Abstract
Background
Myocardial dysfunction is a critical cause of post‐cardiac arrest hemodynamic instability and circulatory failure that may lead to early mortality after resuscitation. Trimetazidine is a metabolic agent that has been demonstrated to provide protective effects in myocardial ischemia. However, whether trimetazidine protects against postresuscitation myocardial dysfunction is unknown.
Methods and Results
Cardiopulmonary resuscitation was initiated after 8 minutes of untreated ventricular fibrillation in Sprague‐Dawley rats. Animals were randomized to 4 groups immediately after resuscitation (n=15/group): (1) normothermia control (NTC); (2) targeted temperature management; (3) trimetazidine‐normothermia; (4) trimetazidine‐targeted temperature management. TMZ was administered at a single dose of 10 mg/kg in rats with trimetazidine. The body temperature was maintained at 34.0°C for 2 hours and then rewarmed to 37.5°C in rats with targeted temperature management. Postresuscitation hemodynamics, 96‐hours survival, and pathological analysis were assessed. Heart tissues and blood samples of additional rats (n=6/group) undergoing the same experimental procedure were collected to measure myocardial injury, inflammation and oxidative stress‐related biomarkers with ELISA‐based quantification assays. Compared with normothermia control, tumor necrosis factor‐α, and cardiac troponin‐I were significantly reduced, whereas the left ventricular ejection fraction and 96‐hours survival rates were significantly improved in the 3 experimental groups. Furthermore, inflammation and oxidative stress‐related biomarkers together with collagen volume fraction were significantly decreased in rats undergoing postresuscitation interventions.
Conclusions
Trimetazidine significantly alleviates postresuscitation myocardial dysfunction and improves survival by decreasing oxidative stress and inflammation in a ventricular fibrillation rat model. A single dose of trimetazidine administrated immediately after resuscitation can effectively improve cardiac function, whether used alone or combined with targeted temperature management.
Keywords: cardiac arrest, cardiopulmonary resuscitation, myocardial dysfunction, oxidative stress, targeted temperature management, trimetazidine
Subject Categories: Animal Models of Human Disease, Ventricular Fibrillation
Nonstandard Abbreviations and Acronyms
- CA
cardiac arrest
- NTC
normothermia control
- ROSC
return of spontaneous circulation
- TMZ‐NT
trimetazidine‐normothermia
- TMZ‐TTM
trimetazidine‐targeted temperature management
- TTM
targeted temperature management
Clinical Perspective
What Is New?
This study finds that trimetazidine significantly alleviates postresuscitation myocardial dysfunction and improves survival rate in a rat model of ventricular fibrillation.
The effectiveness of trimetazidine on improving hemodynamic stability and myocardial function was comparable with that of targeted temperature management whether administered alone or combined with targeted temperature management.
A single dose of trimetazidine administrated immediately after resuscitation exerts a cardioprotective effect by decreasing postresusciatation oxidative stress and inflammatory response.
What Are the Clinical Implications?
Trimetazidine may be a promising drug for the treatment of post‐cardiac arrest syndrome by improving cardiac function.
Administration of trimetazidine could have readily apparent advantages in postresuscitation care because it is a time‐saving and effective therapy without affecting the efficacy of targeted temperature management.
Cardiac arrest (CA) is a major and lethal health problem worldwide. 1 Emergency interventions including cardiopulmonary resuscitation (CPR) and defibrillation may be effective in achieving return of spontaneous circulation (ROSC); however, most resuscitated patients do not survive to hospital discharge. 2 , 3 The high morbidity and mortality rate of patients who initially achieve ROSC can be attributed to a unique pathophysiological process that is termed as post‐cardiac arrest syndrome (PCAS). A key component of PCAS is myocardial dysfunction, which is mainly manifested as hemodynamic instability and circulatory failure. 4 Treatment of postresuscitation myocardial dysfunction may potentially improve cardiac function and survival outcomes. 5
Cardiovascular ischemia/reperfusion injury and oxidative stress damage are the 2 major pathways leading to myocardial dysfunction after CA. 6 Oxidative stress induced by excessive reactive oxygen species (ROS) plays a key role in deterioration of cardiac function. 7 The increased ROS would not only drive lipid peroxidation but also exacerbate inflammatory infiltration which results in elevated cell death. 8 As a standard of care strategy after resuscitation recommended by the guidelines for adult CPR and emergency cardiovascular care, 9 targeted temperature management (TTM) has been shown to improve survival outcomes not only by mitigating cerebral injury, but also preventing damage to the myocardium. 10 However, many questions persist about its therapeutic application. On one hand, the barriers against a streamlined TTM protocol includes selection of the appropriate patient population, optimal target temperature, ideal window of therapy time, most effective duration of treatment, and rate of cooling or rewarming. 11 On the other hand, the disadvantages and adverse events that may affect the effectiveness includes sluggish induction of the target temperature, complicated or even invasive specialized cooling device, hypoglycemia, shivering, bradycardia, electrolyte abnormalities, and infection. 12 , 13
Trimetazidine is a piperazine‐derived metabolic agent that could maintain cellular homeostasis, thereby increasing cell tolerance to ischemia. 14 It has a direct effect on myocardial ischemia and would not induce hemodynamic changes. 15 Animal experiments have shown that trimetazidine could improve myocardial performance in vivo regional ischemia‐reperfusion, heart failure, and myocardial infarction animal models. 16 , 17 , 18 Meanwhile, clinical trials have demonstrated that trimetazidine could reduce attack frequency in patients with stable angina, improve left and right ventricular functions in patients with heart failure, and reduce the incidence of ST‐segment exacerbation in patients with myocardial infarction. 19 , 20 , 21 However, whether trimetazidine protects against postresuscitation myocardial dysfunction has not been investigated.
The objective of the present study was to determine whether or not trimetazidine can improve postresuscitation myocardial dysfunction and 96‐hours survival in a ventricular fibrillation (VF) rat model. Meanwhile, the role of trimetazidine in antioxidant and anti‐inflammation, and the influence of hypothermia on trimetazidine’s cardioprotective effect were explored.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
This prospective, randomized, masked, and placebo‐controlled animal study was approved by Laboratory Animal Welfare and Ethics Committee of the Army Medical University (AMUWEC20191521). The study was in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines and all animals received humane care in compliance with the Principles of Laboratory Animal Care and Guide for the Care and Use of Laboratory Animals.
Experimental Animals and Sample Size Estimates
The healthy male Sprague‐Dawley rats weighing between 293.5 and 408.0 g were provided by the laboratory animal center of the Army Medical University.
The primary outcome measure was 96‐hours survival rate and a sample size of 15 rats in each group is required to detect a significant difference between treatment and control group (survival rate of control group is 40%, the anticipated rate of experimental group is 80%, type I error rate is 5%, and type II error rate is 20%). Sixty rats were used for effectiveness exploration since there were 3 experimental groups to compare with the control group. The secondary outcome measure was the concentration of oxidative stress‐related biomarkers, and 24 rats were used for the mechanism exploration. Additional 12 sham‐operated (Sham) rats were served as reference (6 for pathology examination and 6 for biochemical test). Thus, a total of 96 animals were used in this study.
