Abstract
Background
Sedentary behavior is associated with cardiovascular disease, but its association with incident atrial fibrillation is not well studied. Our aim was to measure the association between objectively measured sedentary behavior and incident atrial fibrillation.
Methods and Results
Sedentary behavior was measured by a triaxial accelerometer worn on a belt for 1 week. Incident atrial fibrillation was ascertained from Medicare claims. The associations between total sedentary time (or patterns of sedentary behavior) and incident atrial fibrillation were assessed using Cox proportional hazards models adjusted for demographic and clinical covariates. Among 2675 participants (mean age, 78.2 years), there were 268 (10.0%) cases of incident atrial fibrillation at a rate of 31 cases per 1000 person‐years. Greater total sedentary time was associated with a higher risk of incident atrial fibrillation after adjustment for age, race and ethnicity, body mass index, education, smoking history, hypertension, diabetes, stroke, heart disease, and other chronic conditions (quartile 4 versus quartile 1: hazard ratio, 1.20, [95% CI, 0.81–1.78]; P for trend=0.05). After adjusting for physical function and self‐rated health, this was no longer statistically significant. Both longer mean sedentary bout duration and more continuous sedentary periods (versus frequent breaks in sedentary time) were also associated with higher risks of incident atrial fibrillation, but these associations were also attenuated with serial adjustment.
Conclusions
Total sedentary time and prolonged patterns of sedentary accumulation were associated with a higher risk of atrial fibrillation in this prospective study of community‐dwelling older women, but these associations were attenuated by adjustment for physical function and self‐reported health. This suggests that associations between sedentary behavior and atrial fibrillation may be attributable to global measures of overall function and health.
Keywords: aging, atrial Fibrillation, exercise, healthy lifestyle, women
Subject Categories: Atrial Fibrillation, Aging, Exercise, Risk Factors, Women
Nonstandard Abbreviations and Acronyms
- FFS A+B
Medicare Fee‐for‐Service Parts A and B
- OPACH
Objective Physical Activity and Cardiovascular Health
- WHI
Women’s Health Initiative
Clinical Perspective
What Is New?
This is one of the first studies to assess the association between objectively measured sedentary behavior and incident atrial fibrillation, and it uniquely uses laboratory‐calibrated accelerometers to define and measure both total sedentary time and patterns of sedentary behavior.
We found that in community‐dwelling older women, higher total sedentary time and patterns of prolonged sedentary bouts were associated with a higher risk of incident atrial fibrillation.
What Are the Clinical Implications?
Sedentary behavior may impact risk of incident atrial fibrillation in other populations.
Interventions that limit sedentary behavior may be able to reduce rates of atrial fibrillation in older women.
Atrial fibrillation (AF) is the most prevalent arrhythmia in the United States, and is an independent predictor of stroke and mortality. 1 , 2 The prevalence of AF increases with age, and although AF is less common in women, the effect of AF on the risks of stroke and death is greater in women than in men. 3 , 4 Therefore, the identification of risk factors for AF in older women has important implications for prevention and control of the large burden of morbidity and mortality associated with the disease. Current known modifiable risk factors for AF in older women include hypertension, obesity, and diabetes. 5
Sedentary behavior has emerged as a risk factor for various health outcomes including cardiovascular disease and mortality. 6 , 7 , 8 , 9 The health implications of sedentary behavior are distinct from those related to a lack of exercise, because sedentary time has been shown to be associated with cardiometabolic health even after statistically accounting for exercise and physical activity. In addition to total sedentary time, the manner in which sedentary time is accumulated is also associated with cardiovascular risk, with prolonged sedentary periods being more detrimental than interrupted sedentary time. 10 Despite the growing literature on sedentary behavior as a cardiovascular risk factor, AF has not been well studied as an outcome. Our objective was to measure the association between objectively measured sedentary time and the risk of incident AF.
Methods
Participants and Study Design
Because of the sensitive nature of the data collected for this study, requests to access this data set from qualified researchers trained in human subject confidentiality protocols may be sent to the WHI (Women’s Health Initiative) at helpdesk@whi.org. The WHI is a large prospective study of postmenopausal women, the details of which have been published. 11 , 12 From 2012 to 2014, the OPACH (Objective Physical Activity and Cardiovascular Health) study enrolled 7048 community‐dwelling WHI participants aged 63 to 99 years to investigate the association between accelerometer‐measured physical activity and cardiovascular disease. 13 For this study, we included OPACH participants for whom accelerometer data were successfully collected and for whom claims data could be obtained from Medicare for AF ascertainment. We excluded those who did not meet requirements for acceptable accelerometer wear time, those without Medicare Fee‐for‐Service data, and those with a history of AF before OPACH baseline. The original protocol for OPACH was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, and all participants provided informed consent. This secondary analysis was also approved by institutional review boards at the University of California, San Diego and Stanford University.
