Abstract
Background
The prognostic value of early repolarization pattern (ERP) remains controversial. We aim to test the hypothesis that temporal changes in ERP are associated with increased risks for sudden cardiac death (SCD) and cardiovascular death.
Methods and Results
A total of 14 679 middle‐aged participants from the prospective, population‐based cohort were included in this analysis, with ERP status recorded at baseline and during 3 follow‐up visits in the ARIC (Atherosclerosis Risk in Communities) study. We related baseline ERP, time‐varying ERP, and temporal changes in ERP to cardiovascular outcomes. Cox models were used to estimate the hazard ratios (HRs) adjusted for possible confounding factors. With a median follow‐up of 22.5 years, there were 5033 deaths, 1239 cardiovascular deaths, and 571 SCDs. Time‐varying ERP was associated with increased risks of SCD (HR, 1.59 [95% CI, 1.25–2.02]), cardiovascular death (HR, 1.70 [95% CI, 1.44–2.00]), and death from any cause (HR, 1.16 [95% CI, 1.05–1.27]). Baseline ERP was also associated with 3 outcomes. Compared with those with consistently normal ECG findings, subjects with new‐onset ERP or consistent ERP experienced increased risks of developing SCD and cardiovascular death. The time‐varying ERP in women, White subjects, and anterior leads and J‐wave amplitudes ≥0.2 mV appeared to indicate poorer cardiovascular outcomes.
Conclusions
Our findings suggest that baseline ERP, time‐varying ERP, new‐onset ERP, and consistent ERP were independent predictors of SCD and cardiovascular death in the middle‐aged biracial population. Repeated measurements of the ERP might improve its use as a risk indicator for SCD.
Keywords: early repolarization, electrocardiography, epidemiology, J wave, sudden cardiac death
Subject Categories: Arrhythmias, Sudden Cardiac Death
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- ERP
early repolarization pattern
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
Baseline early repolarization pattern (ERP) and time‐varying ERP were associated with increased risks of sudden cardiac death, cardiovascular death, and death from any cause.
Regarding temporal changes in ERP, new‐onset ERP and consistent ERP were associated with increased risks of sudden cardiac death and cardiovascular death.
The time‐varying ERP in women, White subjects, and anterior leads and J‐wave amplitudes ≥0.2 mV indicate poorer cardiovascular outcomes.
What Are the Clinical Implications?
Repeated measurements of the ERP might improve its use as a risk indicator for sudden cardiac death.
Early repolarization pattern (ERP), a common finding characterized by elevation of the QRS‐ST junction (J wave) on a standard 12‐lead ECG relative to the T‐P baseline, has recently been reported to increase the vulnerability to ventricular fibrillation (VF) and sudden cardiac death (SCD). 1 , 2 , 3 Nevertheless, other studies have shown negative or neutral relationships between ERP and cardiovascular outcomes. 4 , 5 As a result, considerable confusion remains concerning the prognostic significance of ERP, its risk stratification, and whether additional evaluation or treatment is warranted.
ERP is a modifiable factor that may change over time in response to the interaction of genes and environmental factors and resting heart rate as well as clinical conditions and medical treatment. 6 Few studies have assessed the associations of changes in ERP over time with mortality. There are several case reports and case‐control studies reporting day‐to‐day variations of J‐wave amplitudes proximate to the VF episodes. 1 , 7 , 8 , 9 However, the association of changes in ERP over time with the risk of cardiovascular death has not been assessed in population studies.
Therefore, the aim of this study was to prospectively examine the association of changes occurring over years in ERP with cardiovascular outcomes in participants of the ARIC (Atherosclerosis Risk in Communities) study, a large, multicenter, biracial, community‐based cohort, with outcome surveillance spanning >2 decades.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design and Population
The design of the ARIC study has been described previously. 10 In brief, participants aged between 45 and 64 years were recruited from 4 communities across the United States (Washington County, MD; Forsyth County, NC; Jackson, MS; and suburban Minneapolis, MN). Participants underwent a standardized evaluation of cardiovascular risk factors and returned for 4 follow‐up visits (visit 1 in 1987–1989, visit 2 in 1990–1992, visit 3 in 1993–1995, and visit 4 in 1996–1998). Thus, the median time interval between visits for participants was a median of 3.0 years, with an interquartile range (IQR) of 2.9 to 4.0 years. In addition to visits to the research center, participants were continuously and actively monitored for major morbidity and mortality through semiannual telephone calls. The examination included a 12‐lead ECG, from which the data for ERP were derived. The study was approved by the institutional review boards at all participating institutions, and all participants provided written informed consent at enrollment. This research has been conducted using publicly available data sets. Deidentified data were used, and no additional ethical approval was required.
We obtained the cohort data sets from the National Institutes of Health BioLINCC (Biologic Specimen and Data Repository Information Coordinating Center). 11 , 12 For the present analysis, we excluded the following participants: (1) subjects for whom ECGs were missing or incomplete; (2) subjects with major ventricular conduction abnormalities (eg, complete left or right bundle branch blocks), pacemakers, Wolff‐Parkinson‐White syndrome, Brugada syndrome, or QRS duration ≥120 ms; (3) subjects with acute chest pain accompanied by ST‐segment elevation on ECG; and (4) the few ARIC participants with races other than Black race or White race.
ECG Measurement
Digital 12‐lead ECGs were obtained at baseline as described previously. 13 At the examination, a standard resting supine 12‐lead ECG using MAC PC ECG machines (Marquette Electronics, Milwaukee, WI) was obtained for each subject a minimum of 1 hour after any smoking or caffeine ingestion. Tracings were sent via a phone modem to be computer coded at the ARIC ECG Reading Center. All records with significant Minnesota Code findings as determined by the computer as well as a random sample of tracings were independently visually coded by 2 trained coders (blinded to outcome status). Final decisions about the discrepancies between the computer code and visual code were made by another senior coder.
According to a recently published consensus article, ERP is present if all of the following criteria are met 14 : (1) an end‐QRS notch or slur on the final 50% of the downslope of a prominent R wave, (2) J‐wave amplitudes ≥0.1 mV in ≥2 contiguous leads of the 12‐lead ECG, and (3) QRS duration is <120 ms. ERP with J wave was detected automatically using signal processing and functional data analysis techniques provided by Wang et al. 15 This method had a sensitivity of 100% and a specificity of 94% to detect the presence of a J wave. 15
Measurement of Temporal Changes in ERP
To evaluate temporal changes in ERP, we considered EPR status as a time‐varying exposure variable so that an individual could have a different value for different segments of observation time. The data from visits 1 to 4 were used, and therefore ERP status was updated up to 3 times after visit 1.
