Abstract
Background
To investigate the dose‐response association between physical activity and lower respiratory tract infection (LoRI) outcomes in patients with cardiovascular disease.
Methods and Results
Using the Korean National Health Insurance data, we identified individuals aged 18 to 99 years (mean age, 62.6±11.3 years; women, 49.6%) with cardiovascular disease who participated in health screening from January 1, 2009, to December 31, 2012 (n=1 048 502), and were followed up until 2018 for mortality and until 2019 for hospitalization. Amount of physical activity was assessed using self‐reported questionnaires and categorized into 5 groups: 0 (completely sedentary), <500, 500 to 999, 1000 to 1499, and ≥1500 metabolic equivalents of task min/wk. After controlling for various confounders, adjusted hazard ratios (95% CIs) were 1.00 (reference), 0.74 (0.70–0.78), 0.66 (0.62–0.70), 0.52 (0.47–0.57), and 0.54 (0.49–0.60) for LoRI mortality, and 1.00 (reference), 0.84 (0.83–0.85), 0.77 (0.76–0.79), 0.72 (0.70–0.73), and 0.71 (0.69–0.73) for LoRI hospitalization among those engaging in physical activity of 0, <500, 500 to 999, 1000 to 1499, and ≥1500 metabolic equivalents of task min/wk, respectively. Assuming linear association between 0 and 2000 metabolic equivalents of task min/wk, each 500–metabolic equivalents of task min/wk increase of physical activity was associated with reduced LoRI mortality and hospitalization by 22% and 13%, respectively. The negative association was stronger in the older population than in the younger population (P for interaction <0.01).
Conclusions
In patients with cardiovascular disease, engaging in even a low level of physical activity was associated with a decreased risk of mortality and hospitalization from LoRI than being completely sedentary, and incremental risk reduction was observed with increased physical activity.
Keywords: cardiovascular disease, dose‐response, physical activity, respiratory tract infection
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise, Lifestyle
Nonstandard Abbreviations and Acronyms
- LoRI
lower respiratory tract infection
- MVPA
moderate to vigorous physical activity
- NHIS
National Health Insurance Service
Clinical Perspective
What Is New?
The present study demonstrated a graded negative dose‐response association of physical activity on mortality and hospitalization attributable to lower respiratory tract infection in individuals with cardiovascular disease, including older individuals.
Engaging in even a low amount of physical activity was associated with a reduced risk compared with being completely sedentary.
Furthermore, incremental risk reduction was demonstrated with increasing physical activity.
What Are the Clinical Implications?
The dose‐response association analyses support the need to limit sedentary behaviors and gradually increase physical activity as medical condition allows to meet the guideline‐suggested goal.
This could be an attainable approach among patients with cardiovascular disease, especially for older patients.
Evidence has shown that physical inactivity increases the risk of various diseases and all‐cause mortality. 1 , 2 , 3 , 4 On the basis of these data, recent guidelines recommend engaging in physical activity to achieve better health outcomes. 5 , 6 However, the effect of physical activity on infection or immune function has been relatively unexplored and remains unclear. 7 Lower respiratory tract infection (LoRI) is a leading cause of morbidity and mortality, contributing to ≈5% of all deaths worldwide in 2017, and its outcome is particularly worse among older and immunocompromised populations. 8 , 9
Patients with cardiovascular disease (CVD), who tend to have a more sedentary lifestyle, 10 were shown to have worse clinical outcomes when they had LoRI. 8 , 9 Currently, limited studies are available on the association between physical activity and LoRI among patients with CVD. Previous studies on respiratory tract infection and physical activity are mostly for general population and mainly focused on upper respiratory tract infection with a benign clinical course. 11 , 12 , 13 , 14 , 15 , 16 , 17 Among the studies on LoRI, inconsistent data exist. 11 , 12 , 13 , 15 Specifically, it is not clear whether a low level of physical activity, performed less than the minimum amount suggested in the guidelines, 5 , 6 could lower the risk of LoRI. Clarifying the dose‐response association between physical activity and LoRI would be helpful to guide patients with CVD about proper physical activity. Moreover, during the recent outbreak of the COVID‐19 pandemic, individuals have been advised to stay indoors and limit their physical/social activity. Although such restrictions may be useful to control the spread of COVID‐19, they can unintentionally decrease the amount of physical activity. Therefore, elucidating the relationship between physical activity and LoRI outcomes in patients with CVD would be of value for improving prognoses in future pandemics and related LoRIs. 18
Using a population‐based cohort study with >1 million patients with CVD, we aimed to investigate the dose‐response association between physical activity and LoRI outcomes (mortality and hospitalization). Specifically, we sought to identify whether there is still some beneficial effect associated with a lower level of physical activity than the minimum level suggested in the guidelines (<500 metabolic equivalents of task [MET] min/wk). 5 In addition, we evaluated whether the association differs by age, with a focus on older patients.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to National Health Insurance Service (NHIS) of Korea at https://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do.
