Abstract
Background
Excess mortality from cardiovascular disease during the COVID‐19 pandemic has been reported. The mechanism is unclear but may include delay or deferral of care, or differential treatment during hospitalization because of strains on hospital capacity.
Methods and Results
We used emergency department and inpatient data from a 12‐hospital health system to examine changes in volume, patient age and comorbidities, treatment (right‐ and left‐heart catheterization), and outcomes for patients with acute myocardial infarction (AMI) and heart failure (HF) during the COVID‐19 pandemic compared with pre‐COVID‐19 (2018 and 2019), controlling for seasonal variation. We analyzed 27 427 emergency department visits or hospitalizations. Patient volume decreased during COVID‐19 for both HF and AMI, but age, race, sex, and medical comorbidities were similar before and during COVID‐19 for both groups. Acuity increased for AMI as measured by the proportion of patients with ST‐segment elevation. There were no differences in right‐heart catheterization for patients with HF or in left heart catheterization for patients with AMI. In‐hospital mortality increased for AMI during COVID‐19 (odds ratio [OR], 1.46; 95% CI, 1.21–1.76), particularly among the ST‐segment–elevation myocardial infarction subgroup (OR, 2.57; 95% CI, 2.24–2.96), but was unchanged for HF (OR, 1.02; 95% CI, 0.89–1.16).
Conclusions
Cardiovascular volume decreased during COVID‐19. Despite similar patient age and comorbidities and in‐hospital treatments during COVID‐19, mortality increased for patients with AMI but not patients with HF. Given that AMI is a time‐sensitive condition, delay or deferral of care rather than changes in hospital care delivery may have led to worse cardiovascular outcomes during COVID‐19.
Keywords: acute myocardial infarction, COVID‐19, heart failure, spillover
Subject Categories: Coronary Artery Disease, Heart Failure, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- LHC
left‐heart catheterization
- RHC
right‐heart catheterization
Clinical Perspective
What Is New?
During the first year of the COVID‐19 pandemic, admissions and emergency department visit volume decreased for acute myocardial infarction and heart failure compared with prior years, but there were no observed changes in patient age, comorbidities, or treatments offered.
However, among patients with acute myocardial infarction, there was significantly higher acuity (proportion of ST‐segment–elevation myocardial infarction, proportion of admissions originating in the emergency department) during COVID‐19 and higher mortality.
What Are the Clinical Implications?
Given that we did not see differences in age, comorbidities, or treatment, but did see higher acuity for AMI, we hypothesize that delay or deferral of care as opposed to disruption of hospital care may have led to the observed differences in acute myocardial infarction mortality during COVID‐19.
Educating patients on the importance of seeking necessary medical care and providing safe means of accessing care may serve as an important intervention to reduce negative spillover effects of the COVID‐19 pandemic on cardiovascular care and outcomes.
The COVID‐19 pandemic has led to 46 million infections and >700 000 deaths in the United States alone. However, its impact also affected non‐COVID‐19 medical conditions. Prior studies suggest that there was significant excess mortality from causes other than COVID‐19 during the pandemic, including from cardiovascular disease. 1 , 2 , 3 , 4 , 5
However, the cause for increased cardiovascular mortality during the COVID‐19 pandemic remains unclear. One hypothesis is that the higher mortality is attributable to delay or deferral of care. 5 , 6 Perhaps in part because of fear of exposure to COVID‐19, patients may have presented later to hospital systems, and as such were sicker and had more adverse outcomes while hospitalized. 6 , 7 For example, one recent study showed that heart failure (HF) hospitalizations decreased by ≈41% in an Australian cohort. 8 Hospitalizations for acute coronary syndromes appear to have decreased during the COVID‐19 pandemic in multiple areas as well. 9 , 10 , 11
Another possibility is that patients who did present to the hospital were treated differently because of hospital limitations at the time, whether related to bed shortages, staff shortages, or changes in protocol. 12 , 13 For example, one German study showed that the rate of percutaneous coronary intervention decreased for patients with acute coronary syndromes during COVID‐19, 14 and a recent Swiss and Spanish study showed similar findings. 15 Another study showed that patients with HF hospitalized during the COVID‐19 pandemic were less likely to receive guideline‐directed medical therapy for left ventricular dysfunction on hospital discharge compared with prior years. 8
Understanding whether delays in presentation or differences in treatment were associated with worse outcomes is important as clinical leaders and policymakers seek to deal with ongoing and future surges of COVID‐19 or other challenges to the health care system in a way that protects patients. We therefore set out to answer 4 research questions using emergency department (ED) and inpatient hospitalization data from a 12‐hospital health care system in the Midwest, examining both patients with acute myocardial infarction (AMI) and patients with HF:
Were there changes in volume of ED visits and hospitalizations for AMI or HF during COVID‐19 compared with similar calendar months in the 2 years prior?
