Abstract
Background
Recent studies have shown improved outcomes in cardiogenic shock through protocols directed toward early identification and initiation of mechanical circulatory support. However, objective therapeutic targets—based on clinical and/or laboratory data—to guide real‐time clinical decision making are lacking. Lactate clearance has been suggested as a potential treatment target because of its independent association with mortality.
Methods and Results
In a post hoc analysis of the DOREMI (Dobutamine Compared to Milrinone in the Treatment of Cardiogenic Shock) trial—a randomized, double‐blind, controlled trial comparing milrinone to dobutamine in the treatment of cardiogenic shock—we used prospectively collected lactate data to evaluate lactate clearance as a surrogate marker for in‐hospital mortality. In total, 82 (57.7%) patients survived to hospital discharge (survivors). In multivariate logistic regression analysis, complete lactate clearance, percentage lactate clearance, and percentage lactate clearance per hour were independently associated with survival beginning as early as 8 hours after enrollment. Complete lactate clearance was the strongest predictor of survival at all time points, with odds ratios ranging between 2.46 (95% CI, 1.09–5.55; P=0.03) at 8 hours to 5.44 (95% CI, 2.14–13.8; P<0.01) at 24 hours.
Conclusions
Complete lactate clearance is a strong and independent predictor of in‐hospital survival in patients with cardiogenic shock. Together with previously published data, these results further support the validity of lactate clearance as an appropriate surrogate for mortality and as a potential therapeutic target in future cardiogenic shock trials.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03207165.
Keywords: cardiogenic shock, lactate clearance, mortality, surrogate end point
Subject Categories: Biomarkers, Mechanisms, Metabolism, Heart Failure, Prognosis, Mortality/Survival, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- CLC
complete lactate clearance
- CS
cardiogenic shock
- DOREMI
Dobutamine Compared to Milrinone in the Treatment of Cardiogenic Shock
- LC
lactate clearance
- RRT
renal replacement therapy
- SCAI
Society for Coronary Angiography and Intervention
Clinical Perspective
What Is New?
This is an original study evaluating the association between lactate clearance and survival in patients with cardiogenic shock (CS).
In a substudy of the DOREMI (Dobutamine Compared to Milrinone in the Treatment of Cardiogenic Shock) trial, lactate clearance was found to be an independent predictor of in‐hospital survival in a cohort of patients with CS who were randomly assigned to treatment with dobutamine or milrinone.
These results provide evidence in support of lactate clearance as a surrogate for mortality in CS.
What Are the Clinical Implications?
In the setting of CS, elevated blood lactate levels are the result of severe hemometabolic disarray and a marker of poor prognosis.
Lactate clearance may be an appropriate therapeutic target used to guide clinical decision making in patients with CS.
Using lactate clearance as a surrogate outcome may improve the feasibility and execution of future clinical trials evaluating CS treatments.
Cardiogenic shock (CS) management strategies have evolved considerably during the past 2 decades as temporary mechanical circulatory support devices have grown in both number and availability. 1 , 2 , 3 , 4 However, this shift in clinical practice has yet to translate into improved patient outcomes, with clinical trials continuing to report mortality rates of 30% to 60%. 5 , 6 , 7 , 8
Although there are many reasons for the lack of progress in CS outcomes, among the most important has been the absence of clear criteria for diagnosing and risk‐stratifying patients, leading to a wide variability in CS management. 9 The recent Society for Coronary Angiography and Intervention (SCAI) CS classification system has addressed this deficiency in part by providing a framework that can be used to risk‐stratify patients according to their hemometabolic profile. 10 Using baseline patient characteristics, this classification system has demonstrated a consistent association between increasing SCAI shock stage and mortality. 3 , 11 , 12 , 13 , 14
Among the clinical, hemodynamic, and biochemical parameters that have been incorporated into the SCAI classification, lactate is perhaps the most widely used for prognosticating outcomes in clinical practice. Certainly, there is ample evidence among large cohorts of critically ill patients demonstrating a direct correlation between baseline lactate levels and patient outcomes.