We also assessed temporal changes in ERP status from the preceding visit by comparing the ERP value at the last visit and the value from the preceding visit. 16 We defined the following binary categories: (1) subjects with no ERP at both measurements (normal–normal or consistently normal), (2) subjects with no ERP at the first measurement but presented with ERP at the second measurement (normal–ERP or new‐onset ERP), (3) subjects with ERP at the first measurement but with no ERP at the second measurement (ERP–normal or transient ERP), and (4) subjects with ERP for both visits (ERP–ERP or consistent ERP). According to our definition of temporal changes in ERP status, the binary categories were based on ERP status in the last 2 exams. For example, if ERP status on the first exam was normal, on a second exam showed ERP, on a third exam showed normal, and a fourth showed ERP, this subject would be labeled as new‐onset ERP.
Ascertainment of Outcomes
The primary study end point was physician‐adjudicated SCD. The methods for ascertainment of SCD events have been described previously. 13 To identify SCD, all fatal cardiovascular events in ARIC were reviewed by an independent panel of physicians. Pairs of physician adjudicators blinded to clinical data and follow‐up status independently assessed the death information using a standard coding form. In case of divergent results, the case was recoded by paired investigators. If further disagreement occurred, a preliminary decision was achieved by consensus. In the present study, SCD was defined as a sudden pulseless condition that was fatal (within 24 hours) and that was consistent with a ventricular tachyarrhythmia occurring in the absence of a known noncardiac condition as the proximate cause of the death.
The secondary end point was cardiovascular death because we hypothesized that the incidence of cardiovascular death should be increased if ERP was proarrhythmic, particularly in a cohort at high risk for atherosclerotic heart disease. In addition, we included an analysis of death from any cause to guard against differential misclassification of deaths. 17
Statistical Analysis
Characteristics of the participants at each visiting time point were compared across categories of ERP status. Baseline characteristics of participants are presented as means (SDs) for continuous variables and percentages for categorical variables. Tests for differences in means were assessed using unpaired t tests for continuous variables and χ2 tests for independence for categorical variables.
We related baseline ERP, time‐varying ERP, and temporal changes in ERP to several outcomes outlined previously. Follow‐up of baseline ERP and time‐varying ERP was defined as the time between the baseline visit until the outcome of interest, death, loss to follow‐up, or end of follow‐up, whichever occurred first. Follow‐up time of temporal changes in ERP started from the visit when the last ERP value was recorded. We used Cox’s proportional hazards models to obtain multivariate adjusted hazard ratios (HRs) for all study outcomes. The multivariable analysis adjusted for the established predictors of outcome. These included sex and race and time‐dependent variables such as age, hypertension, smoking status, diabetes, coronary heart disease or chronic heart failure, body mass index, blood pressure, total cholesterol, fasting blood glucose, heart rate, QTc duration, presence of left ventricular hypertrophy on ECG, and use of cardiac medications. Kaplan–Meier survival curves were plotted for different outcomes. Missing values on the covariates were imputed, and models were run for every imputation and then averaged using Rubin's rules. 18 To evaluate the incremental prognostic value of the addition of baseline ERP or time‐varying ERP to the basic model, we calculated the area under the receiver operating characteristic curve.
ERP status was stratified according to the amplitude of J wave (0.1–0.19 mV or ≥0.2 mV) and lead territories (inferior, lateral, or anterior). Subgroup analyses were also examined in the study participants across several prespecified clinical factors that might be associated with cardiovascular outcomes, and the P values for interaction were calculated in each subgroup. These risk factors included sex, race, age, hypertension, diabetes, dyslipidemia, smoking status, and use of cardiac medications. Bonferroni’s corrected α for the multiple comparison tests was set at 0.005 (0.05/10).
We used Stata version 15.0 for all analyses. A 2‐tailed P value <0.05 was considered to indicate statistical significance unless otherwise specified.
Results
Baseline Characteristics
The baseline characteristics of the study group at visits 1 to 4 are presented in Table 1. At visit 1, of the 14 679 subjects who met the inclusion criteria, the mean (SD) age at baseline was 54.3 (5.8) years, 6700 (45.6%) were men, and 10 932 (74.5%) were White subjects. A total of 5136 (35.0%) of the population at baseline had hypertension, 1778 (12.1%) had diabetes, and 3884 (26.5%) were current smokers. With regard to cardiac medications, 533 (3.6%) were taking calcium‐channel blockers, 244 (1.7%) were taking digitalis, 799 (5.4%) were taking β‐blockers, and 116 (0.8%) were taking antiarrhythmics.
Table 1.
Characteristics of subjects at Visits 1 to 4
| Characteristic | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| No. of subjects | 14 679 | 12 945 | 11 779 | 10 657 |
| Age, y, mean (SD) | 54.3 (5.8) | 57.1 (5.7) | 60.1 (5.7) | 62.9 (5.7) |
| Male subjects, n (%) | 6700 (45.6) | 5892 (45.5) | 5337 (45.3) | 4800 (45.0) |
| White race, n (%) | 10 932 (74.5) | 10 035 (77.5) | 9275 (78.7) | 8445 (79.2) |
| Education, n (%) | ||||
| <High school | 3500 (23.8) | 2777 (21.5) | 2380 (20.2) | 2035 (18.1) |
| High school/vocational school | 5995 (40.8) | 5406 (41.8) | 4922 (41.8) | 4504 (42.3) |
| College, graduate, or professional school | 5184 (35.3) | 4762 (36.8) | 4477 (38.0) | 4118 (38.6) |
| Smoking status, n (%) | ||||
| Never | 6027 (41.1) | 5124 (39.6) | 4822 (40.9) | 4366 (41.2) |
| Former | 4768 (32.5) | 4918 (38.0) | 4866 (41.3) | 4633 (43.8) |
| Current | 3884 (26.5) | 2903 (22.4) | 2091 (17.8) | 1589 (15.0) |
| Hypertension, n (%) | 5136 (35.0) | 4553 (35.2) | 4771 (40.5) | 5034 (47.2) |
| Diabetes, n (%) | 1778 (12.1) | 1493 (11.5) | 1821 (15.5) | 1773 (16.6) |
| Coronary heart disease, n (%) | 736 (5.0) | 739 (5.7) | 845 (7.2) | 931 (8.7) |
| Body mass index, kg/m2, mean (SD) | 27.7 (5.4) | 27.9 (5.4) | 28.5 (5.5) | 28.8 (5.6) |
| Blood pressure, mm Hg, mean (SD) | ||||
| Systolic | 121.3 (19.1) | 121.4 (18.5) | 124.6 (19.1) | 127.6 (19.1) |
| Diastolic | 73.6 (11.4) | 72.0 (10.2) | 71.7 (10.5) | 70.9 (10.4) |
| Fasting glucose, mmol/L, mean (SD) | 6.1 (2.3) | 6.3 (2.4) | 6.2 (2.4) | 6.2 (2.1) |
| Total cholesterol, mmol/L, mean (SD) | 5.6 (1.1) | 5.4 (1.0) | 5.4 (1.0) | 5.2 (1.0) |
| Low‐density lipoprotein cholesterol, mmol/L, mean (SD) | 3.6 (1.0) | 3.5 (1.0) | 3.3 (0.9) | 3.2 (0.9) |
| High‐density lipoprotein cholesterol, mmol/L, mean (SD) | 1.3 (0.4) | 1.3 (0.4) | 1.3 (0.5) | 1.3 (0.4) |
| Triglycerides, mmol/L, mean (SD) | 1.5 (1.0) | 1.5 (1.0) | 1.6 (1.0) | 1.6 (1.0) |
| ECG findings | ||||
| Heart rate, bpm, mean (SD) | 66.3 (10.4) | 65.4 (10.1) | 65.3 (10.1) | 62.6 (10.2) |
| QTc duration, ms, mean (SD) | 416.4 (20.4) | 417.1 (19.8) | 420.0 (22.1) | 420.7 (21.6) |
| Left ventricular hypertrophy on ECG, n (%) | 332 (2.3) | 308 (2.4) | 303 (2.6) | 259 (2.4) |
| Early repolarization pattern, n (%) | 1922 (13.1) | 1460 (11.3) | 1289 (10.9) | 1203 (11.3) |
| Medication, n (%) | ||||
| Calcium antagonist | 533 (3.6) | 920 (7.1) | 1384 (11.8) | 1427 (13.4) |
| Digitalis | 244 (1.7) | 249 (1.9) | 285 (2.4) | 285 (2.7) |
| β‐blocker | 799 (5.4) | 758 (5.9) | 845 (7.2) | 983 (9.2) |
| Antiarrhythmics | 116 (0.8) | 86 (0.7) | 74 (0.6) | 77 (0.7) |
At the follow‐up visits, there were increases in body mass index; systolic blood pressure; QTc duration; prevalence of diabetes; hypertension; coronary heart disease; and the use of calcium antagonists, digitalis, and β‐blockers. There were declines in diastolic blood pressure, total cholesterol, low‐density lipoprotein cholesterol, resting heart rate, and the prevalence of current smoking.
