Abstract
Background
Despite the well‐established capacity of physical activity to reduce blood pressure, the associations between physical activity with cardiovascular disease (CVD) incidence and mortality in people living with hypertension are not well understood. We examine the dose‐response associations of device‐assessed physical activity with all‐cause and CVD mortality and CVD incidence (total, stroke, and coronary heart disease) in adults with hypertension.
Methods and Results
This prospective study included data from 39 294 participants with hypertension in the UK Biobank study who had valid accelerometry data and for whom mortality and CVD followed‐up data were available. We categorized moderate‐to‐vigorous physical activity and total physical activity volume into 4 categories based on the 10th, 50th, and 90th percentiles and used Cox regressions to estimate their associations with CVD mortality and incidence outcomes. Splines were used to assess the dose‐response associations. During a median follow‐up of 6.25 years (241 418 person‐years), 1518 deaths (549 attributable to CVD) and 4933 CVD (fatal and nonfatal) incident events were registered. Compared with the lowest category of moderate‐to‐vigorous physical activity, the relative risks (hazard ratios and 95% CIs) of all‐cause mortality for increasing categories were 0.53 (0.46–0.61), 0.41 (0.34–0.49), and 0.36 (0.26–0.49). We found associations of similar magnitude for total CVD incidence, stroke, and coronary heart disease; and for total physical activity volume across all outcomes. For all outcomes, there were linear or nearly linear inverse dose‐response relationships with no evidence of harms with high levels of physical activity. Results were robust to removing participants who died within the first 2 years.
Conclusions
Our findings underscore the importance of physical activity for people living with hypertension and provide novel insights to support the development of physical activity guideline recommendations for this high‐risk group.
Keywords: activity, aging, exercise, high blood pressure, primary prevention, secondary prevention
Subject Categories: Aging, Cardiovascular Disease, Lifestyle, Exercise, Epidemiology
Clinical Perspective
What Is New?
For the first time, we assessed the dose‐response associations of accelerometer‐derived physical activity with disease incidence and all‐cause and specific mortality in people with hypertension.
What Are the Clinical Implications?
We found no minimal or upper threshold for the beneficial effect of device‐assessed physical activity on risk of cardiovascular mortality and disease incidence.
Hypertension is the leading modifiable cause of cardiovascular disease (CVD) and premature death worldwide. 1 Globally, hypertension affects >1 billion people and is responsible for 10.4 million deaths each year. 2 The prevalence of hypertension and adverse impact on CVD morbidity and mortality are increasing. 3 , 4 It is therefore critical to find cost‐effective strategies to reduce the large‐scale burden of hypertension. 4
Several studies have documented the potential benefits of physical activity to prevent hypertension. 5 , 6 For example, a meta‐analysis of 24 cohort studies and 330 222 individuals reported a 6% reduction in the risk of hypertension among adults meeting the physical activity guidelines when compared with inactive individuals, with further risk reductions observed at higher levels of physical activity. 7 Evidence stemming from experimental and observational studies also shows the beneficial associations of regular participation in physical activities to lower the levels of blood pressure. 5 , 6 Not surprisingly, clinical 4 and public health 8 , 9 guidelines often recommend physical activity for the prevention and management of hypertension.
Fewer studies have investigated the associations of physical activity with CVD incidence and mortality in people with hypertension. A study conducted in 26 643 Finnish men and women with hypertension concluded that moderate or high levels of physical activity reduced CVD mortality. 10 More recently, another study in a sample of 18 974 men and women concluded that higher levels of self‐reported physical activity reduced all‐cause mortality and CVD incidence, independent of baseline blood pressure levels. 11 Despite its relevance, the current evidence relies almost exclusively on self‐reported physical activity assessments. The dose‐response association of physical activity with CVD incidence and mortality in this population remains largely unknown. This gap was flagged by the World Health Organization’s physical activity and sedentary behavior Guidelines Development Group in 2020 12 and the 2018 US Physical Activity Guidelines Advisory Committee. 5 Both initiatives concluded that the current evidence is insufficient and recommended robust cohort studies examining the dose‐response relationship between physical activity and key clinical end points in people with hypertension.