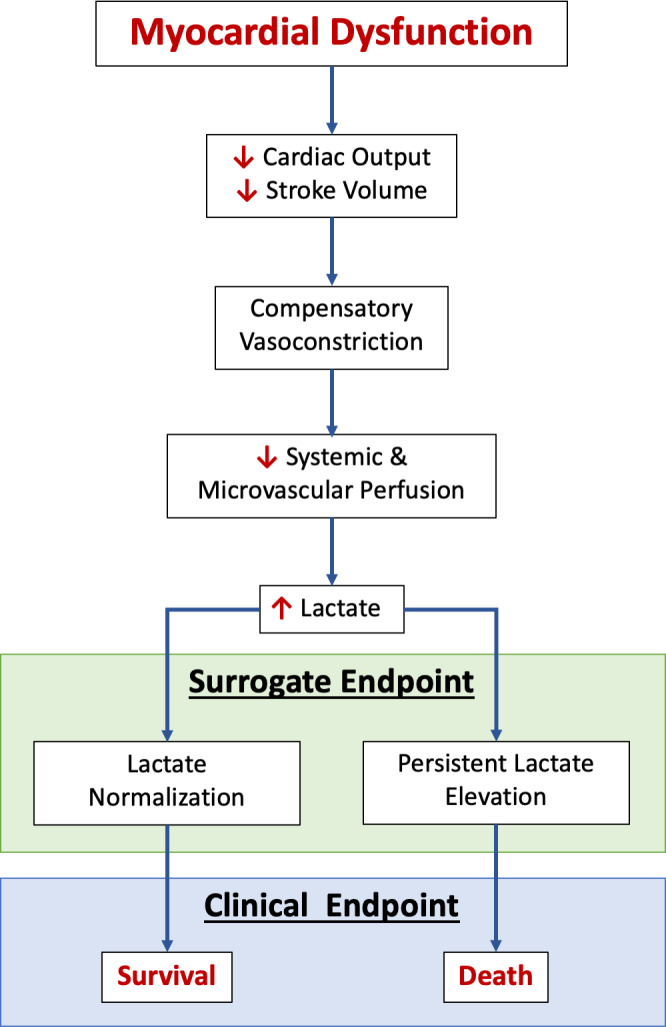
Lactate levels, however, are not static over time, and therefore it has been postulated that temporal lactate trends may increase diagnostic precision and provide a potential therapeutic target for guiding clinical decisions in real time. Zhang and colleagues provided evidence to support this assertion in a large cohort of patients admitted to the intensive care unit, where they demonstrated that the time to lactate normalization provided additional prognostic value beyond baseline lactate. 15 Similarly, early lactate clearance (LC)—within 6 hours of emergency department presentation—has been shown to directly correlate with survival in patients with severe sepsis or septic shock. 16 Although these data support the relationship between LC and survival among a heterogeneous population of critically ill patients, there is little data validating LC as a surrogate for survival in CS. 17 , 18 , 19 , 20 , 21 As such, whether LC may be an appropriate treatment target in CS has not yet been established. In a substudy of the recently published DOREMI (Dobutamine Compared to Milrinone in the Treatment of Cardiogenic Shock) trial, we sought to confirm that LC is directly associated with survival in patients with CS and therefore may be a reasonable therapeutic target for clinicians and surrogate end points for researchers 8 , 22 (Figure 1).
Figure 1. Representation of blood lactate as a surrogate end point in cardiogenic shock, demonstrating the relationship between myocardial dysfunction, lactate levels, and mortality.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
The presented study is a post hoc analysis of the DOREMI trial, an investigator‐initiated, single‐center, double‐blind, randomized clinical trial comparing outcomes in patients with CS randomly assigned to treatment with dobutamine or milrinone. 8 The study was approved by the Ottawa Health Science Network Research Ethics Board, and the study was conducted in accordance with the Helsinki Declaration. All patients or their substitute decision makers provided written informed consent before randomization. The full details of the DOREMI trial protocol and design have been previously published. 23 Briefly, adult patients who were admitted with SCAI stages B through E CS between September 1, 2017, and May 17, 2020, were eligible for inclusion. 12 We excluded patients who were pregnant, presented with an out‐of‐hospital cardiac arrest, had milrinone or dobutamine initiated before enrollment, or were participating in another interventional trial and those for whom written informed consent was not able to be obtained.
Participants were randomly assigned in a 1:1 ratio to milrinone or dobutamine. The randomization was performed using a computer‐generated random sequence. Patients were allocated to treatment groups using sequential, serially numbered, opaque envelopes. Patients, treating physicians, and all other research personnel were blinded to the treatment allocation. Per protocol, once randomly assigned patients were initiated on intravenous dobutamine or milrinone. The inotrope dose was titrated using a standardized staging system, ranging from stages 1 to 5, which corresponded to 2.5, 5.0, 7.5, 10.0, and >10.0 µg/kg per minute and 0.125, 0.250, 0.375, 0.500, and >0.500 µg/kg per minute for dobutamine and milrinone, respectively
Once treatment had been initiated, patients were reassessed by the treating team to determine if the inotrope dose should be maintained, increased, or decreased. Frequency and timing of patient reassessments were prespecified to occur at the following intervals after treatment initiation: 4, 8, 12, 18, 24, 36, 48, 60, and 72 hours then daily. At the time of enrollment as well as at each reassessment, clinical, laboratory, and hemodynamic (when available) data were collected. Pulmonary artery catheters were placed per the primary team and were not required as part of the treatment protocol. All patients enrolled in the Capital DOREMI trial who had elevated blood lactate levels at baseline were included in this analysis, and all data included in the analysis were collected prospectively.
Study Outcomes
The primary goal of this analysis was to determine the utility of LC as a surrogate marker for in‐hospital mortality in patients with CS. Lactate levels were measured at baseline and at each reassessment, as noted previously. Throughout the literature, LC has been defined in several different ways. In an attempt to identify the optimal surrogate marker, we performed all analyses using each of the following definitions, where L° is the baseline lactate level, Lt is the lactate level at time t, and Δt is the time difference between L° and Lt.
LC1 is the percentage reduction in lactate from baseline to time t, and LC2 is the percentage reduction in lactate per hour from baseline to time t. Finally, patients were assessed at each time point for the presence or absence of complete LC (CLC), which was defined by a serum lactate level (<2.0 mmol/L).
Statistical Analysis
Patients were grouped into survivors and nonsurvivors based on in‐hospital mortality. When normally distributed, continuous variables are summarized as mean±SD and otherwise expressed as median (interquartile range [IQR]). Categorical variables are summarized as number (percentage). All data were analyzed according to the intention‐to‐treat principle, which included all patients according to the group to which they were randomly assigned. The Pearson χ2 test was used to compare discrete variables, and continuous variables were compared using either the Student t test or Mann‐Whitney U test, as appropriate.
