Abstract
Background
To investigate the effectiveness and safety of withholding or restarting antithrombotic agents, and different antithrombotic therapies among patients with atrial fibrillation post‐intracranial hemorrhage.
Methods and Results
This is a nationwide retrospective cohort study involving patients with atrial fibrillation receiving antithrombotic therapies who subsequently developed intracranial hemorrhage between January 1, 2011 and December 31, 2017. The risk of ischemic stroke (IS), recurrent intracerebral hemorrhage (ICH), and all‐cause mortality were investigated between patients receiving no treatment versus patients reinitiating oral anticoagulants (OACs) or antiplatelet agents, and warfarin versus non‐vitamin K antagonist OACs. We applied inverse probability of treatment weighting to balance the baseline characteristics and Cox proportional hazards model to estimate the hazard ratios (HRs) of different outcomes of interest. Compared with no treatment, OACs reduced the risk of IS (HR, 0.61; 0.42–0.89), without increase in the risk of ICH (1.15, 0.66–2.02); antiplatelet agent users showed a similar risk of IS (1.13, 0.81–1.56) and increased risk of ICH (1.81, 1.07–3.04). Use of OACs or antiplatelet agents did not reduce the risk of all‐cause mortality (0.85, 0.72–1.01; and 0.88, 0.75–1.03, respectively). Compared with warfarin, non‐vitamin K antagonist OAC users showed a similar risk of IS (0.92, 0.50–1.70), non‐significantly reduced risk of ICH (0.53, 0.22–1.30), and significantly reduced all‐cause mortality (0.60, 0.43–0.84).
Conclusions
OACs are recommended in patients with atrial fibrillation and intracranial hemorrhage because they reduced the risk of IS with no increase in the risk of subsequent ICH. Non‐vitamin K antagonist OACs are recommended over warfarin owing to their survival benefits.
Keywords: antiplatelet therapy, antithrombotic therapy, atrial fibrillation, intracerebral hemorrhage, oral anticoagulant therapy
Subject Categories: Cardiovascular Disease, Quality and Outcomes, Anticoagulants
Nonstandard Abbreviations and Acronyms
- AT
antithrombotic therapy
- ICH
intracerebral hemorrhage
- NOAC
non‐vitamin K antagonist oral anticoagulant
- OAC
oral anticoagulant
Clinical Perspective
What Is New?
Among patients with history of intracranial hemorrhage, anticoagulant therapy reduces the risk of ischemic stroke, without increasing the risk of recurrent intracerebral hemorrhage compared with no treatment.
Among patients with history of intracranial hemorrhage, use of antiplatelet agents is associated with a similar risk of ischemic stroke but increases the risk of intracerebral hemorrhage compared with no treatment.
Non‐vitamin K antagonist oral anticoagulants show similar effectiveness and safety, but lower mortality as compared with warfarin.
What Are the Clinical Implications?
For patients with intracranial hemorrhage history, anticoagulant therapy is recommended over antiplatelet therapy.
Among anticoagulant therapy, non‐vitamin K antagonist oral anticoagulants should be the priority choice.
Patients with atrial fibrillation (AF) have an increased risk of thromboembolism, particularly those with a CHA2DS2‐VASc score ≥2; therefore, oral anticoagulant (OAC) therapy is recommended commonly according to different guidelines. 1 , 2 , 3 However, OAC therapy increases the risk of bleeding. Thus, after the occurrence of an intracranial hemorrhage episode, OAC agents should be discontinued immediately. 4 Reinitiating antithrombotic therapy (AT) after the episode is a difficult decision, which requires balancing the risk of re‐bleeding and occurrence of thromboembolism. 5 To date, there has been little research on whether or when to start AT and the drug of choice.
According to the report of RESTART (Restart or Stop Antithrombotics Randomised Trial) conducted in 2019, reinitiating antiplatelet therapy after an intracerebral hemorrhage (ICH) episode, compared with no treatment, did not increase the risk of ICH and major hemorrhagic events. 6 Nevertheless, <20% of participants in the RESTART trial had AF. 6 Several retrospective observational studies have investigated the effectiveness and safety of reinitiating AT among patients with AF and a history of intracranial hemorrhage. A Danish study showed that reinitiating anticoagulants was associated with a reduced risk of ischemic stroke (IS) and all‐cause mortality compared with no treatment. 7 Another Korean study showed that maintaining time in the therapeutic range of 60% among warfarin starters post‐intracranial hemorrhage, compared with no treatment, significantly reduced the risk of composite outcome of thromboembolic and major bleeding events. 8 Contrarily, a Taiwanese study showed that initiating warfarin, compared with no treatment, significantly reduced the risk of IS but increased the risk of intracranial hemorrhage. 9
Large‐scale, randomized, prospective studies have proven that non‐vitamin K antagonist oral anticoagulants (NOACs) are equivalently effective to warfarin in preventing thromboembolism among patients with AF; additionally, they significantly reduce the risk of intracranial hemorrhage. 10 , 11 , 12 , 13 However, patients with a history of intracranial hemorrhage have been universally excluded from these studies. 11 , 12 , 13 In addition, warfarin has been a major OAC evaluated in prior studies. 7 , 8 , 9 To date, data on the effectiveness and safety of reinitiating NOACs subsequent to an intracranial hemorrhage episode are lacking.
