Abstract
Background
Whether cardiac structure and function abnormalities associated with dysglycemia are sufficient to explain the increased risk of death or heart failure (HF) remains unclear.
Methods and Results
We analyzed 6059 participants (mean age, 75±5 years; 58% women; and 22% Black individuals) who attended the ARIC (Atherosclerosis Risk in Communities) study visit 5 examination (2011–2013). Participants were categorized as no diabetes, pre‐diabetes, and diabetes (on the basis of medical history and glycated hemoglobin values). We assessed whether diabetes modified the association between echocardiographic measures of cardiac structure and function and the composite of all‐cause death or HF hospitalization and then estimated the extent to which the increased risk of the composite outcome associated with diabetes was explained by cardiac structure and function. Diabetes was prevalent in 33.5% of the subjects. Death or HF occurred in 1111 (18%) at a rate of 3.6 per 100 person‐years. Both measures of cardiac structure and function and diabetes status were significantly associated with worse prognosis after accounting for clinical confounders. While diabetes was consistently associated with a higher risk of events, it did not significantly modify the association between cardiac abnormalities and the risk of death or HF, except for subjects with higher left atrial volume who showed higher relative risk of events (P for interaction <0.001). Measures of cardiac structure and function accounted for ≈16% of the increased risk of death or HF associated with diabetes. Similar results were observed analyzing subjects without prevalent heart disease.
Conclusions
In a biracial cohort of older adults, the increased risk of events associated with diabetes was partially explained by cardiac structure and function abnormalities.
Keywords: cardiac structure and function, death, diabetes, echocardiography, heart failure
Subject Categories: Cardiomyopathy; Heart Failure; Remodeling; Echocardiography; Diabetes, Type 2
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
Clinical Perspective
What Is New?
In a community‐based biracial cohort of older adults, the increased risk of all‐cause death or heart failure hospitalization associated with diabetes is partially explained by cardiac structure and function abnormalities.
What Are the Clinical Implications?
Cardiac abnormalities associated with dysglycemia may not fully explain the increased risk of cardiovascular events, suggesting the importance of biological factors related to dysglycemic processes at the molecular and cellular levels, as well as genetic predisposition and specific biomarker pathways.
Diabetes is associated with an increased risk of heart failure (HF) and mortality even after accounting for known cardiovascular risk factors. 1 , 2 Mechanistic hypotheses related to hyperglycemia, advanced glycation end products, or inflammation and oxidative stress have been described 3 and theoretically related to cardiac structure and function. 4 The concept of a specific diabetic cardiomyopathy affecting subjects with dysglycemia resulting in left ventricular (LV) concentric remodeling, increased LV mass and subtle impaired systolic and diastolic function 5 was reported nearly 5 decades ago. However, little is known about the underlying dysglycemia‐related cardiac mechanisms leading to HF in these patients.
Our group has previously shown that subtle alterations in cardiac structure and function were associated with worsening dysglycemia in a large community cohort of individuals free of prevalent heart disease. 6 However, the extent to which these echocardiographic‐based cardiac abnormalities contribute to the increased risk of death or HF associated with diabetes remains unclear.
To estimate the contribution of cardiac structure and function abnormalities to the risk of death or HF across the glycemic spectrum, we analyzed echocardiographic and outcome data from the ARIC (Atherosclerosis Risk in Communities) study among participants, with and without prevalent heart disease, who attended the fifth visit.
Methods
Study Population
The design and sampling of the ARIC study has been described previously. 7 Briefly, individuals were recruited from 4 communities (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD) between 1987 and 1989. Of all 15 792 participants who were enrolled in ARIC at the first examination, a total of 6538 attended the fifth visit between 2011 and 2013 for a standardized physical examination, interviewer‐administered questionnaires, and a comprehensive echocardiographic examination. 8 For the present analysis, we excluded subjects of non‐White or non‐Black race (n=18), without echocardiographic examination (n=418), without available measurement of glycated hemoglobin (HbA1c) (n=35) and follow‐up data (n=8). The study was approved by the Institutional Review Board at all institutions involved, and informed consent was obtained. Data that support the findings of this study are available per ARIC (Atherosclerosis Risk in Communities) study policies (https://sites.cscc.unc.edu/aric/contact_the_coord_center). All participants provided written informed consent, and study procedures were conducted in accordance with institutional guidelines about the protection of human subjects.
Dysglycemia Classification
On the basis of annual telephone interviews, comprehensive questionnaires, medication lists, and results of visit 5 laboratory tests for HbA1c levels, we classified participants into 1 of 3 groups 6 , 9 : (1) diabetes: known diabetes before visit 5 or on antidiabetes medications or visit 5‐HbA1c ≥6.5%; (2) prediabetes: no known diabetes, but visit 5 HbA1c between 5.7% and 6.4%; and (3) normal: no known diabetes at visits 1 to 5 and annual follow‐up data, and visit 5 HbA1c <5.7%. HbA1c was measured using a Tosoh G7 automated high‐performance liquid chromatography analyzer (Tosoh Bioscience, South San Francisco, CA) standardized to the Diabetes Control and Complications Trial assay. The agreement between the HbA1c‐based and the glucose‐based glycemic classification at the ARIC fifth visit was previously described. 6
Echocardiographic Analysis
The ARIC echocardiographic study methods and design at visit 5 have been previously described in detail. 8 Briefly, all studies were prospectively acquired on Philips IE33 machines by trained sonographers according to a study‐specific comprehensive echocardiographic protocol. Analyses of 2‐dimensional, Doppler, and tissue Doppler echocardiography were overread by echocardiographers in a central echo core laboratory.