Animal Preparation
All animals were housed in 12 hours‐light/12 hours‐dark conditions (temperature 22.0°C±2.0°C) with ad libitum access to chow and water. Before the procedure, animals were fasted for 12 hours overnight, but allowed free access to water. Anesthesia was induced with intraperitoneal pentobarbital (45 mg/kg), if required, additional doses (10 mg/mg) would be administered intravenously at ≈1‐hour intervals. After fixing the animals in supine position on a surgical board, 3 subcutaneous needle electrodes were inserted into the limbs for ECG measurement. Mechanical ventilation was initiated with a tidal volume of 0.65 mL/100 g at a FiO2 of 0.21 (ALC‐V8, Alcott Biotech Co. Ltd, Shanghai, China) after tracheal intubation with a 14‐gauge cannula. To monitor arterial pressure, a PE‐50 catheter was advanced into the right femoral artery. Fluids and medications were administered through the left femoral vein inserted with an additional PE‐50 catheter. All of the catheters were flushed intermittently with saline solution containing 2.5 IU/mL of heparin. A probe for measuring body temperature was placed into the esophagus. Body temperature was continuously monitored and maintained at 37.5°C±0.5°C with an overhead‐heating lamp in the preparation phase.
Experimental Procedures
Before the induction of VF, baseline data were collected and mechanical ventilation was ceased. VF was electrically induced by transesophageal stimulation with a 5 mA/50 Hz alternating current. The stimulation was continued for 2 minutes to prevent spontaneous defibrillation. CA was defined as an abrupt decrease in mean arterial pressure (MAP) <20 mm Hg with irregular, chaotic deflections of varying amplitude and shape in ECG waveform. After 8 minutes of untreated CA, mechanical ventilation was initiated with 100% O2. Manual external chest compression was delivered at a rate of 240 compressions/min with equal compression and relaxation duration. Depth of compression was initially adjusted to secure an MAP >20 mm Hg. The first and second doses of epinephrine (0.02 mg/kg) were administered at 1 and 3 minutes after the start of CPR. A single 2 J defibrillation (M‐Series, Zoll Medical Corporation, Chelmsford, MA) was provided after 4 minutes of chest compression. If ROSC was not achieved, an additional dose of epinephrine was administered thereafter and defibrillation was attempted every 30 seconds. CPR was continued until ROSC or the same procedure was repeated for a maximum of 5 cycles. ROSC was defined as a supraventricular rhythm with >60 mm Hg lasting for a minimum of 5 minutes.
Randomization and Intervention
The animals were randomized into 4 groups immediately after resuscitation using a randomized number sequence: (1) normothermia control (NTC); (2) TTM; (3) trimetazidine‐normothermia (TMZ‐NT); (4) trimetazidine‐targeted temperature management (TMZ‐TTM).
In the NTC group, animals were administered with a single dose of 25% glucose as placebo immediately after ROSC and the body temperature was kept at 37.5°C for 5 hours. In the TTM group, animals were administered with a single dose of 25% glucose, meanwhile, the body temperature was reduced to 34.0°C by ice packs and an electric fan and maintained for the first 2 hours of postresuscitation, followed by gradual rewarming until reaching 37.5°C within 2 hours. In the TMZ‐NT group, animals were administered with a single dose of 10 mg/kg trimetazidine and the body temperature was kept at 37.5°C for 5 hours. In the TMZ‐TTM group, animals were administered with a single dose of 10 mg/kg trimetazidine and experienced a temperature management procedure as that in the TTM group. This dosage was proven to be adequate to prevent deleterious effects of ischemia‐reperfusion in previous studies. 22 , 23 For all of the rats, the oxygen concentration was maintained at 100% for 1 hour and then was adjusted to 21% after ROSC (Figure 1). The resuscitated rats received intensive care for 5 hours, then catheters and endotracheal tube were removed and incisions were surgically closed. This time point was chosen because clinical study suggested that postresuscitation care was most effective if the interventions were administrated within 6 to 12 hours. 24 For CA model of rats, this therapeutic time window was 4 hours and the animals would recover from anesthesia and restore spontaneous breathing 5 hours after ROSC. 25 Rats for effectiveness exploration were returned to the cage and carefully observed with small surveillance camera for 96 hours. Rats for mechanism exploration were anesthetized 6 hours after resuscitation and the myocardial tissue and blood samples of these animals were taken for detection of myocardial injury, inflammation, and oxidative stress‐related biomarkers.
Figure 1. Experimental protocol and randomization in each group.
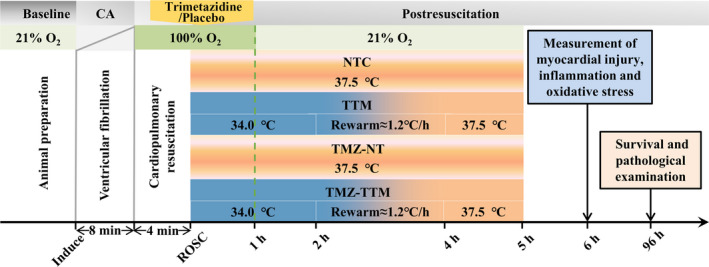
CA indicates cardiac arrest; NTC, normothermia control; ROSC, return of spontaneous circulation; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; and TTM, targeted temperature management.
Measurements
Postresuscitation Hemodynamics
MAP and ECG waveforms were continuously monitored and recorded for 5 hours using a PC‐based data acquisition system supported by WINDAQ software (DATAQ Instruments Inc., Akron, OH). Doppler echocardiographic examination (DC‐6, Mindray Medical International Limited, Shenzhen, China) was performed to calculate left ventricular ejection fraction (LVEF) at baseline and at intervals of 1 hour after resuscitation. Core temperature was constantly monitored and recorded at hourly intervals.
Survival Outcomes
Animals were continuously observed and the recovery condition was regularly evaluated with the aid of a small surveillance camera. For animals died within 96 hours, survival times were recorded. For animals survived to 96 hours, hearts were harvested for subsequent pathological examination.