As the goal of our analysis was to evaluate LC, patients who had normal lactate levels (<2.0 mmol/L) at baseline were excluded. Absolute lactate levels were compared between groups at baseline and 4, 8, 12, 18, 24, and 36 hours. LC1 and LC2 were calculated for survivors and nonsurvivors at 4, 8, 12, 18, 24, and 36 hours, as was the frequency of CLC among survivors and nonsurvivors at each of these time points.
Univariate logistic regression analyses were conducted to assess the unadjusted relationship of survival with baseline demographic and clinical parameters as well as LC1, LC2, and CLC at 4, 8, 12, 18, 24, and 36 hours. Baseline and clinical parameters were chosen for univariate regression analysis based on known or presumptive association with survival in CS. Next, a multivariate logistic regression model was constructed to evaluate the adjusted relationship between LC and survival at each of the aforementioned time points. The multivariate model was constructed using a stepwise forward entry approach, whereby all variables with P<0.10 in univariate analysis were assessed for inclusion. An entry level of P<0.05 was used for inclusion in the final model. Next, LC1, LC2, and CLC were individually forced into the model, thereby creating an individual model for each definition of LC, and receiver operating characteristic curves were calculated for CLC at 8, 12, and 24 hours. Finally, survival curves were plotted for survivors and nonsurvivors according to CLC status at 12 and 24 hours using the Wilcoxon test. All reported P values are 2‐sided, and a value <0.05 was considered statistically significant. Analyses were conducted in R version 4.0.2 (R Core Team, Vienna, Austria).
Results
In total, 192 patients were enrolled in the Capital DOREMI clinical trial, and 116 (60.4%) of them survived to hospital discharge. The median baseline lactate levels were 2.75 mmol/L (IQR, 1.78–4.15 mmol/L) and 3.00 mmol/L (IQR, 2.23–4.50 mmol/L) among survivors and nonsurvivors, respectively (P=0.386). After the exclusion of patients who had normal baseline lactate (N=50), 74% of the total cohort (N=142) was included in the present analysis (Figure 2). Baseline characteristics and clinical outcomes between patients with normal compared with elevated baseline lactate are compared in Table S1. Of the patients who had a normal baseline lactate, 48% (N=24) of them subsequently had a rise in lactate, and 25% of those patients (N=6) died.
Figure 2. Patient flow.
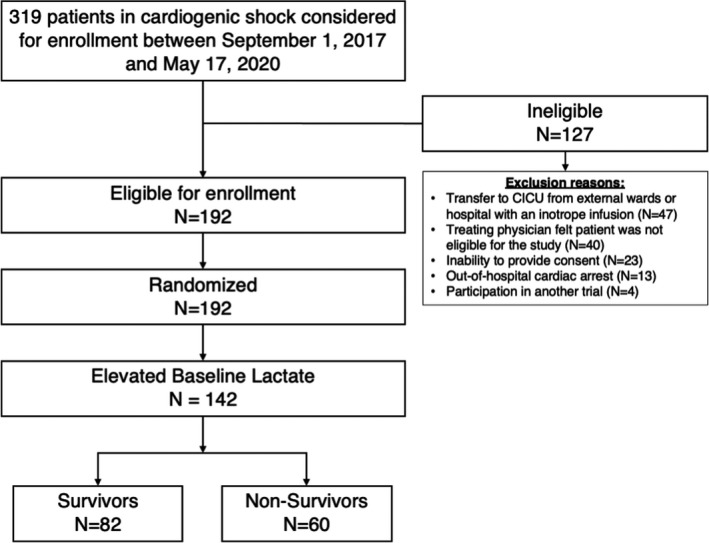
CICU indicates cardiac intensive care unit.
Of the 142 patients included in our analysis, 82 (57.7%) were discharged alive from the hospital (survivors). The overall mean±SD patient age was 70.1±13.1 years; however, survivors were significantly younger than nonsurvivors (66.8±13.0 years versus 74.5±11.9 years; P<0.001). Men comprised the majority of survivors (68.3%) and nonsurvivors (65.0%), but there was no significant difference in the proportion of men between groups (P=0.68). Otherwise, the baseline demographics and medical histories were similar between survivors and nonsurvivors.
With respect to baseline clinical characteristics, survivors had higher heart rates (97.5±23.2 bpm versus 88.1±20.3 bpm; P=0.01) and mean arterial blood pressures (80.5±13.4 mm Hg versus 73.9±13.3 mm Hg; P=0.007) than nonsurvivors. They were also less likely to require baseline intravenous vasopressors (35.4% versus 59.3%; P=0.01) and mechanical ventilation (9.8% versus 28.3%; P=0.01; Table 1). There was limited initiation or escalation of mechanical circulatory support in both survivors and nonsurvivors. Among patients who survived, 7 intra‐aortic balloon pumps and 4 Impella devices were placed. In nonsurvivors, 8 intra‐aortic balloon pumps were placed, and venoarterial extracorporeal membrane oxygenation was initiated in 1 patient.
Table 1.
