillary microcirculation. We 25 , 26 and others 27 , 28 , 29 , 30 , 31 , 32 , 33 have used the OCTA to image patients with hypertension and reported the associations of rarefaction of the retinal capillary network with higher blood pressure and lower kidney function. Whether OCTA measurements of microcirculation are associated with adverse LV remodeling remains to be determined.
In this study, we examined the associations between quantitative OCTA parameters and measures of LV remodeling on CMR in patients with hypertension. We hypothesize that patients with hypertension with reduction in the density of the retinal microvasculature (rarefaction) will have adverse cardiac remodeling on CMR.
Methods
Study Patients
The data that support the findings of this study are available from the corresponding author upon reasonable request. We conducted a cross‐sectional observational study in a clinic setting, detailed in Figure S1. Asymptomatic patients with essential hypertension were enrolled from the REMODEL study (Response of the myocardium to hypertrophic conditions in the adult population; NCT02670031). 6 , 25 The aim of REMODEL is to examine the role of cardiovascular magnetic resonance in patients with hypertension. Briefly, patients with essential hypertension on antihypertensive medications, aged ≥18 years, were recruited from a tertiary cardiac center and primary care clinics in Singapore, from 2018 to 2021. Patients with secondary causes of hypertension, any ongoing unstable medical conditions, previously diagnosed significant coronary artery disease, strokes, atrial fibrillation, women who are pregnant or breastfeeding, individuals with impaired renal function of estimated glomerular filtration rate (eGFR) <30 mL/min per 1.73 m2 and metallic implant were excluded. All studies were approved by the SingHealth Centralized Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Written Informed consent was obtained from all patients.
Examination Procedures
A questionnaire was used to collect demographic data, and medical history (eg, smoking, and diabetes) and number of anti‐hypertensives. Twenty‐four‐hour ambulatory blood pressure monitoring was performed on the patients. Patients’ height was measured in centimeters using a wall‐mounted measuring tape, and weight was measured in kilograms using a digital scale (SECA, model 782 2321009, Germany). Body mass index was calculated as body weight (in kilograms) divided by body height (in meters) squared. Body surface area was calculated using the DuBois formula. 34
CMR Protocol and Image Analysis
All patients underwent CMR scans (Siemens Aera 1.5T, Siemens Healthineers, Erlangen, Germany). Image analyses were performed by trained individuals at the National Heart Research Institute of Singapore CMR Core Laboratory using CVI42 software (Circle Cardiovascular Imaging, Calgary, Canada). Balanced steady‐state free precession cine images were acquired in the standard long‐axis (2‐, 3‐, and 4‐chamber) and short‐axis views (acquired voxel size: 1.6×1.3×8.0 mm slice thickness; 2 mm gap; 30 phases per cardiac cycle).
Left ventricular volumes, function, and mass were measured using standardized protocols and indexed to body surface area. 35 End‐diastolic myocardial wall thickness was measured semi‐automatically in the short‐axis views (50 chords per myocardial slice), according to the standard 16‐segment model. Short‐axis slices that did not have complete ring of myocardium were excluded in the wall thickness analysis. We have recently developed a novel marker (Remodeling Index [RI]) of adverse remodeling based on the Laplace Law of Wall Stress. 6 This index was calculated as . A lower RI value was associated with worse LV remodeling. The presence of left ventricular hypertrophy and low RI was defined according to age and sex‐specific indexed LV mass and RI ranges, respectively. 6 , 35
Myocardial fibrosis was assessed using 2 approaches: (1) presence of replacement myocardial fibrosis using late gadolinium enhanced (LGE) imaging and (2) quantification of diffuse interstitial fibrosis using myocardial T1 mapping. LGE imaging started at 8 minutes after administration of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Pharma AG, Germany). An inversion‐recovery fast gradient echo sequence was used, and the inversion time was optimized to achieve appropriate nulling of the myocardium. Myocardial T1 mapping was performed using the Modified Look‐Locker inversion‐recovery sequence (heartbeat acquisition scheme of 5(3)3 and 4(1)3(1)2 for native and post‐contrast myocardial T1, respectively). Extracellular volume fraction (ECV), which represents the extracellular matrix as a proportion of total LV myocardial volume, was estimated from the native and 15‐minute post‐contrast T1 map. 5 , 36 Hematocrit for ECV calculation was sampled on the day of CMR. Indexed interstitial volume was defined as ECV×LV end‐diastolic myocardial volume normalized to body surface area. 37
Global longitudinal strain (GLS) was analyzed using the semi‐automated feature tracking analysis module in CVI42 software. LV epi‐ and endocardial borders were manually traced in the 2‐, 3‐, and 4‐chambers long‐axis views at end‐diastole. The contours were then automatically propagated throughout the cardiac cycle. Higher (less negative) GLS values indicate worse myocardial contractile function.
Ocular Examinations
Fundus photography and OCTA were performed ≈30 minutes after topical instillation of 2 drops of 1% tropicamide, given 5 minutes apart. Fundus photography was then performed using a retinal camera (Canon CR‐DGi with a 10‐DSLR back; Canon, Tokyo, Japan). Patients with eye diseases (glaucoma, vascular or nonvascular retinopathies, age‐related macular degeneration) were excluded from the study.