Among patients who did present with AMI or HF, was there evidence of a higher number of comorbidities or advanced age?
Among admitted patients with AMI or HF, were treatments such as cardiac catheterization used differently during COVID‐19?
Were there differences in outcomes for AMI or HF during COVID‐19?
Methods
Data
In this observational study, we used claims data to examine all ED and inpatient hospitalization visits without a documented COVID‐19 diagnosis to 12 hospitals in the BJC Healthcare organization, a large health system in the St. Louis metropolitan region, between January 1, 2018, and September 23, 2020. These data include check‐in and discharge or death date, as well as patient age; sex; race; insurance payor (primary); and International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis and procedure codes. As 97% of our patient population was composed of Black and White patients, other races were not included in our final analyses because of small sample size.
This study was approved by the Washington University Office of Human Subjects Protection. Requirement for informed consent was waived because of the observational nature of the study and the deidentified nature of the data. Because the data contain identifiable information on individual patients, they cannot be made publicly available and cannot be shared without specific institutional approval. However, researchers wishing to access deidentified parts of the data set should contact the corresponding author.
Analytic Data Set Description
Our sample included 27 427 ED and inpatient visits over the time period from January 1, 2018, through September 22, 2020, in the BJC Healthcare system, which includes 12 of 13 hospitals (we excluded St. Louis Children’s Hospital to focus on our target population, age >18 years). We divided the data set into seasons to account for any seasonal variation noted previously for HF hospitalizations 16 , 17 and AMI hospitalizations, 18 , 19 categorizing winter (December 21 – March 20), spring (March 21 to June 20), summer (June 21–September 20), and fall (September 21–December 20) in each year. Of note, the St. Louis City and County “Stay at Home” ordinances were put in place on March 23, 2020, and discontinued on May 19, 2020. COVID‐19 events were defined as ED visits and hospitalizations occurring between March 21, 2020, and September 22, 2020.
Predictors
Our primary predictor was whether or not the clinical event took place during COVID‐19. We used an indicator variable to describe ED and inpatient hospitalizations before and following the St. Louis City and St. Louis County Stay at Home orders from March 21, 2020. Clinical comorbidities were defined using the Elixhauser approach, which is a validated method to risk‐adjust in‐hospital outcomes.
Outcomes
Outcome measures included volume (the daily number of non‐COVID‐19 ED and inpatient hospitalizations), patient age and medical comorbidities, in‐hospital treatments, and mortality. For in‐hospital treatments, we examined the use of left‐heart catheterization (LHC) for patients with AMI, and right‐heart‐catheterization (RHC) for patients with HF. We counted hospitalizations as having an LHC if the claim contained an ICD‐10 procedure code for LHC or percutaneous coronary intervention. WhileRHC is not a routine part of HF care, it can be of utility for particularly ill or otherwise challenging patients. An increase in use might indicate that patients were sicker during COVID‐19; a decrease might indicate more hesitancy to perform procedures.
Statistical Analysis
Patient characteristics were summarized by season and COVID‐19 time period, and compared using chi‐squared tests and t‐tests as appropriate. Graphical representations of trends in mortality rates were created using locally estimated scatterplot smoothing regression, fitting a locally weighted model surrounding every week that describes the proportion of hospitalizations that ended in death and how it varies over time. Raw rates of characteristics of presentation and in‐hospital treatments and outcomes were similarly summarized by season and COVID‐19 time period and compared using chi‐squared tests and t‐tests. As a falsification test, we evaluated whether any of these key elements differed between 2018 and 2019. To determine whether COVID‐19 was associated with receipt of specific treatments (RHC/LHC) or with higher mortality in either cohort, logistic regression models fit using generalized estimating equations were used to control for key medical comorbidities, age, race, insurance, and seasonal variation, as well as to account for within‐site clustering.
P values <0.05 were considered statistically significant. All statistical analyses were performed using R version 4.0.2. This study was approved by the Office of Human Research Protection at the Washington University School of Medicine. The requirement for informed consent and Health Insurance Portability and Accountability Act notification were waived because of the deidentified nature of the data.
Results
Changes in Volume and Patient Demographics and Comorbidities
For the HF cohort, there were 21 262 total visits during the study period (Table 1). Daily patient volume decreased in COVID‐19 compared with pre‐COVID‐19 (20.7–22.3 events/day pre‐COVID‐19, 17.0–18.3 events/day during COVID‐19; P<0.001). While sex distribution was unchanged, patients during COVID‐19 were more often Black, more often insured by Medicaid or self‐pay, and tended to have a higher prevalence of liver disease and fluid and electrolyte disorders, though differences were small and of uncertain clinical significance.
Table 1.
