Abstract
Background
Inadequate pulmonary vascular growth results in morbidity for many children with single‐ventricle heart disease (SVHD). Endothelin 1 (ET1) is a potent vasoconstrictor and stimulator of pulmonary artery smooth muscle proliferation. Circulating ET1 levels and their association with outcomes have not been studied during early SVHD palliation. We aimed to define circulating levels of ET1 in patients with SVHD undergoing stage 2 palliation and evaluate their relationship to postoperative hypoxemia. We hypothesized that patients with SVHD with higher ET1 concentration would have a greater post–stage 2 hypoxemia.
Methods and Results
Prospective cohort study of 55 subjects with SVHD undergoing stage 2 palliation and 50 controls. Samples for ET1 analysis were collected at preoperation (systemic and pulmonary vein) and 2, 24, and 48 hours postoperation for cases and a single time point for controls. The primary outcome was percentage of first 48 postoperative hours with clinically significant hypoxemia (saturation, <70%). ET1 concentration was lower in preoperative cases than controls (2.2 versus 2.7 pg/mL; P=0.0015) and in the pulmonary vein than systemic vein (1.7 versus 2.2 pg/mL; P<0.001). ET1 level increased by 2 hours postoperation and trended back to baseline by 48 hours. Higher preoperative pulmonary vein ET1 and 2 hours postoperative ET1 were associated with larger hypoxemia burden (10.6% versus 2.7% [P=0.0081]; and 7.6% versus 3.2% [P=0.01], respectively). Multivariable testing demonstrated ET1 concentration and cardiopulmonary bypass time were associated with hypoxemia, whereas catheterization measurements and clinical variables were not.
Conclusions
Infants with SVHD with higher perioperative ET1 concentration experience more post–stage 2 hypoxemia. ET1 activity may be a modifiable risk factor of pulmonary vascular inadequacy for stage 2 palliation.
Keywords: biomarkers, endothelin 1, Glenn operation, hypoxemia, single‐ventricle palliation
Subject Categories: Biomarkers, Endothelium/Vascular Type/Nitric Oxide, Translational Studies, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- ET1
endothelin 1
- PDE5i
phosphodiesterase type 5 inhibitor
- SVHD
single‐ventricle heart disease
Clinical Perspective
What Is New?
Infants with single‐ventricle heart disease undergoing stage 2 palliation experience a moderate burden of postoperative morbidity related to insufficient pulmonary blood flow that is not well predicted by either demographic parameters or preoperative catheterization metrics.
After adjusting for clinical confounders, single‐ventricle infants with higher preoperative and postoperative endothelin 1 concentration experienced a greater burden of postoperative pulmonary vascular morbidity than subjects with lower endothelin 1 levels.
What Are the Clinical Implications?
Interstage infants with single‐ventricle heart disease as a group have lower circulating endothelin 1 levels than similar‐aged healthy infants, suggesting that failure to adequately suppress endothelin 1 may be the key pathophysiologic derangement for the subset who experience postoperative pulmonary vascular inadequacy.
A severe form of congenital heart disease where patients have only one functional pumping chamber, single‐ventricle heart disease (SVHD), affects 2 to 8 per 10 000 live births and is typically fatal without intervention. 1 Although there is no surgical cure for SVHD, 3‐staged palliation allows many patients to survive into adulthood. 2 From the first week of life (after stage 1 palliation for most patients) until the stage 2 operation, subjects are considered “interstage.” During this time, regardless of patient‐specific cardiac anatomical features, subjects with SVHD have an unobstructed pathway from the single ventricle to the body and a pressure‐restrictive source of pulmonary blood flow, commonly a shunt between the aorta or the right ventricle and the pulmonary arteries. The second of the 3 staged operations, the superior cavopulmonary anastomosis (stage 2, bidirectional Glenn, or hemi‐Fontan), is most commonly performed between 4 and 6 months of age. 2 This operation is unique in that the mechanism of blood delivery to the lungs is converted from an active process driven by ventricular contraction to a passive process via a direct connection between the superior vena cava and the central pulmonary arteries. Because of this, the peri–stage 2 period is the optimal time to evaluate both preoperative markers of interstage pulmonary vascular growth and the postoperative adequacy of the pulmonary vasculature to accept passive blood flow.
For many children with SVHD, inadequate pulmonary vascular growth and development result in morbidity before and after stage 2 palliation. 3 Among infants completing stage 2, up to 25% experience complications in the immediate postoperative period directly linked to impaired pulmonary blood flow, including severe hypoxemia, respiratory failure, and persistent pleural effusions. 3 Current evaluation to determine stage 2 readiness is limited to serial oxygen saturation measurements, echocardiograms, cardiac magnetic resonance imaging, computed tomographic angiography, and cardiac catheterizations. 4 There are no validated biomarkers or personalized medicine strategies to identify subjects in this population at risk for failure to tolerate stage 2 physiological features.
Endothelin 1 (ET1) is a potent vasoconstrictor and stimulator of pulmonary artery smooth muscle cell proliferation that is produced by pulmonary vascular endothelial cells. 5 , 6 , 7 It is known to play an important role in the pathogenesis of pulmonary arterial hypertension in children with 2‐ventricle circulation. 8 , 9 Data on the role of ET1 in the pulmonary vasculature of patients with SVHD are highly limited. A small study in older patients with SVHD undergoing stage 3 palliation demonstrated elevated postoperative circulating ET1. 10 Another study found elevated tissue‐level ET1 and endothelin receptor expression associated with increased medial hypertrophy in the pulmonary vasculature of patients who died secondary to failed SVHD circulation. 11 And, treatment of adult patients with SVHD with short courses of endothelin receptor antagonists produces modest benefit in exercise capacity. 12 , 13 , 14 Circulating ET1 levels and their association with pulmonary hemodynamics have not been previously studied early in the staged palliation process when critical vascular growth and development are occurring.
Herein, we present a prospective, cohort study of circulating ET1 levels in infants with SVHD undergoing stage 2 palliation. We hypothesized that interstage infants with SVHD would have different circulating ET1 concentration compared with similar age healthy controls, that the circulating ET1 level would increase immediately after stage 2 palliation, and that patients with SVHD with higher ET1 concentration before and after surgery would have a greater burden of post–stage 2 complications associated with pulmonary vascular inadequacy.