OCTA Imaging
Both eyes of each patient were included in the analysis if the OCTA images from both eyes were of good quality. The OCTA allows a high‐resolution 3‐dimensional visualization of perfused microvasculature in a non‐invasive manner and characterizes vascular information at the retinal and choriocapillaris layers (Figure S2). Patients underwent a 3.0×3.0‐mm2 macular centered imaging using the OCTA (PLEX Elite 9000, Carl Zeiss Meditec, Inc., Dublin, USA; Version 1.7). The OCTA machine provided a signal strength index, ranging from 0 to 10, where only images with an index of 6 and above were accepted. A trained grader masked to the participant characteristics reviewed the quality of OCTA scans. 25 Poor quality scans were excluded from the analysis if one of the following criteria were met: (1) poor clarity images; (2) local weak signal caused by artifacts such as floaters; (3) residual motion artifacts visible as irregular vessel patterns on the en face angiogram; and (4) scans with segmentation errors.
Evaluation of the Retinal Microvasculature and the Choriocapillaris Flow Deficits
OCTA images of the superficial retinal plexus, deep retinal plexus, and choriocapillaris were exported from the built‐in review software (PLEX Elite Review Software, Carl Zeiss Meditec, Inc., Dublin, USA; Version 1.7.1.31492). A list of OCTA metrics (Figure S2), including retinal capillary density at the superficial and deep capillary plexus, choriocapillaris flow deficits density and foveal avascular zone (FAZ) area, can be calculated automatically (MATLAB; The MathWorks, Inc., Natick, MA; Version R2020b). 38 , 39 Capillary density was defined as the total vessel length (in mm) per total imaged area (in mm2). Choriocapillaris flow deficits density was defined as the percentage of flow deficits area per total imaged area. We showed previously that the superficial capillary density (intraclass correlation=0.85), deep capillary density (intraclass correlation=0.82), and choriocapillaris flow deficits density (intraclass correlation=0.87), exhibited good reproducibility. 23 , 38 The FAZ was manually outlined, and the area was computed in mm2.
Statistical Analysis
Primary outcome was OCTA metrics. The Shapiro‒Wilk test was used to assess the normality of the distribution of the continuous variables. Continuous variables that are normally distributed are presented as mean±SD whereas non‐normally distributed variables are presented as median (interquartile range). Comparisons between groups were performed using either the parametric 1‐way ANOVA test or non‐parametric Kruskal‒Wallis test, depending on normality of the distribution. For simplicity, average measurements of both eyes were used for group stratification. We used the single measurements, where each eye (n=197) is considered 1 subject for multivariable linear regression analysis and accounted for correlations between paired eyes of subjects using the generalized estimating equations approach to assess the effect of adverse cardiac remodeling (LV mass index, interstitial volume, RI, and GLS) on each OCTA metric (dependent variable), adjusting for age, sex, systolic blood pressure, diabetes, and signal strength of OCTA scans. The diagnostic accuracy of the superficial capillary density (reference) in differentiating patients with versus without replacement fibrosis and patients with low RI versus normal RI were compared using the area under the receiver operating characteristics curve. Data were analyzed with STATA, version 16; StataCorp LP, SPSS, version 24, IBM, and GraphPad Prism 8.1.2, GraphPad Software, Inc.
Results
We excluded 30 patients because of eye diseases and/or poor quality OCTA images. A total of 197 eyes of 118 patients with hypertension (59 [13] years, 65% male) were available for analysis. Patients were stratified into tertiles based on the average superficial capillary density values of 2 eyes per subject: low (<16.16 mm‐1), medium (from 16.16 to 17.67 mm‐1) and high (>17.67 mm‐1). There was a small but significant difference in the average OCTA quality in patients with low superficial capillary density (9.0±0.9 signal strength), medium superficial capillary density (8.8±0.9 signal strength), and high superficial capillary density (8.5±0.7 signal strength; P=0.006). Patients with low superficial capillary density were older, had higher blood pressures and longer duration of hypertension treatment (Table 1).
Table 1.
Clinical and Cardiovascular Magnetic Resonance Characteristics of Patients With Hypertension Stratified By Vessel Superficial Capillary Density (Stratified Into Tertiles)
|
High (>17.67 mm−1) (n=39) |
Medium (16.16 to 17.67 mm−1) (n=40) |
Low (<16.16 mm−1) (n=39) |
P value | |
|---|---|---|---|---|
| Clinical | ||||
| Age, y | 55±10 | 56±10 | 60±8 | 0.022* |
| Men, n (%) | 25 (64) | 26 (65) | 26 (67) | 0.971 |
| BMI, kg/m2 | 26.5±4.7 | 25.9±5.8 | 26.4±4.0 | 0.866 |
| Duration of treatment, y | 9 [6,12] | 12 [5,18] | 16 [8, 20] | 0.014* |
| No. of anti‐hypertensives | 1 [1,1] | 1 [1,1] | 2 [1,2] | 0.011* |
| Diabetes, n (%) | 6 (15) | 9 (23) | 14 (36) | 0.102 |
| Hyperlipidemia, n (%) | 17 (43) | 17 (43) | 24 (62) | 0.167 |
| Systolic blood pressure, mm Hg | 122±11 | 129±15 | 133±14 | 0.022* |
| Diastolic blood pressure, mm Hg | 76±8 | 80±9 | 80±8 | 0.071 |
| Romhilt Estes ECG score | 3 [1,4] | 3 [1,4] | 3 [1,4] | 0.707 |
| Cardiovascular magnetic resonance | ||||
| LV mass, g/m2 | 49±8 | 55±22 | 53±10 | 0.167 |
| LV end diastolic volume, mL/m2 | 72±9 | 72±13 | 72±15 | 0.975 |
| LV end systolic volume, mL/m2 | 29±5 | 29±8 | 29±9 | 0.850 |
| LV ejection fraction, % | 59±5 | 61±6 | 61±7 | 0.277 |
| Remodeling index | 6.11±0.93 | 5.76±1.12 | 5.58±0.88 | 0.060 |
| Global longitudinal strain, % | −17.6±2.3 | −17.0±3.4 | −16.7±2.3 | 0.325 |
| Late gadolinium enhancement, n (%) | 5 (13) | 12 (30) | 10 (26) | 0.169 |
| Indexed interstitial volume, mL/m2 | 11.5±2 | 13.6±6.0 | 12.9±2.4 | 0.065 |
| Extracellular volume, % | 24.5 [22.8,26.7] | 25.6 [23.7,27.1] | 25.9 [23.8,27.5] | 0.316 |
Data presented are number (%) or mean±SD or median [interquartile range], as appropriate. BMI indicates body mass index; and LV, left ventricular.