Baseline Characteristics
Age, race and ethnicity, and education were obtained at WHI enrollment. Body mass index was obtained from in‐home examinations at OPACH baseline. Smoking history was obtained from combining self‐reported smoking history at WHI enrollment with reported current smoking status at OPACH baseline. Measurement of chronic health conditions present at OPACH baseline included both self‐reported and physician‐adjudicated diagnoses. 14 Eight of these conditions (cancer, cognitive impairment, frequent falls, chronic obstructive pulmonary disease, osteoarthritis, depression, incontinence, and sensory impairment) were summarized in a comorbidity index equal to the number of conditions present in a given individual. This index is similar to one previously described, 14 with the difference being that heart disease (which includes coronary artery disease and congestive heart failure) and stroke, both of which were previously part of the composite index, were included in these analyses as separate variables, because they are known to be associated with AF. Hip fracture was excluded because of a high rate of missing values, as has been done in prior studies with this cohort. 15 , 16 Self‐reported health status and physical functioning (assessed with the RAND‐36 survey instrument for health‐related quality of life) were obtained from questionnaires at OPACH baseline. Prevalent AF was determined by meeting any of the following criteria: (1) presence of AF on a 12‐lead ECG at WHI enrollment (1993–1998), (2) self‐reported physician diagnosis of AF at WHI enrollment or in subsequent annual follow‐up questionnaires before OPACH baseline, or (3) presence of AF diagnosis in Medicare claims before OPACH baseline.
Accelerometer Data
An ActiGraph GT3X+ (ActiGraph, Pensacola, FL) triaxial accelerometer was distributed at OPACH baseline. Participants were instructed to wear the accelerometer on the hip for 7 days at all times except when bathing or swimming. In‐bed and out‐of‐bed times were based on sleep logs and non–wear time was identified using the common Choi algorithm. 17 An adherent day was considered to be ≥10 hours of out‐of‐bed wear time, and inclusion for final analysis required participants to have had ≥4 adherent days.
Processing of the raw accelerometer data into sedentary behavior variables has been described in detail elsewhere. 15 The specific cut points for accelerometer activity counts that best distinguished levels of physical activity intensity were chosen based on a separate laboratory calibration study of older women. 18 Each 15‐second interval of time was classified as sedentary behavior, light intensity activity, or moderate‐to‐vigorous physical activity. Total sedentary time and total moderate‐to‐vigorous physical activity time were derived from these 15‐second‐level data, whereas all sedentary accumulation pattern variables were derived from minute‐level data using a previously validated method. 18 , 19 , 20 The 15‐second‐level data were not used for these pattern variables because they were overly sensitive to breaks in sedentary time. Three sedentary accumulation pattern metrics were examined in this analysis: (1) mean sedentary bout duration, (2) mean number of daily breaks in sedentary time, and (3) α metric. A break in sedentary time was defined as any transition from a sedentary to a nonsedentary bout, regardless of bout duration. The α metric was calculated according to the methods described by Chastin et al, 20 , 21 and simultaneously captures the frequency and duration of all sedentary bouts. A lower α indicates a prolonged accumulation pattern with many long bouts, whereas a higher α indicates an interrupted accumulation pattern with many short bouts.
Outcomes
Ascertainment of incident AF in WHI participants has been described, 5 , 22 and for this study was based on linked Medicare claims through December 2016. A case of AF was defined as having at least a single AF diagnosis code (427.31 in the International Classification of Diseases, Ninth Revision [ICD‐9]; I48.0, I48.1, I48.2, or I48.91 in the Tenth Revision [ICD‐10]) while the participant was enrolled in Medicare Fee‐for‐Service Parts A and B (FFS A+B). Participants who enrolled in FFS A+B after WHI enrollment were evaluated with a 2‐year look‐back period to assess for possible preexisting AF at the time of first joining FFS A+B. Participants who were free of AF for the duration of the look‐back period were included in the analysis at the time of completion of the look‐back period. Participants who dropped out of FFS A+B and then returned for a subsequent FFS A+B interval were not required to undergo another look‐back period if they had been established as AF‐free on their initial inclusion in the analysis. The outcome of death was ascertained from WHI records, which by design were ensured to contain identical death dates as those recorded by Medicare.
Statistical Analysis
Baseline characteristics are presented with means and standard deviations for continuous variables, and with frequencies and percentages for categorical variables. P values for comparisons of characteristics across quartiles of total sedentary time were derived from ANOVA for continuous variables or χ2 tests for categorical variables. Measures of total sedentary time and total moderate‐to‐vigorous physical activity time were adjusted for total awake wear time using the residuals method described by Willett and Stampfer. 23 The daily number of breaks in sedentary time was similarly adjusted for total sedentary time. Competing risks methods (cmprsk package in R version 2.2‐7; R Foundation for Statistical Computing, Vienna, Austria) were used to estimate the cumulative incidence of AF. Death was considered a competing risk, whereas loss of FFS A+B follow‐up was a censoring event. The Gray test was used for comparisons of cumulative incidence across quartiles of total sedentary time. 24
In our primary analysis, hazard ratios (HRs) for incident AF were computed for quartiles of total sedentary time using prespecified multivariable Cox proportional hazards models. Model 1 adjusted for age, race and ethnicity, and body mass index. Model 2, our primary model, adjusted for Model 1 covariates, as well as education, smoking history, hypertension, diabetes, stroke, heart disease, and comorbidity index. Model 3 adjusted for Model 2 covariates with the addition of self‐rated health and physical function. P values for trend were calculated from separate models that included total sedentary time as a continuous variable to assess linearity across the entire distribution of the exposure and to maximize statistical power. Our analyses examining patterns of sedentary accumulation were similar to those described above, except total sedentary time was replaced with the accumulation pattern variable being tested.
