Abstract
Background
Left ventricular (LV) involvement is frequently observed in arrhythmogenic cardiomyopathy (ACM). We investigated the association of LV myocardial assessment using cardiac magnetic resonance (CMR) with clinical outcomes including heart failure (HF)‐related events in ACM.
Methods and Results
We retrospectively analyzed 60 patients with ACM between 2005 and 2020 according to the 2010 Task Force Criteria and assessed HF‐related events (HF hospitalization, heart transplantation, and cardiac death) and ventricular tachycardia events. We analyzed CMR findings including late gadolinium enhancement (LGE) in all subjects and obtained mapping values (native T1, extracellular volume, and T2) on 30 (50%) patients out of them. Among the study population (mean age 49 years, 77% male), 41 (68%) patients had LV LGE. During a median follow‐up of 34 months, there were 13 (22%) HF‐related events, and 20 (30%) ventricular tachycardia events. Kaplan‐Meier survival analysis revealed that HF‐related events occurred only in patients with LV LGE (+) (versus LV LGE (‐), log‐rank P=0.006), and the events were not significantly different regarding right ventricular LGE (log‐rank P>0.999). When categorized by median value for each mapping parameter, HF‐related events occurred more in patients with higher native T1 (versus lower native T1, log‐rank P=0.002), and higher T2 (versus lower T2, log‐rank P=0.002), higher extracellular volume (versus lower extracellular volume, log‐rank P=0.002). However, regarding ventricular tachycardia events, there were no significant differences according to these CMR markers.
Conclusions
LV myocardial assessment using CMR with LGE imaging and native T1, T2, and extracellular volume markers were significantly associated with HF‐related event risk in patients with ACM.
Keywords: arrhythmogenic right ventricular cardiomyopathy, cardiac magnetic resonance, heart failure, late gadolinium enhancement, left ventricular involvement
Subject Categories: Magnetic Resonance Imaging (MRI), Heart Failure, Cardiomyopathy
Nonstandard Abbreviations and Acronyms
- ACM
arrhythmogenic cardiomyopathy
- ECV
extracellular volume
- ICD
implantable cardioverter‐defibrillator
- LGE
late gadolinium enhancement
Clinical Perspective
What Is New?
Left ventricular (LV) involvement is frequently seen in patients with arrhythmogenic cardiomyopathy (ACM) when analyzing cardiac magnetic resonance (CMR) images.
The patients with ACM who experienced heart failure (HF)‐related events (eg, HF hospitalization, heart transplantation, and cardiac death due to HF) tended to have more abnormalities in the LV on CMR images.
The CMR features were the presence of late gadolinium enhancement, higher values of tissue mapping parameters such as native T1, extracellular volume) fraction, and T2; these parameters were analyzed in LV myocardium, and they were significantly correlated with more HF events in patients with ACM.
What Are the Clinical Implications?
Advanced fibro‐fatty replacement of LV myocardium accounts for more late gadolinium enhancement on CMR in ACM, but it was not associated with ventricular arrhythmia outcomes.
Unlike ventricular arrhythmia events, the HF‐related events occurred more in patients with ACM with LV late gadolinium enhancement and higher native T1, extracellular volume, and T2 values.
CMR features including late gadolinium enhancement and mapping parameters for LV myocardium can be used in prediction for HF events in patients with ACM.
Arrhythmogenic cardiomyopathy (ACM) is a genetic heart disease characterized by progressive fibro‐fatty replacement of myocardium and arrhythmogenic features. 1 The Task Force Criteria revised in 2010 has been used to diagnose ACM, focusing primarily on right ventricular (RV) structural alterations and arrhythmogenic features. 2 However, there has been a growing body of evidence for left‐sided involvement in ACM. 3 A recent report about autopsy findings in sudden cardiac death subjects revealed that 12% of these sudden cardiac death cases were diagnosed with ACM, 87% of them had histopathologic left ventricular (LV) involvement, and 17% were isolated LV disease. 4 Thus, the recent international expert report emphasized that the current classification of ACM includes the “biventricular disease phenotype” and the “left‐dominant phenotype,” 5 and they proposed “Padua criteria” to upgrade the criteria for the entire spectrum of ACM. 6 In this context, there have been several reports which investigated the cardiovascular magnetic resonance (CMR) imaging features of the LV phenotype in ACM and the association with clinical outcomes. 7 , 8 , 9 However, most of these reports focused on arrhythmia‐related outcomes and did not pay attention to heart failure (HF) outcomes. Recently, Zghaib et al showed that LV abnormalities on CMR were not associated with arrhythmic outcomes in patients with ACM. 10 Although LV involvement is known to be a poor prognostic factor in ACM, 11 , 12 there have been little data about HF outcomes assessed by CMR in these subjects. Moreover, there are limited data on the assessment of ACM using T1 and T2 mapping techniques, which are used widely for myocardial characterization in various cardiomyopathies. The present study aimed to assess LV involvement using CMR including mapping techniques and its clinical impact on adverse cardiovascular outcomes, especially on HF‐related outcomes in patients presenting with ACM.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Clinical Diagnosis
We retrospectively investigated the patients with clinically diagnosed ACM who underwent CMR between March 2005 and June 2020 at a single tertiary university hospital. The ACM diagnosis was established as definite, borderline, or possible diagnosis according to the 2010 modified Task Force Criteria which were based on both imaging and non‐imaging criteria. 2 In addition, we classified them in type “dominant‐right/biventricular/or dominant‐left” according to the “Padua criteria” as previously reported. 6 The clinical data including patient information and outcomes were collected by electronic medical record. Even though the clinical characteristics met the Task Force Criteria for ACM, the subject was excluded when the CMR findings strongly suggested other pathology (eg, cardiac sarcoidosis). Finally, 60 patients with ACM were analyzed. Coronary angiography or coronary CT angiography was performed in 53 (88%) subjects and treadmill test was done in 5 (8%) subjects for evaluation of ischemic heart disease. All data were analyzed by 2 independent expert cardiologists (K.H.C., J.O.). The present study (CArdiovascular Mri Union in Severance, CAMUS‐001) was approved by the Institutional Review Board of the Yonsei University Health System (4‐2019‐0764). Given the retrospective nature of this study, written informed consent was waived per recommendation of our institution’s institutional review board for this study.