Capitalizing on a large prospective sample of adults assessed with accelerometers, the aim of the current study was to examine the dose‐response associations of device‐assessed physical activity with all‐cause and CVD mortality and CVD incidence in people with hypertension.
Methods
Data, Materials, and Code Disclosure Statement
Data and material used in this study are publicly available and can be accessed at https://www.ukbiobank.ac.uk/. The code used to analyze the data in this article is accessible on request from the corresponding author.
Study Design and Sample
This prospective investigation used data from the UK Biobank study. Briefly, the UK Biobank recruited 502 547 participants, aged 40 to 69 years, from 22 centers across the United Kingdom between 2006 and 2010. All participants provided consent for access to their national health‐related hospital and death records. The National Health Service and the National Research Ethics Service have approved the UK Biobank (reference 11/NW/0382). Further details about the UK Biobank are available elsewhere. 13 For the purpose of this study, we included participants with valid accelerometer data and who were considered to have hypertension according to hospitalization records (International Classification of Diseases, Tenth Revision [ICD‐10], codes I10.0–I15.9), blood pressure results (ie, >90/140 mm Hg for diastolic and systolic blood pressure, respectively), or self‐reported hypertension medication. Participants with missing data on any covariates were excluded.
Assessment of Physical Activity
Between June 2013 and December 2015, 103 684 UK Biobank participants wore an Axivity AX3 (Axivity, York, UK) accelerometer on their dominant wrist for 7 consecutive days. The Axivity AX3 is a small (23×32.5×8.9 mm) and lightweight (11 g) monitor that measures acceleration along 3 orthogonal axes. The sampling rate is configurable between 12.5 and 3200 Hz, and the dynamic range is between ±2 and ±16 g. For the current study, the accelerometers were initialized to collect data at 100 Hz with a dynamic range of ±8 g. Participants were excluded if the accelerometer could not be calibrated because of a lack of sufficient orientation changes or if they had implausible acceleration values, defined as an average vector magnitude acceleration >100 milligravities or <10 milligravities. Participants were also excluded if they wore the monitors for <3 days or did not have data for every 1‐hour period from midnight to midnight over the course of the 7‐day wear period.
As previously described, total time spent in light, moderate, and vigorous intensity was derived from time spent in 45 to 100 milligravities, 100 to 400 milligravities, and >400 milligravities, respectively. 14 , 15 Time spent in sedentary behavior was obtained by subtracting the time spent <45 milligravities from the participants’ reported duration spent sleeping per night. Total physical activity was estimated as the average acceleration while awake in milligravities as the time between 8 am and 8 pm over the 7‐day wear period.
Assessment of Mortality and CVD
The date and the cause of death (both primary and contributory) were obtained through the data linkage with either the National Health Service Digital of England and Wales or the National Health Service Central Register and National Records of Scotland. The inpatient hospitalization data were provided by the Hospital Episode Statistics for England, the Patient Episode Database for Wales, or the Scottish Morbidity Record for Scotland. Both the cause of death and the inpatient admission were coded with ICD‐10. We defined CVD (codes I00–I99) as diseases of the circulatory system, except for hypertensive diseases. Two CVD subtypes were further identified: stroke (codes I60–I64) and coronary heart disease (CHD) (codes I20–I25). Participants were followed up from the baseline measurement (between June 2013 and December 2015) until an event (death or incident disease) or the censoring date, whichever came first. Because of the nature of rolling updates of the data linkage, censoring dates varied between resources and outcomes: for mortality outcomes, censoring dates were February 12, 2021, March 21, 2021, and March 6, 2021, for Wales, Scotland, and England, respectively. Correspondent dates for hospitalization records were February 11, 2021, for Wales and December 14, 2020, for Scotland and England.