Patient Characteristics
| Heart failure cohort | Pre‐COVID‐19 (January 1, 2018–March 20, 2020) | COVID‐19 (March 21, 2020–September 21, 2020) | P value | ||||
|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Fall | Spring | Summer | ||
| (N=5948) | (N=4163) | (N=3844) | (N=4016) | (N=1565) | (N=1685) | ||
| Daily volume | 23.6 (6.30) | 22.3 (6.02) | 20.7 (5.71) | 22.3 (5.84) | 17.0 (5.75) | 18.3 (4.94) | <0.001 |
| Sex | |||||||
| Female | 2907 (48.9) | 2007 (48.2) | 1981 (51.5) | 1958 (48.8) | 759 (48.5) | 793 (47.1) | 0.05 |
| Male | 3041 (51.1) | 2156 (51.8) | 1863 (48.5) | 2058 (51.2) | 806 (51.5) | 892 (52.9) | |
| Race | |||||||
| Black | 2397 (40.3) | 1731 (41.6) | 1613 (42.0) | 1699 (42.3) | 690 (44.1) | 746 (44.3) | 0.02 |
| White | 3551 (59.7) | 2432 (58.4) | 2231 (58.0) | 2317 (57.7) | 875 (55.9) | 939 (55.7) | |
| Insurance | |||||||
| Commercial | 653 (11.0) | 457 (11.0) | 412 (10.7) | 433 (10.8) | 158 (10.1) | 184 (10.9) | <0.001 |
| Medicaid | 737 (12.4) | 524 (12.6) | 478 (12.4) | 497 (12.4) | 249 (15.9) | 263 (15.6) | |
| Medicare | 4394 (73.9) | 3084 (74.1) | 2858 (74.3) | 2978 (74.2) | 1107 (70.7) | 1178 (69.9) | |
| Self pay | 164 (2.8) | 98 (2.4) | 96 (2.5) | 108 (2.7) | 51 (3.3) | 60 (3.6) | |
| Comorbidities | |||||||
| Renal failure | 3423 (57.5) | 2348 (56.4) | 2189 (56.9) | 2377 (59.2) | 870 (55.6) | 982 (58.3) | 0.77 |
| Liver disease | 326 (5.5) | 258 (6.2) | 215 (5.6) | 259 (6.4) | 127 (8.1) | 111 (6.6) | 0.01 |
| Diabetes | 2419 (40.7) | 1655 (39.8) | 1491 (38.8) | 1617 (40.3) | 610 (39.0) | 671 (39.8) | 0.92 |
| Valvular heart disease | 1656 (27.8) | 1088 (26.1) | 1009 (26.2) | 1072 (26.7) | 399 (25.5) | 425 (25.2) | 0.37 |
| Hypertension | 5424 (91.2) | 3786 (90.9) | 3550 (92.4) | 3716 (92.5) | 1438 (91.9) | 1552 (92.1) | 0.53 |
| Chronic pulm. disease | 2526 (42.5) | 1844 (44.3) | 1712 (44.5) | 1730 (43.1) | 682 (43.6) | 720 (42.7) | 0.23 |
| Fluid/electrolyte disorder | 1994 (33.5) | 1347 (32.4) | 1273 (33.1) | 1342 (33.4) | 628 (40.1) | 654 (38.8) | <0.001 |
| Obesity | 1573 (26.4) | 1151 (27.6) | 986 (25.7) | 1046 (26.0) | 416 (26.6) | 492 (29.2) | 0.18 |
| AMI cohort | Pre‐COVID‐19 (January 1, 2018–March 20, 2020) | COVID‐19 (March 21, 2020–September 21, 2020) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Fall | Spring | Summer | |||
| (N=1645) | (N=1234) | (N=1128) | (N=1186) | (N=450) | (N=505) | |||
| Daily volume | 6.55 (2.70) | 6.63 (2.54) | 6.1 (2.26) | 6.59 (2.72) | 4.89 (2.28) | 5.49 (2.31) | <0.001 | |
| Sex | ||||||||
| Female | 630 (38.3) | 468 (37.9) | 458 (40.6) | 459 (38.7) | 163 (36.2) | 196 (38.8) | 0.41 | |
| Male | 1015 (61.7) | 766 (62.1) | 670 (59.4) | 727 (61.3) | 287 (63.8) | 309 (61.2) | ||
| Race | ||||||||
| Black | 369 (22.4) | 258 (20.9) | 239 (21.2) | 250 (21.1) | 97 (21.6) | 116 (23.0) | 0.45 | |
| White | 1276 (77.6) | 976 (79.1) | 889 (78.8) | 936 (78.9) | 353 (78.4) | 389 (77.0) | ||
| Insurance | ||||||||
| Commercial | 454 (27.6) | 310 (25.1) | 296 (26.2) | 320 (27.0) | 114 (25.3) | 131 (25.9) | 0.94 | |
| Medicaid | 142 (8.6) | 94 (7.6) | 94 (8.3) | 75 (6.3) | 40 (8.9) | 42 (8.3) | ||
| Medicare | 977 (59.4) | 757 (61.3) | 671 (59.5) | 729 (61.5) | 273 (60.7) | 297 (58.8) | ||
| Self‐pay | 72 (4.4) | 73 (5.9) | 67 (5.9) | 62 (5.2) | 23 (5.1) | 35 (6.9) | ||
| Comorbidities | ||||||||
| Renal failure | 476 (28.9) | 328 (26.6) | 337 (29.9) | 358 (30.2) | 140 (31.1) | 159 (31.5) | 0.08 | |