Electrocardiographic and Transthoracic Echocardiographic Data
All subjects underwent an ECG and we analyzed the baseline echocardiography to investigate the features suggestive of classic ACM and distinctive features LV involvement, as previously described. 2 , 12 , 13 , 14 In addition to the analysis of conventional ECG parameters, the following ECG features were investigated: separated duration of the QRS complex in right precordial leads (V1‐3) and left precordial leads (V4‐6); T‐wave inversion in right precordial leads (V1‐3), left precordial leads (V4‐6); epsilon waves in right precordial leads (V1‐3), defined as reproducible low amplitude signals between the end of the QRS complex and the onset of the T wave; terminal activation duration (TAD) of QRS ≥55 ms measured from the nadir of the S wave to the end of the QRS, including R’, in V1, V2, or V3, in the absence of complete right bundle‐branch block (RBBB); and RBBB. All these parameters were analyzed by the two expert cardiologists and were investigated for clinical diagnosis of ACM and study analysis.
All subjects underwent transthoracic echocardiography (Vivid 7, GE Medical Systems, Milwaukee, WI, USA). Regional wall motion abnormalities of the RV (akinesia, dyskinesia, or aneurysm) were documented, and chamber sizes, ejection fraction of LV, fractional area change of RV, and E velocity over mitral tissue Doppler velocity (e’) ratio (E/e’) were measured.
CMR Protocol and Image Analysis
CMR was performed using a 3‐T MR scanner (Magnetom Trio Tim, Siemens Healthcare, Erlangen, Germany) with a six‐channel anterior body matrix coil and a spine matrix coil array. Cine images were acquired in the short‐axis (SA) plane orientation covering both ventricles by using a retrospectively ECG‐gated balanced steady‐state free precession (TrueFISP) sequence. Cardiac localization was achieved using a steady‐state free‐precession sequence under ECG gating.
Native T1 mapping images were acquired in three SA planes (basal, mid, and apical LV) by using a modified look‐locker inversion‐recovery (MOLLI) sequence at the end‐expiratory phase with “5(3)3” scheme. A nonselective inversion pulse, a TrueFISP single‐shot readout sequence in the mid‐diastolic phase, was employed. Fully automated, non‐rigid motion correction was applied to register individual T1 images before inline T1 fitting was performed using a mono‐exponential three‐parameter fit.
After T1 mapping, T2 mapping images were acquired before contrast injection using a T2‐prepared single‐shot TrueFISP sequence, along the same planes as those used for T1 mapping. T2 pixel maps were generated by fitting pixel intensities onto a two‐parameter mono‐exponential signal model after automatic in‐plane non‐rigid motion correction.
Post‐contrast T1 mapping images were acquired at least 15 minutes after injection along the same three SA planes as those used for pre‐T1 mapping. The “4(1)3(1)2” scheme using 3 inversion pulses were used for post‐contrast T1 mapping. Hematocrit values were acquired on the same day before CMR imaging. Native and post T1 values of the LV blood pool were measured using a circular region of interest larger than 10 mm2, avoiding the papillary muscle.
Extracellular volume (ECV) fraction was calculated using the native and post‐contrast T1 values of the LV myocardium and blood pool with the following equation:
The global T1, T2, and ECV were measured using the mean values of 16 segments of LV for the analysis. The edges of the myocardium were excluded by applying a 10% offset to minimize the partial volume artifact.
Late gadolinium enhancement (LGE) magnetic resonance images were acquired 10 minutes after contrast injection of a 0.2‐mmol/kg intravenous dose of a gadolinium contrast agent (Dotarem, Guerbet, France) using a magnitude‐ and phase‐sensitive inversion‐recovery‐prepared TrueFISP sequence, with the inversion time adjusted to null, thus representing the normal myocardium. These LGE images were obtained along the same axis plane covering the whole LV. The appropriate inversion time before LGE MR imaging was determined using the fast low‐angle shot sequence with varying inversion times (from 150 to 650 ms to null). The quantification of LGE was determined using a signal threshold versus reference mean method with a signal intensity threshold of 5 standard deviations (SD) above that of a normal‐appearing myocardium. For the measurement of native T1 values LGE (‐) area, T1 values were measured manually in a well‐defined region of interest in the mid‐ventricle, avoiding the LGE (+) area. T1 dispersion was calculated as the SD of native T1 in all segments as previously described. 15 Our previous study showed the normal reference value measured by the same MRI protocol for native T1, T2, and ECV as 1205.4±37.4 ms, 48.6±5.6 ms, and 25.7%±2.4%, respectively. 16 The native T1, T2, and ECV measurements were done only in a subpopulation 30/60 (50%) subjects because these methods were not available before 2011 in our institution.
LV fat infiltration was identified by a high signal intensity with india‐ink etching artifact cine image, 17 , 18 and LGE on both ventricles were visually identified by two radiologists.
For functional analysis, SA cine images were transferred to the software (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada). Endocardial borders of the RV and endocardial and epicardial borders of the LV wall on end‐diastolic and end‐systolic images were delineated automatically. Some correction was done if needed. Both ventricular volume and systolic functions were calculated.
All patients with sub‐endocardial or transmural involvement patterns of LV LGE on CMR and patients with a history of percutaneous coronary artery intervention were examined again to exclude ischemic cardiomyopathy. The reference values of each mapping parameter in healthy subjects using this protocol were described previously. 16 CMR images were analyzed by two expert radiologists (Y.J.H. and Y.J.K.) who were blinded to baseline patient information and clinical outcomes. When diagnosing ACM using CMR with imaging criteria of Task Force Criteria, the diagnosis was based on analyzing the regional wall motion abnormality on the cine image, RV volume, and ejection fraction, not LGE or mapping data.