LV dimensions (body surface area (BSA)‐indexed LV end‐diastolic diameter) and interventricular septum were obtained from the parasternal long‐axis view. 8 LV mass was calculated by the linear method and indexed to BSA. LV volumes (BSA‐indexed LV end‐diastolic volume, BSA‐indexed LV end‐systolic volume), LV ejection fraction and BSA‐indexed left atrial (LA) volume index were assessed by the modified Simpson’s rule. Right ventricular function was assessed by the fractional area change. LV hypertrophy was defined as LV mass index (LV mass/BSA) >115 g/m2 in men and >95 g/m2 in women. 8 Speckle tracking analysis was performed using TomTec Cardiac Performance Analysis package. Global longitudinal strain was obtained from apical 4‐chamber and 2‐chamber views. Pulsed‐wave Doppler of mitral inflow was used to measure early and late mitral inflow peak velocities (E wave and A wave) and their ratio. The average ratio of E wave and the early mitral annulus tissue Doppler velocity (E/e′) was then calculated. In a sensitivity analysis, we also included maximum tricuspid Doppler jet velocity (available in 3535 subjects [58%]) (Table S1).
Outcomes
The primary end point for this analysis was the composite of all cause death or HF hospitalization. We also conducted additional sensitivity analyses for HF hospitalization alone. HF events underwent physician adjudication 10 and included events after visit 5. All‐cause mortality was ascertained by ARIC surveillance or the National Death Index.
Statistical Analysis
Baseline characteristics were compared across glycemic categories using nonparametric trend test and χ 2 test for trend for continuous 11 and binary variables as needed. Echocardiographic data are presented as unadjusted and multivariable‐adjusted means with P values estimated from linear regression for trend across the categories. The adjusted model for each echocardiographic parameter included age, sex, race, total cholesterol and low‐density lipoprotein cholesterol (samples collected from participants at visit 5), statins medication, history of hypertension, systolic blood pressure, heart rate, QRS interval, estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation, body mass index (BMI), smoking status, coronary artery disease (including history of myocardial infarction), prevalent HF, heart valve disease, history of implantable cardiac defibrillator/pacemaker. If a significant trend across categories was detected, then pairwise comparisons of dysglycemia categories were made. A time‐to‐event analysis with Cox proportional hazards models was used to assess the association of each measure of cardiac structure and function with the outcome of interest on each glycemic group. The multivariable model was stratified by age and history of coronary artery disease and included sex, race, log‐transformed total cholesterol, log‐transformed low‐density lipoprotein cholesterol, statins medication, history of hypertension, systolic blood pressure, heart rate, QRS interval, estimated glomerular filtration rate, body mass index, smoking status, prevalent HF, heart valve disease, and history of implantable cardiac defibrillator/pacemaker. Proportional‐hazard assumption was tested without evidence of violation. The continuous association between event rates and echocardiographic measures was estimated using Poisson regression, with each participant’s duration of follow‐up included as an exposure term. These associations were estimated separately for each glycemic group. For selected echocardiographic measurements demonstrating a robust association with clinical outcomes in adjusted analysis, the flexible continuous relationship with the primary outcome on each glycemic group was displayed using restricted cubic splines models with 3 knots.
We also assessed whether glycemic status modified the relationship between measures of cardiac structure and function and outcomes adjusting for demographic confounders and clinical confounders.
In addition, we quantified the extent to which echocardiographic measures account for the association between diabetes and outcomes. We determined the proportional reduction in magnitude of the β coefficient for diabetes in a Cox proportional hazards regression model, after accounting for measures of cardiac structure and function that were linked to both dysglycemia and outcomes. CIs were derived using bootstrap methods with 2000 replications. A sensitivity analysis was also performed excluding subjects with prevalent heart disease at baseline (prevalent HF, coronary artery disease, and moderate/severe valvular disease). Statistical analysis was performed using Stata version 14.2 (StataCorp LP, College Station, TX), with P values <0.05 considered to indicate statistical significance.
Results
Overall, 6059 ARIC visit 5 participants (mean age, 75.5±5.1 years; 58% women; and 22% Black individuals) were included in this analysis. Diabetes was prevalent in 2033 subjects (33.5%), and it was previously undiagnosed in 94 subjects (1.5%). Prediabetes was prevalent in 1818 subjects (30%). Tables 1 and 2 show participants’ clinical and echocardiographic characteristics according to dysglycemia status. Subjects with diabetes were more likely to be Black and have a history of hypertension, coronary artery disease, prevalent HF, and chronic kidney disease. Compared with subjects with diabetes or prediabetes, those in the normal glycemia category had lower body mass index, heart rate, C‐reactive protein, high‐sensitivity troponin, and higher low‐density lipoprotein cholesterol and high‐density lipoprotein levels (Table 1).
Table 1.