The aim of our study was to investigate the effectiveness and safety of withholding or reinitiating AT in patients with AF and a history of intracranial hemorrhage. In addition, we compared different treatment strategies: OAC therapy versus no treatment, antiplatelet therapy versus no treatment, OAC therapy versus antiplatelet therapy, and NOAC versus warfarin.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Health and Welfare Data Science Center, Ministry of Health and Welfare of Taiwan at stdlwu@mohw.gov.tw.
Data Source
The population‐level data source of the Health and Welfare Database in Taiwan was used for this study. The database contains administrative claims data from the National Health Insurance program and registration files. The National Health Insurance program was launched in 1995 and covers ≈99.9% of Taiwan’s population. 14 The population‐level data contain de‐identified information on beneficiaries’ enrollment, demographics, inpatient and outpatient service use, diagnosis, prescriptions, and cause of death. Data from January 1, 2011 to December 31, 2017 were used in this study.
Study Design and Participants
The study was a nationwide retrospective cohort study. We enrolled patients aged ≥20 years who had an intracranial hemorrhage event including ICH, subarachnoid hemorrhage, or other intracranial hemorrhage after being diagnosed for AF between January 1, 2011 and December 31, 2017. The patients who received at least 1 prescription of antithrombotic agent within 3 months before the intracranial hemorrhage event. Participants who did not receive an antithrombotic agent before intracranial hemorrhage were presumed to have the characteristics of not using antithrombotic agents, and these factors may affect the decision to reinitiate AT after ICH as well as risk outcomes. Antithrombotic agents have been categorized as NOACs (dabigatran, rivaroxaban, apixaban, and edoxaban), warfarin, and antiplatelet agents (aspirin, clopidogrel, prasugrel, and ticagrelor).
Participants were assigned to the following groups based on their exposure status on the index date: OAC agents (OAC users), antiplatelet agents (antiplatelet users), and no treatment with any antithrombotic agents (non‐AT users). The effectiveness and safety in OAC users versus non‐AT users, antiplatelet users versus non‐AT users, and OAC users versus antiplatelet users were compared. The index date was defined as 90 days from hospital discharge after an intracranial hemorrhage event. Moreover, we compared the effectiveness and safety in NOAC users versus warfarin users; the index date was defined as the first prescription of NOACs or warfarin after an intracranial hemorrhage event.
Despite absence of any current guideline on the optimal timing of resuming anticoagulation therapy after an ICH episode, 4 the period of 90 days was selected based on: (1) the clinical observation that most patients reinitiate AT within 3 months of discharge after an intracranial hemorrhage episode, and (2) the recommendation of at least 1‐month delay for post‐ICH anticoagulant therapy as the risk of stroke is minimized when OAC is reinitiated at 10 weeks after an ICH event. 4 , 15 This design also eliminates the potential time bias between AT users and non‐users. In addition, we excluded cases of IS or ICH occurring during the 90‐day period, because these events might be related to antithrombotic agent interruption or re‐bleeding of the prior intracranial hemorrhage. 16
Patients were excluded if they had either of the following conditions: valvular AF, structural brain disease, pregnancy, end‐stage renal disease requiring dialysis, or used OACs and antiplatelet agents concurrently on the index date. Valvular AF was defined as mitral stenosis or artificial heart valves. 17 Structural brain disease was defined as malformation of precerebral and cerebral vessels, dissection of cerebral arteries, cerebral aneurysm, or moyamoya disease. The International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD‐9‐CM, ICD‐10‐CM) codes for inclusion and exclusion criteria and the Anatomical Therapeutic Chemical (ATC) Classification codes for the study drugs are listed in Table S1 and S2.
Covariates
Data for baseline characteristics and concurrent medications were collected within the 6‐month pre‐index period. Information on the following comorbidities were collected: congestive heart failure, hypertension, diabetes, coronary artery disease, myocardial infarction, peripheral artery disease, IS, transient ischemic attack, peripheral arterial occlusive disease, venous thromboembolism, pulmonary embolism, gastrointestinal bleeding, other bleeding events, liver disease, and renal disease. Information on the following concurrent medications were collected: calcium channel blockers, renin‐angiotensin system inhibitors, 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors, non‐steroidal anti‐inflammatory drugs, class I and III anti‐arrhythmic drugs, β blockers, digoxin, and proton pump inhibitors. History of antithrombotic agent use was collected for the 3‐month period before admission for intracranial hemorrhage. A claims‐based stroke severity index was used to define intracranial hemorrhage severity, which included the predictors for disease severity such as existence of an invasive catheter, length of hospital stay, and specific treatment procedure or drug. The stroke severity index was calculated based on billing codes and was significantly correlated with the National Health Insurance (NHI) Stroke Scale (r=0.73; 95% CI, 0.71–0.76). 18 The ICD‐9‐CM and ICD‐10‐CMcodes for comorbidities, the NHI codes for stroke severity index, and the ATC codes for concurrent medications are listed in Table S2 through S4.