Study Population and Follow‐Up
The current study used data from the NHIS. The NHIS provides mandatory health insurance, covering up to 97% of the Korean population. Details about the database are described elsewhere. 19 Among participants of NHIS health screening from 2009 to 2012, those aged 18 to 99 years with prevalent CVD, defined by ischemic heart disease (International Classification of Diseases, Tenth Revision [ICD‐10], code of I20–I25), heart failure (ICD‐10 code of I11, I13, I255, I42, or I50), or cerebrovascular disease (ICD‐10 code of I60–I69), including stroke (ICD‐10 code of I60–I64 or I69), were identified as the study cohort (n=1 188 635). These 3 major CVDs were chosen as they are frequently used as clinical end point or study population. 10 , 20 We considered individuals to have prevalent disease at baseline if they visited a medical institution for the disease at least once within 12 months before the baseline health examination date.
Among the study cohort (n=1 188 635), we excluded 136 438 people with preexisting cancers and other miscellaneous diseases, and 3695 individuals because of missing information on the date of health examination and risk factors, including body mass index, fasting glucose, and systolic blood pressure. Finally, for the remaining 1 048 502 patients, follow‐up for death and hospitalization attributable to LoRI (ICD‐10 code of J09–J22; influenza, pneumonia, and other LoRI) was performed until December 31, 2018, through national death records, and until December 31, 2019, through the NHIS claim database. The Institutional Review Board of Catholic Kwandong University, Republic of Korea, approved the study; and the informed consent was waived because anonymized data were provided by the NHIS according to a strict confidentiality protocol.
Data Collection
Body mass index (kg/m2) was calculated as measured weight divided by the square of measured height. Blood pressure was measured in a seated position after resting for at least 5 minutes. Fasting glucose was assayed by enzymatic methods. Smoking history, alcohol use, and physical activity were self‐reported via a questionnaire at the time of health examination. Health examination and data collection followed a standard protocol by the government. The medical institutions for screening that met the standards of personnel, facilities, and equipment were selected according to the law. 19 The external quality validation process for clinical chemistry was performed regularly. Information on prevalent diseases (eg, CVD and chronic obstructive pulmonary disease [ICD‐10 codes of J40–J44 or J47; outpatient visit or hospitalization]) and previous hospitalization for LoRI within 12 months before the baseline health examination date was assessed through the NHIS claims database.
Categorization of Physical Activity Amount and Frequency
Physical activity was assessed using the NHIS questionnaire based on the modified International Physical Activity Questionnaire, which has shown moderately good validity and reliability compared with accelerometer assessment. 21 The expected associations between physical activity (assessed with the NHIS‐modified International Physical Activity Questionnaire questionnaire) and several outcomes have been reported in previous studies. 10 , 22 The English‐translated questionnaire is available elsewhere. 22 In brief, based on 7‐day recall method, the questionnaire evaluated the frequency of each of the following 3 intensity leisure time physical activities (expressed as 0 to 7 days per week): (1) number of days performing light‐intensity physical activity for at least 30 minutes (eg, walking at a leisurely pace), (2) number of days performing moderate‐intensity physical activity for at least 30 minutes (eg, fast walking, tennis, or bicycle riding), and (3) number of days performing vigorous‐intensity physical activity for at least 20 minutes (eg, running, aerobics, high‐speed cycling, or mountain hiking). We calculated the total energy expenditure (MET min/wk) by summing the product of frequency, duration, and assigned values for each of the 3 intensity physical activities. For the intensity of each physical activity, we assigned 2.9, 4.0, and 7.0 MET for light, moderate, and vigorous physical activity, respectively. 10 For the main analyses, study populations were categorized into 5 groups by their amount of physical activity: 0 (completely sedentary), <500, 500 to 999, 1000 to 1499, and ≥1500 MET min/wk. In addition, to characterize the association between moderate to vigorous physical activity (MVPA) and outcome, the study population was categorized into 4 groups by their MVPA frequency: 0, 1 to 2, 3 to 4, and ≥5 times/week.
Statistical Analysis
The hazard ratios (HRs) and CIs associated with physical activity for deaths and hospitalization attributable to LoRI were calculated using a Cox proportional hazards model. In the multivariable models, adjustments were made for age at baseline (continuous variable; within each age group), sex, smoking status (current smoker, former smoker, nonsmoker, and missing information), frequency of alcohol use (none, <10, 10–39, or ≥40 g ethanol/d, and missing information), beneficiary income status (quantiles), body mass index (<18.5, 18.5–24.9, 25–29.9, or ≥30 kg/m2), hypertension (systolic blood pressure <120, 120–139, or ≥140 mm Hg or prevalent hypertension), diabetes (fasting glucose <100, 100–125, or ≥126 mg/dL or prevalent diabetes), prevalent chronic obstructive pulmonary disease, and previous admission attributable to LoRI. In the Cox model for LoRI mortality, the cause‐specific hazard method was used for handling competing risks; individuals who experienced a competing event (other causes of death) or reached the end of follow‐up were treated as censored. Schoenfeld residuals were used to test the proportional hazard assumption. Subgroup analyses by age strata, sex, and CVD subtypes were also performed. To validate the age‐specific association (particularly associations in older patients with CVD), we stratified patients by age strata (18–64, 65–74, and 75–99 years). Sensitivity analyses were conducted that excluded the first 2 years of follow‐up. Analysis using physical activity as a continuous variable (per 500‐MET min/wk increase) was performed. The Cochran Q statistic was used as the interaction test to examine the difference in the effect size of each 500‐MET min/wk increment between age groups. HRs of restricted cubic spline transformation of MET min/wk with 4 knots (5th, 50th, 85th, and 97.5th percentile) and 0 MET min/wk as a reference were also plotted. All P values were 2 sided. All analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Baseline Demographic Findings
During median 8.5 and 9.3 years of follow‐up, 8237 deaths and 116 281 hospitalizations from LoRI occurred, respectively, among 1 048 502 patients with CVD. Mean age was 62.6±11.3 years, and 49.6% were women. Among them, 397 822 patients were diagnosed with ischemic heart disease, 354 525 were diagnosed with heart failure, and 369 756 were diagnosed with cerebrovascular disease. Over half of the population did not meet the physical activity level suggested by the guideline of ≥500 MET min/wk (30.0% were completely sedentary, and 27.3% had <500 MET min/wk). On the basis of their MVPA frequency, 56.6% never engaged in MVPA. Among the population with physical activity of <500 MET min/wk, a substantial portion (61.4%) did not perform MVPA, indicating they only performed light physical activity. Those who were completely sedentary were more likely to be older, women, a nonsmoker, a nondrinker, with poor income status, and with the highest proportion of both obesity and underweight among the physical activity groups (Table 1).