Clinical Characteristics of ARIC Visit 5 Participants Stratified by Glycemic Status
| No diabetes | Prediabetes | Diabetes | P for trend | |
|---|---|---|---|---|
| n=2208 | n=1818 | n=2033 | ||
| Visit center | ||||
| Forsyth County, NC | 543 (24.6) | 460 (25.3) | 381 (18.8) | <0.001 |
| Jackson, MS | 269 (12.2) | 381 (20.9) | 550 (27.0) | <0.001 |
| Minneapolis, MN | 785 (35.6) | 560 (30.8) | 474 (23.3) | <0.001 |
| Washington County, MD | 611 (27.6) | 417 (23.0) | 628 (30.9) | 0.02 |
| Age, y | 75 (71, 79) | 75 (71, 79) | 75 (71, 79) | 0.47 |
| Male sex | 931 (42.1) | 727 (39.9) | 899 (44.2) | 0.18 |
| Black | 286 (12.9) | 423 (23.2) | 609 (30.0) | <0.001 |
| Hypertension | 1645 (74.5) | 1502 (82.6) | 1903 (93.6) | <0.001 |
| Ever smoker | 1338 (60.6) | 1106 (60.9) | 1282 (63.1) | 0.10 |
| Coronary artery disease | 309 (14.0) | 341 (18.7) | 516 (25.4) | <0.001 |
| History of atrial fibrillation | 156 (7.2) | 153 (8.6) | 226 (11.3) | <0.001 |
| Heart failure | 190 (8.6) | 185 (10.2) | 396 (19.5) | <0.001 |
| Diabetic medications | … | … | 1193 (59.0) | By design |
| Insulin therapy | … | … | 303 (14.9) | By design |
| HbA1c (%) | 5.4 (5.2, 5.5) | 5.9 (5.7, 6.0) | 6.4 (5.9, 7.1) | By design |
| Lipid‐lowering medications | 921 (41.9) | 1035 (57.3) | 1419 (70.1) | <0.001 |
| BMI, kg/m2 | 26.5 (23.8, 29.7) | 27.7 (24.9, 31.2) | 29.8 (26.7, 33.8) | <0.001 |
| SBP, mm Hg | 128 (118, 141) | 129 (118, 140) | 129 (117, 141) | 0.79 |
| Heart rate, bpm | 60 (54, 67) | 61 (55, 68) | 63 (57, 71) | <0.001 |
| QRS interval, ms | 92 (84, 102) | 90 (83, 100) | 92 (84, 104) | 0.97 |
| eGFR, mL/min per 1.73 m2 | 72 (61, 83) | 71 (58, 83) | 69 (54, 83) | <0.001 |
| LDL cholesterol, mg/dL | 108 (87, 131) | 104 (83, 126) | 89 (70, 112) | <0.001 |
| hs‐CRP, mg/L | 1.7 (0.8, 3.6) | 2.1 (1.0, 4.5) | 2.3 (1.1, 4.8) | <0.001 |
| NT‐proBNP, ng/L | 145 (76, 262) | 125 (63, 261) | 131 (65, 298) | 0.05 |
| hs‐troponin, ng/L | 1.0 (0.7, 1.5) | 1.0 (0.7, 1.5) | 1.2 (0.8, 1.9) | <0.001 |
Data displayed as n (%) or median (25th, 75th percentiles). ARIC indicates Atherosclerosis Risk in Communities; BMI body mass index; eGFR, estimated glomerular filtration rate by Chronic Kidney Disease Epidemiology Collaboration equation; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐troponin, high‐sensitivity troponin; LDL, low‐density lipoprotein; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and SBP, systolic blood pressure.
Table 2.
Echocardiographic Characteristics of ARIC Visit 5 Participants Stratified by Glycemic Status
| Mean±SD | Multivariable adjusted mean | |||||||
|---|---|---|---|---|---|---|---|---|
| No diabetes | Prediabetes | Diabetes | P for trend | No diabetes | Prediabetes | Diabetes | P for trend | |
| LV structure | ||||||||
| LVEDDi, cm/m2 | 2.43±0.28 | 2.38±0.30* | 2.36±0.30* , † | <0.001 | 2.40 | 2.38* | 2.39 | 0.56 |
| IVS, cm | 1.02±0.16 | 1.04±0.16* | 1.08±0.17* , † | <0.001 | 1.03 | 1.05* | 1.05* | <0.001 |
| LVMi, g/m2 | 78.3±19.9 | 78.5±20.4 | 83.7±22.7* , † | <0.001 | 79.3 | 79.4 | 81.3* , † | 0.003 |
| LVH, n | 198 (9.0) | 188 (10.5) | 309 (15.4)* , † | <0.001 | 10.0% | 11.1% | 12.5%* | 0.02 |
| LVEDVi, mL/m2 | 44.6±10.8 | 43.2±11.3* | 44.6±12.2 † | 0.18 | 44.5 | 43.8* | 44.5 | 0.83 |
| LVESVi, mL/m2 | 15.7±6.3 | 15.3±6.7 | 16.2±8.0* , † | 0.77 | 15.9 | 15.7 | 15.9 | 0.93 |
| Systolic function | ||||||||
| LVEF, % | 65.2±6.3 | 65.2±6.8 | 64.6±7.4* , † | 0.031 | 65.0 | 64.9 | 65.2 | 0.28 |
| GLS, % | −18.2±2.5 | −17.8±2.7* | −17.4±2.7* , † | <0.001 | −18.0 | −17.7* | −17.8* | 0.008 |
| RV FAC, % | 0.53±0.08 | 0.52±0.08* | 0.52±0.08* | <0.001 | 0.53 | 0.52* | 0.52 | 0.31 |
| Diastolic function | ||||||||
| LAVi, mL/m2 | 26.2±9.4 | 25.8±9.8 | 26.7±9.2 † | 0.046 | 26.7 | 26.2 | 26.0* | 0.029 |
| E‐A ratio | 0.88±0.31 | 0.86±0.29* | 0.83±0.29* , † | <0.001 | 0.87 | 0.86 | 0.85 | 0.08 |
| E/e′ average | 10.8±3.8 | 11.3±3.9* | 12.0±4.5* , † | <0.001 | 11.0 | 11.4* | 11.7* , † | <0.001 |
Adjustment: age, sex, race/center, total cholesterol, low‐density lipoprotein cholesterol;, statins medication, history of hypertension, systolic blood pressure, heart rate, QRS interval, estimated glomerular filtration rate, body mass index, smoking status, history of coronary artery disease, prevalent heart failure, heart valve disease, and history of implantable cardiac defibrillator/pacemaker.