| Liver disease | 85 (5.2) | 58 (4.7) | 51 (4.5) | 48 (4.0) | 29 (6.4) | 25 (5.0) | 0.24 | |
| Diabetes | 487 (29.6) | 354 (28.7) | 332 (29.4) | 358 (30.2) | 123 (27.3) | 145 (28.7) | 0.60 | |
| Valvular heart disease | 330 (20.1) | 204 (16.5) | 203 (18.0) | 220 (18.5) | 83 (18.4) | 86 (17.0) | 0.79 | |
| Hypertension | 805 (48.9) | 572 (46.4) | 542 (48.0) | 586 (49.4) | 216 (48.0) | 251 (49.7) | 0.39 | |
| Chronic pulmonary disease | 420 (25.5) | 325 (26.3) | 301 (26.7) | 268 (22.6) | 113 (25.1) | 124 (24.6) | 0.34 | |
| Fluid/electrolyte disorder | 468 (28.4) | 313 (25.4) | 310 (27.5) | 347 (29.3) | 137 (30.4) | 133 (26.3) | 0.28 | |
| Obesity | 360 (21.9) | 261 (21.2) | 228 (20.2) | 251 (21.2) | 92 (20.4) | 99 (19.6) | 0.68 | |
Variables were reported as numbers and percentages. P value compares spring/summer pre‐COVID‐19 to spring/summer during COVID‐19.
AMI indicates acute myocardial infarction.
For the AMI cohort, there were 6165 total visits in our study period. Similar to the HF cohort, patient volume decreased in COVID‐19 compared with pre‐COVID‐19 (6.1–6.6 events/day pre‐COVID‐19, 4.9–5.5 events/day during COVID‐19; P<0.001; Table 1). However, there were no meaningful differences in sex, race, insurance status, or medical comorbidities.
Falsification testing comparing 2018 with 2019 yielded similar demographics, but small differences in the prevalence of comorbidities in the HF cohort, and no meaningful differences in the AMI cohort (Table S1).
Changes in Hospitalization Characteristics and In‐Hospital Treatments
Among the HF cohort, there was no difference in the proportion of patients presenting with cardiogenic shock, but the proportion of admissions originating in the ED increased slightly during COVID‐19 (83.8%–84.9% pre‐COVID‐19, 85.7%–86.8% during COVID‐19; P=0.04; Table 2). There was no difference in the rate of RHC (8.3%–8.4% pre‐COVID‐19, 8.9%–10.7% during COVID‐19; P=0.43), length of stay (4.78–4.83 days pre‐COVID‐19, 4.76–4.9 days during COVID‐19) or mortality (1.8%–2.2% pre‐COVID‐19, 1.6%–2.7% during COVID‐19; P=0.94). There was a decrease in discharge to postacute care and an increase in discharges home with services.
Table 2.
Changes in Presentation, In‐Hospital Treatments, and Outcomes
| Pre‐COVID‐19 (January 1, 2018–March 20, 2020) | COVID‐19 (March 21, 2020–September 21, 2020) | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Fall | Spring | Summer | |||
| Heart failure cohort | ||||||||
| Presentation severity | ||||||||
| Cardiogenic shock | 215 (3.6) | 165 (4.0) | 135 (3.5) | 174 (4.3) | 61 (3.9) | 62 (3.7) | 215 (3.6) | |
| ED admission | 4998 (84.0) | 3490 (83.8) | 3265 (84.9) | 3380 (84.2) | 1341 (85.7) | 1462 (86.8) | 0.041 | |
| Direct admission | 933 (15.7) | 659 (15.8) | 566 (14.7) | 627 (15.6) | 221 (14.1) | 218 (12.9) | 0.053 | |
| In‐hospital procedures/outcomes | ||||||||
| Right‐heart catheterization | 474 (8.0) | 349 (8.4) | 319 (8.3) | 339 (8.4) | 139 (8.9) | 180 (10.7) | 0.431 | |
| LOS, mean (SD) | 4.85 (6.16) | 4.78 (6.26) | 4.83 (6.31) | 4.96 (7.28) | 4.90 (5.96) | 4.76 (5.33) | 0.973 | |
| Death | 145 (2.4) | 93 (2.2) | 69 (1.8) | 94 (2.3) | 42 (2.7) | 27 (1.6) | 0.944 | |
| Discharge status | ||||||||
| Against medical advice | 110 (1.8) | 87 (2.1) | 92 (2.4) | 68 (1.7) | 38 (2.4) | 55 (3.3) | <0.001 | |
| Home | 3716 (62.5) | 2594 (62.3) | 2404 (62.5) | 2511 (62.5) | 1003 (64.1) | 1070 (63.5) | ||