Table 1.
Characteristics of the Study Population Based on the Amount of Physical Activity
| Variable | Total |
0 MET min/wk |
<500 MET min/wk |
500–999 MET min/wk |
1000–1499 MET min/wk |
≥1500 MET min/wk |
|---|---|---|---|---|---|---|
| Participants, n (%) | 1 048 502 (100) | 314 815 (30.0) | 286 526 (27.3) | 271 661 (25.9) | 107 487 (10.3) | 68 013 (6.5) |
| Sex, n (%) | ||||||
| Men | 528 373 (50.4) | 135 208 (42.9) | 138 664 (48.4) | 147 687 (54.4) | 63 370 (59.0) | 43 444 (63.9) |
| Women | 520 129 (49.6) | 179 607 (57.1) | 147 862 (51.6) | 123 974 (45.6) | 44 117 (41.0) | 24 569 (36.1) |
| Age, y | 62.7±11.3 | 64.9±11.4 | 61.9±11.6 | 61.8±11.0 | 60.5±10.3 | 62.2±9.8 |
| Age categories, y | ||||||
| 18–64 | 569 056 (54.3) | 148 097 (47.0) | 163 472 (57.1) | 153 456 (56.5) | 66 198 (61.6) | 37 833 (55.6) |
| 65–74 | 337 403 (32.2) | 105 846 (33.6) | 86 067 (30.0) | 87 928 (32.4) | 33 193 (30.9) | 24 369 (35.8) |
| 75–99 | 142 043 (13.5) | 60 872 (19.3) | 36 987 (12.9) | 30 277 (11.1) | 8096 (7.5) | 5811 (8.5) |
| Smoking status | ||||||
| Nonsmoker | 683 807 (65.2) | 227 525 (72.3) | 187 400 (65.4) | 166 234 (61.2) | 63 049 (58.7) | 39 599 (58.2) |
| Ex‐smoker | 204 917 (19.5) | 39 997 (12.7) | 54 523 (19.0) | 63 238 (23.3) | 28 563 (26.6) | 18 596 (27.3) |
| Current smoker | 155 882 (14.9) | 45 178 (14.4) | 43 896 (15.3) | 41 562 (15.3) | 15 589 (14.5) | 9657 (14.2) |
| Missing | 3896 (0.4) | 2115 (0.7) | 707 (0.2) | 627 (0.2) | 286 (0.3) | 161 (0.2) |
| Alcohol frequency | ||||||
| <1 Time/wk | 721 567 (68.8) | 243 170 (67.7) | 193 974 (67.7) | 176 218 (64.9) | 65 873 (61.3) | 42 332 (62.2) |
| 1–2 Times/wk | 200 709 (19.1) | 38 686 (12.3) | 61 649 (21.5) | 59 899 (22.0) | 25 681 (23.9) | 14 794 (21.8) |
| 3–4 Times/wk | 72 966 (7.0) | 16 024 (5.1) | 19 095 (6.7) | 21 608 (8.0) | 10 273 (9.6) | 5966 (8.8) |
| ≥5 Times/wk | 45 752 (4.4) | 13 310 (4.2) | 9957 (3.5) | 12 686 (4.7) | 5173 (4.8) | 4626 (6.8) |
| Missing | 7508 (0.7) | 3625 (1.2) | 1851 (0.6) | 1250 (0.5) | 487 (0.5) | 295 (0.4) |
| Income status, quartile | ||||||
| First quartile (low) | 218 111 (20.8) | 69 849 (22.2) | 58 162 (20.3) | 55 609 (20.5) | 21 199 (19.7) | 13 292 (19.5) |
| Second quartile | 172 804 (16.5) | 54 758 (17.4) | 47 105 (16.4) | 44 071 (16.2) | 16 372 (15.2) | 10 498 (15.4) |
| Third quartile | 249 427 (23.8) | 76 389 (24.3) | 68 161 (23.8) | 63 783 (23.5) | 25 271 (23.5) | 15 823 (23.3) |
| Fourth quartile (high) | 408 160 (38.9) | 113 819 (36.2) | 113 098 (39.5) | 108 198 (39.8) | 44 645 (41.5) | 28 400 (41.8) |
| Body mass index, kg/m2 | ||||||
| <18.5 | 22 048 (2.1) | 9711 (3.1) | 5577 (1.9) | 4673 (1.7) | 1283 (1.2) | 804 (1.2) |
| 18.5–24.9 | 564 649 (53.9) | 168 182 (53.4) | 153 019 (53.4) | 148 521 (54.7) | 57 806 (53.8) | 37 121 (54.6) |
| 25–29.9 | 402 898 (38.4) | 117 158 (37.2) | 111 150 (38.8) | 104 451 (38.4) | 43 032 (40.0) | 27 107 (39.9) |