Pathological Examination
Four days after ROSC, surviving rats and 6 Sham animals were anaesthetized. PBS and 10% neutral‐buffered formalin were sequentially administered to animals by transcardiac perfusion. The left ventricle myocardium of apex was excised and post‐fixed in formalin. Myocardial tissues were cut in 3‐mm coronal blocks and embedded in paraffin. Blocks were sectioned at 5 µm and stained with hematoxylin and eosin (Servicebio Technology Co., Ltd, Wuhan, China) and Masson (Servicebio Technology Co., Ltd, Wuhan, China) to observe the changes of myocardial histomorphology. Photomicrographs were taken when myocardial tissue occupied the field of view of the microscope without blank area at ×400 magnification and visually assessed under 10 nonoverlapping fields by 2 investigators masked to the group assignment. Hematoxylin and eosin staining was used to evaluate the degree of myocardial tissue impairment by the prevalence of infiltration of immune cells, myocytolysis, and contraction band necrosis. 26 Each photomicrograph was scored based on the percentage of injury by manual quantification: score 0=no damage; score 1=mild (<25%); score 2=moderate (26%–50%); score 3=severe (51%–75%); and score 4=extensive damage (76%–100%) 27 and took the average value for each group. Masson staining was used to examine the myocardial fibrosis of the heart based on the proportion of myocardial collagen fibers. Since myocardial collagen fibers were stained blue and myocardial fibers were stained red, 28 area of myocardial collagen fiber and total area of myocardium were computer‐assisted quantitative analyzed with ImageJ software respectively (version 1.46r, National Institutes of Health, Bethesda, MD). The collagen volume fraction which was defined as the ratio of myocardial collagen fiber area to total myocardial area was used to evaluate the degree of fibrosis. 29
Myocardial Injury, Inflammatory and Oxidative Stress Markers Detection
Blood samples and myocardial tissue of rats used for mechanism exploration were harvested 6 hours after ROSC. The samples were compared with those of the 6 rats in the Sham group. Blood samples were centrifuged at 3000g at 4.0 °C for 15 minutes and the serum was collected. The myocardial tissue homogenate was centrifuged at 5000 g at 4.0 °C for 10 minutes and the supernatant was collected. Both the serum and the supernatant were frozen at −80.0 °C until assayed. Protein concentrations in serum and tissue were quantified using a bicinchoninic acid protein assay kit (Nanjing JianCheng Technology Co., Ltd, Nanjing, China). Concentrations of creatine kinase‐myocardial band (CK‐MB) (Elabscience Biotechnology Co, LAD, Wuhan, China), cardiac troponin‐I (cTn‐I) (Elabscience Biotechnology Co, LAD, Wuhan, China) and malondialdehyde (Elabscience Biotechnology Co, LAD, Wuhan, China) in plasma, tumor necrosis factor alpha (TNF‐α) (Elabscience Biotechnology Co, LAD, Wuhan, China), interleukin‐6 (Elabscience Biotechnology Co, LAD, Wuhan, China) and hydrogen peroxide (Thermo Fisher Scientific (China) Co., Ltd, Shanghai, China) in myocardial tissue were measured with commercial kits. CK‐MB and cTn‐I are diagnostic factors for identification of myocardial damage. 30 They were measured in the plasma because CK‐MB is mainly produced in cardiomyocyte and cTn‐I is only expressed in myocardium. 31 , 32 Interleukin‐6 and TNF‐α are proinflammatory cytokines that provoke the acute‐phase reaction which leads to inflammation and fever. 33 They were measured in the myocardial tissue because they are not heart‐specific makers. 34 Malondialdehyde and hydrogen peroxide are widely regarded as cytotoxic agents which play a role in oxidative stress and programmed cell death responses. 35 , 36
Statistical Analysis
Data was expressed as mean±SD when normality was confirmed using the Kolmogorov‐Smirnov test, and as median and interquartile range for non‐normally distributed data. The comparisons of normally distributed variables of a single measurement were performed with ANOVA followed by post hoc Bonferroni correction. The interaction of treatment and time in normally distributed variables with multiple measurements was evaluated using the mixed effect model. If the interaction was significant, simple effects of each factor were examined one by one. If the interaction was not significant, main effects of each factor were then performed individually. Non‐normally distributed variables were compared using the Kruskal‐Wallis test followed by the Mann‐Whitney U test for 2‐group comparisons. Kaplan‐Meier analysis and log‐rank tests were used for survival analysis. A P<0.05 was considered to indicate statistically significant differences. Statistical analyses were performed using R statistical software (version 3.1.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics and Resuscitation Data
Baseline characteristics and resuscitation data were not significantly different among the 4 groups (Table). All animals were successfully resuscitated and survived the 5‐hours postresuscitation monitoring period with the exception of one animal that died at 1.9 hours in the NTC group. No exclusion or adverse events were observed during the experimental periods.
Table 1.
Baseline Variables and Characteristics of CPR (mean±SD)
| Variable | NTC | TTM | TMZ‐NT | TMZ‐TTM |
|---|---|---|---|---|
| Body weight, g | 327.6±19.5 | 327.0±28.7 | 328.5±20.0 | 325.4±19.6 |
| Heart rate, bpm | 392.6±31.8 | 381.3±46.3 | 374.9±36.9 | 393.1±38.1 |
| MAP, mm Hg | 126.1±10.2 | 120.5±8.8 | 121.6±9.4 | 123.6±7.2 |
| Temperature, ℃ | 37.5±0.2 | 37.4±0.2 | 37.5±0.1 | 37.4±0.2 |
| LVEF, % | 82.1±2.5 | 81.9±2.4 | 82.9±1.4 | 83.2±2.1 |
| CPR duration, s | 247.8±18.0 | 244.1±35.0 | 249.1±32.3 | 247.1±22.1 |
| No. of shocks, n | 1.1±0.5 | 1.2±0.6 | 1.1±0.9 | 1.1±0.6 |
| Total epinephrine, μg | 13.0±0.9 | 13.1±2.3 | 13.1±1.5 | 13.0±0.7 |
CPR indicates cardiopulmonary resuscitation; LVEF, left ventricular ejection fraction; MAP, mean artery pressure; NTC, normothermia control; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; and TTM, targeted temperature management; n=15 in each group.
Postresuscitation Hemodynamics
The body temperature, heart rate, MAP, and LVEF measurements before and after CA are presented in Figure 2. Interactions between treatment and time had statistical significance for the 4 indexes. Body temperatures in the TTM and TMZ‐TTM were significant lower than the NTC and TMZ‐NT groups during the first 3 hours after ROSC (Figure 2A). Significantly reduced heart rate was observed in the TMZ‐TTM group from 2 to 5 hours after ROSC compared with the NTC group (Figure 2B). MAP was considerably higher within the first 3 hours postresuscitation in the TTM and TMZ‐TTM group than in the NTC group (Figure 2C). LVEF in the first 4 hours after resuscitation in the TTM and TMZ‐TTM groups, and from 2 to 5 hours after resuscitation in the TMZ‐NT group were significantly increased compared with the NTC group (Figure 2D).
Figure 2. Esophageal temperature and hemodynamic data (n=15/group). Temperature (A), heart rate (B), mean arterial pressure (C), and left ventricular ejection fraction (D) were measured at baseline (BL) and postresuscitation.