| Home with services | 933 (15.7) | 694 (16.7) | 603 (15.7) | 643 (16.0) | 293 (18.7) | 314 (18.6) | ||
| Other | 63 (1.1) | 45 (1.1) | 32 (0.8) | 35 (0.9) | 15 (1.0) | 17 (1.0) | ||
| Postacute care | 981 (16.5) | 650 (15.6) | 644 (16.8) | 665 (16.6) | 174 (11.1) | 202 (12.0) | ||
| AMI cohort | ||||||||
| Presentation severity | ||||||||
| Cardiogenic shock | 184 (11.2) | 140 (11.3) | 117 (10.4) | 120 (10.1) | 60 (13.3) | 58 (11.5) | 184 (11.2) | |
| ED admission | 1275 (77.5) | 949 (76.9) | 840 (74.5) | 949 (80.0) | 362 (80.4) | 418 (82.8) | 0.001 | |
| Direct admission | 368 (22.4) | 280 (22.7) | 286 (25.4) | 237 (20.0) | 88 (19.6) | 87 (17.2) | 0.002 | |
| NSTEMI | 1147 (69.7) | 873 (70.7) | 801 (71.0) | 861 (72.6) | 281 (62.4) | 341 (67.5) | 0.005 | |
| STEMI | 498 (30.3) | 361 (29.3) | 327 (29.0) | 325 (27.4) | 169 (37.6) | 164 (32.5) | ||
| Procedures and LOS | ||||||||
| Left heart catheterization | 1130 (68.7) | 879 (71.2) | 801 (71.0) | 843 (71.1) | 315 (70.0) | 362 (71.7) | 0.991 | |
| LOS, mean (SD) | 5.22 (5.92) | 5.17 (6.71) | 5.09 (6.21) | 5.05 (6.94) | 4.98 (6.73) | 4.42 (5.19) | 0.184 | |
| Death | 145 (2.4) | 93 (2.2) | 69 (1.8) | 94 (2.3) | 42 (2.7) | 27 (1.6) | <0.001 | |
| Discharge status | ||||||||
| Against medical advice | 110 (1.8) | 87 (2.1) | 92 (2.4) | 68 (1.7) | 38 (2.4) | 55 (3.3) | <0.001 | |
| Home | 3716 (62.5) | 2594 (62.3) | 2404 (62.5) | 2511 (62.5) | 1003 (64.1) | 1070 (63.5) | ||
| Home with services | 933 (15.7) | 694 (16.7) | 603 (15.7) | 643 (16.0) | 293 (18.7) | 314 (18.6) | ||
| Other | 63 (1.1) | 45 (1.1) | 32 (0.8) | 35 (0.9) | 15 (1.0) | 17 (1.0) | ||
| Post‐acute care | 981 (16.5) | 650 (15.6) | 644 (16.8) | 665 (16.6) | 174 (11.1) | 202 (12.0) | ||
Categorical variables were reported as numbers and percentages, and continuous variables were reported as means and standard deviations. P value compares spring/summer pre‐COVID‐19 to spring/summer during COVID‐19.
AMI indicates acute myocardial infarction; ED, emergency department; LOS, length of stay; NSTEMI, non–ST‐segment–elevation myocardial infarction; and STEMI, ST‐segment–elevation myocardial infarction.
For the AMI cohort, the proportion of admissions originating in the ED increased during COVID‐19 (74.5%–76.9% pre‐COVID‐19, 80.4%–82.8% during COVID‐19; P=0.001; Table 2). A higher proportion of patients with AMI were admitted with ST‐segment–elevation myocardial infarction (STEMI; 29.0%–29.3% pre‐COVID‐19 to 32.5%–37.6% during COVID‐19; P=0.005). Rates of LHC and length of stay were similar pre‐COVID‐19 versus during COVID‐19, but mortality was higher during COVID‐19 (1.8%–2.2% pre‐COVID‐19, 1.6%–2.7% during COVID‐19; P<0.001). Discharge status was more often home with services and less often postacute care during COVID‐19.
Falsification testing comparing 2018 with 2019 yielded no significant differences between the 2 years for either cohort (Table S2).
Odds of Mortality in HF and AMI Cohorts
Raw mortality rates for the AMI and HF cohorts are shown in the Figure 1. After controlling for age, comorbidities, and season, for the AMI group, odds of in‐hospital mortality was increased during COVID‐19 (adjusted odds ratio [aOR], 1.36; 95% CI, 1.15–1.60; P=0.001) compared with the pre‐COVID‐19 time period (Table 3). This was particularly striking in the STEMI subgroup (aOR, 2.57; 95% CI, 2.24–2.96; P<0.001).