A secondary aim of this study was to assess the nonlinearity of the association between total sedentary time and incident AF. For this, a Cox proportional hazards model was fit to include total sedentary time as a restricted cubic spline term with 3 knots, adjusting for Model 2 covariates (rms package in R version 5.1.3). Wald tests were used to assess statistical significance of nonlinearity. To visualize the dose‐response trajectory, a plot was generated using the 10th percentile of the sedentary time distribution (7.2 h/d) as the referent category. All analyses were conducted using R statistical software version 3.4.1.
Results
Baseline Characteristics
Among 7048 participants who were given accelerometers, 6126 participants returned accelerometers and were adherent to wear time requirements. We excluded 2026 participants for having no Medicare exposure at any time, 686 for baseline AF, and an additional 739 participants for dropping out of Medicare before OPACH baseline. Ultimately, we included 2675 adherent accelerometer users who were AF‐free at OPACH baseline and had subsequent Medicare follow‐up. On average, participants were 78.2 years old, had a body mass index of 28.1 kg/m2, and were sedentary for 9.2 hours per day. A comparison of baseline characteristics across quartiles of total sedentary time is shown in Table 1.
Table 1.
Baseline Characteristics Stratified by Total Sedentary Time*
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value | |
|---|---|---|---|---|---|
| N=670 | N=668 | N=667 | N=670 | ||
| Age, y, mean (SD) | 76.19 (6.14) | 77.95 (6.64) | 78.40 (6.51) | 80.38 (6.68) | <0.001 |
| Race and ethnicity, n (%) | <0.001 | ||||
| White | 269 (40.1) | 325 (48.7) | 331 (49.6) | 422 (63.0) | |
| Black | 265 (39.6) | 233 (34.9) | 253 (37.9) | 193 (28.8) | |
| Hispanic | 136 (20.3) | 110 (16.5) | 83 (12.4) | 55 (8.2) | |
| Education, n (%) | 0.04 | ||||
| College graduate or more | 309 (46.1) | 315 (47.6) | 265 (40.2) | 281 (42.0) | |
| Some college | 233 (34.8) | 222 (33.5) | 265 (40.2) | 271 (40.5) | |
| High school or equivalent | 128 (19.1) | 125 (18.9) | 129 (19.6) | 117 (17.5) | |
| Ever smoker, n (%) | 275 (41.5) | 277 (41.7) | 304 (46.2) | 322 (48.4) | 0.024 |
| Body mass index, kg/m2, mean (SD) | 26.30 (4.99) | 27.54 (5.33) | 28.39 (5.35) | 30.06 (6.00) | <0.001 |
| Hypertension, n (%) | 423 (63.1) | 468 (70.1) | 499 (74.8) | 529 (79.0) | <0.001 |
| Diabetes, n (%) | 89 (13.3) | 137 (20.5) | 137 (20.5) | 168 (25.1) | <0.001 |
| Stroke, n (%) | 23 (3.4) | 21 (3.1) | 45 (6.7) | 51 (7.6) | <0.001 |
| Heart disease, n (%) † | 27 (4.0) | 53 (7.9) | 48 (7.2) | 66 (9.9) | 0.001 |
| Comorbidity index, n (%) ‡ | <0.001 | ||||
| 0 | 210 (31.4) | 171 (25.7) | 154 (23.2) | 135 (20.3) | |
| 1 | 270 (40.4) | 277 (41.7) | 277 (41.8) | 249 (37.4) | |
| 2 | 141 (21.1) | 159 (23.9) | 166 (25.0) | 178 (26.7) | |
| 3+ | 47 (7.0) | 58 (8.7) | 66 (10.0) | 104 (15.6) | |
| Self‐rated health, n (%) | <0.001 | ||||
| Excellent | 112 (16.8) | 79 (11.9) | 54 (8.1) | 39 (5.8) | |
| Very good | 318 (47.7) | 267 (40.1) | 306 (45.9) | 271 (40.6) | |
| Good | 212 (31.8) | 269 (40.4) | 251 (37.7) | 272 (40.7) | |
| Fair/poor | 25 (3.7) | 51 (7.7) | 55 (8.3) | 86 (12.9) | |
| Physical function score, mean (SD) § | 80.91 (19.62) | 73.61 (23.25) | 69.77 (25.13) | 58.75 (27.31) | <0.001 |
| Total sedentary time, h/d, mean (SD) | 7.26 (0.81) | 8.78 (0.28) | 9.73 (0.27) | 11.03 (0.64) | <0.001 |
| Moderate‐to‐vigorous physical activity, min/d, mean (SD) | 82.86 (36.58) | 55.90 (27.09) | 41.91 (21.67) | 27.01 (17.98) | <0.001 |
Total sedentary time and time spent in moderate‐to‐vigorous physical activity are each adjusted for accelerometer wear time. Cut points for total sedentary time: quartile 1=3.25–8.27 h/d; quartile 2=8.27–9.27 h/d; quartile 3=9.27–10.22 h/d; quartile 4=10.22–13.86 h/d. Number of missing values excluded from table: education=15, smoking history=25, body mass index=169, comorbidity index=13, self‐rated health=8, physical function score=23.
Heart disease includes coronary artery disease and congestive heart failure.
The 8 chronic conditions included in the comorbidity index are cancer, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, sensory impairment, frequent falls, and incontinence.