METHODS
The Colorado Multiple Institution Review Board approved this study. Written informed consent was obtained from the study subjects’ parents in all cases. The data that support the findings of this study are available from the corresponding author on reasonable request.
Subjects
Inclusion criteria for this cohort have been previously described. 15 Briefly, we prospectively enrolled consecutive subjects, aged 31 days to 2 years, at Children’s Hospital Colorado with SVHD either undergoing pre–stage 2 catheterization or a stage 2 operation without plans for cardiac catheterization. Subjects who underwent stage 2 without sample collection at cardiac catheterization did so because (1) cardiac catheterization was performed before the research team had an opportunity to obtain informed consent or (2) catheterization was deemed not required by the clinical team. Stage 2 palliation included any form of superior cavopulmonary anastomosis (Glenn or hemi‐Fontan operations) independent of whether a patient had a prior stage 1 palliation. Potential subjects with a persistent, additional pulsatile source of pulmonary blood flow after stage 2 (so‐called 1.5‐ventricle repair) were excluded.
We identified similar‐aged control subjects from the surgical schedule at Children’s Hospital Colorado. Control infants had weight >4 kg, were aged 3 to 12 months, and were undergoing anesthesia for elective, noncardiac procedures with clinical need for intravenous access. We excluded potential control subjects if they had any known or suspected cardiac, pulmonary, infectious, or genetic abnormalities.
Clinical Data
Clinical data were extracted from the electronic medical record (Epic Systems, Verona, WI). Postoperative oxygen saturation measurements recorded at 1‐minute intervals (>1400 per 24 hours) were downloaded from the BedMaster monitoring system in the cardiac intensive care unit (Anandic Medical Systems, Feuerthalen, Switzerland). Study data were collected and managed using REDCap electronic data capture tools hosted at University of Colorado.
Prespecified variables were identified to evaluate the relationship between classic clinical predictors of stage 2 readiness, protein biomarker levels, and clinical outcomes. The primary clinical variable of interest was percentage of time in the first 48 postoperative hours with clinically significant hypoxemia, defined as an oxygen saturation <70% (48 hour low sat %). This variable was calculated as the ratio of the number of recorded saturation levels <70%/the total number of saturation recordings in the first 48 postoperative hours, times 100. Secondary variables included mean saturation levels, endotracheal intubation time, chest tube days, volume of chest tube drainage, postoperative length of stay, discharge on a phosphodiesterase type 5 inhibitor (PDE5i), incidence diagnostic catheterization within 3 months after stage 2, and use of NO therapy in the first 24 postoperative hours.
Sample Collection
All preoperative samples were obtained under general anesthesia. For subjects with SVHD enrolled at the time of pre–stage 2 catheterization, systemic venous samples were obtained before any procedural interventions. An additional pulmonary venous sample was collected during catheterization for comparison to the systemic venous samples. For subjects enrolled at other times, a systemic venous sample was obtained on the day of stage 2 palliation before initiation of cardiopulmonary bypass. Postoperative systemic venous samples were obtained at 2, 24, and 48 hours after arrival in the cardiac intensive care unit. Control subject samples were obtained from a systemic vein after induction of anesthesia at the time of after intravenous catheter placement.
Protein Analysis
Serum was extracted from all blood samples at the time of collection, aliquoted, frozen at −80 °C, and analyzed in batches. ET1 concentration was measured in each sample using ELISA, according to manufacturer’s instructions (R&D Systems, Inc, Minneapolis, MN) in the Clinical and Translational Research Center Core Laboratory at Children’s Hospital Colorado, a College of American Pathologists– and Clinical Laboratory Improvement Amendments–accredited facility.
Statistical Analysis
Demographic and clinical variables were summarized using descriptive statistics, as indicated by the distribution of the data. Student t test, Wilcoxon rank sum test, Wilcoxon signed rank test, and Spearman correlation testing were used to compare continuous variables, and χ2 testing was used for categorical variables. JMP pro v.15.1.0 was used for descriptive analysis. P<0.05 was considered statistically significant.
Multivariable modeling was performed in JMP pro v.15.1.0 to assess the relationship between candidate clinical predictor variables, ET1 levels, and the a priori primary clinical outcome (48 hour low sat %). Considered covariates included those we identified as being clinically relevant to postoperative outcomes in the population with SVHD, including cardiopulmonary bypass time, weight, oxygen saturation measured at pre–stage 2 catheterization, ventricular morphological features, pulmonary vascular resistance, mean pulmonary artery pressure, postoperative blood hemoglobin concentration, and postoperative arterial partial pressure of carbon dioxide. We considered clinical covariables with P<0.20 on single variable testing for inclusion in a multivariable model. We fit the multivariable model with all such covariables. From this initial edition of the model, we first simultaneously removed any variables with P>0.15 and then iteratively removed the single variable with the highest P value until only metrics with P<0.05 remained. We noted the effect of removal of each nonsignificant variable on the β estimates of the remaining variables as well as on the performance of the model to evaluate for potential confounding relationships. The reported models reflect this final version with only significant variables included. Because ET1 levels were not expected to be independent of each other at the different time points for a given patient, separate models were created considering the relevant clinical variables and one ET1 level per model.
RESULTS
Study Population
Fifty‐five cases with SVHD and 50 similar aged healthy controls were enrolled. Preoperative systemic vein samples were obtained for all cases and controls. A preoperative pulmonary vein sample was collected for 41 cases. Two subjects were deemed to not be good candidates for continued single‐ventricle palliation and underwent heart transplantation instead of stage 2 palliation. One subject ultimately underwent complete repair with a 2‐ventricle circulation, and 2 additional subjects were still awaiting stage 2 palliation at the time of batch analysis. Fifty cases were therefore included in the postoperative analysis. Demographics and clinical characteristics are presented in Table 1. One patient was on a PDE5i, and one was on an endothelin receptor antagonist, at the time of his/her preoperative sample collection. The endothelin receptor antagonist patient ultimately underwent heart transplantation and is therefore not included in the postoperative cohort. Two additional subjects were started on a PDE5i after their pre–stage 2 catheterization but before stage 2 palliation.