Figure 1. Raw weekly mortality rates during the study period.
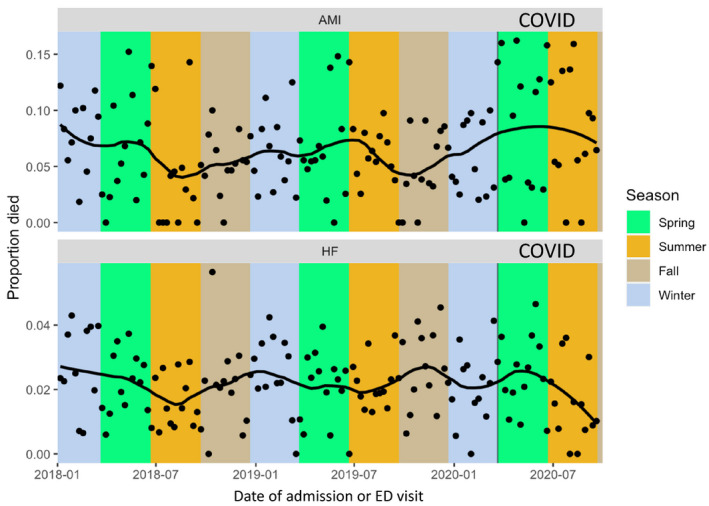
AMI indicates acute myocardial infarction; ED, emergency department; and HF, heart failure.
Table 3.
Odds of Procedure Use and Mortality in HF and AMI Cohorts
| aOR, COVID‐19 vs non‐COVID‐19 | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| AMI | ||||
| Mortality (all) | 1.36 | 1.15 | 1.60 | <0.001 |
| Mortality (STEMI) | 2.57 | 2.24 | 2.96 | <0.001 |
| Left‐heart catheterization (all) | 1.02 | 0.88 | 1.19 | 0.77 |
| Heart failure | ||||
| Mortality | 1.01 | 0.89 | 1.15 | 0.91 |
| Right‐heart catheterization | 1.07 | 0.92 | 1.25 | 0.36 |
Models control for age, race, insurance status, Elixhauser comorbidities, and season. The non‐COVID‐19 time periods serve as the reference group, such that odds ratios >1 indicate a higher odds of the event during COVID‐19.
AMI indicates acute myocardial infarction; aOR, adjusted odds ratio; and STEMI, ST‐segment–elevation myocardial infarction.
Again controlling for age, comorbidities, and season, for the HF group, we saw no difference in the odds of mortality during the COVID‐19 versus pre‐COVID‐19 time period (aOR, 1.01; 95% CI, 0.89–1.15; P=0.91; Table 3).
Discussion
In a large Midwestern hospital system, we found that for both HF and AMI, patient volume decreased markedly during COVID‐19. While patients’ mean age and prevalence of comorbidities were largely unchanged, there was evidence of higher acuity at presentation as reflected in a higher proportion of patients with STEMI in the AMI cohort. The use of key treatments such as LHC was unchanged. Mortality was significantly higher within the AMI cohort during COVID‐19, though it was stable within the HF cohort.
Our data show a decrease in patient volume during the COVID‐19 time period when compared with prior seasons. These data are consistent with multiple studies showing a decrease in both HF and acute coronary syndrome hospitalizations during COVID‐19. 9 , 10 , 11 , 20 , 21 , 22 , 23 The mechanism for this decrease is likely multifactorial, the first of which is a delay or deferral in care because of ED and hospital aversion. This has been described in a number of circumstances both in the United States and abroad. Some patients were likely reacting to local or state‐level orders put in place (eg, Stay at Home ordinances), perhaps unaware that such orders did not apply to people who needed medical care. However, others may have been primarily motivated by fear of contracting COVID‐19, particularly given the images in the popular press of overcrowded EDs and overwhelmed clinicians in areas hard hit by early waves of the pandemic.
We did not find any difference in key treatment modalities (LHC for the AMI group and RHC for the HF group) during versus before COVID‐19. In contrast, other studies have shown reductions in treatments among these patient populations. Specifically, 2 recent studies have shown a decrease in LHC for acute coronary syndrome hospitalizations as a potential mechanism. 10 , 14 In another study, patients were less likely to receive HF guideline‐directed medical therapy on hospital discharge. 8 The difference in our findings and other recent studies may be explained by regional differences in the severity of the pandemic; in greater St. Louis, COVID‐19 volumes never overwhelmed local hospital systems. Our sample may have been able to continue “business as usual” for critically ill patients, even though elective procedures and outpatient care were disrupted.