Statistical significance at the P<0.05 level.
There was a trend, although not statistically significant, towards lower superficial capillary density in patients with more advanced LV remodeling: increased RI, worse myocardial contractile function (assessed by GLS), more myocardial fibrosis (assessed by proportion of patients with replacement fibrosis and interstitial volumes; Table 1; Figure 1). Similar results were observed with stratification approaches using either the maximum or minimum superficial capillary density values between the 2 eyes (Tables S1 and S2).
Figure 1. Lower superficial capillary density in a patient with hypertension (A and B; 14.5 mm‐1) with (C) lower remodeling index, (D) higher interstitial volume and extracellular volume fraction, and (E) worse global longitudinal strain as compared with a denser superficial capillary density in a patient with hypertension (F and G; 21.7 mm‐1) with (H) higher remodeling index, (I) lower interstitial volume, and (J) better global longitudinal strain.

Presence of larger non‐perfused area (blue regions demarcated by red arrows) (B) can be seen in patient with adverse cardiac remodeling markers as compared with (C) a patient with favorable cardiac remodeling markers. Foveal avascular zone area (in black; B and G) and large vessels (seen as a black outline of vessels in A and F) were excluded from the calculation of capillary density. ECV indicates extracellular volume fraction; GLS, global longitudinal strain; and RI, remodeling index.
In the multivariable analysis with generalized estimating equations, patients with significantly lower superficial capillary density had higher LV mass (β=−0.150, 95% CI, −0.290 to −0.010), higher interstitial volume (β=−0.270, 95% CI, −0.535 to −0.020), and worse GLS (β=−0.109, 95% CI, −0.187 to −0.032) (Table 2; Figure 2). No significant association was found between superficial capillary density and RI or ECV values. There were no significant differences between deep capillary density and measures of LV remodeling on CMR (P=0.157; Table 3).
Table 2.
Associations of Cardiac and Renal Characteristics With Superficial Capillary Density (mm−1)
| Characteristics | β | 95% CI | P value |
|---|---|---|---|
| Cardiovascular magnetic resonance characteristics | |||
| Left ventricular mass (per increment of 10 g/m2) | −0.150 | −0.290 to −0.010 | 0.040 † |
| Remodeling index | 0.208 | −0.030 to 0.445 | 0.086 |
| Global longitudinal strain (%) | −0.109 | −0.187 to −0.032 | 0.006 † |
| Indexed interstitial volume (per increment of 5 mL/m2) | −0.270 | −0.535 to −0.020 | 0.050 † |
| Extracellular volume (%) | −0.023 | −0.183 to 0.136 | 0.776 |
| Renal characteristics | |||
| eGFR (per reduction of 10 mL/min per 1.73 m2) | −0.150 | −0.260 to −0.040 | 0.009 † |
eGFR indicates estimated glomerular filtration rate.
Statistical significance at the P<0.05 level.
Figure 2. Scatterplots showing the associations between lower superficial capillary density in patients with hypertension with (A) increased left ventricular mass, (B) increased interstitial volume, and (C) reduced global longitudinal strain, adjusted for age, sex, body mass index, systolic blood pressure, diabetes, and signal strength of scans.
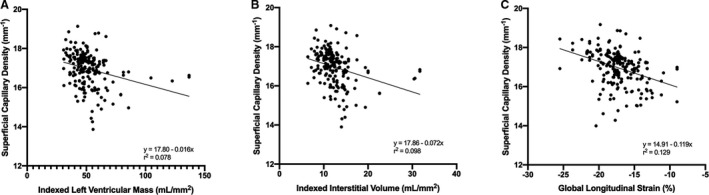
The equation is the fit line of the scattered plot, where y denotes the dependent variable (superficial capillary density), and x represents the independent variable (left ventricular mass/interstitial volume/global longitudinal strain).
Table 3.
Associations of Cardiac and Renal Characteristics With Deep Capillary Density (mm−1)
| Characteristics | β | 95% CI | P value |
|---|---|---|---|
| Cardiovascular magnetic resonance characteristics | |||
| Left ventricular mass (per increment of 10 g/m2) | 0.070 | −0.150 to 0.300 | 0.523 |
| Remodeling index | −0.074 | −0.521 to 0.374 | 0.747 |
| Global longitudinal strain (%) | 0.084 | −0.032 to 0.199 | 0.157 |
| Indexed interstitial volume (per increment of 5mL/m2) | 0.044 | −0.050 to 0.138 | 0.361 |
| Extracellular volume (%) | 0.067 | −0.159 to 0.293 | 0.561 |
| Renal characteristics | |||
| eGFR (per reduction of 10 mL/min per 1.73 m2) | −0.210 | −0.370 to −0.040 | 0.013 † |
eGFR indicates estimated glomerular filtration rate.