ARIC indicates Atherosclerosis Risk in Communities; GLS, global longitudinal strain; IVS, interventricular septum; LAVi, left atrial volume index; LVEDDi, left ventricular end‐diastolic diameter index; LVEDVi, left ventricular end‐diastolic volume index; LVESVi, left ventricular end‐systolic volume index; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMi, left ventricular mass index; and RV FAC, right ventricular fractional area change.
P<0.05 compared with no dysglycemia.
P<0.05 compared with prediabetes.
Interventricular septum thickness, LV mass index, and prevalence of LV hypertrophy were significantly higher in subjects with diabetes than in those without diabetes or prediabetes, even after adjustment for demographic and clinical confounders (Table 2). The adjusted mean of LV mass index of subjects with diabetes (81 g) was statistically higher than that of subjects with prediabetes (79 g) or no diabetes (79 g; P for trend=0.003).
Among measures of LV systolic function, only global longitudinal strain, even if within the normal range, was worse in subjects with diabetes in multivariable analysis (P for trend=0.008). LV ejection fraction was preserved in the majority of participants and was not significantly different across dysglycemia categories (P for trend=0.28). Subjects with prediabetes and diabetes also showed a significant trend for higher E/e′ (P for trend <0.001), suggesting higher LV filling pressures, compared with no‐diabetes subjects.
Risk of Death or HF
Over a median follow‐up of 5.5 years, the composite end point of death or HF occurred in 1111 (18.3%) at a rate of 3.6 per 100 person‐years. HF occurred in 462 participants (1.5 per 100 person‐years) and death in 882 (2.7 per 100 person‐years). Subjects with diabetes, but not those with prediabetes, had a higher event rate and risk for death or HF (4.9 per 100 person‐years; hazard ratio: 1.82, 95% CI, 1.58–2.09 versus 3.1 per 100 person‐years; hazard ratio, 1.13; 95% CI, 0.96–1.32, respectively) compared with subjects with no diabetes (2.7 per 100 person‐years, Figure 1).
Figure 1. Echocardiographic parameters of cardiac structure and function partially contribute to the diabetes associated risk of death or HF in the community.
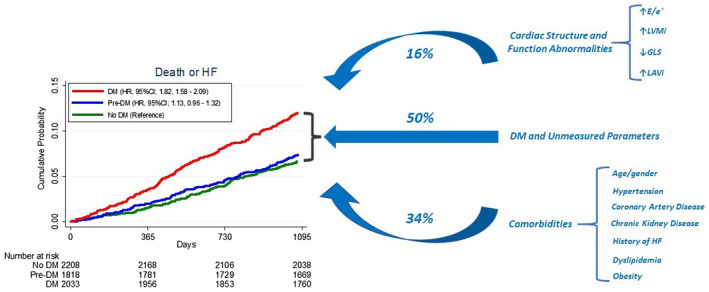
Percentage indicates the proportion of the association between diabetes and the risk of death or HF that can be accounted for by listed parameters of cardiac structure and function or clinical characteristics. DM indicates diabetes; GLS, global longitudinal strain; HF, heart failure; LAVi, left atrial volume index; and LVMi, left ventricular mass index.
Echocardiographic measures of impaired LV structure (ie, higher LV dimension, LV mass index, LV hypertrophy), and that of systolic and diastolic dysfunction such as reduced LV ejection fraction, LV global longitudinal strain, and higher LA volume index and E/e′ were significantly associated with a higher risk of death or HF after adjustment for clinical and demographic confounders in subjects with normal glycemic status, prediabetes or diabetes (Table 3).
Table 3.