Physical function score, dervied from part of the RAND‐36 (a 36‐item survey on health‐related quality of life), has a range of 0 to 100, where higher scores suggest better function.
AF and Total Sedentary Time
Over a median follow‐up of 3.8 years (maximum 4.8 years), there were 268 (10.0%) cases of incident AF at a rate of 31 cases per 1000 person‐years, and 111 all‐cause deaths (4.1%) at a rate of 13 deaths per 1000 person‐years. The cumulative incidence of AF, adjusted for the competing risk of death, was 12.1% at the end of follow‐up. An unadjusted comparison of the cumulative incidence of AF across quartiles of total sedentary time revealed a significant difference between groups (Figure 1, P<0.001).
Figure 1. Cumulative incidence of atrial fibrillation by quartiles of total sedentary time.
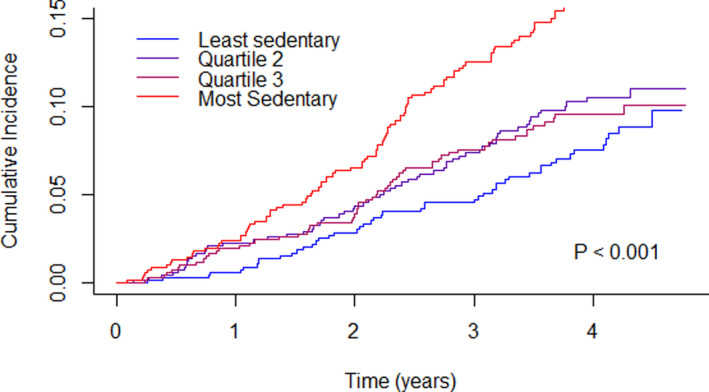
Total sedentary time is adjusted for accelerometer wear time. Cut points for total sedentary time: quartile (1=3.25–8.27 h/d; quartile 2=8.27–9.27 h/d; quartile 3=9.27–10.22 h/d; quartile 4=10.22–13.86 h/day. P value calculated by Gray test.
Table 2 shows the results for serially adjusted models. In Model 2, which adjusted for age, race and ethnicity, body mass index, education, smoking history, hypertension, diabetes, stroke, heart disease, and comorbidity index, greater total sedentary time was associated with more incident AF (P for trend=0.05), with a 20% higher risk of AF in the most sedentary quartile compared with the least sedentary quartile (HR, 1.20 for quartile 4 versus quartile 1 [95% CI, 0.81–1.78]). The addition of self‐rated health and physical function (Model 3) resulted in loss of statistical significance (P for trend=0.12). Addition of moderate‐to‐vigorous physical activity (not shown) also resulted in loss of statistical significance (P for trend=0.11).
Table 2.
Associations Between Total Sedentary Time and Incident Atrial Fibrillation*
| Events | Incidence rate † | Hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 ‡ | Model 2 | Model 3 | |||
| Least sedentary | 48 | 21.6 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 2 | 63 | 28.1 | 1.30 (0.89–1.89) | 1.10 (0.75–1.63) | 1.03 (0.70–1.52) | 0.99 (0.67–1.47) |
| Quartile 3 | 58 | 26.4 | 1.23 (0.84–1.80) | 0.97 (0.65–1.45) | 0.89 (0.59–1.34) | 0.83 (0.55–1.25) |
| Most sedentary | 99 | 46.8 | 2.19 (1.55–3.09) | 1.39 (0.95–2.05) | 1.20 (0.81–1.78) | 1.10 (0.74–1.64) |
| P for trend § | <0.001 | 0.007 | 0.05 | 0.12 | ||
Ref indicates referent.
Total sedentary time is adjusted for accelerometer wear time. Cut points for total sedentary time: quartile 1=3.25–8.27 h/d; quartile 2=8.27–9.27 h/d; quartile 3=9.27–10.22 h/d; quartile 4=10.22–13.86 h/d.
Incidence rate is expressed as events per 1000 person‐years.
Model 1 adjusts for age, race and ethnicity, and body mass index. Model 2 adjusts for Model 1 covariates plus education, smoking, hypertension, diabetes, stroke, heart disease, and comorbidity index. Heart disease includes coronary artery disease and congestive heart failure. The 8 chronic conditions included in the comorbidity index are cancer, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, sensory impairment, frequent falls, and incontinence. Model 3 adjusts for Model 2 covariates plus self‐rated health and physical function score. Event numbers after complete case analysis: unadjusted=268, Model 1=254, Model 2=252, Model 3=251.
P values for trend were derived from separate models including total sedentary time as a continuous variable.
Given the potentially nonlinear relationship between total sedentary time and incident AF, we considered whether there may be a threshold above which accumulating more sedentary time becomes detrimental. Figure 2 plots the risk of incident AF across the distribution of total sedentary time seen in this population, adjusted for the primary model covariates. Formal testing for nonlinearity in our spline model was not statistically significant (P=0.08).
Figure 2. Continuous dose‐response association between total sedentary time and incident atrial fibrillation.