| ≥30 | 58 907 (5.6) | 19 764 (6.3) | 16 780 (5.9) | 14 016 (5.2) | 5366 (5.0) | 2981 (4.4) |
| Hypertension, mm Hg | ||||||
| SBP <120 | 159 754 (15.2) | 45 637 (14.5) | 45 719 (16.0) | 42 072 (15.5) | 16 387 (15.2) | 9939 (14.6) |
| SBP 120–139 | 249 944 (23.8) | 72 846 (23.1) | 68 337 (23.9) | 65 573 (24.1) | 26 339 (24.5) | 16 849 (24.8) |
| Hypertension or SBP ≥140 | 638 804 (60.9) | 196 332 (62.4) | 172 470 (60.2) | 164 016 (60.4) | 64 761 (60.3) | 41 225 (60.6) |
| Diabetes, mg/dL | ||||||
| FBG <100 | 521 446 (49.7) | 156 593 (49.7) | 145 332 (50.7) | 134 919 (49.7) | 52 555 (48.9) | 32 047 (47.1) |
| FBG 100–125 | 291 512 (27.8) | 86 737 (27.6) | 79 462 (27.7) | 75 677 (27.9) | 30 761 (28.6) | 18 875 (27.8) |
| Diabetes or FBG ≥126 | 235 544 (22.5) | 71 485 (22.7) | 61 732 (21.5) | 61 065 (22.5) | 24 171 (22.5) | 17 091 (25.1) |
| COPD | ||||||
| No | 924 361 (88.2) | 274 490 (87.2) | 253 021 (88.3) | 240 868 (88.7) | 95 612 (89.0) | 60 370 (88.8) |
| Yes | 124 141 (11.8) | 40 325 (12.8) | 33 505 (11.7) | 30 793 (11.3) | 11 875 (11.0) | 7643 (11.2) |
| History of admission attributable to lower respiratory tract infection | ||||||
| No | 104 0315 (99.2) | 311 186 (98.8) | 284 515 (99.3) | 269 989 (99.4) | 106 989 (99.5) | 67 636 (88.8) |
| Yes | 8187 (0.8) | 3629 (1.2) | 2011 (0.7) | 1672 (0.6) | 498 (0.5) | 377 (0.6) |
| Frequency of moderate to vigorous physical activity | ||||||
| 0 Times/wk | 593 388 (56.6) | 314 815 (100.0) | 175 863 (61.4) | 102 710 (37.8) | 0 (0.0) | 0 (0.0) |
| 1–2 Times/wk | 136 054 (13.0) | 0 (0.0) | 94 286 (32.9) | 41 768 (15.4) | 0 (0.0) | 0 (0.0) |
| 3–4 Times/wk | 114 752 (10.9) | 0 (0.0) | 16 377 (5.7) | 88 725 (32.7) | 9650 (9.0) | 0 (0.0) |
| ≥5 Times/wk | 204 308 (19.5) | 0 (0.0) | 0 (0.0) | 38 458 (14.2) | 97 837 (91.0) | 68 013 (100.0) |
Data are expressed as mean±SD or number (percentage). COPD indicates chronic obstructive pulmonary disease; FBG, fasting blood glucose; MET, metabolic equivalents of task; and SBP, systolic blood pressure.
Dose‐Response Association Between Physical Activity and LoRI Mortality and Hospitalization
The dose‐response association demonstrated a graded negative association between physical activity and LoRI outcomes. Compared with completely sedentary individuals, a reduced risk was found in those who performed <500 MET min/wk of physical activity. Multivariate‐adjusted HRs (95% CIs) were 1.00 (reference), 0.74 (0.70–0.78), 0.66 (0.62–0.70), 0.52 (0.47–0.57), and 0.54 (0.49–0.60) for LoRI mortality, and 1.00 (reference), 0.84 (0.83–0.85), 0.77 (0.76–0.79), 0.72 (0.70–0.73), and 0.71 (0.69–0.73) for LoRI hospitalization among those engaging in physical activity of 0, <500, 500 to 999, 1000 to 1499, and ≥1500 MET min/wk, respectively (Table 2). Survival plots showed that separation of the curve between those completely sedentary and those with <500 MET min/wk began early (Figure 1).
Table 2.