Statistical significance at the P<0.05 level.
Both superficial capillary density (β=−0.150, 95% CI, −0.260 to −0.040; Table 2) and deep capillary density (β=−0.210, 95% CI, −0.370 to −0.040; Table 3) were related to eGFR. In other words, people with lower eGFR had sparser superficial and deep capillary densities.
Systemic factors were mostly not associated with choriocapillaris flow deficits and FAZ area (P=0.110; Table S3), except between extracellular volume and FAZ area (β=−0.014, 95% CI, −0.023 to −0.005; Table S3).
In the multivariable analysis with generalized estimating equations, lower superficial capillary density was found in patients with hypertension with replacement fibrosis versus no replacement fibrosis (17.04±0.86 mm‐1 versus 16.61±0.76 mm‐1; P=0.004; Figure 3). Indeed, superficial capillary density demonstrated the ability to differentiate patients with versus without replacement fibrosis (c‐statistics=0.718 [95% CI, 0.615 to 0.822]; P=0.004).
Figure 3. Lower superficial capillary density in patients with hypertension with replacement fibrosis vs no replacement fibrosis (P=0.004).
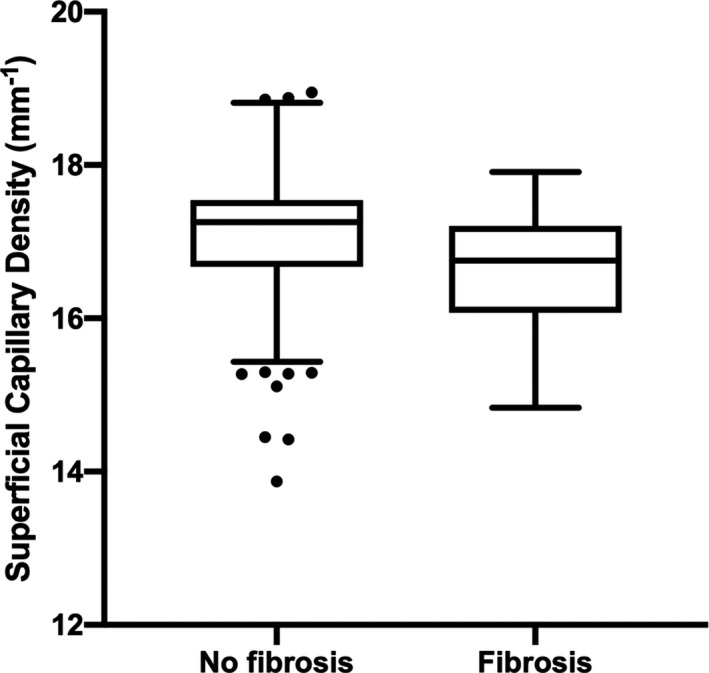
Results presented in box‐and‐whiskers plots (Tukey method), P value adjusted for age, sex, body mass index, systolic blood pressure, diabetes, and signal strength of scans. Scattered data points are outliers.
Discussion
In this study, we described the use of OCTA in people with systemic hypertension to investigate the associations between quantitative retinal capillary network and myocardial abnormalities as measured on CMR. We detailed alterations in the retinal capillary microvasculature that are associated with adverse cardiac remodeling, namely increased LV mass, higher interstitial volume, and worse GLS. Although the associations between superficial capillary density and RI/ECV were not statistically significant (because of small sample size), our findings suggest a trend consistent with our hypothesis that lower superficial capillary density was associated with worse cardiac markers (lower RI and higher ECV). Our results highlight the promising role of OCTA as an accurate, easy to perform and cheaper noninvasive tool to quantify alterations in the microcirculation for risk stratification in clinical settings. To the best of our knowledge, our study is the first, which assessed the associations between quantitative retinal capillary network and myocardial abnormalities based on CMR imaging and using a robust OCTA methodology to collect data.
Conventionally, the diagnosis and management of hypertension relies on blood pressure measurements. Current management of hypertension places great emphasis on achieving blood pressure targets but recommendations on blood pressure targets vary greatly among major guidelines. 40 , 41 Apart from the hemodynamic effect of pressure overload, non‐hemodynamic factors such as neurohormonal, comorbidities, genetics and environmental exposures result in myocardial remodeling. Adverse changes in myocardium eventually lead to LV dysfunction and heart failure. 42 Thus, in recent years, novel strategies have been developed which target on preventing and reversing myocardial remodeling. 43 , 44 , 45 , 46 Previous studies had focused mainly on the retinal vasculature changes with blood pressure control. 25 , 47 To our knowledge, this is the first study that demonstrated the relationships between superficial capillary density and markers of myocardial remodeling. The different markers investigated in this study may reflect distinct domains of the myocardial disease: changes in the interstitium (interstitial fibrosis), cardiomyocyte hypertrophy (LV mass) and myocardial contractile (GLS).