Study Outcomes
The primary outcomes were IS, ICH, and all‐cause mortality. The secondary outcomes were major bleeding and any thromboembolic event. For all participants, the duration of follow‐up was from the index date to the condition which occurred first among the following: outcomes of interest, death, change of exposure status, or the end of the study (December 31, 2017). For participants who reinitiated AT, change in exposure status was defined as a gap ≥42 days between the end date of the last prescription and the date to refill the next prescription. Patients switching between warfarin and NOACs were not censored, except for a comparison of NOACs versus warfarin. For participants who did not reinitiate AT after an intracranial hemorrhage episode, change in exposure status was defined as the initiation of any antithrombotic agent after the index date. The ICD‐9‐CM and ICD‐10‐CM codes for the study outcomes are listed in Table S3.
Statistical Analysis
Inverse probability of treatment weighting (IPTW) was used to balance baseline characteristics between the study groups. Individuals were weighted based on the propensity score to create a pseudo‐population in which the distribution of measured baseline covariates was independent of the treatment assignment. Imbalance of baseline characteristics after weighting was determined by absolute standardized differences. An absolute standardized difference >0.1 indicated a meaningful imbalance in baseline characteristics. 19 No weight trimming or weight truncation was conducted, and there were no extreme weights in the main analysis. The propensity score distribution and weights of different exposure groups are presented in Table S5 and Figure S1. IPTW‐adjusted hazard ratios (HRs) using the Cox proportional hazards model were used to evaluate the relationship between AT exposure and outcomes of interest. Statistical significance was defined as a 2‐sided P value <0.05. All statistical procedures were performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). The study protocol was approved by the Research Ethics Committee B of the National Taiwan University Hospital (NTUH‐REC No. 202002072W), and written informed consent was not required for this retrospective observational investigation based on the de‐identified information in the insurance database.
Sensitivity Analyses
We performed 4 sensitivity analyses. First, for all analyses, we capped the follow‐up time to 2 years to reduce the potential bias caused by different lengths of follow‐up duration. Second, we ensured follow‐up for at least 3 months for each patient. Third, instead of using the first prescription as the index date, we re‐assessed the effectiveness and safety of NOACs versus warfarin within the 90‐day period. Finally, we trimmed the highest and lowest 2.5% of the values and performed all analyses.
Results
Between January 1, 2011 and December 31, 2017, there were 5007 participants with AF who subsequently developed intracranial hemorrhage. Among them, 1369 participants died before discharge, and 22 were admitted to the hospital at the end of the study. A total of 1899 among 3616 (52.52%) participants reinitiated AT after discharge. Among them, antiplatelet monotherapy was the most commonly used alternative as antithrombotic agents (49.34%), followed by NOAC (25.75%) therapy. The median time from discharge to reinitiate AT was 48 days (interquartile range, 12–163 days) for antithrombotic agents, 42 days (interquartile range, 10–127 days) for OACs, and 56 days (interquartile range, 14–204 days) for antiplatelet agents. The study enrollment process is shown in Figure 1.
Figure 1. Sample selection flowchart.
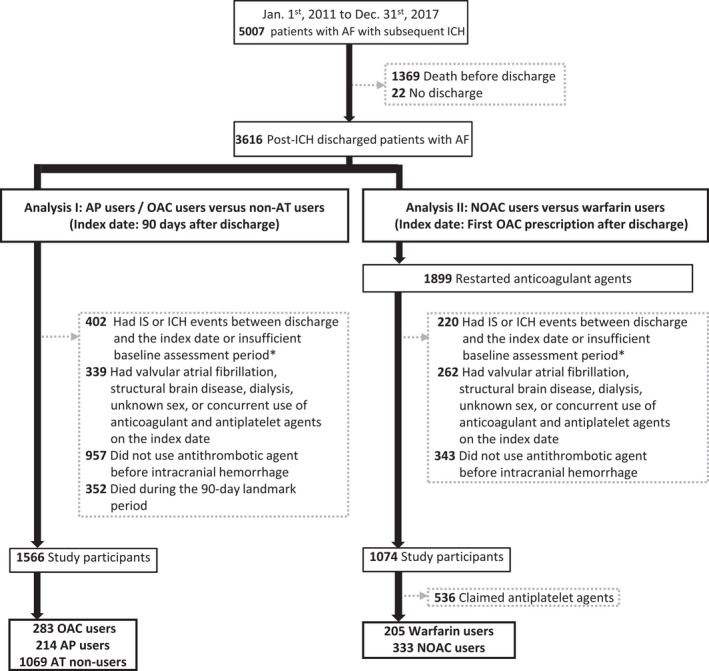
Patients were required to have at least a 6‐month observation period before the index date for baseline characteristic assessment. AF indicates atrial fibrillation; AP, antiplatelet agents; AT, antithrombotic agents; ICH, intracranial hemorrhage; IS, ischemic stroke; NOAC, non‐vitamin K antagonist oral anticoagulants; and OAC, oral anticoagulants.