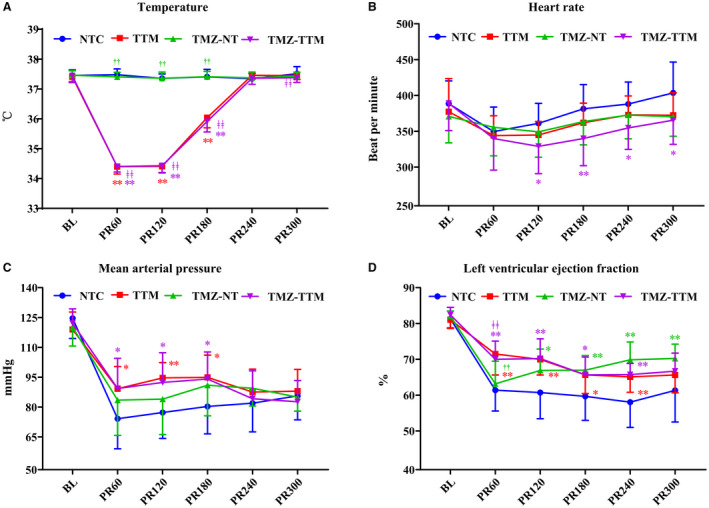
NTC indicates normothermia control; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; and TTM, targeted temperature management. * and ** P<0.05 and P<0.01 compared with NTC; †† P<0.01 compared with TTM; ‡‡ P<0.01 compared with TMZ‐NT. n=15 at each time point except for n=14 from postresuscitation 120 to postresuscitation 360 in the NTC group.
Survival Outcomes
Figure 3 indicates the cumulative 96‐hours survival rates. Compared with the NTC group, 96‐hours survival rates in the TTM (87% versus 40%, P=0.006), TMZ‐NT (93% versus 38%, P=0.003) and TMZ‐TTM (100% versus 38%, P<0.001) groups were significantly increased. However, no significant differences in survival rate were observed among the 3 experimental groups.
Figure 3. Kaplan‒Meier survival curve (n=15/group).
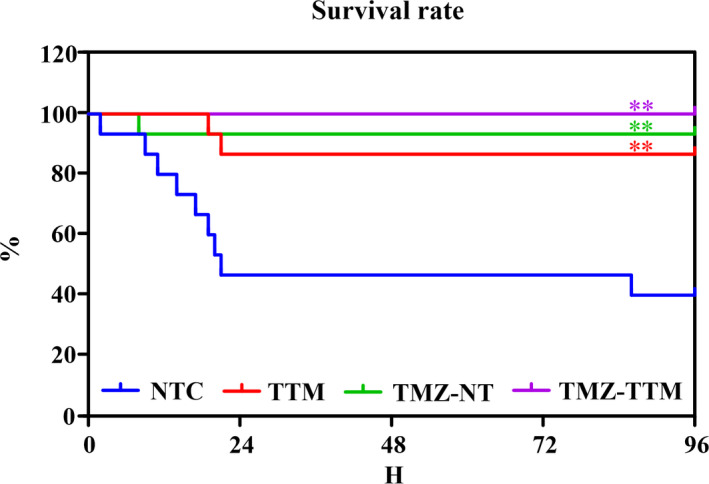
NTC indicates normothermia control; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; and TTM, targeted temperature management. **P<0.01 compared with NTC.
Pathological Analysis
Figure 4 shows the representative photomicrographs of the hematoxylin and eosin ‐stained myocardial tissue from different animals in each group. Microscopic evaluation showed that myocardial cells were arranged orderly with intact nuclei in the Sham group. In the NC group, the arrangement of myocardial cells was irregular and a large number of immune cells aggregated. Compared with the NTC group, fewer myocardial cells were arranged disorderly and inflammatory cell infiltrations were less in the TTM, TMZ‐NT, and TMZ‐TTM groups. Compared with the Sham group, the myocardial damage score was significantly increased in the NTC and the experimental groups. Additionally, myocardial damage score was significantly decreased in the TTM, TMZ‐NT, and TMZ‐TTM groups compared with the NTC group.
Figure 4. Hematoxylin and eosin staining of myocardial tissue (n=6/group). Representative micrographs of the hematoxylin and eosin‐stained myocardial tissue at 96 hours after resuscitation in each group (A through E) (10 quantified fields/animal) and the myocardial damage score (F).
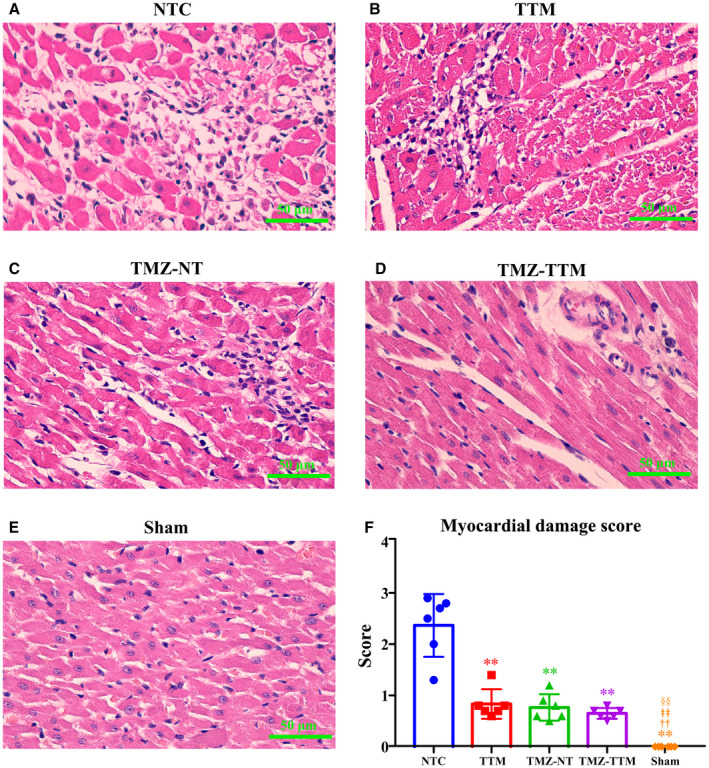
NTC indicates normothermia control; Sham, sham‐operated; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; and TTM, targeted temperature management. ** P<0.01 compared with NTC; †† P<0.01 compared with TTM; ǂǂ P<0.01 compared with TMZ‐NT; §§ P<0.01 compared with TMZ‐TTM.
Representative photomicrographs of the Masson staining of myocardial tissue from different animals in each group are shown in Figure 5. The myocardial fibers displayed an order arrangement with almost no myocardial collagen fibers in the Sham group. However, there was a large amount of collagen deposition in the NTC group. Fewer blue stained myocardial collagen fibers were observed in the myocardial tissue of the 3 experimental groups. Collagen volume fraction was significantly reduced in the TTM, TMZ‐NT, and TMZ‐TTM groups compared with the NTC group.
Figure 5. Masson staining of myocardial tissue (n=6/group). Representative photomicrographs of the Masson staining of myocardial tissue at 96 hours after resuscitation from different animals in each group (A through E) (10 quantified fields/animal) and the collagen volume fraction (F).