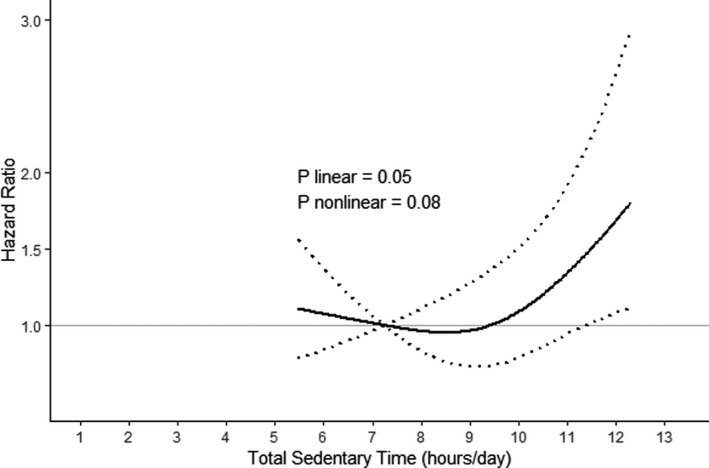
This relationship was modeled using Cox regression with total sedentary time (adjusted for accelerometer wear time) included as a restricted cubic spline term with 3 knots. The solid line represents the hazard ratios relative to the 10th percentile of sedentary time (7.2 hours), adjusted for age, race and ethnicity, body mass index, education, smoking, hypertension, diabetes, stroke, heart disease, and comorbidity index. Heart disease includes coronary artery disease and congestive heart failure. The 8 chronic conditions included in the comorbidity index are cancer, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, sensory impairment, frequent falls, and incontinence. The dotted lines represent the 95% CIs for the hazard ratios. P value for nonlinearity was obtained from the spline model described above. P value for linearity was obtained from a model including total sedentary time as a continuous exposure, adjusted for the same covariates.
AF and Patterns of Sedentary Accumulation
Finally, we assessed the risk of AF associated with various patterns of sedentary accumulation, shown in Table 3. A longer mean sedentary bout was associated with higher risk of incident AF, but this association was attenuated with serial adjustment. The α is a composite measure that takes into account interruptions in sedentary time as well as the duration of sedentary bouts. A lower α, which represents a more prolonged accumulation pattern of sedentary time, was also associated with a higher risk of AF. After adjustment for Model 2 covariates, there was a 50% increased risk of AF in those with the lowest α compared with those with the highest α (HR, 1.50 for Q1 versus Q4 [95% CI, 1.00–2.24]; P for trend=0.02). This association was attenuated by further adjustment for self‐rated health and physical function. Overall, the number of breaks in sedentary time was not independently associated with risk of incident AF in adjusted models.
Table 3.
Associations Between Accumulation Patterns of Total Sedentary Time and Incident Atrial Fibrillation*
| Events | Incidence rate † | Hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 ‡ | Model 2 | Model 3 | |||
| Mean sedentary bout duration § | ||||||
| Shortest bouts | 38 | 17.1 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 2 | 61 | 27.6 | 1.62 (1.08–2.42) | 1.39 (0.91–2.11) | 1.44 (0.94–2.20) | 1.40 (0.92–2.14) |
| Quartile 3 | 72 | 32.8 | 1.92 (1.30–2.84) | 1.41 (0.93–2.15) | 1.37 (0.90–2.09) | 1.31 (0.86–1.99) |
| Longest bouts | 97 | 45.3 | 2.67 (1.84–3.89) | 1.55 (1.02–2.35) | 1.46 (0.96–2.23) | 1.40 (0.92–2.14) |
| P for trend || | <0.001 | 0.02 | 0.05 | 0.14 | ||
| Breaks in sedentary time ¶ | ||||||
| Most breaks | 52 | 24.1 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 3 | 69 | 31.6 | 1.32 (0.92–1.89) | 1.18 (0.82–1.71) | 1.19 (0.83–1.73) | 1.22 (0.84–1.76) |
| Quartile 2 | 63 | 28.2 | 1.18 (0.82–1.70) | 0.92 (0.63–1.35) | 0.92 (0.63–1.36) | 0.92 (0.62–1.35) |
| Least breaks | 84 | 38.5 | 1.61 (1.14–2.27) | 1.15 (0.80–1.65) | 1.16 (0.80–1.67) | 1.16 (0.80–1.67) |
| P for trend | 0.04 | 0.92 | 0.96 | 0.94 | ||
| α # | ||||||
| Highest α | 40 | 18.1 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Quartile 3 | 58 | 26.4 | 1.47 (0.98–2.20) | 1.21 (0.80–1.84) | 1.26 (0.83–1.91) | 1.26 (0.83–1.92) |
| Quartile 2 | 67 | 30.2 | 1.68 (1.14–2.49) | 1.33 (0.88–1.99) | 1.34 (0.89–2.01) | 1.30 (0.86–1.96) |
| Lowest α | 103 | 48.3 | 2.70 (1.87–3.89) | 1.61 (1.08–2.40) | 1.50 (1.00–2.24) | 1.43 (0.96–2.15) |
| P for trend | <0.001 | 0.006 | 0.02 | 0.06 | ||
Each sedentary accumulation pattern was modeled separately. For each sedentary accumulation pattern, the reference quartile was chosen to be the quartile with the lowest risk of incident atrial fibrillation (ie, quartile 1 for mean sedentary bout duration, and quartile 4 for breaks in sedentary time and α metric.
Incidence rate is expressed as events per 1000 person‐years.