OACs Versus No AT After Intracranial Hemorrhage Event
Among all, a total of 283 participants reinitiated oral OACs (OAC users), whereas 1069 did not reinitiate antithrombotic agents (AT non‐users) 90 days after discharge from hospital stay because of intracranial hemorrhage. The baseline characteristics of the participants in different groups before and after IPTW adjustment are listed in Table S6. The median follow‐up duration was 0.7 years for OAC users and 0.5 years for AT non‐users. IS occurred in 10 OAC users (3.5%) and 52 AT non‐users (4.9%). The IPTW‐adjusted HR for IS between OAC users and AT non‐users was 0.61 (95% CI, 0.42–0.89). ICH occurred in 4 OAC users (1.4%) and 17 AT non‐users (1.6%). The adjusted HR for OAC users to AT non‐users was 1.15 (95% CI, 0.66–2.02). OAC users had a significantly lower risk of thromboembolic events than that of AT non‐users (HR, 0.60; 95% CI, 0.43–0.84), but had a similar risk of major bleeding (HR, 1.40; 95% Cl, 0.99–1.98) and all‐cause mortality (HR, 0.85; 95% Cl, 0.72–1.01). The results are shown in Table 1, and the survival curves for ICH and IS are shown in Figure 2.
Table 1.
Event Rate of Different Outcomes According to Treatment Stratification
| Outcome |
No treatment (n=1069) |
Oral anticoagulants (n=283) |
Adjusted HR* |
Antiplatelet therapy (n=214) |
Adjusted HR † |
|---|---|---|---|---|---|
| Ischemic stroke | 0.61 (0.42–0.89) | 1.13 (0.81–1.56) | |||
| No. of events | 52 | 10 | 15 | ||
| Person‐y | 1068.81 | 327.18 | 271.16 | ||
| Event rate (95% CI) | 48.69 (37.10–63.89) | 30.56 (16.45–56.81) | 55.32 (33.35–91.76) | ||
| Thromboembolism | 0.60 (0.43–0.84) | 1.11 (0.84–1.48) | |||
| No. of events | 68 | 12 | 18 | ||
| Person‐y | 1065.08 | 325.29 | 263.12 | ||
| Event rate (95% CI) | 63.84 (50.34–80.97) | 36.89 (20.95–64.96) | 68.41 (43.10–108.58) | ||
| Intracerebral hemorrhage | 1.15 (0.66–2.02) | 1.81 (1.07–3.04) | |||
| No. of events | 17 | 4 | 7 | ||
| Person‐y | 1066.18 | 335.15 | 274.24 | ||
| Event rate (95% CI) | 15.94 (9.91–25.65) | 11.94 (4.48–31.80) | 25.52 (12.17–53.54) | ||
| Major bleeding event | 1.40 (0.99–1.98) | 1.10 (0.77–1.58) | |||
| No. of events | 42 | 11 | 11 | ||
| Person‐y | 1051.00 | 301.22 | 271.86 | ||
| Event rate (95% CI) | 39.96 (29.53–54.07) | 33.50 (18.55–60.50) | 40.46 (22.41–73.06) | ||
| All‐cause mortality | 0.85 (0.72–1.01) | 0.88 (0.75–1.03) | |||
| No. of events | 235 | 48 | 45 | ||
| Person‐y | 1087.31 | 336.22 | 278.89 | ||
| Event rate (95% CI) | 216.13 (190.19–245.61) | 142.77 (107.59–189.45) | 161.35 (120.47–216.11) |
HR indicates hazard ratio.
Hazard ratio between oral anticoagulants and no treatment.
Hazard ratio between antiplatelet therapy and no treatment.
Figure 2. Survival curves of ischemic stroke and intracerebral hemorrhage according to the type of antithrombotic therapy.
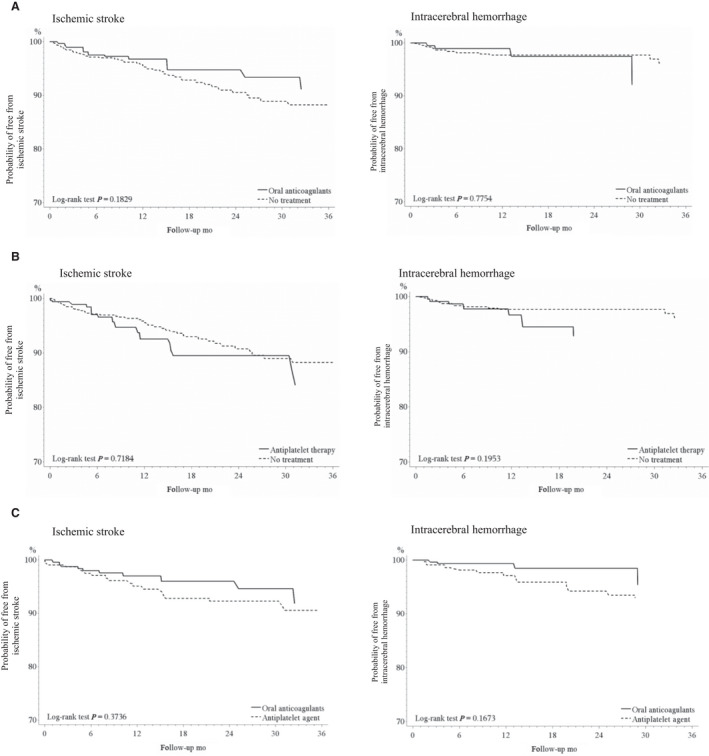
A, Oral anticoagulants versus no treatment; B, Antiplatelet therapy versus no treatment; C, Oral anticoagulants versus antiplatelet therapy. NOACs indicates non‐vitamin K antagonist oral anticoagulants.