Baseline ERP, Time‐Varying ERP, and Cardiovascular Outcomes
With a median follow‐up of 22.5 (IQR, 18.0–23.6) years from visit 1, a total of 5033 (34.3%) subjects died. Of these deaths, 1239 (8.4%) were from cardiovascular causes, and 571 (3.9%) were adjudicated as SCD. After adjusting for multiple cardiovascular risk factors, subjects with ERP at baseline had slightly increased risks for SCD (adjusted HR, 1.34; 95% CI, 1.08–1.68), cardiovascular death (adjusted HR, 1.39; 95% CI, 1.19–1.62), and death from any cause (adjusted HR, 1.10; 95% CI, 1.01–1.19) than did those without ERP (Table 2). The Kaplan–Meier curves for SCD, cardiovascular death, and death from any cause in subjects with baseline ERP are shown in Figure 1.
Table 2.
Unadjusted and Adjusted HRs of Death Associated With Baseline ERP Status and Time‐Varying ERP Status*
| Variable | Baseline ERP | P value | Time‐varying ERP | P value |
|---|---|---|---|---|
| Sudden cardiac death | ||||
| Unadjusted HR (95% CI) | 2.10 (1.72–2.55) | <0.001 | 2.52 (2.04–3.14) | <0.001 |
| Age‐ and sex‐adjusted HR (95% CI) | 1.76 (1.44–2.16) | <0.001 | 2.08 (1.66–2.60) | <0.001 |
| Multivariate adjusted HR (95% CI) | 1.34 (1.08–1.68) | 0.008 | 1.59 (1.25–2.02) | <0.001 |
| Cardiovascular death | ||||
| Unadjusted HR (95% CI) | 1.86 (1.62–2.13) | <0.001 | 2.40 (2.06–2.79) | <0.001 |
| Age‐ and sex‐adjusted HR (95% CI) | 1.64 (1.42–1.89) | <0.001 | 2.08 (1.78–2.43) | <0.001 |
| Multivariate adjusted HR (95% CI) | 1.39 (1.19–1.62) | <0.001 | 1.70 (1.44–2.00) | <0.001 |
| Death from any cause | ||||
| Unadjusted HR (95% CI) | 1.42 (1.32–1.53) | <0.001 | 1.53 (1.40–1.67) | <0.001 |
| Age and sex‐adjusted HR (95% CI) | 1.32 (1.22–1.42) | <0.001 | 1.38 (1.27–1.51) | <0.001 |
| Multivariate adjusted HR (95% CI) | 1.10 (1.01–1.19) | 0.03 | 1.16 (1.05–1.27) | 0.001 |
ERP indicates early repolarization pattern; and HR, hazard ratio.
Variables included in the multivariate analyses were age, race, sex, hypertension, smoking status, diabetes, history of coronary heart disease or chronic heart failure, body mass index, blood pressure, total cholesterol, fasting blood glucose, heart rate, QTc duration, presence of left ventricular hypertrophy on ECG, and use of cardiac medications.
Figure 1. Kaplan–Meier curves for (A) sudden cardiac death, (B) cardiovascular death, and (C) death from any cause in subjects with baseline ERP.
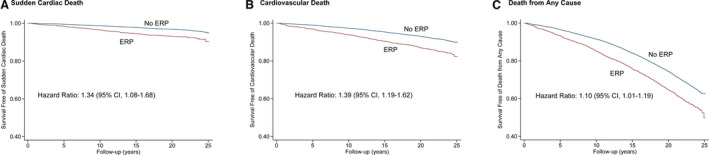
ERP indicates early repolarization pattern.
There was a tendency toward a decrease in the frequency of ERP from visit 1 to visit 4, ranging from 10.9% to 13.1%. However, the risk magnitude of the 3 outcomes associated with time‐varying ERP seems to be higher than the baseline ERP. Compared with subjects without time‐varying ERP, the adjusted HRs of time‐varying ERP for SCD was 1.59 (95% CI, 1.25–2.02), for cardiovascular death was 1.70 (95% CI, 1.44–2.00), and for death from any cause was 1.16 (95% CI, 1.05–1.27; Table 2). On receiver operating characteristic curve analysis, the model for SCD had a significantly higher predictive value when baseline ERP or time‐varying ERP was included (area under the receiver operating characteristic curve, 0.784 [95% CI, 0.763–0.805; P<0.001] for baseline ERP; area under the receiver operating characteristic curve, 0.791 [95% CI, 0.771–0.812; P<0.001] for time‐varying ERP) compared with when it was not (area under the receiver operating characteristic curve, 0.769; 95% CI, 0.747–0.791). Similar results were observed for cardiovascular death and death from any cause (Table 3 and Figure 2).
Table 3.
Incremental Prognostic Value of the Addition of Baseline ERP or Time‐Varying ERP to the Basic Model for Cardiovascular Outcomes
| Basic model* | Basic model+baseline ERP | Basic model+time‐varying ERP | |||
|---|---|---|---|---|---|
| ROC AUC (95% CI) | ROC AUC (95% CI) | P value † | ROC AUC (95% CI) | P value † | |
| Sudden cardiac death | 0.769 (0.747–0.791) | 0.784 (0.763–0.805) | <0.001 | 0.791 (0.771–0.812) | <0.001 |
| Cardiovascular death | 0.768 (0.764–0.793) | 0.779 (0.764–0.793) | <0.001 | 0.787 (0.772–0.802) | <0.001 |
| Death from any cause | 0.764 (0.755–0.773) | 0.769 (0.760–0.778) | <0.001 | 0.778 (0.769–0.787) | <0.001 |
ERP indicates early repolarization pattern; and ROC AUC, area under the receiver operating characteristic curve.