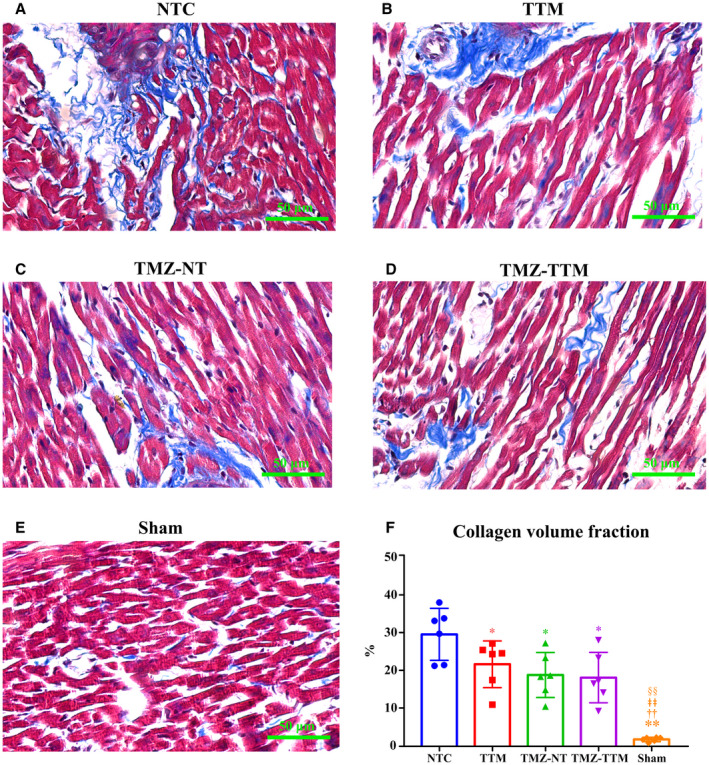
NTC indicates normothermia control; Sham, sham‐operated; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; and TTM, targeted temperature management. * and **P<0.05 and P<0.01 compared with NTC; †† P<0.01 compared with TTM; ǂǂ P<0.01 compared with TMZ‐NT; §§ P<0.01 compared with TMZ‐TTM.
Myocardial Injury, Inflammatory and Oxidative Stress
Levels of myocardial injury, inflammatory and oxidative stress markers are presented in the Figure 6. Compared with the Sham group, concentrations of CK‐MB, interleukin‐6, TNF‐α and hydrogen peroxide in the NTC group, cTn‐I in the NTC and TMZ‐NT groups, and malondialdehyde in the 4 groups were significantly increased. CK‐MB, cTn‐I, and malondialdehyde levels in plasma, and interleukin‐6, TNF‐α, and hydrogen peroxide levels in myocardial tissue were dramatically decreased in the TTM, TMZ‐NT, and TMZ‐TTM groups in comparison with the NTC group, whereas no significant differences were noted among the 3 experimental groups.
Figure 6. The concentrations of myocardial injury, inflammatory and oxidative stress markers (n=6/group). Creatine kinase‐myocardial band (A), cTn‐I (B), interleukin‐6 (C), tumor necrosis factor‐alpha; (D), malondialdehyde, (E) and hydrogen peroxide (F) contents of rats were measured 6 hours after resuscitation.
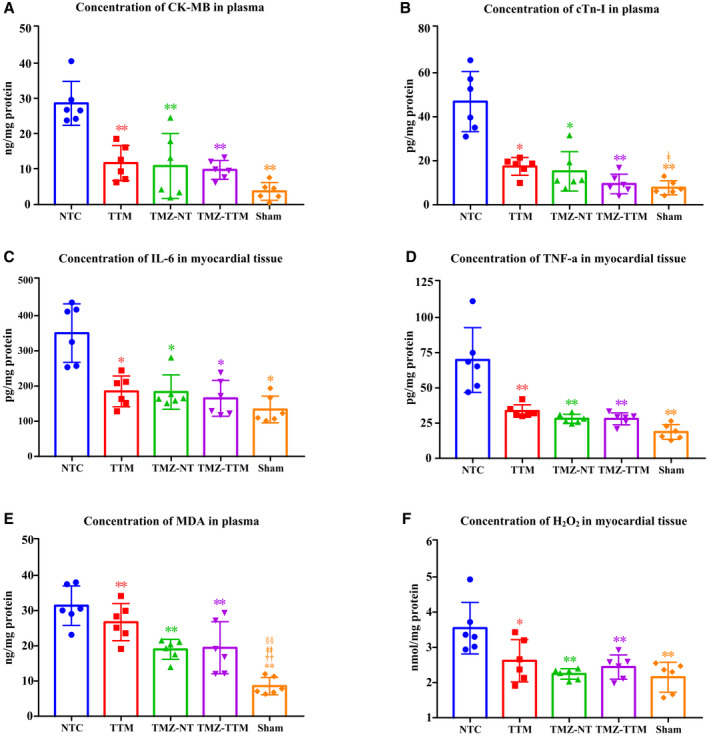
CK‐MB indicates creatine kinase‐myocardial band; cTn‐I, cardiac troponin‐I; H2O2, hydrogen peroxide; IL‐6, interleukin‐6; NTC, normothermia controlSham, sham‐operated; TMZ‐NT, trimetazidine‐normothermia; TMZ‐TTM, trimetazidine‐targeted temperature management; TNF‐α, tumor necrosis factor alpha; and TTM, targeted temperature management. * and ** P<0.05 and P<0.01 compared with NTC; †† P<0.01 compared with TTM; ‡ and ‡‡ P<0.05 and P<0.01 compared with TMZ‐NT; §§ P<0.01 compared with TMZ‐TTM.
Discussion
In the current study, we investigated the effect of trimetazidine on postresuscitation myocardial dysfunction and 96‐hours survival in a VF model of CA. Our results indicated that trimetazidine could ameliorate cardiac function and improve survival rate by inhibiting oxidative stress and inflammation. The effectiveness of trimetazidine monotherapy was comparable with that of TTM either administered alone or combined with TTM.
Postresuscitation myocardial dysfunction is a reversible global dysfunction that is related to the duration of no‐flow time during CA. 37 Deterioration of cardiac function may begin within minutes of the arrest and peaked at 2 to 5 hours after resuscitation which reinforces the need for early intervention and treatment. 5 , 38 Aggressive treatment of myocardial dysfunction early after ROSC is critical since myocardial injury is likely to be irreversible outside the therapeutic time window. 39 The MAP, LVEF, cardiac output, myocardial performance index, and hemodynamic stability would be preserved if an effective therapy is applied. The improvement of cardiac performance could also ameliorate injuries in the specific organs induced by ischemia‐reperfusion. However, the research on postresuscitative cardioprotective strategy is still insufficient and the majority of the studies are focusing on the optimization of cerebral protection. Although earlier postresuscitation myocardial dysfunction was not predictive of the neurological injuries, but subsequent neuroprotective intervention for PCAS might be infeasible during profound shock and unstable hemodynamic status. 40 , 41 Therefore, finding interventions that can effectively improve postresuscitation myocardial dysfunction is of great significance to the prognosis of PCAS.