Dose‐Response Association Between the Amount of Physical Activity and LoRI Mortality and Hospitalization Rates
|
Amount of physical activity, MET min/wk |
No. of events |
Event rate, n/10 000 person‐years |
Age‐ and sex‐adjusted | Multivariate‐adjusted* | Multivariate‐adjusted † | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Mortality | ||||||||
| 0 | 3751 | 15.4 | 1 (Reference) | … | 1 (Reference) | … | 1 (Reference) | … |
| <500 | 1968 | 8.5 | 0.70 (0.67–0.74) | <0.001 | 0.72 (0.68–0.76) | <0.001 | 0.74 (0.70–0.78) | <0.001 |
| 500–999 | 1707 | 7.8 | 0.63 (0.59–0.66) | <0.001 | 0.65 (0.61–0.68) | <0.001 | 0.66 (0.62–0.70) | <0.001 |
| 1000–1499 | 447 | 5.1 | 0.48 (0.43–0.53) | <0.001 | 0.50 (0.45–0.55) | <0.001 | 0.52 (0.47–0.57) | <0.001 |
| ≥1500 | 364 | 6.6 | 0.51 (0.45–0.56) | <0.001 | 0.53 (0.47–0.59) | <0.001 | 0.54 (0.49–0.60) | <0.001 |
| Hospitalization | ||||||||
| 0 | 44 148 | 172.8 | 1 (Reference) | … | 1 (Reference) | … | 1 (Reference) | … |
| <500 | 29 992 | 122.1 | 0.81 (0.80–0.83) | <0.001 | 0.83 (0.82–0.84) | <0.001 | 0.84 (0.83–0.85) | <0.001 |
| 500–999 | 26 660 | 113.7 | 0.74 (0.73–9.76) | <0.001 | 0.77 (0.75–0.78) | <0.001 | 0.77 (0.76–0.79) | <0.001 |
| 1000–1499 | 9153 | 96.6 | 0.68 (0.66–0.69) | <0.001 | 0.70 (0.69–0.72) | <0.001 | 0.72 (0.70–0.73) | <0.001 |
| ≥1500 | 6328 | 106.7 | 0.68 (0.66–0.69) | <0.001 | 0.70 (0.68–0.72) | <0.001 | 0.71 (0.69–0.73) | <0.001 |
HR indicates hazard ratio; LoRI, lower respiratory tract infection; and MET, metabolic equivalents of task.
HRs were calculated by Cox models, after adjusting for age at baseline, sex, smoking status, alcohol consumption frequency, and household income.
HRs were calculated by Cox models, after adjusting for age at baseline, sex, smoking status, alcohol consumption frequency, household income, blood pressure status, fasting glucose status, body mass index, prevalent chronic obstructive pulmonary disease, and history of admission attributable to LoRI.
Figure 1. Survival plots for lower respiratory tract infection outcomes by the amount of physical activity among individuals with cardiovascular diseases.
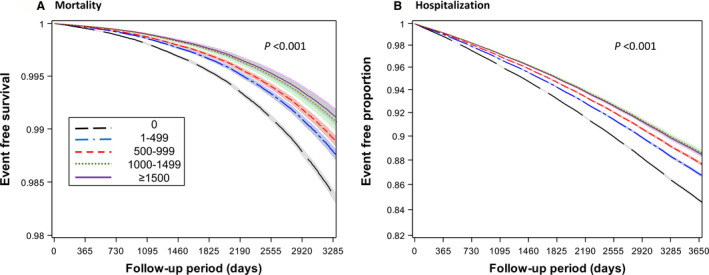
The amount of physical activity was categorized into 5 groups (0, 1–499, 500–999, 1000–1499, and ≥1500 metabolic equivalents of task min/wk). To create the survival plots for lower respiratory tract infection mortality; (A) and lower respiratory tract infection hospitalization (B), multivariate‐adjusted Cox regression was used (adjusting for age at baseline, sex, smoking status, alcohol consumption frequency, household income, blood pressure status, fasting glucose status, body mass index, prevalent chronic obstructive pulmonary disease, and history of admission attributable to lower respiratory tract infection).
A similar dose‐response association was observed for the MVPA frequency. Compared with individuals who never performed MVPA (including individuals who performed only light‐intensity physical activity [n=278 573]), a gradual risk reduction was observed in those who performed MVPA up to 3 to 4 times/week but without further risk reduction in those performed MVPA ≥5 times/week; multivariate‐adjusted HRs (95% CIs) were 1.00 (reference), 0.74 (0.69–0.81), 0.62 (0.56–0.68), and 0.61 (0.56–0.65) for LoRI mortality, and 1.00 (reference), 0.86 (0.84–0.87), 0.77 (0.75–0.78), and 0.77 (0.76–0.79) for LoRI hospitalization among those engaging in MVPA frequency of 0, 1 to 2, 3 to 4, and ≥5 times/week, respectively (Table S1).
Subgroup analyses by age strata, sex, and CVD subtype generally yielded similar results (Tables S2 and S3). Particularly, when participants were stratified by age, a gradual risk reduction was evident among older patients (aged ≥65 years), including those aged 75 to 99 years. Moreover, the effect size was larger in the older than in the younger population (P for interaction [age] <0.001). Sensitivity analyses that excluded the first 2 years of follow‐up were similar to the main analyses (Table S4). Further controlling for prevalent asthma, outpatient clinic visits attributable to LoRI, and previous use of steroids, which act as sensitivity analyses, did not virtually alter the results (Table S5). Assuming linear association (between 0 and 2000 MET min/wk of physical activity), each 500‐MET min/wk increase of physical activity was associated with 22% and 13% reduced risk of LoRI mortality and hospitalization in the overall population, respectively, in the fully adjusted model. The association strength increased as age increased. For example, a 500‐MET min/wk increase of physical activity was associated with 25% and 16% reduced risk of LoRI mortality and hospitalization, respectively, in individuals aged 75 to 99 years (Figure 2 and Table S6).
Figure 2. Age‐specific association of each 500–metabolic equivalents of task (MET) min/wk increase in physical activity on lower respiratory tract infection outcomes.