Table 1.
Demographics
| Variable | SVHD cases (n=55) | Controls (n=50) | P value |
|---|---|---|---|
| Age, mo | 4.45 (2.7) | 7.46 (2.5) | <0.0001* |
| Weight, kg | 5.80 (0.9) | 7.85 (1.2) | <0.0001* |
| Female sex, n (%) | 24 (43.6) | 18 (36.0) | 0.55 |
| Dominant RV, n (%) | 43 (78.1) | ||
| Stage 1 operation, n (%) | |||
| Norwood | 36 (65.5) | ||
| Shunt | 8 (14.5) | ||
| PDA stent | 1 (1.8) | ||
| PA band | 6 (10.9) | ||
| Other | 1 (1.8) | ||
| None | 3 (5.4) | ||
| PA pressure, mm Hg | 12 (12–15) | ||
| PVRi, units×m2 | 2.0 (1.6–2.6) | ||
| Ventricle EDP, mm Hg | 6.0 (5.0–7.0) | ||
| Qp/Qs | 1.1 (0.7–1.6) | ||
| CPB time, min | 133 (84–161) | ||
| ETT duration, h | 16 (8–27) | ||
| Chest tube duration, d | 2 (2–3) | ||
| Pleural drainage, mL | 137 (70–226) | ||
| Discharge PDE5i therapy, n (%) | 17 (31) | ||
| Length of stay, d | 7 (5–20) | ||
| Catheterization within 3 mo, n (%) | 19 (35) | ||
| 48‐h mean sat % | 79.2 (75.6–81.2) | ||
| 48‐h low sat % | 4.10 (2.3–11.5) | ||
Variables are represented as mean (SD), number (percentage), or median (interquartile range). 48‐hour low sat % indicates percentage of oxygen saturation values <70% recorded at 1‐minute intervals during first 48 postoperative hours; 48‐h mean sat %, average of oxygen saturation values recorded at 1‐minute intervals during first 48 postoperative hours; CPB, cardiopulmonary bypass; EDP, end‐diastolic pressure; ETT, endotracheal tube; PA, pulmonary artery; PDA, patent ductus arteriosus; PDE5i, phosphodiesterase type 5 inhibitor; PVRi, indexed pulmonary vascular resistance; Qp/Qs, ratio of pulmonary/systemic blood flow; RV, right ventricle; and SVHD, single‐ventricle heart disease.
Signifies P < 0.05.
Post–Stage 2 Outcomes
The postoperative SVHD population (n=50) experienced moderate morbidity. The median percentage of time in the first 2 postoperative days spent with oxygen saturation <70% (48 hour low sat %) was 4.1% (interquartile range [IQR], 2.3%–11.5%; range, 0.4%–57.4%). Subjects experienced median 2 chest tube days (IQR, 2–3 days) with 137 mL of aggregate pleural drainage (IQR, 70–226 mL). Median postoperative endotracheal intubation time was 16 hours (IQR, 8–27 hours). No patients required an emergent return to the operating room, and all patients survived to hospital discharge. All patients received oxygen therapy throughout their post–stage 2 course and were discharged home on supplemental oxygen (0.25 to 0.5 liters per minute by nasal cannula in almost all cases) per our local protocol. Median length of stay was 7 days (IQR, 5–20 days). At discharge following stage 2, 17 subjects were receiving PDE5i therapy, and no subjects were receiving an endothelin receptor antagonist. Median oxygen saturation concentration at discharge was 83%. Nineteen subjects ultimately underwent diagnostic cardiac catheterization within the first 3 months following stage 2 palliation because of anatomic concerns based on noninvasive imaging or persistent hypoxemia.
We did not identify a significant relationship between conventional catheterization‐derived metrics of pulmonary vascular readiness for stage 2 palliation and postoperative hypoxemia. Specifically, when divided by the population median, subjects with higher versus lower mean pulmonary artery pressure (median, 12 mm Hg) had similar 48 hours low sat % (4.4% versus 3.8%; P=not significant) and 48 hours mean saturation (78.5% versus 80.0%; P=not significant). Dividing the population by preoperative pulmonary vascular resistance (median, 2 Woods units×m2), ventricular end‐diastolic pressure (median, 6 mm Hg) or ratio of pulmonary/systemic blood flow (median, 1.1) also did not identify differences in postoperative hypoxemia.
ET1 Concentration
Pre–stage 2 subjects with SVHD demonstrated a median systemic vein ET1 concentration of 2.2 pg/mL (Figure). Compared with control subjects (median, 2.7 pg/mL), SVHD cases had lower circulating systemic vein ET1 concentration (P=0.0015; Figure – Panel A). The subjects with SVHD demonstrated net pulmonary clearance of ET1 with higher ET1 concentration in the systemic veins than in the pulmonary veins (2.2 versus 1.7 pg/mL; P<0.001; Figure – Panel B). Systemic vein ET1 concentration increased in the immediate postoperative period with a steady return to baseline by 48 hours. Median ET1 level at 2 hours postoperatively was 4.0 pg/mL, with a decline to 2.7 pg/mL by 24 hours and 1.9 pg/mL by 48 hours (Figure – Panel C).
Figure 1. Endothelin 1 (ET1) distribution.