Antiplatelet Agents Versus No AT After Intracranial Hemorrhage Event
A total of 214 participants reinitiated antiplatelet agents (antiplatelet users) 90 days after discharge from hospital stay because of intracranial hemorrhage. The baseline characteristics of the participants in different groups before and after IPTW adjustment are listed in Table S7. The median follow‐up duration was 0.9 years for antiplatelet users and 0.5 for AT non‐users. IS occurred in 15 antiplatelet users (7.0%) and 52 AT non‐users (4.9%). The HR for antiplatelet users to AT non‐users was 1.13 (95% CI, 0.81–1.56). ICH occurred in 7 antiplatelet users (3.3%) and 17 AT non‐users (1.6%). The HR for antiplatelet users to AT non‐users was 1.81 (95% Cl, 1.07–3.04). Compared with AT non‐users (n=1069), antiplatelet users had a similar risk of thromboembolic events (HR, 1.11; 95% CI, 0.84–1.48), major bleeding (HR, 1.10; 95% Cl, 0.77–1.58), and all‐cause mortality (HR, 0.88; 95% Cl, 0.75–1.03), as shown in Table 1 and Figure 2.
OACs Versus Antiplatelet Agents After Intracranial Hemorrhage Event
The baseline characteristics of 283 OAC users and 214 antiplatelet users before and after IPTW adjustment are presented in Table S8. The median follow‐up duration was 0.7 years for OAC users and 0.9 for antiplatelet users. IS occurred in 10 OAC users (3.5%) and 19 antiplatelet users (8.9%). The HR for OAC users to antiplatelet users was 0.66 (95% CI, 0.37–1.19). ICH occurred in 4 OAC users (1.4%) and 11 antiplatelet users (5.1%). The HR for OAC users to antiplatelet users was 0.42 (95% Cl, 0.18–0.99). OAC users had a lower risk of thromboembolic events than that of antiplatelet users (HR, 0.54; 95% CI, 0.32–0.91), and similar risk of major bleeding (HR, 0.97; 95% Cl, 0.57–1.64) and all‐cause mortality (HR, 0.96; 95% Cl, 0.73–1.25), as shown in Table 2. The survival curves for ICH and IS are shown in Figure 2.
Table 2.
Event Rate of Different Outcomes in Oral Anticoagulant Users and Antiplatelet Agent Users
| Outcome |
Oral anticoagulants (n=283) |
Antiplatelet therapy (n=214) |
Adjusted HR |
|---|---|---|---|
| Ischemic stroke | 0.66 (0.37–1.19) | ||
| No. of events | 10 | 19 | |
| Person‐y | 327.18 | 510.83 | |
| Event rate (95% CI) | 30.56 (16.45–56.81) | 37.19 (23.72–58.31) | |
| Thromboembolism | 0.54 (0.32–0.91) | ||
| No. of events | 12 | 25 | |
| Person‐y | 325.29 | 493.60 | |
| Event rate (95% CI) | 36.89 (20.95–64.96) | 50.65 (34.22–74.96) | |
| Intracerebral hemorrhage | 0.42 (0.18–0.99) | ||
| No. of events | 4 | 11 | |
| Person‐y | 335.15 | 532.05 | |
| Event rate (95% CI) | 11.94 (4.48–31.80) | 20.67 (11.45–37.33) | |
| Major bleeding event | 0.97 (0.57–1.64) | ||
| No. of events | 11 | 19 | |
| Person‐y | 328.33 | 526.19 | |
| Event rate (95% CI) | 33.50 (18.55–60.50) | 36.11 (23.03–56.61) | |
| All‐cause mortality | 0.96 (0.73–1.25) | ||
| No. of events | 48 | 76 | |
| Person‐y | 336.22 | 540.83 | |
| Event rate (95% CI) | 142.77 (107.59–189.45) | 140.52 (112.23–175.95) |
HR indicates hazard ratio.