Definitions of Clinical Manifestations and Events During Follow‐Up
Patients with a final diagnosis of ACM underwent follow‐up for HF‐related events (a composite of HF hospitalization, heart transplantation, and cardiac death due to pump failure), and ventricular tachycardia (VT) events.
HF, which is one of the initial manifestation types, was defined as the presence of symptoms or signs (dyspnea, fatigue, peripheral edema, or pulmonary edema on chest x‐ray) suggestive of volume overload with identified structural/functional cardiac abnormalities, as the ACC/AHA guideline suggested. 19 HF hospitalization event was defined as an unexpected admission for worsening symptoms or signs of HF which needed treatment with intravenous diuretics, vasodilators, or inotropic agents after diagnosis of ACM. Heart transplantation was regarded as a HF‐related event if the transplantation was performed because of the patient’s end‐stage HF status. Cardiac death due to pump failure was defined as death occurring with worsening symptoms of HF lasting at least 24 hours without other evidence of identified life‐threatening arrhythmia or acute myocardial infarction.
VT event during follow‐up is a composite of documented sustained VT and VT requiring appropriate therapy from an implantable cardioverter‐defibrillator (ICD). Sustained VT was defined as sustained ventricular beats with >100 bpm for >30 seconds documented by holter monitoring, unexpected hospital visit, or regular ICD interrogation data. Appropriate ICD therapy (anti‐tachycardia pacing or shock) was also regarded as VT event during follow‐up and was identified from ICD interrogation data. Sudden cardiac death, one of the initial manifestations, was defined as resuscitated unexpected cardiac arrest with witnessed prodromal symptoms lasting <24 hours.
Endomyocardial Biopsy and Genetic Analysis
Among the study population, we performed endomyocardial biopsies in 23 (38%) subjects. In addition, the study population was tested using a next‐generation sequencing panel from 12 (20%) subjects to determine whether they had genetic mutations related to ACM. The determination of pathogenicity (pathogenic or likely‐pathogenic) for the variants was based on the American College of Medical Genetics and Genomics guideline. 20
Statistical Analysis
Continuous variables were presented as mean±SD or median (interquartile range [IQR]) and categorical variables were expressed as a percentage of the group total. Because we analyzed a small sample of patients, comparisons between groups were made using the permutation test versions of the t‐test for continuous variables and Fisher’s exact test for categorical variables. Variables that were not normally distributed were log‐transformed for analysis if needed (eg, N‐terminal prohormone brain natriuretic peptide). Survival rates were estimated using the Kaplan‐Meier survival method, and differences were analyzed using a log‐rank test. Mapping values (native T1, T2, ECV) of the variables in the Kaplan‐Meier analysis were divided based on the median value of each variable (higher group versus lower group). All statistical tests were two‐tailed, and 95% CI were calculated. All statistical analyses were performed using R software (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria) with “survminer” and “coin” packages.
Results
Baseline Clinical Characteristics
The baseline characteristics of study patients are summarized in Table 1. Of the 60 patients who were finally analyzed, 33 (55%) patients were categorized as definite ACM, eight (13%) as borderline ACM, and 19 (32%) as possible ACM. The mean age at diagnosis was 49±18 years and 46 (77%) were male. Five patients (8%) presented with sudden cardiac death, 24 (40%) patients presented with VT, and 17 (28%) patients had symptoms or signs of HF at the time of diagnosis. Twenty‐three (38%) patients underwent ICD implantation, and the purpose of ICD implantation was mostly for secondary prevention (91%). To characterize the clinical profile of patients with and without HF‐related events, we divided the study population into two groups based on the occurrence of HF‐related events during the follow‐up (Table 1). Notably, in terms of medication, patients who had HF‐related events were more likely to be treated with loop diuretics (92% versus 26%, P<0.001).
Table 1.
Baseline Characteristics in the Total Patient Group Regarding HF Events
|
Total (n=60) |
HF event (‐) (n=47) |
HF event (+) (n=13) |
P value | |
|---|---|---|---|---|
| Clinical demographics | ||||
| Age at diagnosis, y | 49±18 | 47±17 | 49±18 | 0.185 |
| Male sex, n (%) | 46 (77) | 36 (77) | 10 (77) | >0.999 |
| Body surface area, m2 | 1.8±0.2 | 1.8±0.2 | 1.7±0.2 | 0.193 |
| Hypertension, n (%) | 16 (27) | 12 (26) | 2 (15) | 0.731 |
| Diabetes, n (%) | 7 (12) | 5 (11) | 2 (15) | 0.639 |
| CAD, n (%) | 4 (7) | 3 (6) | 1 (8) | >0.999 |
| Atrial fibrillation, n (%) | 13 (22) | 9 (19) | 3 (31) | 0.450 |
| Initial manifestation, n (%) | ||||
| Sudden cardiac death | 5 (8) | 5 (11) | 0 (0) | 0.575 |
| VT | 24 (40) | 21 (45) | 3 (23) | 0.210 |
| HF | 17 (28) | 9 (19) | 8 (62) | 0.005 |
| Task Force criteria, n (%) | ||||
| Definite | 33 (55) | 24 (51) | 9 (69) | 0.062 |
| Borderline | 8 (13) | 5 (11) | 3 (23) | |
| Possible | 19 (32) | 18 (38) | 1 (8) | |
| Medications, n (%) | ||||
| Beta‐blockers | 35 (58) | 29 (62) | 6 (46) | 0.354 |
| Anti‐arrhythmics | 15 (25) | 12 (26) | 3 (23) | >0.999 |
| ACEi/ARBs | 33 (55) | 23 (49) | 10 (77) | 0.115 |
| MRAs | 21 (35) | 14 (30) | 7 (54) | 0.187 |
| Loop diuretics | 24 (40) | 12 (26) | 12 (92) | <0.001 |
| VT management, n (%) | ||||
| ICD | 23 (38) | 18 (38) | 5 (39) | >0.999 |
| RFA | 6 (10) | 5 (11) | 1 (8) | >0.999 |
Data are presented as mean±standard deviation or number (percentage).