Our OCTA findings agreed with population studies, which have reported the associations between hypertensive retinopathy with incident coronary heart disease, 15 , 16 congestive heart failure, 17 coronary heart disease mortality 18 and cardiovascular 0 mortality. 19 , 20 Retinal arteriolar and venular diameter assessed from fundus photographs have shown the associations of narrower retinal arterioles and wider venules with long‐term risk of mortality and ischemic stroke in both sexes 48 and coronary heart disease in women. 48 , 49 However, the information provided by the fundus photographs is limited to the larger vessels (arterioles and venules) in the superficial layer of the retinal circulation. It thus, may not be the earliest biomarkers of systemic microcirculation. Instead, the OCTA can provide high‐resolution images of the superficial retinal layer of not only the larger (arterioles and venules; 100–300 µm in diameter) and smaller vessels (capillaries; 3.5–6 µm in diameter) of the retina, 50 but also the deeper retinal vascular layer. 21 Our study provided evidence that OCTA can characterize the retinal microvascular abnormalities that may be suggestive of systemic microcirculatory dysfunction in the heart.
The retinal microcirculation is made up of 2 distinct vascular layers, of which the superficial and deep capillary plexus are most differentiated for characterizing diseases. Why the association between adverse markers of cardiac remodeling and superficial but not deep retinal vessels is not established and open to speculation. A recent OCTA study by Rakusiewicz 51 reported similar OCTA findings, where there was a significantly altered vessel density in the superficial capillary plexus but not the deep capillary plexus in children with chronic heart failure because of dilated cardiomyopathy as compared with healthy children. However, Wang 52 observed changes in the vessel densities in both superficial and deep plexuses in patients with chronic heart failure versus those without it. It should be noted that a fifth of the individuals with hypertension individuals in Wang et. Al had diabetes whereas the controls were free from diabetes. 53 Since deep retinal vessels show early alterations in diabetes, the alterations in the deep retinal vessels in the hypertensive cohort may be attributable to diabetes and not hypertension. 54 In another OCTA study by Li, 55 they also observed a significantly lower vessel density in both the superficial and deep capillary plexus in patients with congenital heart disease when compared with control subjects. However, Li and co‐workers did not indicate the diabetes status of their subjects.
Others have reported that patients with chronic kidney disease had reduced vessel densities in both superficial and deep capillary plexuses as compared with healthy controls using either the Optovue OCTA system 56 or the swept‐source OCT system (DRI Triton, Topcon Inc., Japan). 57 In our previous publication 25 using a spectral‐domain OCT system (AngioVue; Optovue, Inc., USA), we reported that retinal capillary density in the deep plexus (and not the superficial plexus) was reduced in people with lower eGFR. The use of a different swept‐source OCT system (Plex Elite 9000, Carl Zeiss Meditec), which provides higher sampling density (100 pixels/mm versus 51 pixels/mm) and also more patients (n=118 versus 77 patients) may explain the relationship of eGFR and retinal vessel rarefaction in both plexuses as compared with our earlier study. 25 Most importantly we studied an entirely different cohort of individuals with hypertension and report that lower eGFR is related to sparser capillary densities in both superficial and deep plexuses.
Alterations in retinal capillary density imaged using the OCTA could be attributable to capillary dropout, capillary closure, or slow rates of blood flow within perfused capillaries. The current OCTA device images capillaries by detecting motion contrast from blood flow. A blood vessel with an exceedingly sluggish flow, below the sensitivity limit of the OCTA will not be detected. Although fast blood flows are observable on OCTA, there is currently no way to quantify perfusion rate with this technology. 58 Therefore, the term “capillary density” is used as a quantitative index of the vascular structures detected that reflects the area occupied by perfused blood vessels.
Clinical Implications
To our knowledge, this is the first study that demonstrated the associations between retinal microcirculation abnormalities and measures of LV adverse remodeling. Although the CMR can accurately assess multiple aspects of LV remodeling in a single examination, its widespread use is limited by cost, availability, long examination, patient selection, and concerns about the effects of gadolinium‐based contrast agents. OCTA microcirculation measurements can be easily acquired during routine eye check, automatically extracted, simple to use and reproducible tool, which could serve as a first‐line risk assessment in patients with hypertension before further investigations with dedicated imaging. OCTA could also be used as a marker to monitor response to targeted therapies and progression of hypertensive heart disease.
Strengths and Limitations
The strength of this study includes a good phenotype cohort of individuals with hypertension who underwent CMR imaging, which is considered the gold standard for evaluation of myocardial volumes and function. Second, we excluded the FAZ area from the calculation of capillary density as it exhibits large interindividual variation in normal eyes 59 and may misconstrue the actual area of non‐perfusion. (Figure 1). Excluding the FAZ area will also mitigate the effect of ocular magnification on capillary density measurements. 60 We excluded larger vessels for calculation of the superficial capillary density as we wanted to capture the presence of capillaries. 61 Third, ocular microvascular dysfunction can be affected by age, eye diseases, or hypertension. We statistically adjusted the confounding effects of age, sex, body mass index, systolic blood pressure, diabetes, and signal strength of OCTA scans in the multivariate analysis model and further excluded eyes with clinically relevant eye diseases. While it is clear that eye diseases, such as glaucoma, 62 diabetic retinopathy, 63 and age‐related macular degeneration, 64 can interfere with the OCTA parameters, we did not exclude eyes with hypertensive retinopathy. This is because hypertensive retinopathy is a biological process of hypertension, particularly in individuals with hypertension with more adverse cardiovascular outcomes. 65 Excluding them might therefore make the results less generalizable. Our study had a few limitations. The cross‐sectional study design does not allow us to infer causality because the temporal sequence is not known. OCTA device was only recently launched, and a follow‐up study is ongoing.