NOACs Versus Warfarin Therapy After Intracranial Hemorrhage Event
The results of the effectiveness and safety of NOACs versus warfarin after ICH are shown in Table 3. After discharge from hospital stay because of intracranial hemorrhage, 333 patients received NOACs, and 205 patients received warfarin. The baseline characteristics of the participants in different groups before and after IPTW adjustment are listed in Table S9. The median follow‐up duration was 0.5 years for both NOAC and warfarin users. IS occurred in 12 NOAC users (3.6%) and 9 warfarin users (4.4%). The adjusted HR for NOAC users to warfarin users was 0.92 (95% CI, 0.50–1.70). ICH occurred in 5 NOAC users (1.5%) and 6 warfarin users (2.9%). The adjusted HR for NOAC users to warfarin users was 0.53 (95% Cl, 0.22–1.30). Compared with warfarin users, NOAC users had a similar risk of thromboembolic events (HR, 0.81; 95% CI, 0.46–1.44), and a significantly low risk of major bleeding (HR, 0.36; 95% Cl, 0.22–0.60) and all‐cause mortality (HR, 0.60; 95% Cl, 0.43–0.84). No further comparisons were made between individual NOAC agents and warfarin because of small sample sizes (apixaban, n=68; dabigatran, n=104; edoxaban, n=10; and rivaroxaban, n=151).
Table 3.
Event Rate of Different Outcomes in NOAC and Warfarin Users
| Outcome |
NOACs (n=333) |
Warfarin (n=205) |
Adjusted HR |
|---|---|---|---|
| Ischemic stroke | 0.92 (0.50–1.70) | ||
| No. of events | 12 | 9 | |
| Person‐y | 275.13 | 196.82 | |
| Event rate (95% CI) | 53.42 (24.66–76.46) | 45.73 (23.79–87.88) | |
| Thromboembolism | 0.81 (0.46–1.44) | ||
| No. of events | 13 | 11 | |
| Person‐y | 275.10 | 196.19 | |
| Event rate (95% CI) | 47.22 (27.42–81.32) | 56.07 (31.05–101.24) | |
| Intracerebral hemorrhage | 0.53 (0.22–1.30) | ||
| No. of events | 5 | 6 | |
| Person‐y | 284.42 | 196.53 | |
| Event rate (95% CI) | 17.58 (7.32–42.24) | 30.53 (13.72–67.96) | |
| Major bleeding event | 0.36 (0.22–0.60) | ||
| No. of events | 13 | 18 | |
| Person‐y | 281.96 | 187.84 | |
| Event rate (95% CI) | 46.11 (26.77–79.40) | 95.83 (60.38–152.10) | |
| All‐cause mortality | 0.60 (0.43–0.84) | ||
| No. of events | 34 | 39 | |
| Person‐y | 285.20 | 198.28 | |
| Event rate (95% CI) | 119.22 (85.18–166.84) | 196.69 (143.77–269.21) |
HR indicates hazard ratio; and NOACs, non‐vitamin K antagonist oral anticoagulants.
Sensitivity Analyses
The results of the sensitivity analyses were consistent with those of the main analyses. Capping the follow‐up duration at 2 years did not substantially affect the study results (Table S10 and S11). The requirement of at least 3 months of follow‐up yielded similar results to those in the main analyses (Table S12 and S13), but a significant increase in risk of major bleeding events was observed in antiplatelet users compared with non‐AT users (HR, 1.56; 95% Cl, 1.01–2.41). Using a 90‐day period for comparison between NOACs and warfarin might have caused to move the point estimates away from the null, but the overall findings remained consistent with the main analyses (Table S14 and S15). Further, trimming the upper and lower 2.5% of the values did not significantly change the results (Table S16 and S17). Antiplatelet users showed higher risk of IS (HR, 1.44; 95% CI, 1.03–2.02) and thromboembolic events (HR, 1.37; 95% CI, 1.01–1.86) than in the group who received no treatment in this analysis, but this association was not statistically significant in prior analyses.
Discussion
Our study compared the effectiveness and safety of different treatment options after an episode of intracranial hemorrhage, including OACs, antiplatelet agents, and no treatment. Our analysis demonstrated that reinitiating OACs, compared with no treatment, significantly reduced the risk of IS and did not increase the risk of ICH. We further showed that OACs significantly reduced the risk of thromboembolic events and ICH compared with antiplatelet agents. Among anticoagulants, effectiveness of NOACs was similar in terms of IS prevention, but survival improved compared with warfarin. Reinitiating antiplatelet therapy did not reduce the risk of IS but increased the risk of ICH compared with no treatment.
ICH is one of the most common and serious adverse effects of OACs. However, patients with history of ICH during AT therapy were not included in large‐scale clinical trials comparing OAC to antiplatelet or to placebo in patients with non‐valvular AF. Physicians may be hesitant to reinitiate OACs in patients with a history of ICH, particularly in the Asian population that is more likely to suffer from OAC‐related ICH than non‐Asian populations. 20 Moreover, Asian race is a risk factor for recurrent ICH. 21 Other risk factors for recurrent ICH are multifocal including age, blood pressure, extent of OAC exposure, presence of multiple cerebral microbleeds, and hematoma size and location. 4 , 5 The risk of re‐bleeding after reinitiating OACs can be reduced by selecting patients carefully, controlling reversible risk factors such as blood pressure, and avoiding off‐label dosing of OACs. 6 Our data showed that patients with a history of intracranial hemorrhage still benefit from reinitiating OACs. Therefore, it is rational to reinitiate OAC in patients with intracranial hemorrhage and at a high risk of thromboembolism. Moreover, among anticoagulants, NOACs are preferred over warfarin because they reduce major bleeding events and improve survival.