HF event is defined as a composite of HF hospitalization, heart transplantation, and cardiac death during follow‐up period. ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; CAD, coronary artery disease; HF, heart failure; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; RFA, radiofrequency ablation, and VT, ventricular tachycardia.
Baseline Laboratory Data, ECG, and Echocardiographic Findings
Baseline laboratory, ECG, and echocardiographic data are shown in Table 2. Patients with HF‐related events had significantly lower hemoglobin and serum sodium levels, and significantly higher N‐terminal prohormone brain natriuretic peptide levels. We analyzed baseline ECGs as previously described, but we found no statistically different ECG findings between the two groups. Patients with HF‐related events had significantly lower left ventricular ejection fraction (LVEF) as assessed by echocardiography, compared with patients without HF‐related events (39%±15% versus 50%±14%, P=0.017).
Table 2.
Laboratory, ECG, and Echocardiographic Findings According to HF Events
|
HF event (‐) (n=47) |
HF event (+) (n=13) |
P value | |
|---|---|---|---|
| Laboratory data | |||
| Hematocrit, % | 43.6±5.1 | 39.4±5.5 | 0.015 |
| BUN, mg/dL | 15.7±7.6 | 17.5±7.5 | 0.470 |
| eGFR, mL/min per 1.73 m2 | 83±13 | 81±15 | 0.730 |
| Serum sodium, mEq/L | 141±2 | 137±5 | <0.001 |
| NT‐proBNP, pg/mL [IQR] | 151 [83; 1047] | 1150 [951; 4090] | <0.001 |
| Log (NT‐proBNP) | 2.3±0.8 | 3.2±0.5 | <0.001 |
| ECG | |||
| RBBB, n (%) | 14 (30) | 4 (31) | >0.999 |
| QTc interval, ms | 454±34 | 468±35 | 0.200 |
| TWI (V1‐3), n (%) | 27 (57) | 5 (39) | 0.347 |
| TWI (V4‐6), n (%) | 19 (40) | 7 (54) | 0.529 |
| Epsilon wave (V1‐3), n (%) | 6 (13) | 4 (31) | 0.201 |
| TAD (V1‐3), n (%) | 13 (28) | 7 (54) | 0.101 |
| Echocardiogram | |||
| RV regional dyskinesia/aneurysm, n (%) | 34 (72) | 8 (62) | 0.504 |
| RV FAC, % | 34±12 | 76±9 | 0.088 |
| RV FAC ≤35%, n (%) | 25 (53) | 11 (85) | 0.056 |
| LVEF, % | 50±14 | 39±15 | 0.017 |
| LVEF ≤40%, n (%) | 12 (26) | 7 (54) | 0.089 |
| LAVI, mL/m2 | 28±17 | 38±19 | 0.049 |
| E/e’ | 11±7 | 18±8 | 0.009 |
BUN indicates blood urea nitrogen; E/e’, early diastolic mitral inflow/early diastolic mitral annular tissue velocity; eGFR, estimated glomerular filtration rate; IQR, interquartile range; HF, heart failure; LAVI, left atrium volume index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; RBBB, right bundle‐branch block; RV, right ventricular; RV FAC, right ventricular fractional area change; TAD, terminal activation delay; and TWI, T‐wave inversion.
CMR Imaging Findings
Table 3 shows the functional, structural, and qualitative/quantitative tissue characteristics examined by CMR. Patients who had HF‐related events had lower LVEF, as assessed by CMR compared with patients without HF‐related events (35%±17% versus 48%±13%, P=0.009), and right ventricular ejection fraction (RVEF) was not significantly different (32%±13% versus 40%±13%, P=0.077). Forty‐one patients (68%) had LV LGE and 36 (60%) patients had RV LGE. There was no significant difference in the presence of RV LGE in the two groups, but LV LGE was more common in patients with HF‐related events than patients without HF‐related events (100% versus 60%, P=0.005). The presence of LV fatty infiltration was not significantly different between the two groups (62% versus 53%, P=0.755). Based on these CMR markers and other clinical findings, we classified these subjects according to the “Padua criteria.” Notably, there was no subject with dominant‐right type among patients who had HF‐related events. We also measured quantitative mapping parameters, including native T1, ECV, and T2 values in available patients (n=30, Table 3). In this analysis, patients with HF‐related events had higher native T1, LGE‐free native T1, ECV, and T2 values. According to the presence of LV LGE (Table 4 and 5), the patients with LV LGE had lower LVEF than those without LGE, which was in line with the recent observations that LV LGE is related to LV systolic dysfunction. 5 , 7 In addition, native T1 values, dispersion of native T1, ECV fraction, and T2 values were significantly increased in patients with LV LGE compared with patients without LV LGE (Table 5).
Table 3.
Cardiac Magnetic Resonance Findings According to HF Events
|
HF Event (‐) (n=47) |
HF Event (+) (n=13) |
P value | |
|---|---|---|---|
| Functional and structural assessment | |||
| LVEF, % | 48±13 | 35±17 | 0.009 |
| LVEF ≤40%, n (%) | 13 (28) | 7 (70) | 0.025 |
| LVEDVi, mL/m2 | 96±32 | 106±17 | 0.294 |
| RVEF, % | 40±13 | 32±13 | 0.077 |
| RVEF ≤35%, n (%) | 16 (35) | 5 (50) | 0.476 |
| RVEDVi, mL/m2 | 130±49 | 166±62 | 0.046 |
| Tissue characterization | |||
| RV LGE, n (%) | 28 (60) | 8 (62) | >0.999 |
| LV LGE, n (%) | 28 (60) | 13 (100) | 0.005 |
| LV LGE amount, % [IQR] (n=36) | 16 [8; 31] | 21 [13; 40] | 0.055 |
| RV fatty infiltration, n (%) | 9 (19) | 2 (15) | >0.999 |
| LV fatty infiltration, n (%) | 25 (53) | 8 (62) | 0.755 |
| Padua criteria, n (%) | |||
| Dominant‐right | 12 (26) | 0 (0) | 0.074 |
| Biventricular | 34 (72) | 12 (92) | |
| Dominant‐left (possible) | 1 (2) | 1 (8) | |
| Quantitative parameters (n=30) | |||
| Native T1 value, ms | 1291±68 | 1433±69 | <0.001 |
| T1 dispersion, ms | 133±49 | 135±32 | 0.910 |
| LGE‐free T1 value, ms | 1274±57 | 1382±75 | <0.001 |
| ECV fraction, % | 29.6±4.7 | 40.9±6.4 | <0.001 |
| T2 value, ms | 50.7±3.7 | 58.2±3.7 | <0.001 |
ECV indicates extracellular volume, HF, heart failure; LGE, late gadolinium enhancement; LV, left ventricular; LVEDVi, indexed LV end‐diastolic volume; LVEF, left ventricular ejection fraction; RV, right ventricular; RVEDVi, indexed RV end‐diastolic volume; and RVEF, RV ejection fraction.