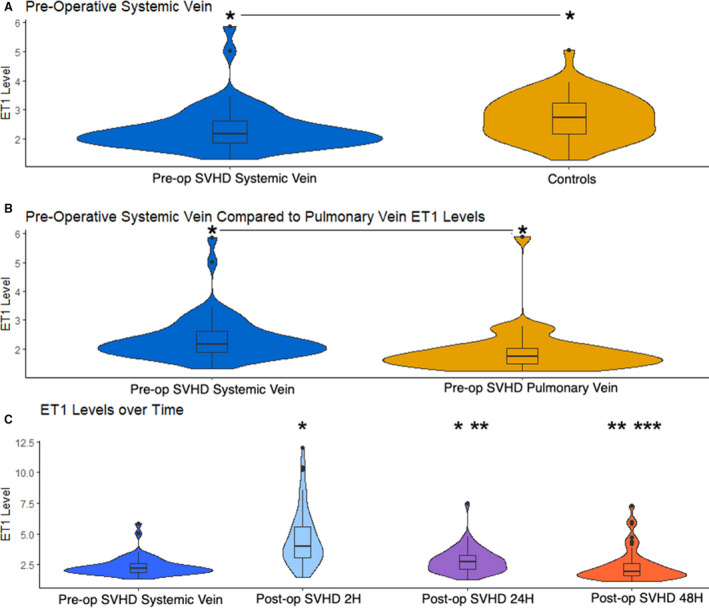
Violin plots depicting ET1 concentration. The upper and lower borders of the box represent the upper and lower quartiles. The middle horizontal line represents the median. The upper and lower whiskers represent the maximum and minimum values of nonoutliers. Extra dots represent outliers. Wider distribution represents a greater proportion of values at that concentration. A, Interstage infants with single‐ventricle heart disease (SVHD) have lower circulating ET1 concentration than similar aged healthy controls. *P<0.05. B, Interstage infants with SVHD demonstrate net pulmonary clearance of ET1 with lower concentration in the pulmonary vein than in the systemic vein sample. *P<0.05. C, ET1 concentrations increase immediately after stage 2 palliation and then trend down to baseline by 48 hours postoperatively (Post‐op). *P<0.05 compared to preoperatively (Pre‐op), **P<0.05 compared to 2 hours, ***P<0.05 compared to 24 hours. All P values reflect results of Wilcoxon test. All ET1 levels reported in pg/mL.
Relationship Between ET1 and Clinical Variables
On single‐variable testing, subjects with higher preoperative pulmonary vein ET1 demonstrated a greater postoperative hypoxemia burden than those with lower ET1 levels (Table 2). Similarly, the mean saturation during the first 48 postoperative hours was lower among subjects with higher pulmonary vein ET1 concentration. Higher preoperative pulmonary vein ET1 subjects also experienced greater incidence of diagnostic catheterization within 3 months after stage 2, more use of NO in the first 24 post–stage 2 hours, more frequent discharge on PDE5i, and a trend toward longer length of stay (Table 2). We did not identify a difference in preoperative catheterization‐derived metrics of pulmonary vascular disease between subjects with higher versus lower pulmonary vein ET1 concentration (Table 2). Two‐hour postoperation ET1 concentration also showed a significant relationship with clinical outcomes (Table 3). Subjects with 2 hours ET1 >4.0 pg/mL demonstrated greater 48 hours low sat % and lower 48 hours mean saturation than those with lower ET1 levels. Similarly, high 2 hours ET1 subjects more frequently received NO during recovery and PDE5i therapy at discharge. Although not reaching statistical significance, they also showed a trend toward longer length of stay and more frequent post–stage 2 diagnostic catheterization requirement (Table 3) than the low 2 hours ET1 group.
Table 2.
Pre–Stage 2 Pulmonary Vein ET1 Concentration
| Variable | Pre‐PV ET1 ≤1.7 pg/mL | Pre‐PV ET1 >1.7 pg/mL | P value |
|---|---|---|---|
| Age, mo | 4.4 (4.0) | 5.2 (3.9) | 0.38 |
| Weight, kg | 5.9 (0.7) | 5.8 (1.2) | 0.95 |
| Female sex, n (%) | 9 (45.0) | 11 (52.4) | 0.64 |
| Single RV, n (%) | 17 (85.0) | 16 (76.2) | 0.70 |
| PA pressure, mm Hg | 12 (11–14) | 14.0 (12–15) | 0.17 |
| PVRi, units×m2 | 1.9 (1.45–2.3) | 2.0 (1.5–3.0) | 0.66 |
| Ventricle EDP, mm Hg | 6.0 (5.0–7.0) | 6.0 (5.0–7.0) | 0.93 |
| Qp/Qs | 1.1 (0.7–1.5) | 1.1 (0.7–1.4) | 0.70 |
| CPB time, min | 95.5 (79.5–143.0) | 140.5 (82.0–191.0) | 0.08 |
| Postoperative hemoglobin | 14.2 (12.9–14.9) | 13.4 (12.4–14.7) | 0.45 |
| Postoperative arterial pco 2 | 48 (43–51) | 48 (45–50) | 0.78 |
| 2‐h Postoperative cerebral NIRS | 57 (50–62) | 51 (35–55) | 0.04* |
| Any NO in first 24 h, % | 21 | 63 | 0.01* |
| ETT duration, h | 10.0 (7.0–18.0) | 20.5 (9.0–38.0) | 0.10 |
| Chest tube duration, d | 2.0 (2.0–2.0) | 2.0 (2.0–4.0) | 0.09 |
| Pleural drainage, mL | 159.5 (36.0–254.0) | 127.0 (91.0–184.0) | 0.97 |
| Length of stay, d | 5 (5–9) | 8 (6–19) | 0.12 |
| Discharge PDE5i therapy, % | 16 | 53 | 0.02* |
| Catheterization within 3 mo, % | 11 | 47 | 0.01* |
| 48‐h mean sat % | 80.8 (79.6) | 75.8 (73.4) | 0.0007* |
| 48‐h low sat % | 2.7 (1.9–5.4) | 10.6 (3.4–24.2) | 0.0081* |
Variables are represented as mean (SD), number (%), or median (interquartile range). 48‐h low sat % indicates percentage of oxygen saturation values <70% recorded at 1‐minute intervals during first 48 postoperative hours; 48‐h mean sat %, average of oxygen saturation values recorded at 1‐minute intervals during first 48 postoperative hours; CPB, cardiopulmonary bypass; EDP, end‐diastolic pressure; ET1, endothelin 1; ETT, endotracheal tube; NIRS, near‐infrared spectroscopy; PA, pulmonary artery; PDE5i, phosphodiesterase type 5 inhibitor; Pre‐PV ET1, ET1 concentration measured preoperatively in the pulmonary vein; PVRi, indexed pulmonary vascular resistance; Qp/Qs, ratio of pulmonary/systemic blood flow; and RV, right ventricle.
Signifies P < 0.05.
Table 3.