Despite the lack of measured differences in comorbidities or treatments, our data show increased in‐hospital mortality for the AMI cohort during COVID‐19, a pattern that was not seen in the HF cohort. In addition to the higher prevalence of STEMI in our cohort, we suspect that patients presenting with AMI also differed compared with prior years in ways that were not easily captured in our claims data. 24 For example, patients may have waited longer to seek care in the ED when experiencing chest pain or other symptoms, and many patients with milder symptoms may have elected not to come in at all. The long‐term effects of these shifts remain to be seen, as it is possible that delay or deferral of care for AMI in the near term may bring higher rates of recurrent ischemia or HF in the longer‐term.
Patterns for HF may differ from those for AMI because these 2 cardiovascular conditions have very different pathophysiologies. Among patients with HF, symptoms are generally gradual in onset; it is possible that some patients who preferred to avoid the ED were instead treated presumptively over the phone, or even did their own medication titration. Patients who did present, even if they were somewhat sicker at the time of arrival, may still have been within a window in which they could respond well to typical treatments. On the other hand, AMI is generally sudden in onset, and treatment far more time sensitive; even small delays in seeking care may be associated with poor outcomes. Interestingly, while others have shown a decrease in HF hospitalizations during COVID‐19, thus far none find an effect on in‐hospital mortality. 8 , 20 , 21 , 23 , 25
There are limitations to our findings. Our study is limited to the patient population studied, a 12‐hospital system in a midsize US city. While likely representative of our region, these data may not generalize to other regional health systems in the United States or to international patient populations. Because we used administrative data, we are limited in our ability to determine clinical presentation severity, as well as treatment elements such as door‐to‐balloon time. For the HF cohort, we are limited in our detection of certain HF treatments (such as goal‐directed medical therapy for left ventricular dysfunction). Low rates of HF mortality may have underpowered our mortality comparison. Additionally, these data are limited to a discrete time period (January 1, 2018, to September 22, 2020) and may not reflect patterns during later time frames; other studies have suggested that the reduction in cardiovascular volume seen with COVID‐19’s initial surge may not have persisted during subsequent waves. 26 Finally, we are limited in our follow‐up and did not capture postdischarge events such as 30‐day mortality; longer‐term study is needed to determine whether there are additional consequences of near‐term delays or deferrals of care.
In conclusion, cardiovascular volume (both AMI and HF) of ED visits and hospitalizations decreased significantly during COVID‐19, but patient age, comorbidities, and treatment patterns were largely unchanged. Mortality was higher for myocardial infarction but stable for HF during the pandemic. Collectively, these data raise the possibility that deferral in care is the most likely mechanism for increased cardiovascular mortality during COVID‐19, and that public health efforts aimed at educating patients on the importance of seeking necessary medical care might be an important intervention to reduce excess mortality associated with COVID‐19.
Sources of Funding
This work was supported by National Institutes of Health T35 National Heart, Lung, and Blood Institute Training Grant HL007815, Bethesda, Maryland.
Disclosures
Dr Joynt Maddox receives research support from the National Heart, Lung, and Blood Institute (R01HL143421) and National Institute on Aging (R01AG060935, R01AG063759, and R21AG065526), and previously did contract work for the US Department of Health and Human Services. She also serves on the Health Policy Advisory Council for the Centene Corporation (St. Louis, MO). The remaining authors have no disclosures to report.
Supporting information
Supplemental Material
Tables S1–S2
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022625
For Sources of Funding and Disclosures, see page 8.
References
- 1. Aladağ N, Atabey RD. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID‐19 patients. Acta Cardiol. 2021;76(2):132–139. doi: 10.1080/00015385.2020.1810914 [DOI] [PubMed] [Google Scholar]
- 2. Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, Guan X, Gary T, Zhu Z. Impact of cardiovascular disease and cardiac injury on in‐hospital mortality in patients with COVID‐19: a systematic review and meta‐analysis. Heart. 2020;106:1142–1147. doi: 10.1136/heartjnl-2020-317062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGonagle D, Plein S, O'Donnell JS, Sharif K, Bridgewood C. Increased cardiovascular mortality in African Americans with COVID‐19. Lancet Respir Med. 2020;8:649–651. doi: 10.1016/S2213-2600(20)30244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID‐19. New Engl J Med. 2020;382:e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Wu J, Mamas MA, Mohamed MO, Kwok CS, Roebuck C, Humberstone B, Denwood T, Luescher T, de Belder MA, Deanfield JE, et al. Place and causes of acute cardiovascular mortality during the COVID‐19 pandemic. Heart. 2021;107:113–119. doi: 10.1136/heartjnl-2020-317912 [DOI] [PubMed] [Google Scholar]