Table 4.
Clinical Characteristics According to LV LGE
| Clinical variable |
LV LGE (‐) (n=19) |
LV LGE (+) (n=41) |
P value |
|---|---|---|---|
| Clinical demographics | |||
| Age at diagnosis, y | 46±16 | 50±19 | 0.486 |
| Male sex, n (%) | 15 (79) | 31 (74) | >0.999 |
| Body surface area, m2 | 1.8±0.2 | 1.8±0.2 | 0.885 |
| Hypertension, n (%) | 3 (16) | 13 (32) | 0.229 |
| Diabetes, n (%) | 1 (5) | 6 (15) | 0.414 |
| CAD, n (%) | 1 (5) | 3 78) | >0.999 |
| Atrial fibrillation, n (%) | 4 (21) | 9 (22) | >0.999 |
| Initial manifestation, n (%) | |||
| Sudden cardiac death | 4 (21) | 1 (2) | 0.031 |
| VT | 9 (47) | 15 (37) | 0.572 |
| HF | 2 (11) | 15 (37) | 0.063 |
| Others | 4 (21) | 10 (24) | |
CAD indicates coronary artery disease; HF, heart failure; LV LGE, left ventricular late gadolinium enhancement; and VT, ventricular tachycardia.
Table 5.
Cardiac Magnetic Resonance Findings According to LV LGE
|
LV LGE (‐) (n=19) |
LV LGE (+) (n=41) |
P value | |
|---|---|---|---|
| Functional and structural assessment | |||
| LVEF, % | 51±9 | 43±16 | 0.032 |
| LVEF ≤ 40%, n (%) | 2 (11) | 96 (49) | 0.007 |
| RVEF, % | 42±13 | 36±13 | 0.094 |
| RVEF ≤ 35%, n (%) | 4 (21) | 17 (46) | 0.086 |
| LVEDVi, mL/m2 | 84±22 | 105±31 | 0.013 |
| RVEDVi, mL/m2 | 127±55 | 142±52 | 0.440 |
| Quantitative parameters (n=30) | |||
| Native T1 value, ms | 1276±65 | 1352±92 | 0.023 |
| T1 dispersion, ms | 96±30 | 153±39 | <0.001 |
| LGE‐free T1 value, ms | 1276±65 | 1307±81 | 0.279 |
| ECV fraction, % | 27.5±3.1 | 35.0±7.1 | 0.002 |
| T2 value, ms | 49.7±2.9 | 54.0±5.1 | 0.016 |
| LV LGE pattern, n (%) | |||
| Subendocardial | … | 8 (20) | … |
| Midmural | … | 7 (17) | … |
| Subepicardial | … | 32 (76) | … |
| Transmural | … | 15 (37) | … |
| Clinical events, n (%) | |||
| Cardiac death | 0 (0) | 1 (2) | >0.999 |
| Heart transplant | 0 (0) | 4 (10) | 0.297 |
| Heart failure hospitalization | 0 (0) | 13 (32) | 0.005 |
| Ventricular arrhythmia events | 8 (42) | 12 (29) | 0.384 |
| Sudden cardiac death | 0 (0) | 0 (0) | >0.999 |
| Sustained VT | 3 (16) | 11 (39) | 0.414 |
| Appropriate ICD therapy | 7 (37) | 6 (15) | 0.089 |
| All adverse cardiovascular events | 8 (42) | 21 (51) | 0.585 |
ECV indicates extracellular volume, ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; LVEDVi, indexed LV end‐diastolic volume; LVEF, left ventricular ejection fraction; RV, right ventricular; RVEDVi, indexed RV end‐diastolic volume; RVEF, RV ejection fraction; and VT, ventricular tachycardia.
Genetic Analysis, ACM Phenotype, and Mapping Values: Supplemental Analysis
Genetic analysis revealed that 4 out of 12 patients had pathogenic or likely pathogenic ACM‐related variants. Table S1 shows these subjects with ACM‐related genetic mutations, their phenotypes, and mapping values. The results cannot show statistical significance due to the small number of samples, but we observed that the native T1, ECV, and T2 values were higher in patients with DSP/TMEM43 mutation (biventricular type) than those with PKP2 mutation (dominant‐right type).
Clinical Outcomes
During the median follow‐up period of 34 months (IQR, 15–63 months), there was a total of 13 (22%) HF‐related events and a total of 20 (33%) VT events (Table 5). All heart transplantations (7%) were performed in patients with end‐stage HF, and one cardiac death (2%) event was also related to HF. Among 13 HF‐related events, 9 (69%) were re‐hospitalization for HF, while the other 4 (31%) were the first HF‐related events for each. All HF‐related events occurred in patients with LV LGE (Figure 1A) (log‐rank P=0.0055). Furthermore, there was no significant difference in the rate of HF‐related events in patients with and without RV LGE (Figure 1B) (log‐rank P>0.999). According to the measurable CMR parameters for the systolic function of each ventricle, HF‐related events more occurred in patients with reduced LVEF (Figure 1C, log‐rank P=0.0014), but the events were not different regarding RVEF (Figure 1D).