In this study, trimetazidine was chosen because it is a widely used anti‐ischemic agent that could improve heart function. 42 Our data showed a significant improvement in MAP and contractile function accompanied with a prominent reduction of myocardial injury markers concentration in animals administrated with a single dose of trimetazidine immediately after ROSC. These finding was consistent with previous studies investigating the cardioprotective effect of trimetazidine. Ruixing et al showed that MAP and left ventricular systolic pressure in animals fed with 2 mg/kg per day trimetazidine were significantly higher than those in the control group in ischemia‐reperfusion rabbit model. 43 Tabbi‐Anneni I et al. reported that cardiac hypertrophy was significantly reduced in heart failure rats fed with 7.5 mg/day trimetazidine. 17 El‐Kady et al. demonstrated that receiving trimetazidine 20 mg 3 times daily lead to an increase in LVEF in patients with ischemic left ventricular dysfunction and multivessel coronary artery disease. 44 These results suggest that trimetazidine may ameliorate cardiac function by improving the contractile response of left ventricular myocardium.
The mechanism by which trimetazidine exerts cardioprotective effect has been explored in numerous animal and clinical studies. The beneficial effect of trimetazidine is attributed to the regulation of cardiac energy metabolism. 45 As a competitive fatty acid oxidation inhibitor, trimetazidine inhibits the enzymes of fatty acid β‐oxidation and stimulates total glucose utilization, including both glycolysis and glucose oxidation. 46 Because utilization of glucose oxidation is more efficient than fatty acid oxidation, the preserved myocardial high‐energy phosphate levels result in improvement of left ventricular function. 47 In this study, myocardial injury markers were significantly reduced and postresuscitation LVEF was significantly improved in the trimetazidine treated animals. This was consistent with previous studies investigating the effects of trimetazidine on cardioprotection. 48 Additionally, trimetazidine may protect against myocardial dysfunction by inhibiting oxidative damage and decreasing myocardial fibrosis. 49 The finding that biomarkers of the oxidative stress and inflammation were markedly reduced after administration of trimetazidine was also in accordance with previous studies. 50 , 51 The enhanced generation of ROS instigates a wide array of pathways that lead to ischemia reperfusion injury, including the regulation of inflammatory reaction. Trimetazidine reduces the generation of ROS by maintaining energy production with less oxygen consumption. 52 However, the antioxidant mechanisms responsible for cardioprotection has not been fully elucidated. In a rat model of myocardial infarction, Khan et al. reported that the effect of trimetazidine was mediated by the activation of p38 mitogen‐activated protein kinase and Akt signaling pathway. 53 Using the same animal model, Liu et al. revealed that the protective effect of trimetazidine was through the activation of AMP‐activated protein kinase and extracellular signal‐regulated kinase signaling pathway. 16 Meanwhile, the reduction of oxygen‐derived free radicals may also mitigate inflammatory response since oxidative stress and inflammation are interdependent and interconnected processes. 54 A single dose of trimetazidine administrated immediately after resuscitation considerably decreased the concentrations of oxidative stress and inflammatory biomarkers which further contributed to a significant ameliorative myocardial pathology damage. Since the different mechanism of measurements of biomarkers and pathological examination and the different time point of the sample extraction, the final results may not be completely consistent between biochemical analysis and pathological analysis. Therefore, trimetazidine could offer a cardioprotective effect presented as decreasing postresusciatation oxidative stress and inflammatory response, thereby significantly improving survival.
To our knowledge, this is the first study to investigate the effect of trimetazidine on postresuscitation, myocardial dysfunction, and survival outcome in a CA model of rats. We found that a single dose of trimetazidine administrated immediately after resuscitation could attenuate myocardial dysfunction. The efficacy of trimetazidine is comparable with that of TTM and its cardioprotective effect is independent of TTM. Trimetazidine may be a promising and easily applicable solution for the management of myocardial dysfunction in patients after CA. On the one hand, the therapeutic time window for myocardial dysfunction after CA is limited, but previous studies found that it would take at least 4 hours to achieve the target temperature in patients with TTM. 12 Administration of trimetazidine could exhibit readily apparent advantages in the treatment because it is a time‐saving and effective therapy without affecting the efficacy of TTM and other subsequent therapeutic measure. On the other hand, it is feasible for intravenous injection of trimetazidine immediately after ROSC in clinical practice since intravenous access would be established rapidly for giving emergency pharmacotherapy in patients with CA with the recommendation of the Guidelines. 55
There are some limitations in this study. First, this study induced VF in healthy animals without any comorbidity therefore it was not capable to imitate the clinical scenario of CA completely. Second, this study did not investigate the effect of trimetazidine on neurological outcomes, so the improved survival was directly from the improved cardiac function or indirectly by the improved neurological recovery is unknown. Thirdly, this study did not compare the effects of different dosage and timing of trimetazidine administration on myocardial function and survival. Fourthly, although we observed that biomarkers of oxidative stress and inflammation were significantly reduced in animals treated with trimetazidine, we did not measure the phosphocreatine and adenosine triphosphate intracellular level and did not explore the mechanism on how trimetazidine exert antioxidative effects and alleviates the inflammatory response.
Conclusions
A single dose of trimetazidine administrated immediately after resuscitation significantly alleviates postresuscitation myocardial dysfunction and improves survival by decreasing oxidative stress and inflammation in a VF rat model. The cardioprotective effect of trimetazidine was comparable with TTM, either used alone or combined with TTM.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (NSFC31771070).