Baseline Demographic and Clinical Characteristics
| Baseline characteristics | Total, N=142 | Survivors, n=82 | Nonsurvivors, n=60 | P Value |
|---|---|---|---|---|
| Age, y | 70.1±13.1 | 66.8±13.0 | 74.5±11.9 | <0.001 |
| Male sex | 95 (66.9) | 56 (68.3) | 39 (65.0) | 0.680 |
| Body mass index, kg/m2 | 27.4±5.73 | 27.2±5.6 | 27.7±6.2 | 0.593 |
| Race and ethnicity | 0.869 | |||
| White patients | 123 (86.6) | 70 (85.4) | 53 (88.3) | |
| Black patients | 4 (2.8) | 3 (3.7) | 1 (1.7) | |
| Asian patients | 7 (4.9) | 5 (6.0) | 2 (5.3) | |
| Middle Eastern patients | 6 (4.2) | 3 (3.7) | 3 (5.0) | |
| Other races or ethnicities | 2 (1.4) | 1 (1.2) | 1 (1.7) | |
| Medical history | ||||
| Diabetes | 73 (51.4) | 43 (52.4) | 30 (50.0) | 0.774 |
| Previous myocardial infarction | 48 (33.8) | 29 (35.4) | 19 (31.7) | 0.645 |
| Previous coronary bypass grafting | 31 (21.8) | 16 (19.5) | 15 (25.0) | 0.434 |
| Previous stroke or TIA | 22 (15.5) | 13 (15.9) | 9 (15.0) | 0.890 |
| Previous percutaneous coronary intervention | 35 (24.6) | 23 (28.0) | 12 (20.0) | 0.272 |
| Atrial fibrillation | 74 (52.1) | 48 (58.5) | 26 (43.3) | 0.073 |
| Chronic kidney disease | 33 (23.2) | 19 (23.2) | 14 (23.3) | 0.982 |
| Chronic obstructive pulmonary disease | 19 (13.4) | 13 (15.9) | 6 (10.0) | 0.311 |
| Chronic liver disease | 8 (5.6) | 5 (6.1) | 3 (5.0) | 0.779 |
| Home medications before presentation | ||||
| Aspirin | 86 (60.6) | 48 (58.5) | 38 (68.3) | 0.563 |
| P2Y12 inhibitor | 64 (45.1) | 34 (41.5) | 30 (50.0) | 0.313 |
| Warfarin | 19 (13.4) | 11 (13.4) | 8 (13.3) | 0.989 |
| Direct oral anticoagulant | 36 (25.4) | 23 (28.0) | 13 (21.7) | 0.388 |
| β‐blocker | 69 (48.6) | 41 (50.0) | 28 (46.7) | 0.695 |
| Statin | 90 (63.4) | 54 (65.9) | 36 (60.0) | 0.474 |
| ACEi, ARB, or ARNI | 63 (44.4) | 39 (47.6) | 24 (40.0) | 0.370 |
| Mineralicoid receptor antagonist | 20 (14.1) | 12 (14.6) | 8 (13.3) | 0.826 |
| Nitrates and/or hydralazine | 17 (12.0) | 10 (12.2) | 7 (11.7) | 0.924 |
| Diuretic | 109 (76.8) | 64 (78.0) | 45 (75.0) | 0.671 |
| Digoxin | 10 (7.0) | 6 (7.3) | 4 (6.7) | 0.881 |
| Baseline clinical characteristics | ||||
| Etiology of left ventricular dysfunction | 0.282 | |||
| Ischemic cardiomyopathy | 94 (66.2) | 57 (69.5) | 37 (61.7) | |
| Nonischemic cardiomyopathy | 48 (33.8) | 25 (30.5) | 23 (38.3) | |
| Heart rate, bpm | 93.5±22.5 | 97.5±23.2 | 88.1±20.3 | 0.01 |
| Mean arterial blood pressure, mm Hg | 77.7±13.7 | 80.5±13.4 | 73.9±13.3 | 0.007 |
| Cardiac index, L/min per m2 | 1.54±0.39 | 1.59±0.43 | 1.52±0.39 | 0.769 |
| Systemic vascular resistance, dynes×sec/cm5 | 1914±634 | 1735±739 | 2025±582 | 0.724 |
| SCAI shock class | ||||
| SCAI C | 118 (83.1) | 71 (86.6) | 47 (78.3) | 0.195 |
| SCAI D | 19 (13.4) | 8 (9.8) | 11 (18.3) | 0.138 |
| SCAI E | 4 (2.8) | 2 (2.4) | 2 (3.3) | 0.750 |
| Invasive ventilation | 25 (17.6) | 8 (9.8) | 17 (28.3) | 0.004 |
| Intravenous vasopressors | 64 (45.4) | 29 (35.4) | 35 (59.3) | 0.005 |
| Vasoactive–inotropic score* | 15.3±47.7 | 9.0±16.0 | 24.2±71.1 | 0.006 |
| Mechanical circulatory support | ||||
| Intra‐aortic balloon pump | 10 (7.0) | 4 (4.9) | 6 (10.0) | 0.239 |
| Pulmonary artery catheter | 13 (9.2) | 5 (6.1) | 8 (13.3) | 0.140 |
| Hemoglobin, g/dL | 122±24.1 | 124±25.0 | 119±22.6 | 0.118 |
| Lactate, mmol/L | 4.67±3.21 | 4.38±2.71 | 5.07±3.78 | 0.578 |
| Aspartate transaminase, units/L | 655±1113 | 722±1184 | 571±1020 | 0.535 |
| Renal function | ||||
| Serum creatinine, mmol/L | 192±136 | 173±96.5 | 218±173 | 0.069 |
| Estimated GFR, † mL/min per 1.73 m2 | 72.0±38.5 | 76.3±37.9 | 65.6±38.8 | 0.081 |
Data are presented as mean±SD or number (percentage). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; GFR, glomerular filtration rate; P2Y12 inhibitor indicates P2Y12 platelet receptor inhibitors; SCAI, Society for Cardiovascular Angiography and Interventions; and TIA, transient ischemic attack.