Conclusions
In summary, we showed that patients with hypertension who have CMR documentation of LV remodeling had signs of altered retinal capillary density at the superficial retinal plexus on OCTA. The presence of structural alterations in microcirculation plays an important role in the development of cardiovascular events. Quantitative evaluation of the retinal capillary network using OCTA, which captures capillaries that cannot be observed by fundus photography, may provide insights into the microvascular contribution of cardiovascular disease.
Sources of Funding
This work was funded by grants from the Duke‐NUS Medical School (Duke‐NUS‐KP(Coll)/2018/0009A), the National Medical Research Council (CG/C010A/2017_SERI; NMRC/OFLCG/001c/2017; OFIRG/0048/2017; OFLCG/004c/2018; TA/MOH‐000249‐00/2018 and MOH‐OFIRG20nov‐0014), National Research Foundation Singapore (NRF2019‐THE002‐0006 and NRF‐CRP24‐2020‐0001), A*STAR (A20H4b0141), the Singapore Eye Research Institute & Nanyang Technological University (SERI‐NTU Advanced Ocular Engineering (STANCE) Program), and the SERI‐Lee Foundation (LF1019‐1) Singapore.
Disclosures
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supporting information
Tables S1–S3
Figures S1–S2
Acknowledgments
We would like to thank the study participants for their important contributions to this study.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024226
For Sources of Funding and Disclosures, see page 8.
References
- 1. Dai H, Bragazzi NL, Younis A, Zhong W, Liu X, Wu J, Grossman E. Worldwide trends in prevalence, mortality, and disability‐adjusted life years for hypertensive heart disease from 1990 to 2017. Hypertension. 2021;77:1223–1233. doi: 10.1161/HYPERTENSIONAHA.120.16483 [DOI] [PubMed] [Google Scholar]
- 2. Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 3. Diez J, Gonzalez A, Lopez B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209–216. doi: 10.1038/ncpcardio0158 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez A, Ravassa S, Lopez B, Moreno MU, Beaumont J, San Jose G, Querejeta R, Bayes‐Genis A, Diez J. Myocardial remodeling in hypertension. Hypertension. 2018;72:549–558. doi: 10.1161/HYPERTENSIONAHA.118.11125 [DOI] [PubMed] [Google Scholar]
- 5. Messroghli DR, Moon JC, Ferreira VM, Grosse‐Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn meric. 2017;19:75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goh VJ, Le TT, Bryant J, Wong JI, Su B, Lee CH, Pua CJ, Sim CPY, Ang B, Aw TC, et al. Novel index of maladaptive myocardial remodeling in hypertension. Circ Cardiovasc Imaging. 2017;10. doi: 10.1161/CIRCIMAGING.117.006840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le TT, Lim V, Ibrahim R, Teo MT, Bryant J, Ang B, Su B, Aw TC, Lee CH, Bax J, et al. The remodelling index risk stratifies patients with hypertensive left ventricular hypertrophy. Eur Heart J Cardiovasc Imaging. 2021;22:670–679. doi: 10.1093/ehjci/jeaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frojdh F, Fridman Y, Bering P, Sayeed A, Maanja M, Niklasson L, Olausson E, Pi H, Azeem A, Wong TC, et al. Extracellular volume and global longitudinal strain both associate with outcomes but correlate minimally. JACC Cardiovasc Imaging. 2020;13:2343–2354. doi: 10.1016/j.jcmg.2020.04.026 [DOI] [PubMed] [Google Scholar]
- 9. Camici PG, Crea F. Coronary microvascular dysfunction. New Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889 [DOI] [PubMed] [Google Scholar]
- 10. Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72 [DOI] [PubMed] [Google Scholar]
- 11. Vegsundvag J, Holte E, Wiseth R, Hegbom K, Hole T. Coronary flow velocity reserve in the three main coronary arteries assessed with transthoracic Doppler: a comparative study with quantitative coronary angiography. J Am Soc Echocardiogr. 2011;24:758–767. doi: 10.1016/j.echo.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 12. Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac pet imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 13. Thomson LEJ, Wei J, Agarwal M, Haft‐Baradaran A, Shufelt C, Mehta PK, B. Gill E, Johnson BD, Kenkre T, M. Handberg E, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A national heart, lung, and blood institute‐sponsored study from the women’s ischemia syndrome evaluation. Circ Cardiovasc Imaging. 2015;8:e002481. doi: 10.1161/CIRCIMAGING.114.002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60:1094–1103. doi: 10.1161/HYPERTENSIONAHA.111.189142 [DOI] [PubMed] [Google Scholar]
- 15. Duncan BB, Wong TY, Tyroler HA, Davis CE, Fuchs FD. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br J Ophthalmol. 2002;86:1002–1006. doi: 10.1136/bjo.86.9.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The atherosclerosis risk in communities study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153 [DOI] [PubMed] [Google Scholar]
- 17. Wong TY, Rosamond W, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, Folsom AR, Klein R. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63–69. doi: 10.1001/jama.293.1.63 [DOI] [PubMed] [Google Scholar]
- 18. Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95:391–394. doi: 10.1136/hrt.2008.146670 [DOI] [PubMed] [Google Scholar]
- 19. Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10‐year cardiovascular mortality: a population‐based case‐control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8 [DOI] [PubMed] [Google Scholar]