Adjusted Association of Echocardiographic Characteristics and the Primary End Point According to the Glycemic Status
| No diabetes (317/2208) 2.7 per 100 person‐years | Prediabetes (295/1818) 3.1 per 100 person‐years | Diabetes (499/2033) 4.9 per 100 person‐years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | |Z| | HR | 95% CI | P | |Z| | HR | 95% CI | P | |Z| | |
| LV structure | ||||||||||||
| LVEDDi, per 0.1 cm/m2 increase | 1.09 | 1.04–1.14 | 0.001 | 3.8 | 1.13 | 1.09–1.18 | <0.001 | 6.0 | 1.07 | 1.04–1.11 | <0.001 | 4.1 |
| IVS, per 0.1 cm increase | 1.11 | 1.03–1.21 | 0.006 | 2.7 | 1.10 | 1.02–1.18 | 0.015 | 2.4 | 1.07 | 1.01–1.13 | 0.013 | 2.4 |
| LVMi, per 10 g/m2 | 1.18 | 1.12–1.25 | <0.001 | 5.9 | 1.24 | 1.17–1.31 | <0.001 | 7.7 | 1.28 | 1.08–1.17 | <0.001 | 6.0 |
| LVH | 2.32 | 1.65–3.32 | <0.001 | 4.9 | 2.51 | 1.80–3.51 | <0.001 | 5.4 | 1.76 | 1.38–2.26 | <0.001 | 4.5 |
| EDVi, per 5 mL/m2 increase | 1.12 | 1.06–1.18 | <0.001 | 4.0 | 1.11 | 1.05–1.17 | <0.001 | 3.9 | 1.11 | 1.06–1.16 | <0.001 | 4.8 |
| ESVi, per 5 mL/m2 increase | 1.24 | 1.14–1.34 | <0.001 | 5.3 | 1.16 | 1.07–1.26 | <0.001 | 3.7 | 1.15 | 1.08–1.22 | <0.001 | 4.5 |
| Systolic function | ||||||||||||
| LVEF, per 5% increase | 0.83 | 0.76–0.91 | <0.001 | 3.9 | 0.88 | 0.81–0.96 | 0.004 | 2.8 | 0.89 | 0.83–0.95 | 0.001 | 3.4 |
| GLS, per 1% increase | 1.07 | 1.01–1.12 | 0.007 | 2.7 | 1.06 | 1.00–1.11 | 0.021 | 2.3 | 1.07 | 1.03–1.11 | <0.001 | 3.6 |
| RV FAC, per 5% increase | 1.03 | 0.95–1.12 | 0.42 | 0.8 | 0.96 | 0.88–1.04 | 0.35 | 0.9 | 0.95 | 0.89–1.02 | 0.24 | 1.1 |
| Diastolic function | ||||||||||||
| LAVi, per 5 mL/m2 increase | 1.08 | 1.04–1.12 | <0.001 | 4.3 | 1.16 | 1.10–1.23 | <0.001 | 5.3 | 1.20 | 1.14–1.26 | <0.001 | 7.1 |
| E/A ratio, per 0.1 cm/s increase | 1.09 | 1.05–1.12 | <0.001 | 5.2 | 1.05 | 1.01–1.10 | 0.015 | 2.4 | 1.03 | 1.00–1.07 | 0.017 | 2.4 |
| E/e′ average, per 1 U increase | 1.05 | 1.03–1.08 | <0.001 | 4.1 | 1.06 | 1.03–1.10 | <0.001 | 4.4 | 1.03 | 1.01–1.05 | <0.001 | 4.0 |
Adjustment: sex, race/center, log‐total cholesterol, log‐LDL, statins medication, history of hypertension, systolic blood pressure, heart rate, QRS interval, estimated glomerular filtration rate, body mass index, smoking status, prevalent heart failure, heart valve disease, and history of implantable cardiac defibrillator/pacemaker, stratified by age and history of coronary artery disease. GLS indicates global longitudinal strain; IVS, interventricular septum; LAVi, left atrial volume index; LVEDDi, left ventricular end‐diastolic diameter index; LVEDVi, left ventricular end‐diastolic volume index; LVESVi, left ventricular end‐systolic volume index; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMi, left ventricular mass index; and RV FAC, right ventricular fractional area change.
We observed similar results in sensitivity analyses for the end point of HF alone (Table S2) and of subjects without prevalent heart disease (Table S3).
Cardiac Structure and Function and HF Outcomes by Glycemic Status
Diabetes consistently increased the risk of death or HF associated with measures of cardiac structure and function. However, dysglycemia status at visit 5 did not significantly modify the relationship between cardiac measurements and outcome in adjusted models (Figure 2 and Table S4). Only subjects with diabetes and higher LA volume index showed a significantly higher event rate for the composite end point compared with subjects with prediabetes or no diabetes. For each 5 mL/m2 increase in LA volume index there was a 20% (95% CI, 1.14–1.26) increased risk for death or HF in subjects with diabetes compared with a 16% and 8% increased risk in subjects with prediabetes and no diabetes, respectively (fully adjusted P for interaction <0.001; Figure 2).
Figure 2. Adjusted association of LAVi, GLS, LVMi and E/e′ and death or HF stratified by glycemic groups (Red: diabetes; Blue: Pre‐diabetes; Green: No‐diabetes).