Vasoactive–inotropic score=dopamine dose (mcg/kg per min)+dobutamine dose (mcg/kg per min)+(100×epinephrine dose [mcg/kg per min])+(10×milrinone dose [mcg/kg per min])+(10 000×vasopressin dose [units/kg per min])+(100×norepinephrine dose [mcg/kg per min]).
Modification of Diet in Renal Disease equation.
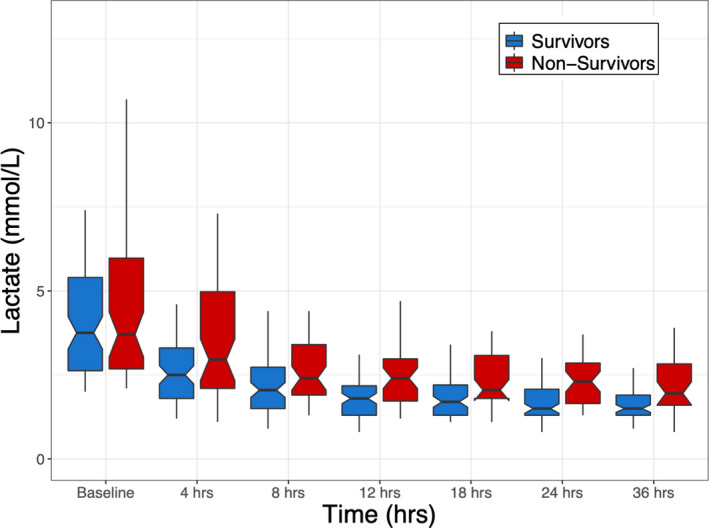
Absolute blood lactate levels were similar between the survivors and nonsurvivors at baseline (survivors, 3.75 mmol/L [IQR, 2.63–5.40 mmol/L] versus nonsurvivors, 3.70 mmol/L [IQR, 2.68–5.98 mmol/L]; P=0.58) and at 4 hours (survivors, 2.50 mmol/L [IQR 1.80–3.33 mmol/L] versus nonsurvivors, 2.95 mmol/L [IQR, 2.10–4.97 mmol/L]; P=0.08); yet, as demonstrated in Figure 3 and Table S2, at all time points from 8 to 36 hours absolute blood lactate levels were significantly lower in survivors.
Figure 3. Box‐and‐whisker plots of absolute lactate levels over time, grouped by survivors and nonsurvivors.
There was no difference between groups in the proportion of patients who had CLC within 4 hours (survivors, 30.5%, versus nonsurvivors, 20.0%; risk ratio, 0.80 [95% CI, 0.61–1.07]; P=0.16), but a significantly greater percentage of patients who survived had CLC at each time point thereafter (Figure 4A and Table S3). Similarly, LC1 and LC2 were significantly greater in survivors at 8, 12, 18, 24, and 36 hours (Table S4). Renal replacement therapy (RRT) was initiated in 20 (33.3%) survivors, and 7 (8.5%) nonsurvivors. Although RRT may partially clear serum lactate, there was no significant difference in the rate of CLC at 24 (RRT, 59.3%, versus no RRT, 75.7%; P=0.09) or 48 hours (RRT, 66.7%, versus no RRT, 81.7%; P=0.08) between patients who did and did not receive RRT.
Figure 4. Association between survival and complete lactate clearance.
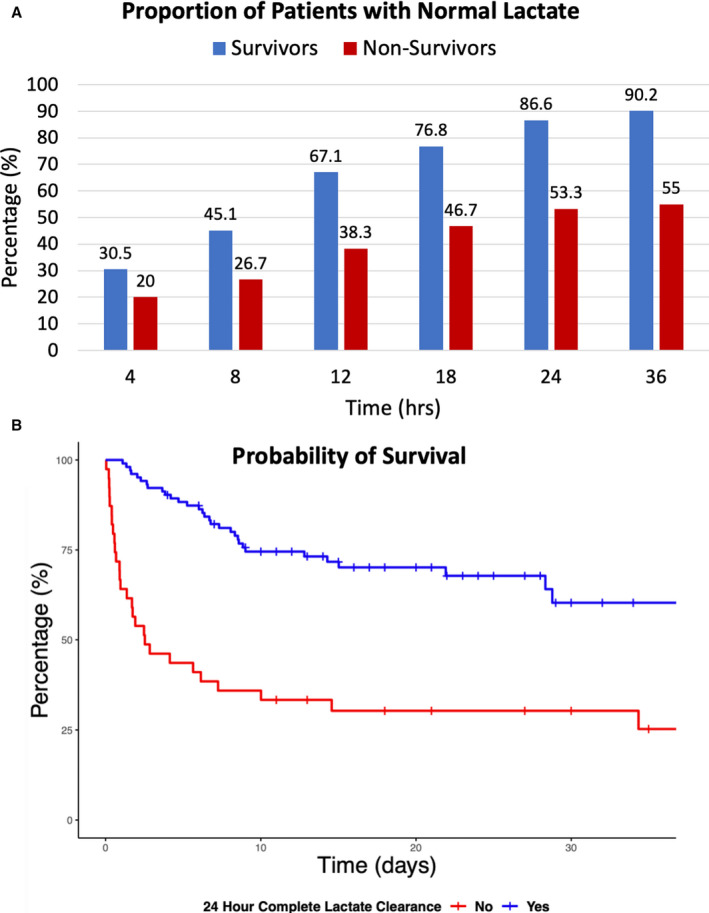
A, Proportion of survivors and nonsurvivors who achieved complete lactate clearance from 4 to 36 hours. B, Probability of survival for patients with and without complete lactate clearance at 24 hours.B, Probability of survival for patients with and without complete lactate clearance at 24 hours.