Disclosures
The authors declare that they have no competing interests.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Wang CH, Huang CH, Chang WT, Tsai MS, Lu TC, Yu PH, Wang AY, Chen NC, Chen WJ. Association between early arterial blood gas tensions and neurological outcome in adult patients following in‐hospital cardiac arrest. Resuscitation. 2015;89:1–7. doi: 10.1016/j.resuscitation.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 2. Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016 [DOI] [PubMed] [Google Scholar]
- 3. Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012;40:3135–3139. doi: 10.1097/CCM.0b013e3182656976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta‐analysis of observational studies. Resuscitation. 2014;85:1142–1148. doi: 10.1016/j.resuscitation.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 5. Huang CH, Wang CH, Tsai MS, Hsu NT, Chiang CY, Wang TD, Chang WT, Chen HW, Chen WJ. Urocortin treatment improves acute hemodynamic instability and reduces myocardial damage in post‐cardiac arrest myocardial dysfunction. PLoS One. 2016;11:e0166324. doi: 10.1371/journal.pone.0166324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chalkias A, Xanthos T. Pathophysiology and pathogenesis of post‐resuscitation myocardial stunning. Heart Fail Rev. 2012;17:117–128. doi: 10.1007/s10741-011-9255-1 [DOI] [PubMed] [Google Scholar]
- 7. Zhao S, Qian J, Wang J, Gong P, Yang Z, Cahoon J, Wu X, Duggal N, Lin C, Tang W. Effects of oxygen concentrations on postresuscitation myocardial oxidative stress and myocardial function in a rat model of cardiopulmonary resuscitation. Crit Care Med. 2015;43:e560–e566. doi: 10.1097/CCM.0000000000001297 [DOI] [PubMed] [Google Scholar]
- 8. Halladin NL. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan Med J. 2015;62:B5054. [PubMed] [Google Scholar]
- 9. Merchant RM, Topjian AA, Panchal AR, Cheng A, Aziz K, Berg KM, Lavonas EJ, Magid DJ. Part 1: executive summary: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S337–S357. doi: 10.1161/CIR.0000000000000918 [DOI] [PubMed] [Google Scholar]
- 10. Rakesh K, Vishnu Bhat B, Adhisivam B, Ajith P. Effect of therapeutic hypothermia on myocardial dysfunction in term neonates with perinatal asphyxia—a randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31:2418–2423. doi: 10.1080/14767058.2017.1344633 [DOI] [PubMed] [Google Scholar]
- 11. Carwell M. Targeted temperature management for improved outcomes: are we there yet? Crit Care Nurs Q. 2018;41:102–108. doi: 10.1097/CNQ.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 12. Kirkegaard H, Søreide E, de Haas I, Pettilä V, Taccone FS, Arus U, Storm C, Hassager C, Nielsen JF, Sørensen CA, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out‐of‐hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318:341–350. doi: 10.1001/jama.2017.8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mody P, Kulkarni N, Khera R, Link MS. Targeted temperature management for cardiac arrest. Prog Cardiovasc Dis. 2019;62:272–278. doi: 10.1016/j.pcad.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 14. McClellan KJ, Trimetazidine PGL. A review of its use in stable angina pectoris and other coronary conditions. Drugs. 1999;58:143–157. [DOI] [PubMed] [Google Scholar]
- 15. Ma N, Bai J, Zhang W, Luo H, Zhang X, Liu D, Qiao C. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac miRNA‐21 expression, Akt and the Bcl‐2/Bax pathway. Mol Med Rep. 2016;14:4216–4222. doi: 10.3892/mmr.2016.5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Chen J‐M, Huang H, Kuznicki M, Zheng S, Sun W, Quan N, Wang L, Yang H, Guo H‐M, et al. The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway. Metabolism. 2016;65:122–130. doi: 10.1016/j.metabol.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabbi‐Anneni I, Helies‐Toussaint C, Morin D, Bescond‐Jacquet A, Lucien A, Grynberg A. Prevention of heart failure in rats by trimetazidine treatment: a consequence of accelerated phospholipid turnover? J Pharmacol Exp Ther. 2003;304:1003–1009. doi: 10.1124/jpet.102.042143 [DOI] [PubMed] [Google Scholar]
- 18. Gong W, Ma Y, Li A, Shi H, Nie S. Trimetazidine suppresses oxidative stress, inhibits mmp‐2 and mmp‐9 expression, and prevents cardiac rupture in mice with myocardial infarction. Cardiovasc Ther. 2018;36:e12460. doi: 10.1111/1755-5922.12460 [DOI] [PubMed] [Google Scholar]
- 19. Glezer MG, Vygodin VA. Effectiveness of trimetazidine in patients with stable angina pectoris of various durations: results from ODA. Cardiol Ther. 2020;9:395–408. doi: 10.1007/s40119-020-00174-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gunes Y, Guntekin U, Tuncer M, Sahin M. Improved left and right ventricular functions with trimetazidine in patients with heart failure: a tissue doppler study. Heart Vessels. 2009;24:277–282. doi: 10.1007/s00380-008-1118-x [DOI] [PubMed] [Google Scholar]
- 21. Steg PG, Grollier G, Gallay P, Morice M‐C, Karrillon GJ, Benamer H, Kempf C, Laperche T, Arnaud P, Sellier P, et al. A randomized double‐blind trial of intravenous trimetazidine as adjunctive therapy to primary angioplasty for acute myocardial infarction. Int J Cardiol. 2001;77:263–273. doi: 10.1016/S0167-5273(00)00443-5 [DOI] [PubMed] [Google Scholar]
- 22. Elimadi A, Settaf A, Morin D, Sapena R, Lamchouri F, Cherrah Y, Tillement JP. Trimetazidine counteracts the hepatic injury associated with ischemia‐reperfusion by preserving mitochondrial function. J Pharmacol Exp Ther. 1998;286:23–28. [PubMed] [Google Scholar]
- 23. Mahfoudh Boussaid A, Selmi R, Bejaoui M, Hadj Ayed K, Zaouali MA, Ben AH. Effectiveness of a single versus repeated administration of trimetazidine in the protection against warm ischemia/reperfusion injury of rat liver. Turk J Med Sci. 2016;46:1258–1264. doi: 10.3906/sag-1505-102 [DOI] [PubMed] [Google Scholar]
- 24. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand council on resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652 [DOI] [PubMed] [Google Scholar]
- 25. Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011;39:1423–1430. doi: 10.1097/CCM.0b013e318212020a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai C, Chen G, Chen B, Wang J, Yin C, Wang J, Gong Y, Wei L, Huang Y, Li Y. Repetitive anodal transcranial direct current stimulation improves neurological outcome and survival in a ventricular fibrillation cardiac arrest rat model. Brain Stimul. 2019;12:659–667. doi: 10.1016/j.brs.2018.12.974 [DOI] [PubMed] [Google Scholar]
- 27. Liu XW, Lu MK, Zhong HT, Wang LH, Fu YP. Panax notoginseng saponins attenuate myocardial ischemia‐reperfusion injury through the HIF‐1α/BNIP3 pathway of autophagy. J Cardiovasc Pharmacol. 2019;73:92–99. doi: 10.1097/FJC.0000000000000640 [DOI] [PubMed] [Google Scholar]