- 20. Sairenchi T, Iso H, Yamagishi K, Irie F, Okubo Y, Gunji J, Muto T, Ota H. Mild retinopathy is a risk factor for cardiovascular mortality in Japanese with and without hypertension: the Ibaraki prefectural health study. Circulation. 2011;124:2502–2511. doi: 10.1161/CIRCULATIONAHA.111.049965 [DOI] [PubMed] [Google Scholar]
- 21. Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, Shi Y, Wang RK. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. doi: 10.1016/j.preteyeres.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hong J, Tan B, Quang ND, Gupta P, Lin E, Wong D, Ang M, Lamoureux E, Schmetterer L, Chua J. Intra‐session repeatability of quantitative metrics using widefield optical coherence tomography angiography (OCTA) in elderly subjects. Acta Ophthalmol. 2019;98:e570–e578. doi: 10.1111/aos.14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. You Q, Freeman WR, Weinreb RN, Zangwill L, Manalastas PIC, Saunders LJ, Nudleman E. Reproducibility of vessel density measurement with optical coherence tomography angiography in eyes with and without retinopathy. Retina. 2017;37:1475–1482. doi: 10.1097/IAE.0000000000001407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chua J, Chin CWL, Hong J, Chee ML, Le TT, Ting DSW, Wong TY, Schmetterer L. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J Hypertens. 2019;37:572–580. doi: 10.1097/HJH.0000000000001916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun C, Ladores C, Hong J, Nguyen DQ, Chua J, Ting D, Schmetterer L, Wong TY, Cheng CY, Tan ACS. Systemic hypertension associated retinal microvascular changes can be detected with optical coherence tomography angiography. Sci Rep. 2020;10:9580. doi: 10.1038/s41598-020-66736-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Q, Sun H, Huang X, Qu Y. Retinal microvascular metrics in untreated essential hypertensives using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2021;259:395–403. doi: 10.1007/s00417-020-04714-8 [DOI] [PubMed] [Google Scholar]
- 28. Donati S, Maresca AM, Cattaneo J, Grossi A, Mazzola M, Caprani SM, Premoli L, Docchio F, Rizzoni D, Guasti L, et al. Optical coherence tomography angiography and arterial hypertension: a role in identifying subclinical microvascular damage? Eur J Ophthalmol. 2021;31:158–165. doi: 10.1177/1120672119880390 [DOI] [PubMed] [Google Scholar]
- 29. Terheyden JH, Wintergerst MWM, Pizarro C, Pfau M, Turski GN, Holz FG, Finger RP. Retinal and choroidal capillary perfusion are reduced in hypertensive crisis irrespective of retinopathy. Transl vis Sci Technol. 2020;9:42. doi: 10.1167/tvst.9.8.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng Q, Hu Y, Huang M, Wu Y, Zhong P, Dong X, Wu Q, Liu B, Li C, Xie J, et al. Retinal neurovascular impairment in patients with essential hypertension: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci. 2020;61:42. doi: 10.1167/iovs.61.8.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hua D, Xu Y, Zeng X, Yang N, Jiang M, Zhang X, Yang J, He T, Xing Y. Use of optical coherence tomography angiography for assessment of microvascular changes in the macula and optic nerve head in hypertensive patients without hypertensive retinopathy. Microvasc Res. 2020;129:103969. doi: 10.1016/j.mvr.2019.103969 [DOI] [PubMed] [Google Scholar]
- 32. Hua D, Xu Y, Zhang X, He T, Chen C, Chen Z, Xing Y. Retinal microvascular changes in hypertensive patients with different levels of blood pressure control and without hypertensive retinopathy. Curr Eye Res. 2021;46:107–114. doi: 10.1080/02713683.2020.1775260 [DOI] [PubMed] [Google Scholar]
- 33. Frost S, Nolde JM, Chan J, Joyson A, Gregory C, Carnagarin R, Herat LY, Matthews VB, Robinson L, Vignarajan J, et al. Retinal capillary rarefaction is associated with arterial and kidney damage in hypertension. Sci Rep. 2021;11:1001. doi: 10.1038/s41598-020-79594-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du Bois D & Du Bois EF A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989; 5:303‐311; discussion 312‐303 [PubMed]
- 35. Le TT, Tan RS, De Deyn M, Goh EP, Han Y, Leong BR, Cook SA, Chin CW. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn meric. 2016;18:21. doi: 10.1186/s12968-016-0236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chin CW, Semple S, Malley T, White AC, Mirsadraee S, Weale PJ, Prasad S, Newby DE, Dweck MR. Optimization and comparison of myocardial T1 techniques at 3T in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2014;15:556–565. doi: 10.1093/ehjci/jet245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1320–1333. doi: 10.1016/j.jcmg.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong J, Ke M, Tan B, Lau A, Wong D, Yao X, Liu X, Schmetterer L, Chua J. Effect of vessel enhancement filters on the repeatability of measurements obtained from widefield swept‐source optical coherence tomography angiography. Sci Rep. 2020;10:22179. doi: 10.1038/s41598-020-79281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin E, Ke M, Tan B, Yao X, Wong D, Ong L, Schmetterer L, Chua J. Are choriocapillaris flow void features robust to diurnal variations? A swept‐source optical coherence tomography angiography (OCTA) study. Sci Rep. 2020;10:11249. doi: 10.1038/s41598-020-68204-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the TASK force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 41. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 42. Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792 [DOI] [PubMed] [Google Scholar]
- 43. Gonzalez A, Ravassa S, Beaumont J, Lopez B, Diez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–1843. doi: 10.1016/j.jacc.2011.06.058 [DOI] [PubMed] [Google Scholar]