Variables included in the basic model were age, race, sex, hypertension, smoking status, diabetes, history of coronary heart disease or chronic heart failure, body mass index, blood pressure, total cholesterol, fasting blood glucose, heart rate, QTc duration, presence of left ventricular hypertrophy on ECG, and use of cardiac medications.
P value compared with basic model.
Figure 2. Comparison of area under the receiver operating characteristic curve (AUC) for (A) sudden cardiac death, (B) cardiovascular death, and (C) death from any cause between the full model without and with baseline ERP or time‐varying ERP.
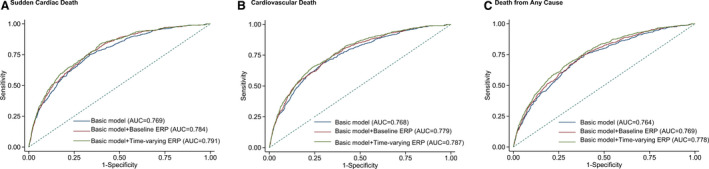
ERP indicates early repolarization pattern.
We performed sensitivity analyses by excluding subjects with a history of coronary heart disease or chronic heart failure, and the risk magnitude seemed to be decreased for ERP at baseline, which was only associated with a slightly increased risk of cardiovascular death (adjusted HR, 1.21; 95% CI, 1.01–1.45). However, for time‐varying ERP, the results were similar to the results obtained from the main analysis (adjusted HR, 1.42 [95% CI, 1.06–1.90] for SCD; adjusted HR, 1.55 [95% CI, 1.27–1.89] for cardiovascular death; Table S1).
Temporal Changes in ERP and Cardiovascular Outcomes
Baseline characteristics of subjects according to changes in ERP status are shown in Table S2. Of the 13 357 subjects with at least 2 measurements, 11 359 (85.0%) subjects had consistent normal ECG findings, 477 (3.6%) had transient ERP, 470 (3.5%) had new‐onset ERP, and 1051 (7.9%) had consistent ERP ECG findings in both measurements.
Compared with those with consistent normal ECG findings, subjects with new‐onset ERP experienced increased risks of developing SCD (adjusted HR, 1.61; 95% CI, 1.09–2.40) and cardiovascular death (adjusted HR, 1.67; 95% CI, 1.27–2.19), but new‐onset ERP did not seem to be associated with death from any cause. For those with transient ERP, the risks for the 3 outcomes were not significantly increased compared with the consistent normal group in the multivariable adjusted model. In addition, subjects with consistent ERP also had increased risks for SCD (adjusted HR, 1.50; 95% CI, 1.13–1.99), cardiovascular death (adjusted HR, 1.52; 95% CI, 1.24–1.86), and death from any cause (adjusted HR, 1.16; 95% CI, 1.04–1.29; Table 4). The Kaplan–Meier curves for cardiovascular outcomes associated with temporal changes in ERP are shown in Figure 3.
Table 4.
Unadjusted and Adjusted HR of Cardiovascular Outcomes Associated With Temporal Changes in ERP Status*
| Variable | Normal–normal (n=11 359) | ERP–normal (n=477) | P value | Normal–ERP (n=470) | P value | ERP–ERP (n=1051) | P value |
|---|---|---|---|---|---|---|---|
| Sudden cardiac death | |||||||
| No. of deaths | 340 | 17 | 28 | 74 | |||
| Unadjusted HR (95% CI) | 1.00 | 1.24 (0.76–2.01) | 0.40 | 2.11 (1.44–3.10) | <0.001 | 2.58 (2.00–3.31) | <0.001 |
| Age‐ and sex‐adjusted HR (95% CI) | 1.00 | 1.01 (0.62–1.65) | 0.97 | 1.74 (1.18–2.56) | 0.005 | 1.98 (1.18–2.56) | <0.001 |
| Multivariate adjusted HR (95% CI) | 1.00 | 0.91 (0.55–1.48) | 0.70 | 1.61 (1.09–2.40) | 0.02 | 1.50 (1.13–1.99) | 0.005 |
| Cardiovascular death | |||||||
| No. of deaths | 759 | 39 | 60 | 140 | |||
| Unadjusted HR (95% CI) | 1.00 | 1.26 (0.92–1.74) | 0.15 | 2.01 (1.55–2.62) | <0.001 | 2.21 (1.84–2.64) | <0.001 |
| Age‐ and sex‐adjusted HR (95% CI) | 1.00 | 1.08 (0.78–1.49) | 0.65 | 1.73 (1.33–2.26) | <0.001 | 1.79 (1.49–2.16) | <0.001 |
| Multivariate adjusted HR (95% CI) | 1.00 | 1.02 (0.74–1.42) | 0.89 | 1.67 (1.27–2.19) | <0.001 | 1.52 (1.24–1.86) | <0.001 |
| Death from any cause | |||||||
| No. of deaths | 3430 | 163 | 166 | 451 | |||
| Unadjusted HR (95% CI) | 1.00 | 1.18 (1.01–1.38) | 0.04 | 1.25 (1.07–1.46) | 0.005 | 1.59 (1.44–1.75) | <0.001 |
| Age‐ and sex‐adjusted HR (95% CI) | 1.00 | 1.04 (0.89–1.22) | 0.61 | 1.11 (0.95–1.30) | 0.19 | 1.35 (1.22–1.49) | <0.001 |
| Multivariate adjusted HR (95% CI) | 1.00 | 0.97 (0.82–1.14) | 0.70 | 1.09 (0.93–1.28) | 0.29 | 1.16 (1.04–1.29) | 0.006 |
ERP indicates early repolarization pattern; and HR, hazard ratio.
Variables included in the multivariate analyses were age, race, sex, hypertension, smoking status, diabetes, history of coronary heart disease or chronic heart failure, body mass index, blood pressure, total cholesterol, fasting blood glucose, heart rate, QTc duration, presence of left ventricular hypertrophy on ECG, and use of cardiac medications.
Figure 3. Kaplan–Meier curves for (A) sudden cardiac death, (B) cardiovascular death, and (C) death from any cause in subjects with temporal changes of ERP.
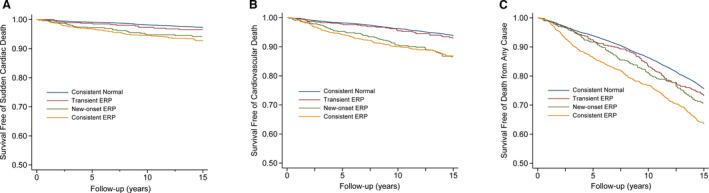
ERP indicates early repolarization pattern.