- 28. Yu SY, Dong B, Fang ZF, Hu XQ, Tang L, Zhou SH. Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating mir‐204‐3p and inhibiting autophagy. J Cell Mol Med. 2018;22:4886–4898. doi: 10.1111/jcmm.13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pickering JG, Boughner DR. Fibrosis in the transplanted heart and its relation to donor ischemic time. Assessment with polarized light microscopy and digital image analysis. Circulation. 1990;81:949–958. doi: 10.1161/01.CIR.81.3.949 [DOI] [PubMed] [Google Scholar]
- 30. Guo H, Tang L, Xu J, Lin C, Ling X, Lu C, Liu Z. Microrna‐495 serves as a diagnostic biomarker in patients with sepsis and regulates sepsis‐induced inflammation and cardiac dysfunction. Eur J Med Res. 2019;24:37. doi: 10.1186/s40001-019-0396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grossman SH, Sellers DS. Subunit conformation and dynamics in a heterodimeric protein: studies of the hybrid isozyme of creatine kinase. Biochim Biophys Acta. 1998;1387:447–453. doi: 10.1016/S0167-4838(98)00132-0 [DOI] [PubMed] [Google Scholar]
- 32. Martínez Díaz F, Rodríguez‐Morlensín M, Pérez‐Cárceles MD, Noguera J, Luna A, Osuna E. Biochemical analysis and immunohistochemical determination of cardiac troponin for the postmortem diagnosis of myocardial damage. Histol Histopathol. 2005;20:475–481. doi: 10.14670/HH-20.475 [DOI] [PubMed] [Google Scholar]
- 33. Ambler DR, Fletcher NM, Diamond MP, Saed GM. Effects of hypoxia on the expression of inflammatory markers IL‐6 and TNF‐a in human normal peritoneal and adhesion fibroblasts. Syst Biol Reprod Med. 2012;58:324–329. doi: 10.3109/19396368.2012.713439 [DOI] [PubMed] [Google Scholar]
- 34. Çakır M, Tekin S, Okan A, Çakan P, Doğanyiğit Z. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture‐induced sepsis model in rats. Int Immunopharmacol. 2020;78:105978. doi: 10.1016/j.intimp.2019.105978 [DOI] [PubMed] [Google Scholar]
- 35. Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol. 2005;168:17–20. doi: 10.1083/jcb.200409170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen TT, Ngo LQ, Promsudthi A, Surarit R. Salivary lipid peroxidation in patients with generalized chronic periodontitis and acute coronary syndrome. J Periodontol. 2016;87:134–141. doi: 10.1902/jop.2015.150353 [DOI] [PubMed] [Google Scholar]
- 37. Topjian AA, de Caen A, Wainwright MS, Abella BS, Abend NS, Atkins DL, Bembea MM, Fink EL, Guerguerian A‐M, Haskell SE, et al. Pediatric post‐cardiac arrest care: a scientific statement from the American Heart Association. Circulation. 2019;140:e194–e233. doi: 10.1161/CIR.0000000000000697 [DOI] [PubMed] [Google Scholar]
- 38. Laurent I, Monchi M, Chiche J‐D, Joly L‐M, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, et al. Reversible myocardial dysfunction in survivors of out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/S0735-1097(02)02594-9 [DOI] [PubMed] [Google Scholar]
- 39. Huang CH, Tsai MS, Hsu CY, Su YJ, Wang TD, Chang WT, Chen WJ. Post‐cardiac arrest myocardial dysfunction is improved with cyclosporine treatment at onset of resuscitation but not in the reperfusion phase. Resuscitation. 2011;82:S41–S47. doi: 10.1016/S0300-9572(11)70150-2 [DOI] [PubMed] [Google Scholar]
- 40. Cerchiari EL, Safar P, Klein E, Cantadore R, Pinsky M. Cardiovascular function and neurologic outcome after cardiac arrest in dogs. The cardiovascular post‐resuscitation syndrome. Resuscitation. 1993;25:9–33. doi: 10.1016/0300-9572(93)90003-9 [DOI] [PubMed] [Google Scholar]
- 41. Kilgannon JH, Roberts BW, Jones AE, Mittal N, Cohen E, Mitchell J, Chansky ME, Trzeciak S. Arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest*. Crit Care Med. 2014;42:2083–2091. doi: 10.1097/CCM.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 42. Fan Q, Niu Z, Ma L. Meta‐analysis of trimetazidine treatment for cardiomyopathy. Biosci Rep. 2018;38:BSR20171583. doi: 10.1042/BSR20171583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruixing Y, Wenwu L, Al‐Ghazali R. Trimetazidine inhibits cardiomyocyte apoptosis in a rabbit model of ischemia‐reperfusion. Transl Res. 2007;149:152–160. doi: 10.1016/j.trsl.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 44. El‐Kady T, El‐Sabban K, Gabaly M, Sabry A, Abdel‐Hady S. Effects of trimetazidine on myocardial perfusion and the contractile response of chronically dysfunctional myocardium in ischemic cardiomyopathy: a 24‐month study. Am J Cardiovasc Drugs. 2005;5:271–278. doi: 10.2165/00129784-200505040-00006 [DOI] [PubMed] [Google Scholar]
- 45. Di Napoli P, Taccardi AA, Barsotti A. Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart. 2005;91:161–165. doi: 10.1136/hrt.2003.031310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long‐chain 3‐ketoacyl coenzyme a thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.RES.86.5.580 [DOI] [PubMed] [Google Scholar]
- 47. Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio A, Margonato A. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–948. doi: 10.1093/eurheartj/ehi816 [DOI] [PubMed] [Google Scholar]
- 48. He C, Cao S, Tong Z, Wang W, Zhang Y, Guo C. Trimetazidine ameliorates myocardial ischemia‐reperfusion injury. Pak J Pharm Sci. 2018;31:1691–1696. [PubMed] [Google Scholar]
- 49. Marzilli M, Vinereanu D, Lopaschuk G, Chen Y, Dalal JJ, Danchin N, Etriby EL, Ferrari R, Gowdak LH, Lopatin Y, et al. Trimetazidine in cardiovascular medicine. Int J Cardiol. 2019;293:39–44. doi: 10.1016/j.ijcard.2019.05.063 [DOI] [PubMed] [Google Scholar]
- 50. Dhote V, Balaraman R. Anti‐oxidant activity mediated neuroprotective potential of trimetazidine on focal cerebral ischaemia‐reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35:630–637. doi: 10.1111/j.1440-1681.2008.04845.x [DOI] [PubMed] [Google Scholar]
- 51. Su Q, Li L, Zhao J, Sun Y, Yang H. Effects of trimetazidine on PDCD4/NF‐κB/TNF‐α pathway in coronary microembolization. Cell Physiol Biochem. 2017;42:753–760. doi: 10.1159/000478067 [DOI] [PubMed] [Google Scholar]
- 52. Tsioufis K, Andrikopoulos G, Manolis A. Trimetazidine and cardioprotection: facts and perspectives. Angiology. 2015;66:204–210. doi: 10.1177/0003319714530040 [DOI] [PubMed] [Google Scholar]
- 53. Khan M, Meduru S, Mostafa M, Khan S, Hideg K, Kuppusamy P. Trimetazidine, administered at the onset of reperfusion, ameliorates myocardial dysfunction and injury by activation of p38 mitogen‐activated protein kinase and Akt signaling. J Pharmacol Exp Ther. 2010;333:421–429. doi: 10.1124/jpet.109.165175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGarry T, Biniecka M, Veale DJ, Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042 [DOI] [PubMed] [Google Scholar]
- 55. Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S366–S468. [DOI] [PubMed] [Google Scholar]