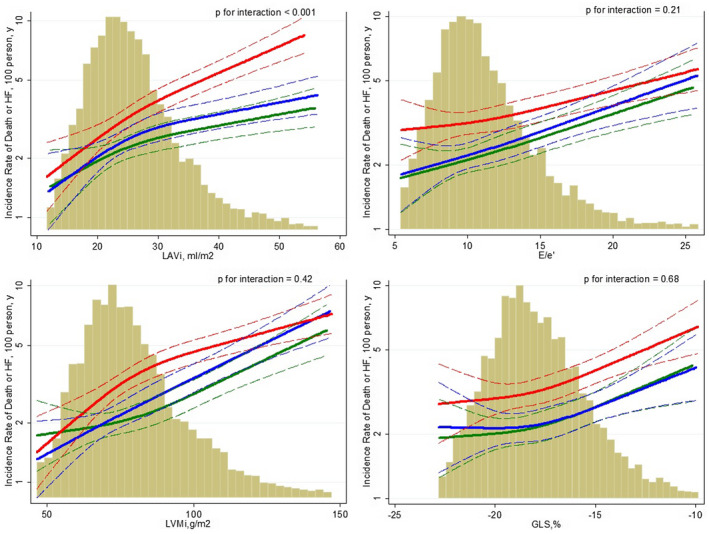
Multivariable adjustment: sex, race/center, log‐total cholesterol, log‐LDL, statins medication, history of hypertension, systolic blood pressure, heart rate, QRS interval, estimated glomerular filtration rate, body mass index, smoking status, history of coronary artery disease, prevalent HF, heart valve disease, and history of implantable cardiac defibrillator/pacemaker, stratified by age and history of coronary artery disease. The histograms on the background show the population distribution of the cardiac measure analyzed. The dashed lines indicate the 95% CIs for the reported incidence rate. GLS indicates global longitudinal strain; LAVi, left atrial volume index; and LVMi, left ventricular mass index.
For all other echocardiographic measures, the presence of diabetes consistently shifted the risk of death or HF upward without significant interaction in unadjusted and adjusted models. Similar results were observed in sensitivity analyses for HF alone (Table S5) and in subjects without prevalent heart disease (Table S6).
Diabetes‐Related Risk Explained by Cardiac Structure and Function
After adjusting for demographic variables (age, sex, race/center), diabetes was associated with an increased risk of death or HF (hazard ratio, 1.71; 95% CI, 1.51–1.92; Table 4) compared with non‐diabetes. Each echocardiographic parameter further attenuated that risk. Even after adjusting for all measures of cardiac structure and function, diabetes remained significantly associated with a significantly increased risk of death or HF, albeit attenuated (hazard ratio, 1.58; 95% CI, 1.39–1.79; Table 4). Approximately 16% (95% CI, 6%–29%) of the increased risk associated with diabetes was explained by measures of cardiac structure and function when accounting for demographics (Table 4 and Figure 1). Similar results were observed in a sensitivity analysis including estimation of pulmonary systolic pressure among measures of cardiac function (19%; 95% CI, 6%–41%). Demographic and other clinical variables accounted for ≈34% (95% CI, 18%–54%) of the mortality and HF risk associated with diabetes. Similar results were observed excluding subjects with prevalent heart disease (Table S7).
Table 4.
Contribution of Cardiac Structure and Function and Comorbidities to the Risk of death or HF in the Population With Diabetes
| HR (95% CI) | Attenuation of association (95% CI), % | |
|---|---|---|
| Diabetes (vs no diabetes) | 1.71 (1.51–1.92) | Reference |
| + LV structure | 1.56 (1.38–1.77) | 15 (8 to 24) |
| + Systolic function | 1.63 (1.44–1.85) | 20 (14 to 29) |
| + Diastolic function | 1.68 (1.48–1.90) | 6 (−0.1 to 15) |
| + All cardiac structure and function | 1.58 (1.39–1.79) | 16 (6 to 29) |
| + Comorbidities | 1.42 (1.24–1.63) | 34 (18 to 54) |
Attenuation of association is the proportion of the association between diabetes and the risk of death or HF that can be accounted for by listed parameters of cardiac structure and function or clinical characteristics, adjusting for demographics. Analyses are restricted to participants with available measurements of cardiac structure and function. Demographics: age, sex, race/center. Left ventricular structure parameters include left ventricular hypertrophy, left ventricular mass, and interventricular septum. Systolic function parameters include global longitudinal strain. Diastolic function parameters include left atrial volume index and E/e′. Comorbidities include demographics+log‐total cholesterol, log low‐density lipoprotein cholesterol, statins medication, history of hypertension, systolic blood pressure, heart rate, QRS interval, estimated glomerular filtration rate, body mass index, smoking status, history of coronary artery disease, prevalent HF, heart valve disease, and history of implantable cardiac defibrillator/pacemaker. HF indicates heart failure; HR, hazard ratio; and LV, left ventricle.
Discussion
In a large community‐based biracial cohort of older adults, we show that the association between cardiac abnormalities and the risk of death or HF was not modified by the glycemic status, except for subjects with LA dilation and diabetes who showed a particularly worse prognosis (P for interaction <0.001). The increased risk of all‐cause death or HF hospitalization associated with diabetes was partially explained by cardiac structure and function abnormalities. These findings suggest that cardiac abnormalities associated with dysglycemia may not fully explain the increased risk of death or HF.