Figure 1. Survival curves categorized by LGE and ejection fraction for each ventricle.
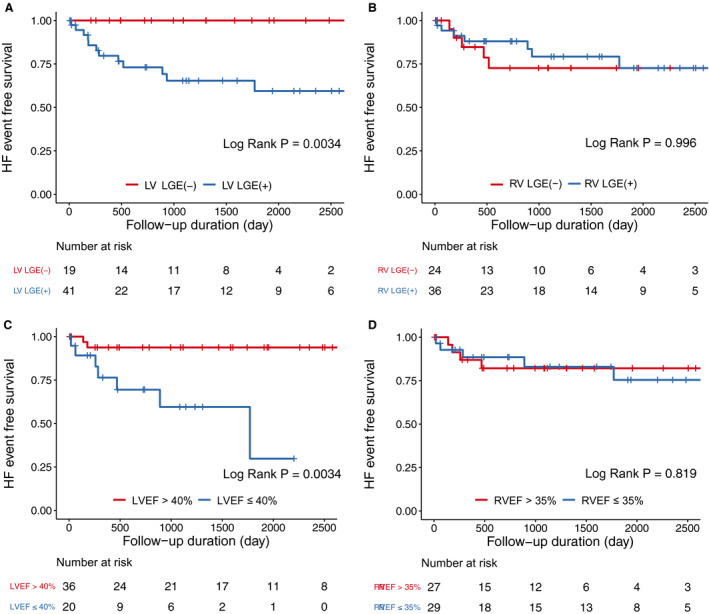
Kaplan‐Meier survival curves for heart failure‐related events according to the presence of late gadolinium enhancement (LGE) in the LV (A), and in the RV (B), and ejection fraction of the LV (C), and the RV (D). EF indicates ejection fraction HF, heart failure; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; RVEF, right ventricular ejection fraction.
We also analyzed the clinical outcomes using mapping parameters in the measurable subgroup. Since the mapping value of the subjects in this study was higher than the normal reference value (Native T1: 1205.4±37.4 ms, ECV: 25.7±2.4%, T2: 48.6±5.6 ms) measured by the same MRI protocol as our study, 16 the values of the variables in the Kaplan‐Meier survival analysis for relative comparative analysis were divided based on the median value of each variable. At first, we divided the patients into two groups according to the median value of each native T1 (1308ms), ECV fraction (30.2%), and T2 value (52.5ms) (higher group versus lower group) for analysis. Interestingly, HF‐related events occurred more in subjects with higher native T1, T2, and ECV groups (Figure 2). In addition, similar trends were observed when analyzing HF‐related events with LGE or mapping parameters including T1, T2, and ECV and LV dysfunction according to LVEF measured by CMR (Figure S1). Figure 3 shows representative cases of ACM with and without LV involvement as assessed by CMR. However, regarding VT events, there were no significant differences according to LGE in both ventricles, native T1, or T2 values (Figure S2).
Figure 2. Survival curves categorized by T1, T2, and ECV.
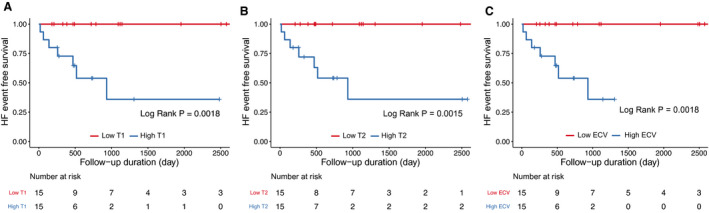
The survival curves the events according to native T1 mapping value (A) (high vs low, cut‐off value: 1308 ms, as a median value), T2 mapping values (B) (high vs low, cut‐off value: 52.5 ms, as a median value), and extracellular volume (ECV) fraction (C) (high vs low, cut‐off value: 30.2%, as a median value). HF indicates heart failure.
Figure 3. LV LGE and tissue characteristics on CMR in patients with ACM.
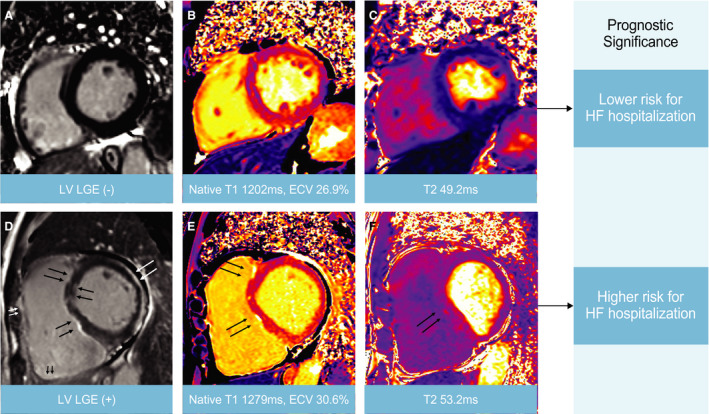
The prognostic significance of LV late gadolinium enhancement (LGE) and mapping parameters on cardiac magnetic resonance (CMR) in patients with arrhythmogenic cardiomyopathy (ACM). A 66‐year‐old male presented with ventricular tachycardia (A through C), and a 54‐year‐old male presented with heart failure (D through F). Arrows (black, white) indicate focal LGEs. HF indicates heart failure; LV, left ventricular.
Discussion
The principal findings of the current study were as follows: (1) patients with LV LGE had more HF‐related events and (2) higher native T1, T2, and ECV values could predict a higher risk of HF‐related events in patients with ACM.