Multivariate logistic regression modeling identified age, mean arterial blood pressure, and mechanical ventilation to be independent predictors of survival. LC was independently associated with survival at each time point from 8 to 36 hours, irrespective of the definition used. Among the definitions of LC evaluated, CLC was the strongest predictor of survival at all time points, with odds ratios ranging between 2.46 (95% CI, 1.09–5.55; P=0.03) at 8 hours to 5.44 (95% CI, 2.14–13.8; P<0.01) at 24 hours (Table 2 and Table S5).
Table 2.
Multivariate Logistic Regression for In‐Hospital Mortality According to Lactate Clearance Definition
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| 6–8 h | |||
| Age | 0.95 | 0.92–0.98 | <0.01 |
| Baseline MAP | 1.03 | 1.00–1.07 | 0.03 |
| Mechanical ventilation | 0.28 | 0.10–0.79 | 0.02 |
| Complete lactate clearance | 2.46 | 1.09–5.55 | 0.03 |
| 12 h | |||
| Age | 0.94 | 0.91–0.94 | <0.01 |
| Baseline MAP | 1.04 | 1.00–1.07 | 0.03 |
| Mechanical ventilation | 0.31 | 0.11–0.89 | 0.03 |
| Complete lactate clearance | 3.98 | 1.76–8.99 | <0.01 |
| 18 h | |||
| Age | 0.95 | 0.91–0.98 | <0.01 |
| Baseline MAP | 1.03 | 1.00–1.07 | 0.04 |
| Mechanical ventilation | 0.29 | 0.10–0.84 | 0.02 |
| Complete lactate clearance | 3.68 | 1.62–8.38 | <0.01 |
| 24 h | |||
| Age | 0.95 | 0.92–0.99 | <0.01 |
| Baseline MAP | 1.03 | 0.99–1.07 | 0.05 |
| Mechanical ventilation | 0.25 | 0.08–0.74 | 0.01 |
| Complete lactate clearance | 5.44 | 2.14–13.8 | <0.01 |
MAP indicates mean arterial blood pressure.
As CLC was the most powerful surrogate marker, we plotted receiver operating characteristic curves for CLC at 8, 12, and 24 hours, which demonstrated a modest improvement in area under the curve during this time frame (Figure 5). Finally, survival curves were plotted for patients with and without CLC at 24 hours (Figure 4B).
Figure 5. Receiver operator curves of multivariate logistic regression for association between complete lactate clearance and death at 8 hours, 12 hours, and 24 hours.
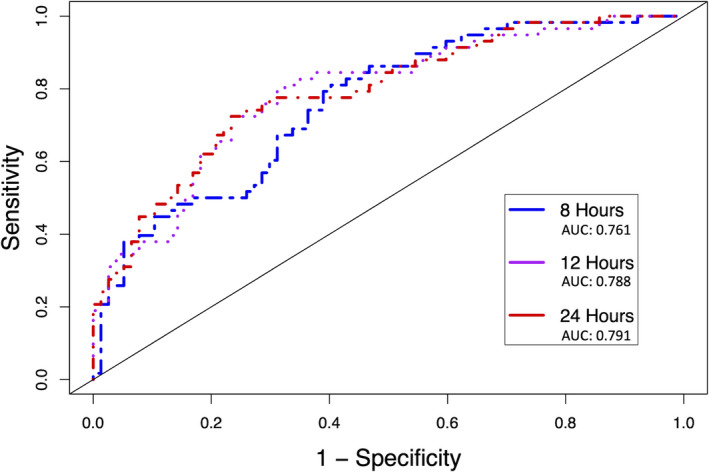
Receiver operator curves of multivariate logistic regression for association between complete lactate clearance and death at 8 hours (Blue), 12 hours (Purple), and 24 hours (Red). AUC – area under the curve.
Discussion
In a cohort of patients with CS randomly assigned to treatment with dobutamine or milrinone, LC was an independent predictor of in‐hospital survival. The independent association between survival and LC was observed at all time points evaluated between 8 and 36 hours, irrespective of how LC was defined. Moreover, these results remained consistent in both adjusted and unadjusted analyses. When compared to one another, CLC was the most powerful definition of LC with respect to predicting in‐hospital survival.
Lactic acid is produced as a byproduct of anaerobic glycolysis, which functions to provide glucose in the setting of hypoxia. 24 Although a normal physiologic process, the delicate balance between lactate production and clearance becomes significantly impaired in the setting of CS. Decreased cardiac output resulting in tissue hypoperfusion, reduced oxygen use attributed to microcirculatory dysfunction, and the release of catecholamines and other inflammatory mediators all contribute to increased lactate production in CS. 24 Also, concomitant renal and hepatic injury serve to impair LC, thereby further raising serum lactate levels. The prognostic importance of this relationship between tissue hypoperfusion, elevated lactate levels, and mortality has been understood for decades. 25 , 26 , 27
Recently, investigators have determined that LC may be a more reliable surrogate marker then absolute lactate levels. Although the majority of evidence in support of this theory originates from non‐CS populations (ie, sepsis, trauma), its mechanistic rationale applies equally to CS. However, the appropriateness of LC‐guided resuscitation in CS is yet to be established. That said, observational data supporting LC as a surrogate for mortality in CS have steadily grown in recent years. In 1 study of 139 patients treated with extracorporeal membrane oxygenation for refractory CS, LC 24 hours after extracorporeal membrane oxygenation initiation was predictive of 30‐day mortality. 28 Tongers and colleagues found that LC 12 hours after the initiation of combined therapy with Impella (Abiomed, Danvers, MA) and extracorporeal membrane oxygenation was independently associated with survival; Attaná et al demonstrated similar results among patients with ST‐segment–elevation myocardial infarction. 20 , 29 Finally, in a recent systematic review and meta‐analysis evaluating the association between LC and survival in patients with CS, there was a significant difference in LC between survivors and nonsurvivors as early as 6 to 8 hours after diagnosis. 30 However, it is important to note that none of the studies included in this analysis implemented treatment protocols targeting LC. Therefore, the utility of LC as a target for resuscitation efforts remains to be directly studied.