Assessment of Outcomes According to Time‐Varying ERP Phenotypes
Compared with subjects without time‐varying ERP, those with J‐wave amplitudes of 0.1 to 0.19 mV experienced nonsignificantly increased risks of SCD and all‐cause death, but a significantly increased risk of cardiovascular death. In addition, subjects with J‐wave amplitudes >0.2 mV had even higher risks for all 3 outcomes (adjusted HR, 1.62 [95% CI, 1.27–2.07] for SCD; adjusted HR, 3.08 [95% CI, 2.23–4.25] for cardiovascular death; adjusted HR, 1.73 [95% CI, 1.40–2.15] for death from any cause; Table 5).
Table 5.
HRs of Various ERP Phenotypes for Cardiovascular Outcomes in subjects With Time‐Varying ERP*
| Sudden cardiac death | Cardiovascular death | Death from any cause | ||||
|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| J‐wave amplitude | ||||||
| 0.1–0.19 mV | 1.32 (0.69–2.50) | 0.40 | 1.53 (1.28–1.83) | <0.001 | 1.10 (0.99–1.21) | 0.05 |
| ≥0.2 mV | 1.62 (1.27–2.07) | <0.001 | 3.08 (2.23–4.25) | <0.001 | 1.73 (1.40–2.15) | <0.001 |
| Lead distributions | ||||||
| Inferior | 2.08 (1.03–4.21) | 0.04 | 1.86 (1.09–3.16) | 0.02 | 1.40 (1.02–1.93) | 0.04 |
| Lateral | 1.00 (0.63–1.57) | 0.99 | 1.13 (0.83–1.54) | 0.44 | 1.07 (0.91–1.27) | 0.40 |
| Anterior | 1.58 (1.24–2.02) | <0.001 | 1.72 (1.45–2.03) | <0.001 | 1.14 (1.04–1.26) | 0.003 |
ERP indicates early repolarization pattern; and HR, hazard ratio.
Variables included in the multivariate analyses were age, race, sex, hypertension, smoking status, diabetes, history of coronary heart disease or chronic heart failure, body mass index, blood pressure, total cholesterol, fasting blood glucose, heart rate, QTc duration, presence of left ventricular hypertrophy on ECG, and use of cardiac medications.
With respect to lead distribution, time‐varying ERP in lateral leads or inferior leads was not significantly associated with increased risks for all 3 outcomes. However, time‐varying ERP in anterior leads seems to confer increased risks for SCD (adjusted HR, 1.58; 95% CI, 1.24–2.02), cardiovascular death (adjusted HR, 1.72; 95% CI, 1.45–2.03), and death from any cause (adjusted HR, 1.14; 95% CI, 1.04–1.26; Table 5).
We also assessed the ERP phenotypes for baseline ERP. Similarly, J‐wave amplitudes ≥0.2 mV seemed to confer higher risk of death than J‐wave amplitudes of 0.1 to 0.19 mV. However, only ERP in anterior leads was associated with increased risks of SCD and cardiovascular death (Table S3).
Stratified Analysis for Time‐Varying ERP
We performed stratified analyses across several prespecified clinical factors that might influence the outcomes (Table 6). The finding of increased risks of SCD and cardiovascular death in subjects with time‐varying ERP was consistently found in all of the stratified analyses. However, significant interactions were present for sex and race. Time‐varying ERP was associated with a greater risk of SCD in women (adjusted HR, 2.32; 95% CI, 1.46 to 3.70) than in men (adjusted HR, 1.45; 95% CI, 1.10 to 1.90; P=0.002 for interaction) and in White subjects (adjusted HR, 2.07; 95% CI, 1.52 to 2.80) than in Black subjects (adjusted HR, 1.21; 95% CI, 0.83 to 1.75; P<0.001 for interaction). subjects with dyslipidemia (adjusted HR, 1.86; 95% CI, 1.33 to 2.61) had a nonsignificantly higher risk of SCD than those without dyslipidemia (adjusted HR, 1.42; 95% CI, 1.01 to 2.00; P=0.03 for interaction). Of note, although use of cardiac medications was associated with a higher prevalence of ERP, it did not seem to influence the association between time‐varying ERP and SCD significantly (P=0.72 for interaction).
Table 6.
Stratified Analysis of HRs of Cardiovascular Outcomes Associated With Time‐Varying ERP*
| Variable | Sudden cardiac death | Cardiovascular death | Death from any cause | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value for interaction | HR (95% CI) | P value for interaction | HR (95% CI) | P value for interaction | |
| Sex | ||||||
| Male | 1.45 (1.10–1.90) | 0.002 | 1.53 (1.26–1.86) | 0.003 | 1.11 (1.00–1.24) | 0.15 |
| Female | 2.32 (1.46–3.70) | 2.33 (1.71–3.19) | 1.32 (1.10–1.58) | |||
| Race | ||||||
| White | 2.07 (1.52–2.80) | <0.001 | 1.84 (1.49–2.28) | 0.31 | 1.19 (1.05–1.34) | 0.95 |
| Black | 1.21 (0.83–1.75) | 1.51 (1.16–1.96) | 1.12 (0.97–1.30) | |||
| Age, y | ||||||
| ≥55 | 1.77 (1.31–2.37) | 0.44 | 1.78 (1.46–2.17) | 0.87 | 1.16 (1.04–1.30) | 0.60 |
| <55 | 1.28 (0.85–1.93) | 1.51(1.11–2.04) | 1.15 (0.98–1.36) | |||
| Hypertension | ||||||
| Yes | 1.60 (1.17–2.20) | 0.57 | 1.73 (1.39–2.14) | 0.95 | 1.26 (1.10–1.43) | 0.29 |
| No | 1.55 (1.07–2.25) | 1.65 (1.28–2.14) | 1.08 (0.95–1.24) | |||
| Diabetes | ||||||
| Yes | 2.00 (1.29–3.10) | 0.82 | 2.28 (1.68–3.10) | 0.33 | 1.49 (1.23–1.81) | 0.34 |
| No | 1.50 (1.12–2.00) | 1.57 (1.28–1.91) | 1.10 (0.99–1.22) | |||
| Dyslipidemia † | ||||||
| Yes | 1.86 (1.33–2.61) | 0.03 | 1.99 (1.57–2.51) | 0.05 | 1.32 (1.14–1.51) | 0.05 |
| No | 1.42 (1.01–2.00) | 1.47 (1.17–1.86) | 1.04 (0.92–1.18) | |||
| Smoking status | ||||||
| Never | 1.50 (0.93–2.44) | 0.87 | 1.74 (1.26–2.41) | 0.40 | 1.29 (1.07–1.55) | 0.46 |
| Former | 1.64 (1.09–2.47) | 1.87 (1.43–2.46) | 1.20 (1.02–1.40) | |||
| Current | 1.56 (1.07–2.28) | 1.54 (1.17–2.02) | 1.06 (0.91–1.23) | |||
| Use of cardiac medications | ||||||
| Yes | 1.52 (0.94–2.48) | 0.72 | 1.54 (1.12–2.13) | 0.39 | 1.28 (1.03–1.59) | 0.71 |
| No | 1.57 (1.19–2.07) | 1.74 (1.43–2.11) | 1.13 (1.02–1.25) | |||
ERP indicates early repolarization pattern; and HR, hazard ratio.