- 6. Boserup B, McKenney M, Elkbuli A. The impact of the COVID‐19 pandemic on emergency department visits and patient safety in the United States. Am J Emerg Med. 2020;38:1732–1736. doi: 10.1016/j.ajem.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westgard BC, Morgan MW, Vazquez‐Benitez G, Erickson LO, Zwank MD. An analysis of changes in emergency department visits after a state declaration during the time of COVID‐19. Ann Emerg Med. 2020;76:595–601. doi: 10.1016/j.annemergmed.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toner L, Koshy AN, Ko J, Driscoll A, Farouque O. Clinical characteristics and trends in heart failure hospitalizations: an Australian experience during the COVID‐19 lockdown. JACC Heart Fail. 2020;8:872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huynh K. Reduced hospital admissions for ACS – more collateral damage from COVID‐19. Nat Rev Cardiol. 2020;17:453. doi: 10.1038/s41569-020-0409-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gitt AK, Karcher AK, Zahn R, Zeymer U. Collateral damage of covid‐19‐lockdown in Germany: decline of NSTE‐ACS admissions. Clin Res Cardiol. 2020;109:1585–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, Secco GG, Campo G, Gallone G, Verardi R, et al. Reduced rate of hospital admissions for ACS during COVID‐19 outbreak in northern Italy. New Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carenzo L, Costantini E, Greco M, Barra FL, Rendiniello V, Mainetti M, Bui R, Zanella A, Grasselli G, Lagioia M, et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID‐19 outbreak in Italy. Anaesthesia. 2020;75:928–934. [DOI] [PubMed] [Google Scholar]
- 13. Zhou W, Wang A, Wang X, Cheke RA, Xiao Y, Tang S. Impact of hospital bed shortages on the containment of COVID‐19 in Wuhan. Int J Environ Res Public Health. 2020;17:8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nef HM, Elsässer A, Möllmann H, Abdel‐Hadi M, Bauer T, Brück M, Eggebrecht H, Ehrlich JR, Ferrari MW, Fichtlscherer S, et al. Impact of the COVID‐19 pandemic on cardiovascular mortality and catherization activity during the lockdown in central Germany: an observational study. Clin Res Cardiol. 2021;110:292–301. doi: 10.1007/s00392-020-01780-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roffi M, Guagliumi G, Ibanez B. The obstacle course of reperfusion for ST‐segment‐elevation myocardial infarction in the COVID‐19 pandemic. Circulation. 2020;141:1951–1953. doi: 10.1161/CIRCULATIONAHA.120.047523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel NJ, Nalluri N, Deshmukh A, Pant S, Shah N, Badheka AO, Asti D, Lafferty JC, Schwartz C. Seasonal trends of heart failure hospitalizations in the United States: a national perspective from 2000 to 2011. Int J Cardiol. 2014;173:562–563. doi: 10.1016/j.ijcard.2014.03.122 [DOI] [PubMed] [Google Scholar]
- 17. Gallerani M, Boari B, Manfredini F, Manfredini R. Seasonal variation in heart failure hospitalization. Clin Cardiol. 2011;34:389–394. doi: 10.1002/clc.20895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chongprasertpon N, Coughlan JJ, Cahill C, Kiernan TJ. Circadian and seasonal variations in patients with acute STEMI: a retrospective, single PPCI center study. Chronobiol Int. 2018;35:1663–1669. doi: 10.1080/07420528.2018.1500478 [DOI] [PubMed] [Google Scholar]
- 19. Hodzic E, Perla S, Iglica A, Vucijak M. Seasonal incidence of acute coronary syndrome and its features. Mater Sociomed. 2018;30:10–14. doi: 10.5455/msm.2018.30.10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez Manzanares R, Pericet Rodriguez C, Gallo Fernandez I, Castillo Dominguez JC, Anguita SM. Heart failure hospitalization during COVID‐19 pandemic. Semergen. 2020;46(Suppl 1):91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bromage DI, Cannata A, Rind IA, Gregorio C, Piper S, Shah AM, McDonagh TA. The impact of COVID‐19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail. 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alvarez‐Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas‐Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID‐19. J Am Coll Cardiol. 2020;76:2334–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall ME, Vaduganathan M, Khan MS, Papadimitriou L, Long RC, Hernandez GA, Moore CK, Lennep BW, McMullan MR, Butler J. Reductions in heart failure hospitalizations during the COVID‐19 pandemic. J Card Fail. 2020;26:462–463. doi: 10.1016/j.cardfail.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia S, Dehghani P, Grines C, Davidson L, Nayak KR, Saw J, Waksman R, Blair J, Akshay B, Garberich R, et al. Initial findings from the North American COVID‐19 myocardial infarction registry. J Am Coll Cardiol. 2021;77:1994–2003. doi: 10.1016/j.jacc.2021.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, Signorovitch J, Thune JJ, Vardeny O, Solomon SD. Clinical outcomes in patients with heart failure hospitalized with COVID‐19. JACC Heart Fail. 2021;9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solomon MD, Nguyen‐Huynh M, Leong TK, Alexander J, Rana JS, Klingman J, Go AS. Changes in patterns of hospital visits for acute myocardial infarction or ischemic stroke during COVID‐19 surges. JAMA. 2021;326:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material
Tables S1–S2