Model 1 adjusts for age, race and ethnicity, and body mass index. Model 2 adjusts for Model 1 covariates plus education, smoking, hypertension, diabetes, stroke, heart disease, and comorbidity index. Heart disease includes coronary artery disease and congestive heart failure. The 8 chronic conditions included in the comorbidity index are cancer, osteoarthritis, depression, chronic obstructive pulmonary disease, cognitive impairment, sensory impairment, frequent falls, and incontinence. Model 3 adjusts for Model 2 covariates plus self‐rated health and physical function score.
Cut points for mean sedentary bout duration: quartile 1=2.63–5.63 min; quartile 2=5.63–6.81 min; quartile 3=6.81–8.35 min; quartile 4=8.35–27.74 min.
P values for trend were derived from separate models including the sedentary accumulation pattern as a continuous variable.
The number of breaks in sedentary time was adjusted for total sedentary time. Cut points for number of breaks in sedentary time: quartile 1=30.3–76.5/d; quartile 2=76.5–86.3/d; quartile 3=86.3–95.6/d; quartile 4=95.6–146.6/d.
Low α represents a prolonged accumulation pattern, with frequent long sedentary bouts and few short sedentary bouts. Cut points for α: quartile 1=1.48–1.77; quartile 2=1.77–1.86; quartile 3=1.86–1.96; quartile 4=1.96–2.7.
Discussion
In our prospective study of community‐dwelling older women, greater accelerometer‐measured total sedentary time was associated with a higher risk of AF. Mean bout duration and α, indicators of how sedentary time is accumulated, were also associated with incident AF. However, these findings were all attenuated after serial adjustment for demographic and clinical covariates.
Although the pathophysiology of AF is not completely understood, structural remodeling of the atria likely plays an important role. Aging, hypertension, obesity, and diabetes are all associated with atrial remodeling that promotes ectopic electrical activity. 25 Sedentary behavior has been linked to higher systolic blood pressure, insulin resistance, and adiposity, which may explain its association with incident AF in a dose‐dependent manner beyond the binary absence or presence of hypertension, diabetes, or obesity. 26 , 27 Besides its relation to these risk factors that may partially mediate the effect of sedentary time on incident AF, sedentary behavior has been associated with inflammation, oxidative stress, and endothelial dysfunction, which are all associated with a higher risk of cardiovascular disease. 27 For AF specifically, oxidative stress is one mechanism by which fibrotic remodeling may occur, and inflammation may be correlated with the incidence and recurrence of AF. 25
The impact of physical activity on the risk of AF in the general population is controversial, with studies suggesting that moderate exercise may be protective or have no impact, 28 , 29 , 30 whereas strenuous physical activity, such as that seen in high‐endurance athletes, may increase risk. 29 , 30 , 31 In older women specifically, higher physical activity is associated with lower incidence of AF and favorably modifies the association between obesity and incident AF. 32 Prior studies have suggested that sedentary behavior may be associated with a higher incidence of AF. 32 , 33 , 34 , 35 , 36 , 37 , 38 However, rather than directly investigate sedentary behavior, these studies often define physical inactivity as a reference group for their main exposure, which is typically the frequency of exercise or an estimate of energy expenditure.
Few studies have examined AF risk and true sedentary behavior, which is not simply the absence of exercise. Kubota et al assessed the dose‐response of sedentary behavior on risk of AF using self‐reported television watching as a surrogate for sedentary time. 35 The authors found a 28% higher risk of AF in those who spent the most time watching television versus those who never or seldom watched television. Palmisano et al used accelerometer measurements from implanted cardiac defibrillators to assess the effects of physical activity on the risk of atrial tachyarrhythmia (most of which likely represents AF), and found that more inactivity time was associated with a higher risk of arrhythmia. 38 In contrast to our study, their accelerometer measurements did not appear to be calibrated to actual energy expenditure, so it is unclear how accurately their binary definition of physical activity (versus inactivity) distinguished true sedentary behavior from various levels of physical activity. Amadid et al found no association between accelerometer‐measured sedentary time and a composite outcome of incident cardiovascular disease that included AF. 37 Notably, they did not find any association between cardiovascular disease and time spent at any physical activity level. Although this study may conflict with some of the existing literature, the statistical analysis used in their study was a tree‐structured survival analysis, which limits comparison to our study and others. Their study also included clinical and laboratory measurements that were not available in our study.
In addition to being one of the first studies to assess the association between sedentary behavior and incident AF, our study has several strengths. Our measure of sedentary behavior is objective and quantitative, rather than based on self‐report. Cut points for our accelerometer measurements were calibrated in a laboratory study to more accurately define sedentary behavior in older women. Our study looks at AF as a sole outcome, rather than as part of a composite outcome; and we study a population of community‐living older women who are at average risk for AF (for their age), rather than a high‐risk population with implanted devices and functional limitations associated with their underlying pathology. Finally, we uniquely assess the impact of patterns of sedentary behavior on incident AF.
Limitations
Despite its strengths, our study is not without limitations. Although our study included racial and ethnic diversity, our findings may not be generalizable to men, younger populations, or those who are not enrolled in Medicare. Additionally, because our study required participants to return accelerometers with usable data, we acknowledge that there may be differences between those who did and did not return accelerometers. Sedentary time ascertainment was limited by the duration of wear time relative to the average follow‐up period, and the accelerometer technique precluded accurate detection of posture. Although we were able to adjust for multiple potential confounders in our analysis, we were unable to adjust for obstructive sleep apnea, which is a known risk factor for AF. 39 Although our sample size was large, our event numbers may have limited statistical precision. We expect the effects observed would only be amplified over a longer follow‐up period. Finally, although we used multiple strategies to maximize the identification of baseline AF, there may be residual confounding from symptomatic undiagnosed AF.