Clinical Outcomes According to CMR Features in ACM
Although the LGE degree varies between studies, there are LV scars with LGE in a majority (60%–70%) of patients with ACM. 7 Abnormal CMR findings, including LGE in the LV and/or RV, have been reported to be associated with ventricular arrhythmic events. 9 , 21 However, the most recent study showed that typically manifested LV abnormalities on CMR were not associated with arrhythmic outcomes in patients with ACM, 10 which is a consistent finding of our results. Our finding could be supported by a recent study of DSP cardiomyopathy (one subtype of ACM) that showed the LV involvement including LV LGE was also not related to VT events. 22 Considering the nature of ACM, a heterogeneous disease, it is thought that the genetic backgrounds and clinical courses of each study may be different. Nevertheless, it is meaningful that we showed that CMR markers (LGE, native T1, ECV, and T2 mapping) were associated with HF‐related outcomes in patients with ACM.
CMR Mapping Techniques and Tissue Characterization in ACM
We analyzed by using mapping techniques on CMR in available patients. Native T1 mapping is a promising technique for detection of earlier stages of cardiomyopathy and quantitative measurement of myocardial change. 23 A recent brief report showed that native T1 values were higher in patients with ACM than control subjects, but it did not show any relationship with clinical outcomes. 15
Together with native T1, ECV also represents diffuse myocardial fibrosis. 24 In ACM, ECV expansion occurs because fibro‐fatty tissue deposits in the extracellular interstitium. Our results showed that CMR‐derived ECV, as well as native T1, value could have a prognostic value for the prediction of clinical outcomes in patients with ACM.
However, native T1 and ECV have some limitations in ACM. The lowering of T1 by fat components can underestimate myocardial fibrosis in the fibro‐fatty area of the myocardium. For this reason, the previous study focused on the diagnostic value of the dispersion of native T1 in ACM. 15 Fat itself also can underestimate CMR‐derived ECV because fat had less gadolinium enhancement than fibrosis. 25 Nevertheless, we showed that ECV and T1 measured in the LV myocardium were related to clinical outcomes in patients with ACM.
In the group with HF‐related events, the T2 value was higher than that in the no‐event group. An elevated T2 value is a well‐known marker of myocardial edema and inflammation. 26 , 27 , 28 Along with T1 and ECV, our results indicated that high T2 value could also be associated with HF‐related events in patients with ACM. We thought that the elevated T2 value in patients who experienced the HF‐related events was attributed to the inflammation as many studies have reported inflammatory infiltration in ACM. 29
The recent studies have shown that ACM with DSP mutations is more associated with LV involvement than traditional ACM (particularly with PKP2 mutations), 30 and PKP2 genotype carriers are more arrhythmic than DSC2/DSG2/DSP or gene‐negative carrier status, whereas reduced LVEF was mostly seen among DSC2/DSG2/DSP carriers. 31 But these studies did not show any comparisons in CMR mapping values between DSP versus PKP2 ACM. For the first time, we found a patient with DSP mutation had high mapping values than those with PKP2 mutation as far as we know, and it can trigger further studies in the future.
From the present results, we showed that LGE, native T1, ECV, and T2 values measured by CMR were associated with HF‐related events in patients with ACM. ACM had long been recognized as a disease of the right side of the heart. It is not completely possible to distinguish whether the hospitalization for HF is due to RV or LV abnormality, but given that ACM is a biventricular disease, it is expected that the importance of understanding the characteristics of the LV myocardium will be increasingly emphasized.
Study Limitations
The main limitation of this study was the small study population. Especially, mapping values were obtained in half of the study population (50%). Due to the variability of CMR protocol in each participant, some portions of CMR data were impossible to analyze including the mapping parameters. In fact, the novel mapping techniques were not available for routine use before 2011 in our institution. Since the study was conducted in such a small number of patients, the results based on the analysis with mapping parameters are not conclusive; however, we suggest that they can be hypothesis‐generating. Also, the lack of genetic testing results is another important limitation of our research. We could obtain a better understanding of ACM by combining CMR data if we performed genetic tests for all subjects. For this reason, subjects classified by the Padua criteria as dominant‐left (possible) in the present results lack the genetic basis to be classified as definite dominant‐left subtype. However, we found an interesting signal for the association of the mapping parameters with different genetic mutations in ACM. Finally, we did not confirm ACM by cardiac biopsy in all subjects. We performed endomyocardial biopsies in just 23 patients. However, in clinical practice, performing a biopsy in ACM is challenging considering the low yield of positive pathologic results. Because of these limitations, findings of LV LGE and elevated T1, T2 values in LV myocardium certainly raise significant concern for the presence of myocarditis and/or cardiac sarcoidosis in the study population. Nevertheless, the present study investigated the clinical significance of CMR findings in patients with ACM satisfying the current diagnostic criteria, so it has significant implications in this population.
Conclusions
The assessment of LV involvement using CMR with LGE imaging is now crucial in patients with clinically diagnosed ACM according to the 2010 Task Force Criteria. From the results of our study, we have shown that the presence of LGE in LV myocardium and higher native T1, ECV, and T2 values measured by CMR were associated with more HF‐related events in patients with ACM. Further research is needed to confirm that these LV myocardial characteristics from CMR are associated with the clinical outcomes in this patient population.
Sources of Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF‐2021R1I1A1A01060135).
Disclosures
None.
Supporting information
Table S1
Figures S1–S2
Acknowledgments
Special thanks to Mun‐ju Jung, publisher, for making figures included.
Supplemental Material for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023167
For Sources of Funding and Disclosures, see page 10.