- 44. Kanellakis P, Dinh TN, Agrotis A, Bobik A. Cd4(+)cd25(+)foxp3(+) regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens. 2011;29:1820–1828. doi: 10.1097/HJH.0b013e328349c62d [DOI] [PubMed] [Google Scholar]
- 45. Fabbiano S, Menacho‐Márquez M, Robles‐Valero J, Pericacho M, Matesanz‐Marín A, García‐Macías C, Sevilla MA, Montero MJ, Alarcón B, López‐Novoa JM, et al. Immunosuppression‐independent role of regulatory T cells against hypertension‐driven renal dysfunctions. Mol Cell Biol. 2015;35:3528–3546. doi: 10.1128/MCB.00518-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan‐dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.CIR.0000017264.66561.3D [DOI] [PubMed] [Google Scholar]
- 47. Lee WH, Park JH, Won Y, Lee MW, Shin YI, Jo YJ, Kim JY. Retinal microvascular change in hypertension as measured by optical coherence tomography angiography. Sci Rep. 2019;9:156. doi: 10.1038/s41598-018-36474-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seidelmann SB, Claggett B, Bravo PE, Gupta A, Farhad H, Klein BE, Klein R, Di Carli M, Solomon SD. Retinal vessel calibers in predicting long‐term cardiovascular outcomes: the atherosclerosis risk in communities study. Circulation. 2016;134:1328–1338. doi: 10.1161/CIRCULATIONAHA.116.023425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gopinath B, Chiha J, Plant AJ, Thiagalingam A, Burlutsky G, Kovoor P, Liew G, Mitchell P. Associations between retinal microvascular structure and the severity and extent of coronary artery disease. Atherosclerosis. 2014;236:25–30. doi: 10.1016/j.atherosclerosis.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 50. Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E. The eye: Basic sciences in practice: Chapter 1: Anatomy of the eye and orbit . 2016;1–102.
- 51. Rakusiewicz K, Kanigowska K, Hautz W, Ziolkowska L. The impact of chronic heart failure on retinal vessel density assessed by optical coherence tomography angiography in children with dilated cardiomyopathy. J Clin Med. 2021;10:2659. doi: 10.3390/jcm10122659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang J, Jiang J, Zhang Y, Qian YW, Zhang JF, Wang ZL. Retinal and choroidal vascular changes in coronary heart disease: an optical coherence tomography angiography study. Biomed Opt Express. 2019;10:1532–1544. doi: 10.1364/BOE.10.001532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chua J, Sim R, Tan B, Wong D, Yao X, Liu X, Ting DSW, Schmidl D, Ang M, Garhöfer G, et al. Optical coherence tomography angiography in diabetes and diabetic retinopathy. J Clin Med. 2020;9:1723. doi: 10.3390/jcm9061723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen QI, Ma Q, Wu C, Tan F, Chen F, Wu Q, Zhou R, Zhuang X, Lu F, Qu J, et al. Macular vascular fractal dimension in the deep capillary layer as an early indicator of microvascular loss for retinopathy in type 2 diabetic patients. Invest Ophthalmol vis Sci. 2017;58:3785–3794. doi: 10.1167/iovs.17-21461 [DOI] [PubMed] [Google Scholar]
- 55. Li C, Zhong P, Yuan H, Dong X, Peng Q, Huang M, Wu Q, Liu B, Xu M, Kuang YU, et al. Retinal microvasculature impairment in patients with congenital heart disease investigated by optical coherence tomography angiography. Clin Exp Ophthalmol. 2020;48:1219–1228. doi: 10.1111/ceo.13846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeung L, Wu IW, Sun CC, Liu CF, Chen SY, Tseng CH, Lee HC, Lee CC. Early retinal microvascular abnormalities in patients with chronic kidney disease. Microcirculation. 2019;26:e12555. doi: 10.1111/micc.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vadala M, Castellucci M, Guarrasi G, Terrasi M, La Blasca T, Mule G. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes Arch Clin Exp Ophthalmol. 2019;257:1687–1698. doi: 10.1007/s00417-019-04358-3 [DOI] [PubMed] [Google Scholar]
- 58. Tan B, Sim R, Chua J, Wong DWK, Yao X, Garhofer G, Schmidl D, Werkmeister RM, Schmetterer L. Approaches to quantify optical coherence tomography angiography metrics. Ann Transl Med. 2020;8:1205. doi: 10.21037/atm-20-3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chui TY, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012;89:602–610. doi: 10.1097/OPX.0b013e3182504227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chua J, Schmetterer L. Letter to the editor on “macular OCT‐angiography parameters to predict the clinical stage of nonproliferative diabetic retinopathy: an exploratory analysis”. Eye (Lond). 2020;34:2341–2342. doi: 10.1038/s41433-020-0788-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chua J, Hu Q, Ke M, Tan B, Hong J, Yao X, Hilal S, Venketasubramanian N, Garhöfer G, Cheung CY, et al. Retinal microvasculature dysfunction is associated with alzheimer’s disease and mild cognitive impairment. Alzheimers Res Ther. 2020;12:161. doi: 10.1186/s13195-020-00724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435. doi: 10.1016/S0140-6736(07)60198-6 [DOI] [PubMed] [Google Scholar]
- 63. Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen S‐J, Dekker JM, Fletcher A, Grauslund J, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Age‐Related Eye Disease Study Research Group . Risk factors associated with age‐related macular degeneration. A case‐control study in the age‐related eye disease study: age‐related eye disease study report number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, Kuller L, Burke G, Tracy RP, Polak JF, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the cardiovascular health study. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