Conclusions
In summary, AF is a significant cause of morbidity and mortality that can be mitigated by identifying modifiable risk factors. In our study, total sedentary time and prolonged patterns of sedentary accumulation were associated with incident AF, but these associations were attenuated after multivariate adjustment. This suggests that associations between sedentary behavior and AF may be attributable to other patient characteristics. Future studies can be done to assess whether interventions that limit sedentary behavior can reduce rates of AF in older women, and to assess the influence of sedentary behavior on incident AF in other populations.
Sources of Funding
The National Heart, Lung, and Blood Institute provided funding for the OPACH study (grant numbers RO1 HL105065 and P01 AG052352 to A.Z.L.) and for studies of AF in the Women’s Health Initiative (grant number 1R01HL136390‐01 to M.V.P.). Funding also came from training grants provided by the National Institutes of Health (grant numbers T32HL079891 to J.B., TL1TR001084 to B.C.B.), and from a grant from the National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Award (grant number UL1TR001085 to B.C.B.). The Women’s Health Initiative program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and US Department of Health and Human Services (contract numbers HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C). The content is solely the responsibility of the authors and does not necessarily represent the official views of these organizations.
Disclosures
None.
Acknowledgments
The authors thank the Women’s Health Initiative participants, staff, and investigators. The full list of Women’s Health Initiative investigators can be found at the following site: https://www.whi.org/doc/WHI‐Investigator‐Long‐List.pdf.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022 [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229 [DOI] [PubMed] [Google Scholar]
- 3. Friberg J, Scharling H, Gadsbøll N, Truelsen T, Jensen GB, Copenhagen City Heart Study . Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol. 2004;94:889–894. doi: 10.1016/j.amjcard.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 4. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. doi: 10.1001/archinte.1995.00430050045005 [DOI] [PubMed] [Google Scholar]
- 5. Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the women’s health initiative observational study. Heart. 2013;99:1173–1178. doi: 10.1136/heartjnl-2013-303798 [DOI] [PubMed] [Google Scholar]
- 6. Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJH. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta‐analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z [DOI] [PubMed] [Google Scholar]
- 7. Ekelund U, Steene‐Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta‐analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310. doi: 10.1016/S0140-6736(16)30370-1 [DOI] [PubMed] [Google Scholar]
- 8. Pandey A, Salahuddin U, Garg S, Ayers C, Kulinski J, Anand V, Mayo H, Kumbhani DJ, de Lemos J, Berry JD. Continuous dose‐response association between sedentary time and risk for cardiovascular disease: a meta‐analysis. JAMA Cardiol. 2016;75390:575–583. doi: 10.1001/jamacardio.2016.1567 [DOI] [PubMed] [Google Scholar]
- 9. Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults a systematic review and meta‐analysis. Ann Intern Med. 2015;162:123–132. doi: 10.7326/M14-1651 [DOI] [PubMed] [Google Scholar]
- 10. Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM, Blair SN, Hooker SP. Patterns of sedentary behavior and mortality in U.S. middle‐aged and older adults. Ann Intern Med. 2017;167:465–476. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson GL, Cummings SR, Freedman LS, Furberg C, Henderson MM, Johnson SR, Kuller LH, Manson JE, Oberman A, Prentice RL, et al. Design of the women’s health initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 12. Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the women’s health initiative study design. Ann Epidemiol. 2003;13:5–17. doi: 10.1016/S1047-2797(03)00043-7 [DOI] [PubMed] [Google Scholar]
- 13. LaCroix AZ, Rillamas‐Sun E, Buchner D, Evenson KR, Di C, Lee I‐M, Marshall S, LaMonte MJ, Hunt J, Tinker LF, et al. The objective physical activity and cardiovascular disease health in older women (OPACH) study. BMC Public Health. 2017;17:192. doi: 10.1186/s12889-017-4065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rillamas‐Sun E, LaCroix AZ, Bell CL, Ryckman K, Ockene JK, Wallace RB. The impact of multimorbidity and coronary disease comorbidity on physical function in women aged 80 years and older: the women’s health initiative. J Gerontol Ser A Biol Sci Med Sci. 2016;71:S54–S61. doi: 10.1093/gerona/glv059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellettiere J, Healy GN, Lamonte MJ, Kerr J, Evenson KR, Rillamas‐Sun E, Di C, Buchner DM, Hovell MF, LaCroix AZ. Sedentary behavior and prevalent diabetes in 6,166 older women: the objective physical activity and cardiovascular health study. Journals Gerontol Ser A. 2019;74:387–395. doi: 10.1093/gerona/gly101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bellettiere J, Lamonte MJ, Evenson KR, Rillamas‐Sun E, Kerr J, Lee IM, Di C, Rosenberg DE, Stefanick ML, Buchner DM, et al. Sedentary behavior and cardiovascular disease in older women: the OPACH study. Circulation. 