Antiplatelet agents can be used as a treatment alternative for patients who are not eligible for reinitiating OAC therapy. The American Heart Association/American Stroke Association guidelines suggest that antiplatelet monotherapy may be considered in patients with a history of ICH event and having concern of bleeding. 4 The results from the RESTART trial showed that antiplatelet agents did not increase the risk of recurrent ICH. Moreover, antiplatelet agents significantly reduced major vascular events such as non‐fatal myocardial infarction and non‐fatal stroke compared with no treatment, although the risk of all major occlusive vascular events is similar. 6 Unlike RESTART, in our study, antiplatelet agents showed no benefit in preventing IS and increased risk in ICH, consistent with the findings of Chao et al. 9 These differences may be attributed to the characteristics of our participants who were patients with AF presenting worse effects of antiplatelet agents than OACs in preventing thromboembolism. 3 , 22 , 23 In addition, our results suggested superior effects of OACs to antiplatelet agents in reducing the risk of thromboembolic events and ICH. Considering no beneficial effects of antiplatelet therapy in IS prevention, which may be discouraging for patients with AF who subsequently developed intracranial hemorrhage, OACs should be considered for post‐intracranial hemorrhage.
Two recent studies investigated the risk and benefit of reinitiating NOACs in patients with AF and a history of intracranial hemorrhage. Both studies showed comparable effectiveness of NOAC in preventing IS to that of warfarin along with reducing the risk of intracranial bleeding. 24 , 25 These studies enrolled participants with strong indications for reinitiating OACs and high average CHA2DS2‐VASc score of 4.0 in the study by Lee et al. 24 (6% with a score of 0 to 1) and 5.5 in the study by Tsai et al. (none with a score of 0 to 1). 25 Our study also enrolled participants with a strong indication for OAC therapy (average CHA2DS2‐VASc score of 5.0, <1% of the participants with a score of 0 to 1). However, the study by Lee et al. enrolled patients with AF who were naïve to OAC therapy before occurrence of intracranial hemorrhage. 24 Thus, the results of that study mainly reflected the effectiveness and safety of OAC in patients with a high baseline risk of bleeding such as spontaneous intracranial hemorrhage. In the study by Tsai et al., the average interval between the diagnosis of AF and intracranial hemorrhage was 5.9 years, which was relatively remote. 25 In contrast to these 2 studies, we focused particularly on patients with AF and subsequent intracranial hemorrhage, and an exposure to AT before occurrence of bleeding events. Our study subjects represent a population that present clinical dilemma about whether to reinitiate AT after an intracranial hemorrhage event. The results of this study provide further evidence on the effectiveness and safety of resuming AT (including OACs and antiplatelet agents) in patients who potentially have AT‐related intracranial hemorrhage.
The optimal time to reinitiate OACs after an ICH event remains unclear. The 2015 American Heart Association/American Stroke Association guidelines on spontaneous ICH recommend reinitiating OACs for at least 4 weeks after the onset of ICH in patients with no mechanical heart valve. 4 Our data demonstrated the median time to start OACs as ≈6 weeks, which is even more conservative than the recommended time. Data on patients with mechanical valves are lacking. An observational study in Germany showed that reinitiating therapeutic anticoagulants among patients with mechanical valve within 2 weeks after an ICH onset increased the risk of re‐bleeding and should be discouraged. 26 Collectively, decision to reinitiate OACs in patients with AF and a history of ICH is difficult. In addition to medical therapy, left atrial appendage occluder implantation can be a treatment alternative according to a recent study that has shown its comparable risk of bleeding as NOACs in patients with a high risk of stroke and bleeding. 27
Our study has the following limitations. First, this is a retrospective study and has the potential for unmeasurable residual confounding bias. Therefore, we used IPTW to balance between‐group differences and applied the 90‐day period to avoid time bias and carryover effect from the previous intracranial hemorrhage episode. In addition, we excluded participants who did not receive an AT agent before the occurrence of intracranial hemorrhage because these patients were presumed to be frail and tended to avoid AT agents after the event. Second, we were unable to determine the optimal time to reinitiate AT post‐intracranial hemorrhage owing to the retrospective design of the study. The time to initiate AT after intracranial hemorrhage is mainly driven by patient characteristics. A prospective study with randomization in the timing of reinitiating therapy is necessary to answer this question. Third, our study was conducted in an Asian population. Since Asian race is a risk factor for ICH and recurrent ICH, 21 , 28 the results of this study may not be generalizable to other ethnic groups. Nevertheless, our results suggest that OAC remains a safe treatment alternative post‐intracranial hemorrhage, even in the Asian population. Therefore, it is plausible that OAC is a safe choice in the non‐Asian population. Finally, we did not check the CHA2DS2‐VASc score for study enrollment. The use of OACs in patients with a score of 0 to 1 is not recommended commonly according to different guidelines. 3 , 29 Nevertheless, the proportion of participants with low CHA2DS2‐VASc was low. Our study represents a population with a strong indication for reinitiating OAC therapy after the occurrence of intracranial hemorrhage.