Several studies have established that diabetes was an independent risk factor for the development of HF independently of the presence of overt cardiovascular disease, 12 , 13 including coronary artery disease and hypertension, leading to the hypothesis that diabetes increases the risk of HF by directly affecting the structure and function of the heart. 3
We have previously shown that dysglycemia was associated with subtle alterations of cardiac structure and function in subjects without prevalent heart disease, 6 and although while statistically strong, the association was clinically faint. For example, a 1‐unit (1%) higher HbA1c was associated with only a 3.0 g increase in LV mass. 6
Dysglycemia may affect the myocardium inducing interstitial fibrosis and impairments in cardiac relaxation, compliance, and contractility. 14 , 15 LV diastolic dysfunction can be detected in more than half of subjects with diabetes. 15 Our analysis shows that in subjects with LA dilation, those with diabetes had a significantly higher risk of death or HF than those without diabetes. LA dilation is a well‐known predictor of cardiovascular outcomes in the general population and in subjects with diabetes, 16 , 17 , 18 , 19 likely reflecting a time‐dependent chronic adaptation in response to metabolic stressors and elevated filling pressure. 20 , 21 , 22 From this perspective, atrial myopathy per se or as an adaptation to chronic higher loading conditions may identify subclinical pathological processes that identify subjects with diabetes at higher risk. 23 It should be further explored whether early prevention strategies aimed to control glycemic status when LA enlargement is detected may attenuate this process and potentially result in improved outcomes.
Nevertheless, except for LA volume, in the current analysis we observed that although diabetes consistently increased the risk of death or HF, there was no interaction between echocardiographic measures of cardiac structure and function and dysglycemia status. It has been shown that following a myocardial infarction, diabetes increases the risk of HF across the entire range of LV ejection fraction, further attenuating the relative lower risk of events among those with preserved ejection fraction. 24 These results suggest the importance of biological factors other than cardiac structure and function contributing to the development of HF and subsequent death in patients with diabetes. These may include biological parameters related to diabetes processes at the molecular and cellular levels including oxidative stress and inflammation, as well as genetic predisposition and specific biomarker pathways. Integration of biological factors along with advanced cardiovascular imaging techniques, such as magnetic resonance or nuclear imaging, able to better identify subclinical myocardial structural and functional abnormalities that may not be detected by standard 2‐dimensional echocardiography, may provide further insights to assess the relationship between dysglycemic processes and cardiac impairment.
While the term diabetic cardiomyopathy has been used to describe the effects of dysglycemia on cardiac structure and function, defining and identifying this entity remains challenging because of the link between diabetes and other associated comorbidities that may impact, on one hand, cardiac structure and function, and on the other hand, the increased risk of death or HF. 25 Indeed, our results show that global measures of cardiac structure and function partially accounted (about 16%) for the heightened risk of death or HF, even when excluding subjects with prevalent heart disease. Comorbidities commonly encountered in subjects with diabetes such as coronary artery disease, obesity, hypertension, and pulmonary and kidney diseases may play a substantial prognostic role in this population. 26 , 27 Consequently, alterations in cardiac structure and function commonly associated with dysglycemia result from multiple pathways related to additional comorbidities, not solely attributable to a glycemic‐related process, that ultimately contribute to the increased cardiovascular risk. Future studies are needed to explore the biological parameters, beyond common cardiovascular risk factors, and to assess more sensitive measures of cardiac structure and function, by the use of advanced cardiovascular imaging techniques compared with standard 2‐dimensional echocardiography, that explain the excess risk of cardiovascular events among diabetes subjects.
Limitations of this analysis should be noted. Survival and ascertainment biases may influence our results. Approximately 42% of the original ARIC participants, and 62% of those alive in 2011 to 2013 attended ARIC visit 5. This should be considered for the interpretation of the results as it may introduce attendance bias. Ascertainment of HF was essentially based on HF hospitalizations, as outpatient diagnosis and management were not uniformly available. 28 Although deaths were systematically ascertained by the ARIC surveillance or the National Death Index, specific cause of death was not available. Available follow‐up time after echocardiography at ARIC visit 5 and the multiple stratification steps may have limited our power to assess the relationship between cardiac measures and outcome across the glycemic categories. Our analysis included subjects with prevalent heart disease (history of HF and coronary artery disease) for having more generalizable results to all elderly subjects. Although this may represent a potential bias, we adjusted for these conditions in the final model and we also performed sensitivity analyses excluding subjects with prevalent heart disease. Classification of dysglycemia was based on a single laboratory value of HbA1c, in addition to self‐reports or history of medication use, and there was no glucose tolerance testing or repeated measurements performed, although self‐reported diabetes is known to be reliable and highly specific. 9 Finally, as with all observational analyses, we cannot rule out the possibility of residual confounding.
Nevertheless, strengths of this study need to be mentioned as well: ARIC is a well‐characterized cohort with a large proportion of women and Black participants. The high prevalence of prediabetes and diabetes in this age group is not uncommon and provides significant statistical power. Echocardiographic analyses were systematic, comprehensive, and of high‐quality. We used contemporary statistical methodology to determine the contribution of echocardiographic measures to the diabetes associated risk of HF.