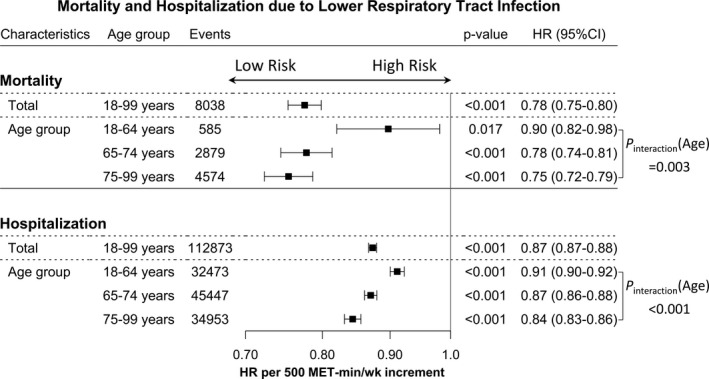
Assuming linear association between 0 and 2000 MET min/wk of physical activity, each 500‐MET min/wk increase of physical activity was associated with 22% and 13% reduced risk of lower respiratory tract infection mortality and hospitalization in the overall population in the fully adjusted model. Of note, the strength of association increased as age increased. HR indicates hazard ratio.
In the restricted cubic spline analyses, the pattern was generally parallel to that from categorical analyses (Figure 3). The risk declined gradually with increased physical activity up to 1500 to 1800 MET min/wk, and the curve flattened beyond that level. The overall pattern was similar, except for a relatively smaller effect size and narrower CI for hospitalization than mortality.
Figure 3. Restricted cubic spline for lower respiratory tract infection outcomes by amount of physical activity.
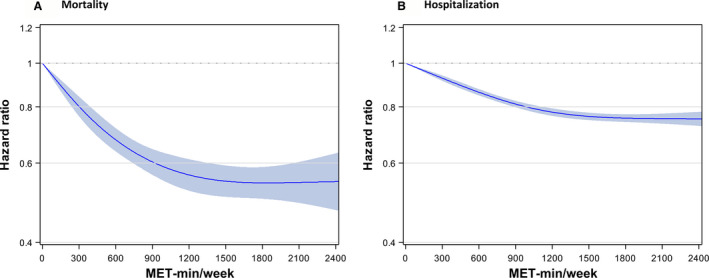
The shape of association between physical activity and lower respiratory infection outcomes for mortality (A) and for hospitalization (B) demonstrated a nonlinear association (L‐shaped association). The risk declined gradually with increased physical activity up to 1500 to 1800 metabolic equivalents of task (MET) min/wk, and the curve flattened beyond that level without additional risk lowering or elevation. Hazard ratios were adjusted for age at baseline, sex, smoking status, alcohol consumption frequency, household income, blood pressure status, fasting glucose status, body mass index, known chronic obstructive pulmonary disease, and history of admission attributable to lower respiratory tract infection.
Discussion
The present study demonstrated a negative dose‐response association of physical activity on mortality and hospitalization attributable to LoRI in >1 million people with CVD. Engaging in even a low amount of physical activity was associated with a reduced risk compared with being completely sedentary. Furthermore, incremental risk reduction was demonstrated with increasing physical activity (Figure 4). Moreover, the dose‐response association was more evident in older individuals than in younger individuals with CVD.
Figure 4. Summary of the study.
CVD indicates cardiovascular disease; and LoRI, lower respiratory tract infection.
The current study evaluated the dose‐dependent association of physical activity with LoRI outcomes. Less attention has been paid to the effect of physical activity on LoRI outcomes. The NHS (Nurses’ Health Study) II demonstrated a graded inverse association between physical activity and pneumonia, although the association was unclear after controlling for obesity, smoking, and alcohol consumption. 11 This might be partly because they enrolled younger adults (aged 27–44 years), and event rates were relatively low. The National Walkers’ and Runners’ Health Studies (NWHS and NRHS) 12 showed an inverse dose‐response relationship between physical activity and mortality from pneumonia after controlling for covariates, similar to our study. Notably, we found that older adults had stronger associations than younger adults. A previous study in Hong Kong reported that the beneficial effect of physical activity for influenza was more evident in younger than in older adults; however, the CIs of the effect between age groups overlapped. 15 The age‐specific association in the current study is noteworthy because older individuals generally have smaller relative effect size of risk or protective factors than the younger population in most epidemiologic studies 23 , 24 ; however, they have a greater absolute effect size. Our study implies that the absolute benefit for older individuals with CVDs from engaging in physical activity would be much greater. Taken together with previous findings showing mortality benefits in older population, 13 health professionals should encourage older individuals with CVDs to engage in physical activity to improve outcomes from LoRI.
A recent cohort study demonstrated a beneficial effect of physical activity on all‐cause and various cause‐specific mortalities, including influenza and pneumonia‐related mortality. 14 However, that study did not evaluate the effect of physical activity that does not satisfy the guideline‐recommended amount. Notably, our study demonstrated that participating in a low level of physical activity is beneficial compared with being completely sedentary, even though it does not meet the physical activity guidelines. This could have clinical implications given that many people with CVD (especially older individuals) did not meet the recommended amount. Indeed, more than half of the study population had <500 MET min/wk, and two thirds of individuals engaging in 1 to 499 MET min/wk only performed light‐intensity physical activity.