Two Hours Post–Stage 2 ET1 Concentration
| Variable | ET1 postop 2 h ≤4.0 pg/mL | ET1 postop 2 h >4.0 pg/mL | P value |
|---|---|---|---|
| Age, mo | 4.2 (3.6) | 3.8 (3.3) | 0.34 |
| Weight, kg | 5.7 (0.7) | 5.7 (0.6) | 0.90 |
| Female sex, n (%) | 13 (52.0) | 9 (36.0) | 0.39 |
| CPB time, min | 97 (84–145) | 147 (92–178.5) | 0.07 |
| Postoperative hemoglobin concentration | 14.5 (13.2–14.9) | 13.8 (12.6–15.1) | 0.37 |
| Postoperative arterial pco 2 | 48 (46–50) | 48 (45–52) | 0.77 |
| 2 h Postoperative cerebral NIRS | 58 (54–62) | 46 (35–55) | 0.0005* |
| Any NO in first 24 h, % | 28 | 60 | 0.02* |
| ETT duration, h | 10 (8–19) | 17 (9–48) | 0.19 |
| Chest tube duration, d | 2 (2–2) | 3 (2–4) | 0.009* |
| Pleural drainage, mL | 167 (101–247) | 130 (70–226) | 0.43 |
| Length of stay, d | 6 (5–11) | 8 (6–30) | 0.10 |
| Discharge PDE5i therapy, % | 16 | 54 | 0.005* |
| Catheterization within 3 mo, % | 28 | 48 | 0.15 |
| 48‐h mean sat % | 80.2 (2.9) | 76.4 (5.1) | 0.0024* |
| 48‐h low sat % | 3.2 (1.7–5.5) | 7.6 (3.3–26.4) | 0.01* |
Variables are represented as mean (SD), number (percentage), or median (interquartile range). 48‐h low sat % indicates percentage of oxygen saturation values <70% recorded at 1‐minute intervals during first 48 postoperative hours; 48‐h mean sat %, average of oxygen saturation values recorded at 1‐minute intervals during first 48 postoperative hours; CPB, cardiopulmonary bypass; ET1, endothelin 1; ET1 post‐op 2 hours, ET1 concentration measured at 2 hours postoperatively in a systemic vein; ETT, endotracheal tube; NIRS, near‐infrared spectroscopy; and PDE5i, phosphodiesterase type 5 inhibitor.
Signifies P < 0.05.
There was no significant relationship identified between the preoperative systemic vein ET1 concentration and the pulmonary vascular resistance, pulmonary artery pressure, pulmonary blood flow, or ventricular end‐diastolic pressure. Postoperative saturations, endotracheal intubation time, chest tube time, and pleural drainage were not significantly different between subjects with higher versus lower preoperative systemic vein ET1 concentration. Similarly, there was no significant relationship identified between systemic to pulmonary vein ET1 difference within an individual patient and the clinical outcomes evaluated.
Multivariable Analysis
To evaluate the linear relationship between candidate predictors and outcomes of interest, we initially performed single‐variable testing (Table 4). We did identify a statistically significant linear association between higher ET1 concentration (as measured in the pulmonary vein preoperatively or in the systemic vein postoperatively at 2 and 24 hours) and greater postoperative hypoxemia burden. There was no significant linear relationship between patient weight, cardiopulmonary bypass time, or catheterization‐derived metrics of pulmonary vascular disease with postoperative hypoxemia.
Table 4.
Linear Correlation With Postoperative Hypoxemia
| Variable | Spearman ρ | P value |
|---|---|---|
| Weight | −0.01 | 0.9210 |
| CPB time | 0.25 | 0.0827 |
| MPAP | 0.03 | 0.8607 |
| PVRi | 0.06 | 0.6729 |
| Systemic saturation at catheterization | −0.28 | 0.0535 |
| Ventricular EDP | 0.18 | 0.2296 |
| Qp/Qs | −0.15 | 0.3042 |
| Postoperative hemoglobin concentration | 0.0002 | 0.9989 |
| Postoperative arterial pco 2 | −0.01 | 0.9507 |
| ET1 SV preoperatively | 0.19 | 0.1945 |
| ET1 PV preoperatively | 0.52 | 0.0010* |
| ET1 2 h postoperatively | 0.36 | 0.0104* |
| ET1 24 h postoperatively | 0.42 | 0.0030* |
| ET1 48 h postoperatively | 0.27 | 0.0671 |
Results of single‐variable linear correlation analysis are shown. Postoperative hypoxemia measured as percentage of oxygen saturation values <70% recorded at 1‐minute intervals during first 48 postoperative hours for all variables. CPB indicates cardiopulmonary bypass; EDP, end‐diastolic pressure; ET1, endothelin 1; MPAP, mean pulmonary artery pressure; PV, pulmonary vein; PVRi, indexed pulmonary vascular resistance; Qp/Qs, ratio of pulmonary/systemic blood flow; and SV, systemic vein.
Signifies P < 0.05.
In multivariable analysis, higher preoperative pulmonary vein ET1, higher 2 hours postoperative ET1, and higher 24 hours postoperative ET1 were independently associated with greater 48 hours low sat % after accounting for clinical covariables (Table 5). Longer cardiopulmonary bypass time was also associated with hypoxemia burden in all models. Weight, oxygen saturation measured at pre–stage 2 catheterization, ventricular morphological features, pulmonary vascular resistance, and mean pulmonary artery pressure were not associated with postoperative hypoxemia.
Table 5.
Multivariable Analysis
| Variables | Estimate | SE | P value |
|---|---|---|---|
| Model 1 | R 2=0.53 | ||
| Intercept | −24.771 | 7.449 | 0.0022 |
| ET1 PV preoperatively | 12.448 | 4.514 | 0.0094 |
| CPB time | 0.103 | 0.026 | 0.0004 |
| Model 2 | R 2=0.40 | ||
| Intercept | −13.67 | 4.936 | 0.0081 |
| ET1 postoperatively at 2 h | 2.586 | 0.769 | 0.0016 |
| CPB time | 0.099 | 0.029 | 0.0016 |
| Model 3 | R 2=0.50 | ||
| Intercept | −21.60 | 5.157 | 0.0001 |
| ET1 postoperatively at 24 h | 7.535 | 1.567 | <0.0001 |
| CPB time | 0.090 | 0.027 | 0.0016 |
Multivariable analysis of the relationship between candidate clinical predictors and percentage of oxygen saturation values <70% recorded at 1‐minute intervals during first 48 postoperative hours. Estimate reflects the strength of association scaled to the absolute value of the variable. Models considered clinical variables, including CPB time, mean pulmonary artery pressure, pulmonary vascular resistance, systemic saturation at catheterization, ventricle end‐diastolic pressure, weight, ventricular morphological features, postoperative hemoglobin, and postoperative arterial pco2 (partial pressure of carbon dioxide). Model 1 considered preoperative PV ET1. Model 2 considered 2 hours postoperative ET1. Model 3 considered 24 hours postoperative ET1. CPB indicates cardiopulmonary bypass; ET1, endothelin 1; and PV, pulmonary vein.