Conclusions
In a large, biracial cohort of older adults, glycemic status did not modify the association between cardiac abnormalities and the risk of death or HF hospitalization. The diabetes‐associated increased risk of death or HF was only partially explained by alterations of cardiac structure and function. These data highlight the importance of factors other than cardiac abnormalities contributing to the development of HF and subsequent death in subjects with diabetes.
Sources of Funding
The Atherosclerosis Risk in Communities Study is performed as a collaborative study supported by the National Heart, Lung, and Blood Institute (contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Disclosures
Dr Cheng reports receiving research support from the National Institutes of Health. Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer‐Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, and Sarepta. Dr Shah reports other from Novartis and personal fees from Philips Ultrasound and Bellerophon Therapeutics, outside the submitted work. Dr Skali received stock options in OptimizeRx for consulting/advisory roles. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions. Drs Inciardi and Skali had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022308
For Sources of Funding and Disclosures, see page 9.
References
- 1. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Itzhaki Ben Zadok O, Kornowski R, Goldenberg I, Klempfner R, Toledano Y, Biton Y, Fisman EZ, Tenenbaum A, Golovchiner G, Kadmon E, et al. Admission blood glucose and 10‐ year mortality among patients with or without pre‐existing diabetes mellitus hospitalized with heart failure. Cardiovasc Diabetol. 2017;16:102. doi: 10.1186/s12933-017-0582-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71:339–351. doi: 10.1016/j.jacc.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 4. Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two‐faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–1727, 1727a–1727c. doi: 10.1093/eurheartj/ehv134 [DOI] [PubMed] [Google Scholar]
- 5. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4 [DOI] [PubMed] [Google Scholar]
- 6. Skali H, Shah A, Gupta DK, Cheng S, Claggett B, Liu J, Bello N, Aguilar D, Vardeny O, Matsushita K, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease the atherosclerosis risk in the community study. Circ Heart Fail. 2015;8:448–454. doi: 10.1161/CIRCHEARTFAILURE.114.001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 8. Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community‐dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self‐reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol. 2012;176:738–743. doi: 10.1093/aje/kws156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuzick J. A Wilcoxon‐type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112 [DOI] [PubMed] [Google Scholar]
- 12. Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–1619. doi: 10.2337/diacare.24.9.1614 [DOI] [PubMed] [Google Scholar]
- 13. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades‐Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Heerebeek L, Hamdani N, Handoko ML, Falcao‐Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJJ, Schalkwijk CG, Bronzwaer JGF, Diamant M, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550 [DOI] [PubMed] [Google Scholar]
- 16. Blomstrand P, Engvall M, Festin K, Lindstrom T, Lanne T, Maret E, Nyström FH, Maret‐Ouda J, Östgren CJ, Engvall J. Left ventricular diastolic function, assessed by echocardiography and tissue Doppler imaging, is a strong predictor of cardiovascular events, superior to global left ventricular longitudinal strain, in patients with type 2 diabetes. Eur Heart J Cardiovasc Imaging. 2015;16:1000–1007. doi: 10.1093/ehjci/jev027 [DOI] [PubMed] [Google Scholar]
- 17. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre‐clinical diastolic dysfunction a population‐based study. J Am Coll Cardiol. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278–285. doi: 10.1093/eurheartj/ehs188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inciardi RM, Giugliano RP, Claggett B, Gupta DK, Chandra A, Ruff CT, Antman EM, Mercuri MF, Grosso MA, Braunwald E, et al. Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail. 2019;21(12):1571–1579. doi: 10.1002/ejhf.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 21. Kadappu KK, Boyd A, Eshoo S, Haluska B, Yeo AET, Marwick TH, Thomas L. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging. 2012;13:1016–1023. doi: 10.1093/ehjci/jes084 [DOI] [PubMed] [Google Scholar]
- 22. Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, Drago A, Guazzi M, Ribichini FL, Cicoira M. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail. 2020;22:499–506. doi: 10.1002/ejhf.1677 [DOI] [PubMed] [Google Scholar]
- 23. Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050 [DOI] [PubMed] [Google Scholar]
- 24. Shah AM, Uno H, Køber L, Velazquez EJ, Maggioni AP, MacDonald MR, Petrie MC, McMurray JJV, Califf RM, Pfeffer MA, et al. The inter‐relationship of diabetes and left ventricular systolic function on outcome after high‐risk myocardial infarction. Eur J Heart Fail. 2010;12:1229–1237. doi: 10.1093/eurjhf/hfq179 [DOI] [PubMed] [Google Scholar]
- 25. Lee MMY, McMurray JJV, Lorenzo‐Almorós A, Kristensen SL, Sattar N, Jhund PS, Petrie MC, et al. Diabetic cardiomyopathy. Heart. 2019;105:337–345. doi: 10.1136/heartjnl-2016-310342 [DOI] [PubMed] [Google Scholar]
- 26. Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1:8–15. doi: 10.1161/JAHA.111.000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. J Am Coll Cardiol HF. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 28. Shah AM, Claggett B, Kitzman D, Biering‐Sørensen T, Jensen JS, Cheng S, Matsushita K, Konety S, Folsom AR, Mosley TH, et al. Contemporary assessment of left ventricular diastolic function in older adults: the Atherosclerosis Risk in Communities study. Circulation. 2017;135:426–439. doi: 10.1161/CIRCULATIONAHA.116.024825 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