Conflicting data exist on the association of high physical activity levels with respiratory tract infection outcomes. 11 , 12 , 13 , 16 , 17 In some studies, an excess risk of extreme physical activity levels was shown for those who underwent strenuous activity, such as running a marathon, particularly among younger adults. However, there was no harm at the highest physical activity level in the present study. This might be because most patients with CVD in our study were older individuals who participated in physical activity for recreational purposes, not for competition. Moreover, the excess risk in those studies was mostly for immediate events after competitive exercise for upper respiratory tract infection, 16 not for LoRI.
To the best of our knowledge, this is the largest cohort study with substantial follow‐up that explicitly explored the association of physical activity with LoRI in individuals with CVD. The dose‐response association analyses support the need to limit sedentary behaviors and gradually increase physical activity (as medical condition allows) to meet the guideline‐suggested goal. 5 , 6 , 25 This could be an attainable approach among patients with CVD and older adults. Although we described the improvement of risk associated with lower amount of physical activity, we should not overlook the incremental benefit of activity that meets the physical activity guidelines. 5 , 6 , 25 In other words, if the lower amount of physical activity is beneficial, sufficient physical activity would be more beneficial for LoRI outcomes. To this end, each patient with CVD should be advised about an appropriate intensity, frequency, and duration of physical activity for better LoRI outcomes. 25 Furthermore, a recent guideline on exercise in patients with CVD also emphasized risk stratification before participation in exercise and individualized physical activity prescription among patients with CVD. 25 The current study did not directly show the association between physical activity and COVID‐19 outcomes. However, a recent study demonstrated that inconsistently as well as consistently performing physical activity was associated with lower risk for severe COVID‐19 outcomes. 26 Encouraging physical activity while following the safety rules (eg, wearing a mask and keeping a safe distance) might be beneficial to improve prognosis during COVID‐19 and future pandemics.
The underlying mechanism for the inverse association of physical activity on LoRI is yet to be determined. Presumably, enhanced immunity, modulated inflammatory response, and improved lung function might play a role. 27 , 28 , 29 , 30 , 31 , 32 Previous studies showed that natural killer cell activity (reflecting innate immune system) is higher in physically active individuals than in sedentary people. 27 Moreover, increased chronic inflammation coupled with oxidative stress could weaken the diaphragm muscle force, 32 which could be recovered by participating in physical activity. Although the immediate effect of a single bout of strenuous physical activity remains controversial, the long‐term effect of physical activity performed on a regular basis is beneficial for both CVD and respiratory infection throughout the lifespan. 28 , 29
The limitations of the present study include the observational study design, which makes causal inference difficult. Although we extensively controlled for various confounders, unavailable potential confounders and residual confounding might affect the study outcomes to some degree. Reverse causality (namely, physical inactivity as a possible reflection of worse disease condition) should be considered. Although the results remained robust after the exclusion of the first 2 years of follow‐up, the possibility of reverse causality cannot be completely eliminated. Furthermore, the level of physical activity might have been changed over time; thus, the baseline physical activity may not be enough to capture the actual physical activity level during the follow‐up. Physical activity assessment was performed using a self‐reported questionnaire. Compared with objective assessment using accelerometers, our assessment was more prone to misclassification. Nonetheless, self‐reported questionnaires were used in large studies as in the current study. 33 There is a chance of misclassification of LoRI outcomes as our study made use of ICD‐10 codes. However, the diagnostic accuracy of ICD‐10 codes for pneumonia, the most important LoRI outcome, was found to be acceptable (85.6%) compared with medical records. 34 In addition, information on muscle strengthening exercise was unavailable. The study results may not be directly applied to all spectrums of CVD (such as valvular heart disease, arrhythmia, and cardiomyopathy). Finally, the findings may be interpreted in the context of the Korean population, showing different metabolic traits and cultural norms. Therefore, subsequent studies of patients with CVD from other races and ethnicities could be valuable.
Conclusions
Physical activity has a graded negative dose‐dependent association with LoRI morbidity and mortality in individuals with CVDs, including older individuals. Of note, our study demonstrated that engaging in even a low level of physical activity leads to a reduced risk than remaining completely sedentary. Moreover, the risk reduction was incremental according to the increasing amount of physical activity.
Sources of Funding
This research was supported by a grant from the Korean Society of CardioMetabolic Syndrome. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Disclosures
None.
Supporting information
Tables S1–S6
Acknowledgments
The authors would like to thank the staff at the Big Data Steering Department at National Health Insurance Service for providing data and support.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023775
For Sources of Funding and Disclosures, see page 10.