DISCUSSION
In this study, we report the novel finding that subjects with higher perioperative ET1 concentration experienced more hypoxemia during their recovery from stage 2 SVHD palliation than those with lower ET1 levels. Our data support a positive linear relationship between ET1 concentration and percentage of the first 48 hours spent with oxygen saturation <70%. The association between perioperative ET1 concentration and postoperative hypoxemia remained significant in multivariable analysis after accounting for relevant clinical variables. More important, the relationship between ET1 concentration and post–stage 2 outcomes is not limited to transient, self‐resolved hypoxemia; high ET1 subjects also experienced greater requirement for inhaled NO, higher rates of discharge on PDE5i therapy, increased incidence of requiring post–stage 2 diagnostic catheterization, and a trend toward longer postoperative length of stay. Furthermore, we did not find a relationship between commonly tracked risk factors of pulmonary vascular inadequacy (such as pulmonary artery pressure, preoperative oxygen saturation, and patient weight) and postoperative hypoxemia.
Hypoxemia in the Stage 2 SVHD Population
Prior studies have demonstrated that, although operative mortality is low in the modern era, the post–stage 2 morbidity burden is significant. 3 This burden includes severe hypoxemia, prolonged length of stay, unplanned reoperation, prolonged chest tube requirement, and endotracheal reintubation. 16 Although prior studies have noted a relationship between the intermediate term outcome hospital length of stay and certain clinical variables, such as lower weight, younger age, higher pulmonary artery pressure, and longer cardiopulmonary bypass time, risk factors and potential mechanisms for early intolerance of stage 2 circulation remain elusive. 3 , 16 , 17 , 18 , 19 In our study, we sought to address this gap by focusing on an immediate postoperative outcome, 48 hours hypoxemia burden, that we expected to be strongly tied to pulmonary vascular adequacy. Interestingly, we were not able to reproduce the relationship between the noted clinical variables and postoperative outcomes in our cohort. We hypothesize, therefore, that pulmonary vascular development may be more significantly affected by patient‐specific biochemical factors, such as ET1 exposure or dysregulation of other pathways, than by chronological factors, such as age.
Perioperative ET1 Activation
ET1 is a potent vasoconstrictor and stimulator of vascular smooth muscle cell proliferation known to cause pathologic changes at the pulmonary arteriolar level in multiple populations. 6 Studies have shown ET1 activity to be crucial to the pulmonary vascular response to hypoxemia. 20 Circulating ET1 levels are known to be elevated in patients with pulmonary arterial hypertension as well as in those with pulmonary hypertension associated with congenital heart disease. 5 , 21 , 22 Given the prior work demonstrating elevated ET1 concentration in subjects with abnormal pulmonary vasculature, it was an unexpected finding that our cohort demonstrated decreased preoperative circulating ET1 compared with healthy controls. It is possible that suppression of ET1 in many infants with SVHD may reflect an adaptive response to maintain adequate pulmonary blood flow during the interstage period and infants with normal ET1 levels represent an at‐risk population. The extent to which the phenotype of ET1 suppression continues into the phase of chronic passive pulmonary blood flow late following stage 2 and the mechanisms underlying this suppression are important areas for future study.
Prior studies have demonstrated that the lung is an important site of both ET1 synthesis and clearance. 23 ET1 concentration can vary between systemic and pulmonary venous sources with some but not all patients demonstrating net pulmonary clearance of ET1. 22 , 24 The clinical significance of the net change in ET1 at different sites in different patient populations is not well delineated. Our population with SVHD showed net clearance of ET1 but no consistent relationship between systemic vein to pulmonary vein ET1 difference and clinical outcomes. Future studies evaluating the relationships among circulating ET1 concentration sampled at different sites, ET1 signaling at the level of the pulmonary endothelium, and clinical phenotype will be important.
Limited data exist on immediate postoperative changes in circulating ET1 among children having cardiac surgery. A small study in patients undergoing stage 3 SVHD palliation showed a postoperative increase in ET1 concentration by 2 to 6 hours after surgery with a subsequent decline by 24 hours. 25 Another study of children with biventricular hearts undergoing surgery with cardiopulmonary bypass also demonstrated a postoperative increase in circulating ET1 with subsequent decline by 24 hours. 26 This ET1 activation may be attributable to vascular endothelial injury induced by cardiopulmonary bypass. 27 Our data mirror these findings, with the highest ET1 levels seen at 2 hours postoperatively, decreased but still elevated from baseline ET1 concentration at 24 hours, and similar to baseline levels at 48 hours. Infants with SVHD with higher 2 and 24 hours postoperative ET1 level had greater hypoxemia burden, suggesting that, in this population, post–cardiopulmonary bypass ET1 activation may be pathologic and merits consideration as a therapeutic target.