References
- 1. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. doi: 10.1056/NEJMra1509267 [DOI] [PubMed] [Google Scholar]
- 2. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sen‐Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left‐dominant arrhythmogenic cardiomyopathy: an under‐recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 4. Miles C, Finocchiaro G, Papadakis M, Gray B, Westaby J, Ensam B, Basu J, Parry‐Williams G, Papatheodorou E, Paterson C, et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli‐Ducci C, et al., International Experts . Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–1429. doi: 10.1093/eurheartj/ehz669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD, Migliore F, Pilichou K, Rampazzo A, Rigato I, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin S, Pilichou K, Motta R, Aliberti C, Thiene G, et al. Arrhythmogenic right ventricular cardiomyopathy: characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J Am Heart Assoc. 2020;9:e014628. doi: 10.1161/JAHA.119.014628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen MT, Yang ZG, Diao KY, Jiang L, Zhang Y, Liu X, Gao Y, Hu BY, Huang S, Guo YK. Left ventricular involvement in arrhythmogenic right ventricular dysplasia/cardiomyopathy predicts adverse clinical outcomes: a cardiovascular magnetic resonance feature tracking study. Sci Rep. 2019;9:14235. doi: 10.1038/s41598-019-50535-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aquaro GD, Pingitore A, Di Bella G, Piaggi P, Gaeta R, Grigoratos C, Altinier A, Pantano A, Strata E, De Caterina R,. et al. Prognostic role of cardiac magnetic resonance in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2018;122:1745–1753. doi: 10.1016/j.amjcard.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 10. Zghaib T, Te Riele ASJM, James CA, Rastegar N, Murray B, Tichnell C, Halushka MK, Bluemke DA, Tandri H, Calkins H, et al. Left ventricular fibro‐fatty replacement in arrhythmogenic right ventricular dysplasia/cardiomyopathy: prevalence, patterns, and association with arrhythmias. J Cardiovasc Magn Reson. 2021;23:58. doi: 10.1186/s12968-020-00702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–1884. doi: 10.1161/01.CIR.0000143375.93288.82 [DOI] [PubMed] [Google Scholar]
- 12. Gilotra NA, Bhonsale A, James CA, te Riele ASJ, Murray B, Tichnell C, Sawant A, Ong CS, Judge DP, Russell SD, et al. Heart failure is common and under‐recognized in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Heart Fail. 2017;10:e003819. doi: 10.1161/CIRCHEARTFAILURE.116.003819 [DOI] [PubMed] [Google Scholar]
- 13. di Gioia CR, Giordano C, Cerbelli B, Pisano A, Perli E, De Dominicis E, Poscolieri B, Palmieri V, Ciallella C, Zeppilli P, et al. Nonischemic left ventricular scar and cardiac sudden death in the young. Hum Pathol. 2016;58:78–89. doi: 10.1016/j.humpath.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 14. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, et al. Nonischemic left ventricular scar as a substrate of life‐threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. doi: 10.1161/CIRCEP.116.004229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bourfiss M, Prakken NHJ, van der Heijden JF, Kamel I, Zimmerman SL, Asselbergs FW, Leiner T, Velthuis BK, Te Riele A. Diagnostic value of native T1 mapping in arrhythmogenic right ventricular cardiomyopathy. JACC Cardiovasc Imaging. 2019;12:1580–1582. doi: 10.1016/j.jcmg.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 16. Hong YJ, Park CH, Kim YJ, Hur J, Lee H‐J, Hong SR, Suh YJ, Greiser A, Paek MY, Choi BW, et al. Extracellular volume fraction in dilated cardiomyopathy patients without obvious late gadolinium enhancement: comparison with healthy control subjects. Int J Cardiovasc Imaging. 2015;31:115–122. doi: 10.1007/s10554-015-0595-0 [DOI] [PubMed] [Google Scholar]
- 17. Aquaro GD, Nucifora G, Pederzoli L, Strata E, De Marchi D, Todiere G, Andrea B, Pingitore A, Lombardi M. Fat in left ventricular myocardium assessed by steady‐state free precession pulse sequences. Int J Cardiovasc Imaging. 2012;28:813–821. doi: 10.1007/s10554-011-9886-2 [DOI] [PubMed] [Google Scholar]
- 18. Aquaro GD, Todiere G, Strata E, Barison A, Di Bella G, Lombardi M. Usefulness of India ink artifact in steady‐state free precession pulse sequences for detection and quantification of intramyocardial fat. J Magn Reson Imaging. 2014;40:126–132. doi: 10.1002/jmri.24335 [DOI] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 20. Hershberger RE, Givertz MM, Ho CY, Judge DP, Kantor PF, McBride KL, Morales A, Taylor MRG, Vatta M, Ware SM, et al. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018;20:899–909. doi: 10.1038/s41436-018-0039-z [DOI] [PubMed] [Google Scholar]
- 21. Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N, Lesizza P, Grigoratos C, Minati M, Dell’Omodarme M, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2020;75:2753–2765. doi: 10.1016/j.jacc.2020.04.023 [DOI] [PubMed] [Google Scholar]
- 22. Wang W, Murray B, Tichnell C, Gilotra NA, Zimmerman SL, Gasperetti A, Scheel P, Tandri H, Calkins H, James CA. Clinical characteristics and risk stratification of desmoplakin cardiomyopathy. Europace. 2021. doi: 10.1093/europace/euab183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol. 2016;71:768–778. doi: 10.1016/j.crad.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 24. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, et al, Society for Cardiovascular Magnetic Resonance I, Cardiovascular Magnetic Resonance Working Group of the European Society of C . Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18:89. doi: 10.1186/s12968-016-0308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montant P, Sigovan M, Revel D, Douek P. MR imaging assessment of myocardial edema with T2 mapping. Diagn Interv Imaging. 2015;96:885–890. doi: 10.1016/j.diii.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 27. Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–278. doi: 10.1016/j.jcmg.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S, Simonetti OP, Raman SV. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. 2012;5:102–110. doi: 10.1161/CIRCIMAGING.111.967836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campuzano O, Alcalde M, Iglesias A, Barahona‐Dussault C, Sarquella‐Brugada G, Benito B, Arzamendi D, Flores J, Leung TK, Talajic M, et al. Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol. 2012;65:1077–1083. doi: 10.1136/jclinpath-2012-201022 [DOI] [PubMed] [Google Scholar]
- 30. Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC, Agarwal PP, Arscott P, Dellefave‐Castillo LM, Vorovich EE, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christensen AH, Platonov PG, Jensen HK, Chivulescu M, Svensson A, Dahlberg P, Madsen T, Frederiksen TC, Heliö T, Lie ØH, et al. Genotype‐phenotype correlation in arrhythmogenic right ventricular cardiomyopathy‐risk of arrhythmias and heart failure. J Med Genet. 2021. doi: 10.1136/jmedgenet-2021-107911 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S2