References
- 1. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT; Lancet Physical Activity Series Working Group . Effect of physical inactivity on major non‐communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high‐income, middle‐income, and low‐income countries: the PURE study. Lancet. 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3 [DOI] [PubMed] [Google Scholar]
- 3. Ekelund U, Tarp J, Steene‐Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, et al. Dose‐response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta‐analysis. BMJ. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose‐response relationship. JAMA Intern Med. 2015;175:959–967. doi: 10.1001/jamainternmed.2015.0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bull FC, Al‐Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput J‐P, Chastin S, Chou R, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayes BH, Haberling DL, Kennedy JL, Varma JK, Fry AM, Vora NM. Burden of pneumonia‐associated hospitalizations: United States, 2001–2014. Chest. 2018;153:427–437. doi: 10.1016/j.chest.2017.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GBD . 2017 Causes of Death Collaborators. Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeong SW, Kim SH, Kang SH, Kim HJ, Yoon CH, Youn TJ, Chae IH. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 2019;40:3547–3555. doi: 10.1093/eurheartj/ehz564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neuman MI, Willett WC, Curhan GC. Physical activity and the risk of community‐acquired pneumonia in US women. Am J Med. 2010;123:281.e7–281.e11. doi: 10.1016/j.amjmed.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams PT. Dose‐response relationship between exercise and respiratory disease mortality. Med Sci Sports Exerc. 2014;46:711–717. doi: 10.1249/MSS.0000000000000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ukawa S, Zhao W, Yatsuya H, Yamagishi K, Tanabe N, Iso H, Tamakoshi A. Associations of daily walking time with pneumonia mortality among elderly individuals with or without a medical history of myocardial infarction or stroke: findings from the Japan Collaborative Cohort Study. J Epidemiol. 2019;29:233–237. doi: 10.2188/jea.JE20170341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao M, Veeranki SP, Magnussen CG, Xi B. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ. 2020;370:m2031. doi: 10.1136/bmj.m2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siu E, Campitelli MA, Kwong JC. Physical activity and influenza‐coded outpatient visits, a population‐based cohort study. PLoS One. 2012;7:e39518. doi: 10.1371/journal.pone.0039518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ekblom B, Ekblom O, Malm C. Infectious episodes before and after a marathon race. Scand J Med Sci Sports. 2006;16:287–293. doi: 10.1111/j.1600-0838.2005.00490.x [DOI] [PubMed] [Google Scholar]
- 17. Martin SA, Pence BD, Woods JA. Exercise and respiratory tract viral infections. Exerc Sport Sci Rev. 2009;37:157–164. doi: 10.1097/JES.0b013e3181b7b57b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laddu DR, Lavie CJ, Phillips SA, Arena R. Physical activity for immunity protection: inoculating populations with healthy living medicine in preparation for the next pandemic. Prog Cardiovasc Dis. 2021;64:102–104. doi: 10.1016/j.pcad.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seong SC, Kim Y‐Y, Park SK, Khang YH, Kim HC, Park JH, Kang H‐J, Do C‐H, Song J‐S, Lee E‐J, et al. Cohort profile: the National Health Insurance Service‐National Health Screening Cohort (NHIS‐HEALS) in Korea. BMJ Open. 2017;7:e016640. doi: 10.1136/bmjopen-2017-016640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417 [DOI] [PubMed] [Google Scholar]
- 21. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 22. Jeong HG, Kim DY, Kang DW, Kim BJ, Kim CK, Kim Y, Yang W, Park ES, Lee SH. Physical activity frequency and the risk of stroke: a Nationwide Cohort Study in Korea. J Am Heart Assoc. 2017;6:e005671. doi: 10.1161/JAHA.117.005671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jung MH, Yi SW, An SJ, Balkau B, Yi JJ, Kim H. Complex interaction of fasting glucose, body mass index, age and sex on all‐cause mortality: a cohort study in 15 million Korean adults. Diabetologia. 2020;63:1616–1625. doi: 10.1007/s00125-020-05160-1 [DOI] [PubMed] [Google Scholar]
- 24. Jung MH, Yi SW, An SJ, Yi JJ. Age‐specific associations between systolic blood pressure and cardiovascular mortality. Heart. 2019;105:1070–1077. doi: 10.1136/heartjnl-2019-314697 [DOI] [PubMed] [Google Scholar]
- 25. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet J‐P, Corrado D, Drezner JA, Halle M, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605 [DOI] [PubMed] [Google Scholar]
- 26. Sallis R, Young DR, Tartof SY, Sallis JF, Sall J, Li Q, Smith GN, Cohen DA. Physical inactivity is associated with a higher risk for severe COVID‐19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021;55:1099–1105. doi: 10.1136/bjsports-2021-104080 [DOI] [PubMed] [Google Scholar]
- 27. Kusaka Y, Kondou H, Morimoto K. Healthy lifestyles are associated with higher natural killer cell activity. Prev Med. 1992;21:602–615. doi: 10.1016/0091-7435(92)90068-s [DOI] [PubMed] [Google Scholar]
- 28. Spielmann G, McFarlin BK, O'Connor DP, Smith PJ, Pircher H, Simpson RJ. Aerobic fitness is associated with lower proportions of senescent blood T‐cells in man. Brain Behav Immun. 2011;25:1521–1529. doi: 10.1016/j.bbi.2011.07.226 [DOI] [PubMed] [Google Scholar]
- 29. Campbell JP, Turner JE. Debunking the myth of exercise‐induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gielen S, Adams V, Möbius‐Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti‐inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9 [DOI] [PubMed] [Google Scholar]
- 31. Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac‐specific overexpression of tumor necrosis factor‐alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102:1690–1696. doi: 10.1161/01.cir.102.14.1690 [DOI] [PubMed] [Google Scholar]
- 33. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population‐based surveys with 1.9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 34. Park EC, Jang SI, Cheon SY, Lee SA, Lee JE, Choi DW. Enhancement and Assessment of the Agreement Between Medical Records and Disease Codes of National Health Insurance Claim Data: the Final Report [in Korean]. Health Insurance Review & Assessment Service; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6