Relationship Between ET1 and Clinical Outcomes
Multiple prior studies have demonstrated the significance of ET1 activity in regulating the pulmonary vascular tone of children with different forms of unrepaired congenital heart disease. 28 , 29 Few studies, however, have described changes in ET1 concentration in the immediate postoperative period or their effects on markers of pulmonary blood flow. In the previously mentioned cohort of stage 3 subjects described by Hiramatsu et al, the degree of ET1 concentration increase after surgery did correlate with elevated postoperative pulmonary vascular resistance, suggesting a potential role for ET1 in modulating postbypass physiological features. 25
In our cohort, we report the finding that increased circulating levels of both preoperative and postoperative ET1 are independently associated with post–stage 2 hypoxemia. Single‐variable testing also identified a relationship between ET1 concentration and the secondary outcomes of PDE5i therapy at discharge, NO therapy use in the intensive care unit, and need for diagnostic catheterization within 3 months following stage 2 palliation. These data support a role for ET1 concentration as a clinical biomarker of pulmonary vascular risk for stage 2 palliation. Our study also raises the possibility that ET1 blockade could be a therapeutic target to improve pulmonary blood flow and vascular development in a subset of the young population with SVHD with increased endogenous ET1 exposure. Recruiting a validation cohort to confirm ET1 as a predictor of adverse outcomes and determining whether those outcomes can be mitigated effectively through endothelin receptor blockade will be important areas for future study. Similarly, evaluation of the extent to which ET1 changes may affect not only immediate recovery from surgery but also long‐term pulmonary vascular growth and development will be important.
Mechanistically, we speculate that for some subjects with SVHD, failure to adequately suppress ET1 in the interstage period may be the inciting pathogenic event. In the context of SVHD, even what would otherwise be considered normal ET1 exposure could lead to a sufficient increase in pulmonary vascular tone and smooth muscle cell hypertrophy to predispose affected patients to intolerance of passive pulmonary blood flow. A significant increase in circulating ET1 immediately following surgery, caused by endothelial injury from cardiopulmonary bypass, could further worsen pulmonary blood flow by acutely increasing vascular tone and leading to significant hypoxemia and potentially further increases in ET1 levels. Clarifying the causal pathway linking preoperative and postoperative circulating ET1 to recovery from stage 2 palliation is an important area for future study.
Limitations
This study has several important limitations. Our center is located ≈5000 feet above sea level, potentially affecting generalizability to centers at lower elevation. Despite being the largest cohort of its type, as a single‐center study focusing on a rare disease, the sample size is modest. Variations in clinical practice between subjects, including vasoactive medication supplemental oxygen use, may confound our results. Although all postoperative samples were drawn from a central systemic vein, based on surgeon preference, the location of the central venous line that postoperative samples were drawn from was variable, with some samples derived from the femoral vein and others from the internal jugular vein. We are also, therefore, not reliably able to report superior vena cava or pulmonary artery pressures during the postoperative period in this cohort. As this study is focused entirely on the peri–stage 2 period, longitudinal studies evaluating the relationship between change in ET1 over time and clinical outcomes will also be of great interest. Because of the challenge of obtaining blood samples from healthy infants, we were not able to age and sex match controls to SVHD cases; rather, we opted to include a “similar age” control cohort. In addition, despite similar inclusion criteria, the enrolled control subjects were slightly older than the SVHD cases, and this could be a confounder. Although we show an association between circulating ET1 and clinical outcomes, tissue‐level ET1 activity is not captured by our method and may differ from circulating levels in important ways.
CONCLUSIONS
We report the novel finding that interstage subjects with SVHD have lower circulating ET1 concentration than healthy controls and net pulmonary clearance of ET1. ET1 concentration increases significantly in the postoperative period and then trends toward baseline by 48 hours. Subjects with SVHD with higher preoperative and postoperative ET1 concentration experienced greater post–stage 2 hypoxemia than those with lower ET1 concentrations. This relationship is significant independent of multiple clinical metrics on multivariable testing. We speculate that ET1 may be clinically relevant as both a biomarker of pulmonary vascular readiness for stage 2 palliation and a treatment target to improve postoperative oxygen saturations.
Sources of Funding
This study was supported by the American Heart Association (20CDA35310498 and AHA18IPA34170070) and the National Institutes of Health (NIH) (NIH/National Center for Advancing Translational Science Colorado Clinical and Translational Science Awards, No. UL1 TR001082, and NIH/National Heart Lung and Blood Institute 1K23HL123634).
Disclosures
None.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 2. Ohye RG, Schranz D, D'Udekem Y. Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation. 2016;134:1265–1279. doi: 10.1161/CIRCULATIONAHA.116.022816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kogon BE, Plattner C, Leong T, Simsic J, Kirshbom PM, Kanter KR. The bidirectional Glenn operation: a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136:1237–1242. doi: 10.1016/j.jtcvs.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 4. Brown DW, Gauvreau K, Powell AJ, Lang P, del Nido PJ, Odegard KC, Geva T. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: long‐term follow‐up of a prospective randomized trial. J Thorac Cardiovasc Surg. 2013;146:1172–1178. doi: 10.1016/j.jtcvs.2012.12.079 [DOI] [PubMed] [Google Scholar]
- 5. Allen SW, Chatfield BA, Koppenhafer SA, Schaffer MS, Wolfe RW, Abman SA. Circulating immunoreartive endothelin‐l in children with pulmonary hypertension. Am Rev Respir Dis. 1993;148:519–522. doi: 10.1164/ajrccm/148.2.519 [DOI] [PubMed] [Google Scholar]