Variables included in the multivariate analyses were age, race, sex, hypertension, smoking status, diabetes, history of coronary heart disease or chronic heart failure, body mass index, blood pressure, total cholesterol, fasting blood glucose, heart rate, QTc duration, presence of left ventricular hypertrophy on ECG, and use of cardiac medications.
Dyslipidemia was defined as total cholesterol ≥5.7 mmol/L or low‐density lipoprotein cholesterol ≥3.6 mmol/L or high‐density lipoprotein cholesterol <1.0 mmol/L or triglycerides ≥1.7 mmol/L or pharmacological treatment for dyslipidemia, according to US National Institutes of Health guidelines for dyslipidemia.
Similarly, women with time‐varying ERP had a higher risk of cardiovascular death than men. No significant interaction effects were identified for age, history of hypertension, diabetes, or smoking status (Table 6).
Discussion
In a large, community‐based study comprising a biracial population, subjects with baseline ERP, time‐varying ERP, new‐onset ERP, and consistent ERP were at significantly increased risks of SCD and cardiovascular death. However, for those with transient ERP, the ERP seems to be a benign phenomenon. In those with time‐varying ERP, J‐wave amplitudes >0.2 mV appeared to indicate poor cardiovascular outcomes. A trend toward an increased risk associated with time‐varying ERP in White subjects and women was observed in the outcome of SCD.
In agreement with previous reports of a prevalence of ERP of 2.3% to 20.0%, the overall prevalence of ERP at baseline in our study was 13.1% (8.8% for White subjects, 25.7% for Black subjects). 2 , 5 Variation in the prevalence of ERP might be associated with disparity in trait definition, detection technique, and demographical characteristics of the study samples. For instance, previous reports describing the clinical correlates and heritability of ERP that used ST‐segment elevation as a criterion found a prevalence of only 2.3% in the general population. 5 However, other studies that used an approved definition of ERP proposed by experts, which did not include ST‐segment elevation as a required criterion, found that 23.9% to 29.3% of healthy control subjects manifested ERP, which compares with our finding of 13.1% in the general population. 19 , 20
The past few years have seen a rapidly growing interest in assessing the association between ERP and cardiovascular outcomes. In the largest cohort study by Tikkanen and colleagues, ERP in inferior leads was associated with modestly higher rates of cardiovascular death and arrhythmia death. 2 However, other studies reported no association between ERP and cardiovascular death. 4 , 5 In our previous analysis of the ARIC cohort, ERP at baseline was also associated with slightly increased risks of SCD and death from coronary heart disease. 21 Nevertheless, this study only used complete data with a relatively shorter follow‐up duration. Furthermore, the approach applied in the previous study was to assess the ERP status at 1 point only, which might not adequately address the nature of ERP.
To our knowledge, no previous population‐based study has assessed the temporal changes in ERP in relation to the cardiovascular outcomes in the general population. Our results indicated that time‐varying ERP was more strongly associated with cardiovascular outcomes than baseline ERP. However, the new‐onset ERP and consistent ERP recorded in the serial ECGs were the strongest predictors of SCD or cardiovascular death. Transient ERP seems to be an innocuous finding. As ERP is not uncommon in the general population, especially in young male subjects, the majority of subjects with ERP are likely not at risk for developing VF in the future. Our observations suggest that monitoring the J‐wave amplitude over time as a biomarker of health status may be useful in identifying individuals at greatest risk of SCD.
In previous cohort studies, ERP was considered to be a stable ECG finding rather than a transient phenomenon. However, in clinical practice, the phenotype of ERP can change over time, in accordance with the changes of vagal tone, heart rate, sport activities, reproductive hormones, and health conditions. 22 , 23 , 24 Therefore, ERP could be a permanent phenomenon for some subjects but a transient ECG pattern for others. 24 A large number of case reports noted that J‐wave amplitude was dramatically augmented only during peri‐event periods of VF, and the amplitude of J wave remote from the VF events did not differ from that seen in healthy controls, indicating that temporal elevation of the J wave might be an important risk factor for the prediction of malignant tachyarrhythmia. 7 , 8 , 9
Recent experimental evidence suggested that ERP might be caused by a transient ventricular transmural voltage gradient owing to a prominent Ito‐mediated action potential notch in the epicardium, potentially leading to phase 2 reentry and causing fatal arrhythmias. 25 Yet results of the study raise the following question: why would new‐onset ERP be associated with an increased risk for SCD? One possible explanation is that transient ERP is associated with younger age, lower heart rate, better cardiovascular fitness, and higher vagal tone, which generally protect against malignant ventricular arrhythmias. Elevation of vagal tone could cause regional dispersion of repolarization, which is manifest as ERP on ECG. This type of ERP tends to disappear with the reduction of vagal activity and increase in sympathetic tone. 26 In the Coronary Artery Risk Development in Young Adults Study, Walsh and colleagues 24 found that lower heart rate and longer exercise duration were associated with the presence of ERP, but they were not predictors of ERP maintenance over time.
Consistent ERP might be a phenotypic manifestation of heart disease or genetic susceptibility. Defects in 1 of the repolarization currents may not suffice to produce phase 2 reentry in these conditions. However, when other factors affecting repolarization are present, the repolarization reserve may become exhausted, and subjects with ERP could be at an increased risk for malignant arrhythmias. 26 The new‐onset ERP might be representative of acute conditions that could lead to dramatic repolarization changes, such as autonomic nervous system perturbances, acute ischemia, hypokalemia, drugs, and acquired ion channel deficiencies, and thus increase the vulnerability to life‐threatening ventricular arrhythmias. 27 , 28 These hypotheses were supported by the higher risks of both SCD and cardiovascular death among subjects with new‐onset J‐waves ≥0.2 mV than among those with J‐wave amplitudes of 0.1 to 0.19 mV.
Moreover, more recent clinical studies have suggested that J waves associated with ERP can be attributed to either depolarization or repolarization abnormalities or both. 29 , 30 There is also the possibility that the risk difference between the transient and consistent/new‐onset ERP observed in this study might in fact be attributed to these different potential origins of the J wave. That is, a consistent/new‐onset ERP could be associated with microstructural abnormalities resulting in delayed depolarizations than more transient functional abnormalities in ionic channels impacting repolarization.