To the best of our knowledge, a substudy of the IABP‐SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) trial is the only prior evaluation of LC as a surrogate for mortality in a randomly assigned cohort of patients with CS. 31 In this analysis of 671 patients, absolute lactate at 8 hours and LC2 were both independent predictors of mortality. Furthermore, the authors determined that a minimum LC of 3.45% per hour in the first 8 hours was the optimal cutoff for predicting mortality. Our data fit with these conclusions, as the median LC in survivors and nonsurvivors at 8 hours was 5.55% and 3.06% per hour, respectively. Although not addressed in the IABP‐SHOCK II substudy, there was no difference in LC between survivors and non survivors in our analysis at 4 hours. While this suggests that failure to clear lactate within the first 4 hours of treatment is not necessarily detrimental to a patient's overall prognosis, it should push clinicians to determine if adequate support is being provided or if escalation is required.
The IABP‐SHOCK II analysis focused on evaluating LC within the first 8 hours, and the authors did not specifically evaluate the prognostic significance of CLC, which was the strongest predictor of survival in our cohort. In fact, we found that only 45% of patients who survived had CLC at 8 hours, whereas this increased to 67.1% at 12 hours and 86.6% by 24 hours. Thus, although the probability of improved survival with CLC first becomes apparent at 8 hours, a significant portion of survivors will normalize their lactate between 8 and 24 hours. Interestingly, the absolute lactate data reported in the IABP‐SHOCK II analysis tell a similar story, with the median lactate level among survivors being in the normal range (<2 mmol/L) at 16 and 24 hours, and remaining >3 mmol/L in nonsurvivors.
This current study has several limitations. First, this is a post hoc analysis of a randomized trial. That said, all data analyzed were collected prospectively during the conduct of the Capital DOREMI trial. Second, the overall trial sample size is quite small at 192 patients. In addition, a significant proportion of the enrolled patients had normal lactate levels at baseline. As the purpose of the present analysis was to evaluate LC as a surrogate for mortality, these patients were excluded from our analysis. The exclusion of these patients led to a reduction in our sample size by ≈20%, in addition to limiting the generalizability of our results to patients with CS with elevated lactate levels at the time of presentation. As demonstrated in Table S1, nearly a quarter of the patients excluded from this analysis were SCAI stage B at the time of enrollment, whereas all included patients were SCAI stages C, D, or E. Consequently, many lower risk patients were excluded, which likely explains the numerically lower, although not statistically significant, reduction in mortality among those who were excluded. Nevertheless, patients with CS with a normal baseline lactate had an in‐hospital mortality rate of >30%, signifying that although LC may be an important prognostic marker in CS, a normal baseline lactate by itself is not sufficient to determine prognosis in these patients.
As discussed previously, the relationship between LC and mortality as well as the biochemical rationale for this relationship is well established. The data presented here are consistent with previous randomized and observational literature demonstrating an independent association between LC and mortality in patients with CS. Together, these facts further support the establishment of LC as a valid surrogate end point in CS, which has the potential to significantly improve the feasibility of developing and successfully completing future CS trials. 32
Sources of Funding
This work was supported by the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario.
Disclosures
None.
Supporting information
Tables S1–S5
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023322
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Goldberg RJ, Makam RCP, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade‐long trends (2001–2011) in the incidence and hospital death rates associated with the in‐hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–125. doi: 10.1161/CIRCOUTCOMES.115.002359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon Venu, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 3. Thayer KL, Zweck E, Ayouty M, Garan AR, Hernandez‐Montfort J, Mahr C, Morine KJ, Newman S, Jorde L, Haywood JL, et al. Invasive hemodynamic assessment and classification of in‐hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13:e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann F‐J, Ferenc M, Olbrich H‐G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 6. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 7. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 8. Mathew R, Di Santo P, Jung RG, Marbach JA, Hutson J, Simard T, Ramirez FD, Harnett DT, Merdad A, Almufleh A, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. 2021;385:516–525. doi: 10.1056/NEJMoa2026845 [DOI] [PubMed] [Google Scholar]