In conclusion, our study demonstrated that reinitiating OACs after the occurrence of intracranial hemorrhage reduces the risk of IS without affecting the risk of recurrent ICH. NOACs are recommended over warfarin owing to the benefit of survival. Antiplatelet agents have not shown any benefit in preventing IS, moreover, they increase the risk of ICH, and thus may not be an appropriate treatment option.
Sources of Funding
This study was funded by research grant from the Ministry of Science and Technology in Taiwan (MOST 109‐2636‐B‐002‐002). The funder had no role in the research design or results interpretation.
Disclosures
None.
Supporting information
Tables S1–S17
Figure S1
Acknowledgments
We thank Ms. Ling‐Ya Huang from the School of Pharmacy, College of Medicine, National Taiwan University for assistance in data management and analysis.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Chiang C‐E, Wu T‐J, Ueng K‐C, Chao T‐F, Chang K‐C, Wang C‐C, Lin Y‐J, Yin W‐H, Kuo J‐Y, Lin W‐S, et al. 2016 guidelines of the Taiwan heart rhythm society and the Taiwan society of cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;115:893–952. doi: 10.1016/j.jfma.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 4. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 5. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, et al. The 2018 European Heart Rhythm association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
- 6. Al‐Shahi Salman R, Dennis MS, Sandercock P, Sudlow C, Wardlaw JM, Whiteley WN, Murray GD, Stephen J, Newby DE, Sprigg N, et al. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open‐label trial. Lancet. 2019;393:2613–2623. doi: 10.1016/S0140-6736(19)30840-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen PB, Larsen TB, Skjøth F, Gorst‐Rasmussen A, Rasmussen LH, Lip GYH. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735 [DOI] [PubMed] [Google Scholar]
- 8. Park YA, Uhm JS, Pak HN, Lee MH, Joung B. Anticoagulation therapy in atrial fibrillation after intracranial hemorrhage. Heart Rhythm. 2016;13:1794–1802. doi: 10.1016/j.hrthm.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 9. Chao T‐F, Liu C‐J, Liao J‐N, Wang K‐L, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, Tuan T‐C, Chung F‐P, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133:1540–1547. doi: 10.1161/CIRCULATIONAHA.115.019794 [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 11. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 12. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 13. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 14. National Health Insurance Administration, Ministry of Helath and Welfare, Taiwan (R.O.C.) . National Health Insurance Annual Report 2018‐2019. 2018.
- 15. Majeed A, Kim Y‐K, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke. 2010;41:2860–2866. doi: 10.1161/STROKEAHA.110.593087 [DOI] [PubMed] [Google Scholar]
- 16. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 17. Fauchier L, Philippart R, Clementy N, Bourguignon T, Angoulvant D, Ivanes F, Babuty D, Bernard A. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108:530–539. doi: 10.1016/j.acvd.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 18. Hung L‐C, Sung S‐F, Hsieh C‐Y, Hu Y‐H, Lin H‐J, Chen Y‐W, Yang Y‐K, Lin S‐J. Validation of a novel claims‐based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol. 2017;27:24–29. doi: 10.1016/j.je.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098 [DOI] [PubMed] [Google Scholar]
- 21. Leasure AC, King ZA, Torres‐Lopez V, Murthy SB, Kamel H, Shoamanesh A, Al‐Shahi Salman R, Rosand J, Ziai WC, Hanley DF, et al. Racial/ethnic disparities in the risk of intracerebral hemorrhage recurrence. Neurology. 2020;94:e314–e322. doi: 10.1212/WNL.0000000000008737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Connolly SJ, Eikelboom J, Joyner C, Diener H‐C, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432 [DOI] [PubMed] [Google Scholar]
- 23. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 24. Lee S‐R, Choi E‐K, Kwon S, Jung J‐H, Han K‐D, Cha M‐J, Oh S, Lip GYH. Oral anticoagulation in Asian patients with atrial fibrillation and a history of intracranial hemorrhage. Stroke. 2020;51:416–423. doi: 10.1161/STROKEAHA.119.028030 [DOI] [PubMed] [Google Scholar]
- 25. Tsai C‐T, Liao J‐N, Chiang C‐E, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, Tuan T‐C, Chung F‐P, Chao T‐F, et al. Association of ischemic stroke, major bleeding, and other adverse events with warfarin use vs non‐vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation with a history of intracranial hemorrhage. JAMA Network Open. 2020;3:e206424. doi: 10.1001/jamanetworkopen.2020.6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuramatsu JB, Sembill JA, Gerner ST, Sprügel MI, Hagen M, Roeder SS, Endres M, Haeusler KG, Sobesky J, Schurig J, et al. Management of therapeutic anticoagulation in patients with intracerebral haemorrhage and mechanical heart valves. Eur Heart J. 2018;39:1709–1723. doi: 10.1093/eurheartj/ehy056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, Poloczek M, Stasek J, Haman L, Branny M, et al. Left atrial appendage closure versus direct oral anticoagulants in high‐risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:3122–3135. doi: 10.1016/j.jacc.2020.04.067 [DOI] [PubMed] [Google Scholar]
- 28. An SJ, Kim TJ, Yoon B‐W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S17
Figure S1