It is of note that, compared with Black subjects and men, White subjects and women tend to have a higher risk of SCD associated with time‐varying ERP. The largely consistent directionality of this effect decreases the probability that the results were attributed to chance. One explanation is that there are underlying genetic differences among races and sexes that result in different types of ERP. Some genotypes might inherently increase the risk of fatal ventricular arrhythmias, whereas others could be relatively benign while producing the same ECG findings. 31 It is also possible that ERP in Black subjects and men result from a higher vagal tone, and it is thus considered exercise related and relatively benign. Furthermore, the higher risk of death observed in women than in men might be attributed to loss of menstruation or onset of estrogen use, which has been identified to increase cardiovascular events. 32 , 33 , 34 Further studies, including well‐designed clinical trials and experiment studies, are warranted to elucidate the specific pathogenic mechanisms.
First, the major strengths of our study include the prospective cohort of a healthy biracial population, the relatively large sample size, the long‐term and virtually complete follow‐up, and the detailed risk factors and ECG information. Second, the application of an entirely automated method to detect ERP eliminates bias and enables us to compare the prognostic significance of this pattern. Third, the cause of death was classified using a strict adjudication process. Fourth, our previous study and other reports have identified that ERP could be a marker of underlying structural heart diseases (ischemic, cardiomyopathy, etc) and not just a pure electrical phenomenon. 35 , 36 We thus performed sensitivity analyses by excluding subjects with coronary heart disease or chronic heart failure, but the results did not materially change. This might further corroborate our hypothesis that dynamic ERP could be an independent risk factor for cardiovascular outcomes.
Some limitations of our analysis should be noted. First, this study included only individuals aged between 45 and 64 years, and thus we cannot comment on the influence of ERP outside this age range. Second, because of the strictly epidemiologic nature of our investigation, we cannot further clarify the pathophysiologic and pathogenetic circumstances underlying ERP. Third, although we controlled for potentially confounding factors in the multivariable‐adjusted analyses, the possibility of residual confounding cannot be completely ruled out. For example, ejection fraction was an important prognostic risk factor for cardiovascular death and could have impacted the results of this study, but the limited data available precluded our further analysis. Fourth, when assessing the role of temporal changes in ERP, we used only the last 2 available exams. Given the variable availability of ERP data, this approach may be biased. However, as the results indicated, the most recent value of ERP before an event or at the end of the follow‐up period could be more informative of outcome than the baseline ERP value in the general population. Fifth, the results from a given study for the multiple comparisons are not independent, and there is the possibility that results with P values closer to 0.05 may be false positives, whereas those with much lower P values are likely true positives. Therefore, we used Bonferroni correction for the 10 interactions that yielded a threshold of 0.005, which might to some extent reduce the possibility of a type I error.
In summary, our study shows that baseline ERP, time‐varying ERP, new‐onset ERP, and consistent ERP, rather than transient ERP, might be promising markers of risk for SCD in the general population. In particular, much attention should be paid to the population with high‐amplitude, time‐varying ERP and time‐varying ERP in anterior leads. Future clinical and experimental studies should focus on understanding the exact mechanisms of temporal ERP and reasons for the trends found based on sex and race.
Sources of Funding
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts. The study was also financially supported by grants from the National Natural Science Foundation of China (81600260), Guangdong Natural Science Foundation (2016A030313210), Guangdong Basic and Applied Basic Research Foundation (2021A1515010405), the project of Guangdong Province Science and Technology Plan (2017A020215174), the Fundamental Research Funds for the Central Universities in Sun Yat‐Sen University (18ykpy08), the project of Kelin new star of the First Affiliated Hospital of Sun Yat‐Sen University (Y50186), and the clinical research plan of the Eastern Hospital of the First Affiliated Hospital of Sun Yat‐Sen University (2019007).
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study and Biologic Specimen and Data Repository Information Coordinating Center for their important contributions.
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Xue‐Qiong Deng, Email: dengxueq@mail.sysu.edu.cn.
Yun‐Jiu Cheng, Email: chyjiu@mail.sysu.edu.cn.
REFERENCES
- 1. Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié J‐L, Nogami A, Babuty D, Yli‐Mayry S, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968 [DOI] [PubMed] [Google Scholar]
- 2. Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589 [DOI] [PubMed] [Google Scholar]
- 3. Cheng YJ, Mei WY, Chen XM, Liu LJ, Zheng DD, Ji CC, Tang K, Wu SH. Long‐term prognosis associated with early repolarisation pattern in Chinese population with atherosclerotic risk factors. Heart. 2017;103:910–916. doi: 10.1136/heartjnl-2016-310259 [DOI] [PubMed] [Google Scholar]
- 4. Pargaonkar VS, Perez MV, Jindal A, Mathur MB, Myers J, Froelicher VF. Long‐term prognosis of early repolarization with J‐wave and QRS slur patterns on the resting electrocardiogram: a cohort study. Ann Intern Med. 2015;163:747–755. doi: 10.7326/M15-0598 [DOI] [PubMed] [Google Scholar]
- 5. Uberoi A, Jain NA, Perez M, Weinkopff A, Ashley E, Hadley D, Turakhia MP, Froelicher V. Early repolarization in an ambulatory clinical population. Circulation. 2011;124:2208–2214. doi: 10.1161/CIRCULATIONAHA.111.047191 [DOI] [PubMed] [Google Scholar]
- 6. Cheng YJ, Lin XX, Ji CC, Chen XM, Liu LJ, Tang K, Wu SH. Role of early repolarization pattern in increasing risk of death. J Am Heart Assoc. 2016;5:e003375. doi: 10.1161/JAHA.116.003375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kitamura T, Fukamizu S, Hojo R, Aoyama Y, Komiyama K, Nishizaki M, Sakurada H, Hiraoka M. Early repolarization pattern and its day‐to‐day dynamic change as markers for ventricular fibrillation in patients with vasospastic angina. Europace. 2016;18:1252–1258. doi: 10.1093/europace/euv281 [DOI] [PubMed] [Google Scholar]