2019;139:1036–1046. doi: 10.1161/CIRCULATIONAHA.118.035312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evenson KR, Wen F, Herring AH, Di C, LaMonte MJ, Tinker LF, Lee IM, Rillamas‐Sun E, LaCroix AZ, Buchner DM. Calibrating physical activity intensity for hip‐worn accelerometry in women age 60 to 91years: the women’s health initiative OPACH calibration study. Prev Med Reports. 2015;2:750–756. doi: 10.1016/j.pmedr.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chastin SFM, Winkler EAH, Eakin EG, Gardiner PA, Dunstan DW, Owen N, Healy GN. Sensitivity to change of objectively‐derived measures of sedentary behavior. Meas Phys Educ Exerc Sci. 2015;19:138–147. doi: 10.1080/1091367X.2015.1050592 [DOI] [Google Scholar]
- 21. Chastin SFM, Granat MH. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture. 2010;31:82–86. doi: 10.1016/j.gaitpost.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 22. Perez MV, Wang PJ, Larson JC, Virnig BA, Cochrane B, Curb JD, Klein L, Manson JE, Martin LW, Robinson J, et al. Effects of postmenopausal hormone therapy on incident atrial fibrillation: the women’s health initiative randomized controlled trials. Circ Arrhythmia Electrophysiol. 2012;5:1108–1116. doi: 10.1161/CIRCEP.112.972224 [DOI] [PubMed] [Google Scholar]
- 23. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366 [DOI] [PubMed] [Google Scholar]
- 24. Gray RJ. A Class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. http://www.jstor.org/stable/2241622 doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 25. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- 26. Young DR, Hivert MF, Alhassan S, Camhi SM, Ferguson JF, Katzmarzyk PT, Lewis CE, Owen N, Perry CK, Siddique J, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation. 2016;134:e262–e279. doi: 10.1161/CIR.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 27. Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND. Sedentary behavior and cardiovascular disease risk: mediating mechanisms. Exerc Sport Sci Rev. 2017;45:80–86. doi: 10.1249/JES.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 28. Ofman P, Khawaja O, Rahilly‐Tierney CR, Peralta A, Hoffmeister P, Reynolds MR, Gaziano JM, Djousse L. Regular physical activity and risk of atrial fibrillation: a systematic review and meta‐analysis. Circ Arrhythmia Electrophysiol. 2013;6:252–256. doi: 10.1161/CIRCEP.113.000147 [DOI] [PubMed] [Google Scholar]
- 29. Drca N, Wolk A, Jensen‐Urstad M, Larsson SC. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart. 2014;100:1037–1042. doi: 10.1136/heartjnl-2013-305304 [DOI] [PubMed] [Google Scholar]
- 30. Myrstad M, Løchen ML, Graff‐Iversen S, Gulsvik AK, Thelle DS, Stigum H, Ranhoff AH. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long‐term endurance sport practice. Scand J Med Sci Sport. 2014;24:238–244. doi: 10.1111/sms.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A Systematic Review and meta‐analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197 [DOI] [PubMed] [Google Scholar]
- 32. Azarbal F, Stefanick ML, Salmoirago‐Blotcher E, Manson JAE, Albert CM, LaMonte MJ, Larson JC, Li W, Martin LW, Nassir R, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3. doi: 10.1161/JAHA.114.001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, Gokoglan Y, Di Biase L, Natale A. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta‐analysis. J Cardiovasc Electrophysiol. 2016;27:1021–1029. doi: 10.1111/jce.13023 [DOI] [PubMed] [Google Scholar]
- 34. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kubota Y, Alonso A, Shah AM, Chen LY, Folsom AR. Television watching as sedentary behavior and atrial fibrillation: the atherosclerosis risk in communities study. J Phys Act Heal. 2018;15:895–899. doi: 10.1123/jpah.2018-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giaccardi M, Macchi C, Colella A, Polcaro P, Zipoli R, Cecchi F, Valecchi D, Sofi F, Petrilli M, Molino‐Lova R. Postacute rehabilitation after coronary surgery. Am J Phys Med Rehabil. 2011;90:308–315. doi: 10.1097/phm.0b013e31820f9535 [DOI] [PubMed] [Google Scholar]
- 37. Amadid H, Johansen NB, Bjerregaard AL, Brage S, Færch K, Lauritzen T, Witte DR, Sandbæk A, Jørgensen ME, Vistisen D. The role of physical activity in the development of first cardiovascular disease event: a tree‐structured survival analysis of the Danish ADDITION‐PRO cohort. Cardiovasc Diabetol. 2018;17:1–12. doi: 10.1186/s12933-018-0769-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palmisano P, Guerra F, Ammendola E, Ziacchi M, Pisanó ECL, Dell’Era G, Aspromonte V, Zaccaria M, Di Ubaldo F, Capucci A, et al. Physical activity measured by implanted devices predicts atrial arrhythmias and patient outcome: results of IMPLANTED (Italian Multicentre Observational Registry on Patients With Implantable Devices Remotely Monitored). J Am Heart Assoc. 2018;7:1–10. doi: 10.1161/JAHA.117.008146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Youssef I, Kamran H, Yacoub M, Patel N, Goulbourne C, Kumar S, Kane J, Hoffner H, Salifu M, McFarlane SI. Obstructive sleep apnea as a risk factor for atrial fibrillation: a meta‐analysis. J Sleep Disord Ther. 2018;07: doi: 10.4172/2167-0277.1000282 [DOI] [PMC free article] [PubMed] [Google Scholar]