- 9. Kapur NK, Thayer KL, Zweck E. Cardiogenic shock in the setting of acute myocardial infarction. Methodist Debakey Cardiovasc J. 2020;16:16–21. doi: 10.14797/mdcj-16-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv off J Soc Card Angiogr Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 11. Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sörensen NA, Goßling A, Becher PM, Grahn H, Wagner T, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96:E213–E219. doi: 10.1002/ccd.28707 [DOI] [PubMed] [Google Scholar]
- 12. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117–2128. doi: 10.1016/j.jacc.2019.07.077 [DOI] [PubMed] [Google Scholar]
- 13. Parlow S, Di Santo P, Mathew R, Jung RG, Simard T, Gillmore T, Mao B, Abdel‐Razek O, Ramirez FD, Marbach JA, et al. CAPITAL DOREMI investigators . The association between mean arterial pressure and outcomes in patients with cardiogenic shock: insights from the DOREMI trial. Eur Heart J Acute Cardiovasc Care. 2021;10:712–720. doi: 10.1093/ehjacc/zuab052 [DOI] [PubMed] [Google Scholar]
- 14. Jung R, Di Santo P, Mathew R, Abdel‐Razek O, Parlow S, Simard T, Marbach J, Gillmore T, Mao B, Bernick J, et al. Implications of myocardial infarction on management and outcome in cardiogenic shock: a sub‐analysis from the CAPITAL‐DOREMI study. J Am Heart Assoc. 2021;10. doi: 10.1161/JAHA.121.021570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Z, Chen K, Ni H, Fan H. Predictive value of lactate in unselected critically ill patients: an analysis using fractional polynomials. J Thorac Dis. 2014;6:995–1003. doi: 10.3978/j.issn.2072-1439.2014.07.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.CCM.0000132904.35713.A7 [DOI] [PubMed] [Google Scholar]
- 17. Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A, Hanson I, Almany S, Timmis S, Dixon S, et al. National Cardiogenic Shock Initiative Investigators . Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv off J Soc Card Angiogr Interv. 2019;93:1173–1183. doi: 10.1002/ccd.28307 [DOI] [PubMed] [Google Scholar]
- 18. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 19. Li C‐L, Wang H, Jia M, Ma N, Meng X, Hou X‐T. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. 2015;149:1445–1450. doi: 10.1016/j.jtcvs.2014.11.052 [DOI] [PubMed] [Google Scholar]
- 20. Attaná P, Lazzeri C, Chiostri M, Picariello C, Franco G, Valente S. Lactate clearance in cardiogenic shock following ST elevation myocardial infarction: a pilot study. Acute Card Care. 2012;14:20–26. doi: 10.3109/17482941.2011.655293 [DOI] [PubMed] [Google Scholar]
- 21. Sugiura A, Abe R, Nakayama T, Hattori N, Fujimoto Y, Himi T, Sano K, Oda S, Kobayashi Y. Predictors of successful weaning from venoarterial extracorporeal membrane oxygenation after coronary revascularization for acute myocardial infarction complicated by cardiac arrest: a retrospective multicenter study. SHOCK. 2019;51:690–697. doi: 10.1097/SHK.0000000000001220 [DOI] [PubMed] [Google Scholar]
- 22. Di Santo P, Mathew R, Jung RG, Simard T, Skanes S, Mao B, Ramirez FD, Marbach JA, Abdel‐Razek O, Motazedian P, et al. CAPITAL DOREMI investigators . Impact of baseline beta‐blocker use on inotrope response and clinical outcomes in cardiogenic shock: a subgroup analysis of the DOREMI trial. Crit Care Lond Engl. 2021;25:289. doi: 10.1186/s13054-021-03706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hibbert B. Comparison of Milrinone Versus Dobutamine in Critically Ill Patients . Available at https://clinicaltrials.gov/ct2/show/NCT03207165. Accessed February 2, 2021.
- 24. Attanà P, Lazzeri C, Picariello C, Dini CS, Gensini GF, Valente S. Lactate and lactate clearance in acute cardiac care patients. Eur Heart J Acute Cardiovasc Care. 2012;1:115–121. doi: 10.1177/2048872612451168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009;13:R90. doi: 10.1186/cc7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Juneja D, Singh O, Dang R. Admission hyperlactatemia: causes, incidence, and impact on outcome of patients admitted in a general medical intensive care unit. J Crit Care. 2011;26:316–320. doi: 10.1016/j.jcrc.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 27. Henning RJ, Weil MH, Weiner F. Blood lactate as prognostic indicator of survival in patients with acute myocardial infarction. Circ Shock. 1982;9:307–315. [PubMed] [Google Scholar]
- 28. Slottosch I, Liakopoulos O, Kuhn E, Scherner M, Deppe A‐C, Sabashnikov A, Mader N, Choi Y‐H, Wippermann J, Wahlers T. Lactate and lactate clearance as valuable tool to evaluate ECMO therapy in cardiogenic shock. J Crit Care. 2017;42:35–41. doi: 10.1016/j.jcrc.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 29. Tongers J, Sieweke J‐T, Kühn C, Napp LC, Flierl U, Röntgen P, Schmitto JD, Sedding DG, Haverich A, Bauersachs J, et al. Early escalation of mechanical circulatory support stabilizes and potentially rescues patients in refractory cardiogenic shock. Circ Heart Fail. 2020;13:1–10. doi: 10.1161/CIRCHEARTFAILURE.118.005853 [DOI] [PubMed] [Google Scholar]
- 30. Marbach JA, Stone S, Schwartz B, Pahuja M, Thayer KL, Faugno AJ, Chweich H, Rabinowitz JB, Kapur NK. Lactate clearance is associated with improved survival in cardiogenic shock: a systematic review and meta‐analysis of prognostic factor studies. J Card Fail. 2021;27:1082–1089. doi: 10.1016/j.cardfail.2021.08.012 [DOI] [PubMed] [Google Scholar]
- 31. Fuernau G, Desch S, de Waha‐Thiele S, Eitel I, Neumann F‐J, Hennersdorf M, Felix SB, Fach A, Böhm M, Pöss J, et al. Arterial lactate in cardiogenic. Shock. 2020;13:9. doi: 10.1016/j.jcin.2020.06.037 [DOI] [PubMed] [Google Scholar]
- 32. Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