- 8. Tan VH, Duff H, Gerull B, Sumner G. Early repolarization syndrome: a case report focusing on dynamic electrocardiographic changes before ventricular arrhythmias and genetic analysis. Heart Rhythm Case Rep. 2015;1:213–216. doi: 10.1016/j.hrcr.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karashima S, Tsuda T, Wakabayashi Y, Kometani M, Demura M, Ichise T, Kawashiri MA, Takeda Y, Hayashi K, Yoneda T. Ventricular fibrillation associated with dynamic changes in J‐point elevation in a patient with silent thyroiditis. J Endocr Soc. 2018;2:135–139. doi: 10.1210/js.2017-00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11. Giffen CA, Wagner EL, Adams JT, Hitchcock DM, Welniak LA, Brennan SP, Carroll LE. Providing researchers with online access to NHLBI biospecimen collections: the results of the first six years of the NHLBI BioLINCC program. PLoS One. 2017;12:e178141. doi: 10.1371/journal.pone.0178141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giffen CA, Carroll LE, Adams JT, Brennan SP, Coady SA, Wagner EL. Providing contemporary access to historical biospecimen collections: development of the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Biopreserv Biobank. 2015;13:271–279. doi: 10.1089/bio.2014.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neal WT, Singleton MJ, Roberts JD, Tereshchenko LG, Sotoodehnia N, Chen LY, Marcus GM, Soliman EZ. Association between QT‐interval components and sudden cardiac death: the ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2017;10:e005485. doi: 10.1161/CIRCEP.117.005485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macfarlane PW, Antzelevitch C, Haissaguerre M, Huikuri HV, Potse M, Rosso R, Sacher F, Tikkanen JT, Wellens H, Yan GX. The early repolarization pattern: a consensus paper. J Am Coll Cardiol. 2015;66:470–477. doi: 10.1016/j.jacc.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 15. Wang YG, Wu HT, Daubechies I, Li Y, Estes EH, Soliman EZ. Automated J wave detection from digital 12‐lead electrocardiogram. J Electrocardiol. 2015;48:21–28. doi: 10.1016/j.jelectrocard.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 16. Vazir A, Claggett B, Cheng S, Skali H, Shah A, Agulair D, Ballantyne CM, Vardeny O, Solomon SD. Association of resting heart rate and temporal changes in heart rate with outcomes in participants of the atherosclerosis risk in communities study. JAMA Cardiol. 2018;3:200–206. doi: 10.1001/jamacardio.2017.4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng YJ, Jia YH, Yao FJ, Mei WY, Zhai YS, Zhang M, Wu SH. Association between silent myocardial infarction and long‐term risk of sudden cardiac death. J Am Heart Assoc. 2021;10:e17044. doi: 10.1161/JAHA.120.017044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haruta D, Matsuo K, Tsuneto A, Ichimaru S, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12‐lead electrocardiogram. Circulation. 2011;123:2931–2937. doi: 10.1161/CIRCULATIONAHA.110.006460 [DOI] [PubMed] [Google Scholar]
- 20. Heng SJ, Clark EN, Macfarlane PW. End QRS notching or slurring in the electrocardiogram: influence on the definition of "early repolarization". J Am Coll Cardiol. 2012;60:947–948. doi: 10.1016/j.jacc.2012.03.061 [DOI] [PubMed] [Google Scholar]
- 21. Cheng YJ, Zhao XX, Pan SP, Pan JM, Zhang M, Li ZY. Association of early repolarization pattern with cardiovascular outcomes in middle‐aged population: a cohort study. Clin Cardiol. 2020;43:1601–1608. doi: 10.1002/clc.23488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rezus C, Floria M, Moga VD, Sirbu O, Dima N, Ionescu SD, Ambarus V. Early repolarization syndrome: electrocardiographic signs and clinical implications. Ann Noninvasive Electrocardiol. 2014;19:15–22. doi: 10.1111/anec.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Junttila MJ, Tikkanen JT, Porthan K, Oikarinen L, Jula A, Kentta T, Salomaa V, Huikuri HV. Relationship between testosterone level and early repolarization on 12‐lead electrocardiograms in men. J Am Coll Cardiol. 2013;62:1633–1634. doi: 10.1016/j.jacc.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 24. Walsh JR, Ilkhanoff L, Soliman EZ, Prineas R, Liu K, Ning H, Lloyd‐Jones DM. Natural history of the early repolarization pattern in a biracial cohort: CARDIA (Coronary Artery Risk Development in Young Adults) Study. J Am Coll Cardiol. 2013;61:863–869. doi: 10.1016/j.jacc.2012.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J. 2012;76:1054–1065. doi: 10.1253/circj.CJ-12-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aagaard P, Baranowski B, Aziz P, Phelan D. Early repolarization in athletes: a review. Circ Arrhythm Electrophysiol. 2016;9:e3577. doi: 10.1161/CIRCEP.115.003577 [DOI] [PubMed] [Google Scholar]
- 27. Naruse Y, Tada H, Harimura Y, Hayashi M, Noguchi Y, Sato A, Yoshida K, Sekiguchi Y, Aonuma K. Early repolarization is an independent predictor of occurrences of ventricular fibrillation in the very early phase of acute myocardial infarction. Circ Arrhythm Electrophysiol. 2012;5:506–513. doi: 10.1161/CIRCEP.111.966952 [DOI] [PubMed] [Google Scholar]
- 28. Cheng YJ, Li ZY, Yao FJ, Xu XJ, Ji CC, Chen XM, Liu LJ, Lin XX, Yao H, Wu SH. Early repolarization is associated with a significantly increased risk of ventricular arrhythmias and sudden cardiac death in patients with structural heart diseases. Heart Rhythm. 2017;14:1157–1164. doi: 10.1016/j.hrthm.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 29. Haïssaguerre M, Nademanee K, Hocini M, Cheniti G, Duchateau J, Frontera A, Sacher F, Derval N, Denis A, Pambrun T, et al. Depolarization versus repolarization abnormality underlying inferolateral J‐wave syndromes: new concepts in sudden cardiac death with apparently normal hearts. Heart Rhythm. 2019;16:781–790. doi: 10.1016/j.hrthm.2018.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haïssaguerre M, Nademanee W, Hocini M, Duchateau J, André C, Lavergne T, Takigawa M, Sacher F, Derval N, Pambrun T, et al. The spectrum of idiopathic ventricular fibrillation and J‐wave syndromes: novel mapping insights. Card Electrophysiol Clin. 2019;11:699–709. doi: 10.1016/j.ccep.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 31. Perez MV, Uberoi A, Jain NA, Ashley E, Turakhia MP, Froelicher V. The prognostic value of early repolarization with ST‐segment elevation in African Americans. Heart Rhythm. 2012;9:558–565. doi: 10.1016/j.hrthm.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Zhao D, Wang M, Sun J‐Y, Liu J, Qi Y, Hao Y‐C, Deng Q‐J, Liu J, Liu J, et al. Combined effect of menopause and cardiovascular risk factors on death and cardiovascular disease: a cohort study. BMC Cardiovasc Disord. 2021;21:109. doi: 10.1186/s12872-021-01919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matthews KA, Chen X, Barinas‐Mitchell E, Brooks MM, Derby CA, Harlow S, Jackson EA, Thurston RC, El KS. Age at menopause in relationship to lipid changes and subclinical carotid disease across 20 years: study of women's health across the nation. J Am Heart Assoc. 2021;10:e21362. doi: 10.1161/JAHA.121.021362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El KS, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 35. Mei WY, Liu LJ, Xu Q, Zheng DD, Cheng YJ. Additional value of early repolarization pattern in prediction of obstructive coronary artery disease as assessed by coronary angiography. Int Heart J. 2019;60:296–302. doi: 10.1536/ihj.18-416 [DOI] [PubMed] [Google Scholar]
- 36. Chan CS, Lin YJ, Chang SL, Lo LW, Hu YF, Chao TF, Chung FP, Liao JN, Chen YJ, Chen SA. Early repolarization of surface ECG predicts fatal ventricular arrhythmias in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy and symptomatic ventricular arrhythmias. Int J Cardiol. 2015;197:300–305. doi: 10.1016/j.ijcard.2015.06.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