- 6. Vignon‐Zellweger N, Heiden S, Miyauchi T, Emoto N. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci. 2012;91:490–500. doi: 10.1016/j.lfs.2012.03.026 [DOI] [PubMed] [Google Scholar]
- 7. Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, Temma S, Shirakami G, Imura H, Mikawa H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Circulation. 1991;84:2280–2285. doi: 10.1161/01.cir.84.6.2280 [DOI] [PubMed] [Google Scholar]
- 8. Yanagisawa M, Kurihara H, Kimura S, Tornobe Y, Kobayashi M, Mitsui Y, Yazak Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0 [DOI] [PubMed] [Google Scholar]
- 9. Chester AH, Yacoub MH. The role of endothelin‐1 in pulmonary arterial hypertension. Glob Cardiol Sci Pract. 2014;2014:62–78. doi: 10.5339/gcsp.2014.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamagishi M, Kurosawa H, Hashimoto K, Nomura K, Kitamura N. The role of plasma endothelin in the Fontan circulation. J Cardiovasc Surg. 2002;43:793–797. [PubMed] [Google Scholar]
- 11. Ishida H, Kogaki S, Ichimori H, Narita J, Nawa N, Ueno T, Takahashi K, Kayatani F, Kishimoto H, Nakayama M, et al. Overexpression of endothelin‐1 and endothelin receptors in the pulmonary arteries of failed Fontan patients. Int J Cardiol. 2012;159:34–39. doi: 10.1016/j.ijcard.2011.02.021 [DOI] [PubMed] [Google Scholar]
- 12. Cedars AM, Saef J, Peterson LR, Coggan AR, Novak EL, Kemp D, Ludbrook PA. Effect of ambrisentan on exercise capacity in adult patients after the Fontan procedure. Am J Cardiol. 2016;117:1524–1532. doi: 10.1016/j.amjcard.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 13. Hebert A, Mikkelsen UR, Thilen U, Idorn L, Jensen AS, Nagy E, Hanseus K, Sørensen KE, Søndergaard L. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo‐Controlled, Double‐Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130:2021–2030. doi: 10.1161/CIRCULATIONAHA.113.008441 [DOI] [PubMed] [Google Scholar]
- 14. Schuuring MJ, Vis JC, van Dijk APJ, van Melle JP, Vliegen HW, Pieper PG, Sieswerda GT, de Bruin‐Bon RHACM, Mulder BJM, Bouma BJ. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. Eur J Heart Fail. 2013;15:690–698. doi: 10.1093/eurjhf/hft017 [DOI] [PubMed] [Google Scholar]
- 15. Frank BS, Khailova L, Silveira L, Mitchell MB, Morgan GJ, Hsieh EWY, DiMaria MV, Twite M, Klawitter J, Davidson JA. Proteomic profiling identifies key differences between inter‐stage infants with single ventricle heart disease and healthy controls. Transl Res. 2021;229:24–37. doi: 10.1016/j.trsl.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francois K, Vandekerckhove K, De Groote K, Panzer J, De Wolf D, De Wilde H, Bové T. Current outcomes of the bi‐directional cavopulmonary anastomosis in single ventricle patients: analysis of risk factors for morbidity and mortality, and suitability for Fontan completion. Cardiol Young. 2016;26:288–297. doi: 10.1017/S1047951115000153 [DOI] [PubMed] [Google Scholar]
- 17. Anderson JB, Beekman RH III, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, Eghtesady P, Marino BS. Lower weight‐for‐age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404.e1. doi: 10.1016/j.jtcvs.2009.02.033 [DOI] [PubMed] [Google Scholar]
- 18. Malhotra SP, Ivy DD, Mitchell MB, Campbell DN, Dines ML, Miyamoto S, Kay J, Clarke DR, Lacour‐Gayet F. Performance of cavopulmonary palliation at elevated altitude: midterm outcomes and risk factors for failure. Circulation. 2008;118:S177–S181. doi: 10.1161/CIRCULATIONAHA.107.751784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran S, Sullivan PM, Cleveland J, Kumar SR, Takao C. Elevated pulmonary artery pressure, not pulmonary vascular resistance, is an independent predictor of short‐term morbidity following bidirectional cavopulmonary connection. Pediatr Cardiol. 2018;39:1572–1580. doi: 10.1007/s00246-018-1932-6 [DOI] [PubMed] [Google Scholar]
- 20. Kim FY, Barnes EA, Ying L, Chen C, Lee L, Alvira CM, Cornfield DN. Pulmonary artery smooth muscle cell endothelin‐1 expression modulates the pulmonary vascular response to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2015;308:L368–L377. doi: 10.1152/ajplung.00253.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Low A, George S, Howard L, Bell N, Millar A, Tulloh RMR. Lung function, inflammation, and endothelin‐1 in congenital heart disease‐associated pulmonary arterial hypertension. J Am Heart Assoc. 2018;7:e007249. doi: 10.1161/JAHA.117.007249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selimovic N, Andersson B, Bergh CH, Sakiniene E, Carlsten H, Rundqvist B. Endothelin‐1 across the lung circulation in patients with pulmonary arterial hypertension and influence of epoprostenol infusion. J Heart Lung Transplant. 2009;28:808–814. doi: 10.1016/j.healun.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 23. Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin‐1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol. 1996;81:1510–1515. doi: 10.1152/jappl.1996.81.4.1510 [DOI] [PubMed] [Google Scholar]
- 24. Dupuis J, Rouleau J, Cernacek P. Reduced pulmonary clearance of endothelin‐1 contributes to the increase of circulating levels in heart failure secondary to myocardial infarction. Circulation. 1998;98:1684–1687. doi: 10.1161/01.CIR.98.16.1684 [DOI] [PubMed] [Google Scholar]
- 25. Hiramatsu T, Imai Y, Takanashi Y, Seo K, Terada M, Aoki M, Nakazawa M. Time course of endothelin‐1 and adrenomedullin after the Fontan procedure. Ann Thorac Surg. 1999;68:169–172. doi: 10.1016/S0003-4975(99)00374-4 [DOI] [PubMed] [Google Scholar]
- 26. Kageyama K, Hashimoto S, Nakajima Y, Shime N, Hashimoto S. The change of plasma endothelin‐1 levels before and after surgery with or without Down syndrome. Paediatr Anaesth. 2007;17:1071–1077. doi: 10.1111/j.1460-9592.2007.02296.x [DOI] [PubMed] [Google Scholar]
- 27. Hiramatsu T, Imai Y, Takanashi Y, Hoshino S, Yashima M, Tanaka SA, Chang D, Nakazawa M. Time course of endothelin‐1 and nitrate anion levels after cardiopulmonary bypass in congenital heart defects. Ann Thorac Surg. 1997;63:648–652. doi: 10.1016/S0003-4975(96)01055-7 [DOI] [PubMed] [Google Scholar]
- 28. Beghetti M, Black SM, Fineman JR. Endothelin‐1 in congenital heart disease. Pediatr Res. 2005;57:16–20. doi: 10.1203/01.PDR.0000160447.83332.13 [DOI] [PubMed] [Google Scholar]
- 29. Vincent JA, Ross RD, Kassab J, Hsu JM, Pinksy WW. Relation of elevated plasma endothelin in congenital heart disease to increased pulmonary blood flow. Am J Cardiol. 1993;71:1204–1207. doi: 10.1016/0002-9149(93)90646-T [DOI] [PubMed] [Google Scholar]