Covariates
Covariates in the fully adjusted models included age, sex, education, device‐assessed sedentary behavior, sleep pattern, obesity, smoking status, and alcohol use. Additional models were also adjusted for diet, 16 , 17 blood pressure, and preexisting CVD/hemoglobin A1c (Figure S1). Education was dichotomized by whether a participant had a college/university degree. Sleep pattern was calculated as the count of healthy characteristics: morning chronotype, adequate sleep duration (7–8 h/night), never or rare insomnia, never or rare snoring, and infrequent daytime sleepiness; and categorized into 3 groups (healthy, ≥4; intermediate, 2–3; and poor, ≤1). 18 , 19 Obesity was defined as body mass index ≥30 kg/m2. 20 Smoking status was categorized as never, previous, or current smoker. Alcohol use was categorized on the basis of drinking habits and the UK recommendation (14 units=140 mL of alcohol per week) into 6 groups: never, previous, occasional, within guidelines, double guidelines, and above double guidelines. 21
Statistical Analysis
We categorized total physical activity volume and time spent in moderate‐to‐vigorous physical activity into 4 categories on the basis of the 10th, 50th, and 90th percentiles of their distribution in the analytic samples (ie, the knots used in our spline regressions, 22 as described below). We described the sample by categories of total physical activity volume using the mean (SD) and percentages for continuous and categorical variables, respectively. For reference, we only computed descriptive statistics for the analytical sample pertaining to mortality outcomes (ie, the larger sample; Table S1 shows descriptive characteristics for moderate‐to‐vigorous physical activity categories).
We used multivariable‐adjusted Cox proportional hazard regressions to estimate the hazard ratios (HRs) and 95% CIs for the associations of categories of time spent in moderate‐to‐vigorous physical activity and total physical activity volume (with lowest category as the reference) with mortality and incidence outcomes. We found no violation of the proportional hazards function assumptions (ie, P values for Schonfeld global tests were all >0.05). For cause‐specific mortality (ie, CVD, stroke, and CHD) and disease incidence (ie, total CVD, stroke, and CHD), we used Fine and Grey models to account for competing risks. 23
We assessed the dose‐response associations of time spent in moderate‐to‐vigorous physical activity and total physical activity volume with all‐cause and cause‐specific mortality and CVD (total, stroke, and CHD) incidence outcomes using a restricted cubic spline model to allow for potential nonlinearity. For the purpose of this analysis, we trimmed observations <5% and >95% of the distribution. We prespecified knots placed at the 10th, 50th (reference), and 90th percentiles of the exposure distribution. 22 We assumed linearity for values <10th percentile and for values >90th percentile. Departure from linearity was assessed by a Wald test examining the null hypothesis that the coefficient of the second spline was equal to zero. When spline models suggested nonlinear associations, we estimated the minimal dose (ie, exposure value at which the risk reduction was statistically significant), the maximal dose (ie, exposure value at which the maximum significant risk reduction was observed), and the optimal dose (ie, exposure value at which the risk reduction was 50% of the observed maximum significant risk reduction).
To test the robustness of our estimates to reverse causation, we repeated the main mortality and CVD incidence outcomes models after excluding participants who died within the first 2 years of follow‐up. 24 Additional models were also adjusted for diet, hypertension medication, and preexisting CVD/hemoglobin A1c. Two additional models were conducted, estimating total physical activity as the average acceleration while awake in milligravities as the time between 7 am to 9 pm and 6 am to 10 pm over the 7‐day wear period.
We used R 4.2.1 (R Core Team, 2017) with package rms to conduct spline regressions and survival to conduct Cox regressions. We did all statistical testing at a 2‐tailed α level of 0.05. This study was reported as per the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (Table S2).
Results
For mortality outcomes, the final analytic sample consisted of 39 294 participants with a median follow‐up of 6.25 years (241 418 person‐years). Of these, 1518 participants died during the follow‐up period, 549 of whom died of CVD causes (93 were attributable to stroke and 251 were attributable to CHD) and 969 died of other causes. For CVD incidence, we further excluded participants with preexisting major CVD conditions at baseline (CHD and stroke). For total CVD incidence, the final sample consisted of 31 968 participants with complete data who entered the study free of CVD. Correspondent samples for stroke and CHD incidence analyses were 38 978 and 36 669 participants without stroke and CHD at study entry, respectively. The flow diagram of the participants in this study is shown in Figure S2.
The Table outlines the core baseline sample characteristics (n=39 294) stratified by category of the total volume of physical activity. All the selected covariates showed significant differences (P<0.001) between categories. Participants who were older, were men, were obese, had a university/college degree, had more sedentary time, experienced poorer sleep, smoked, and drank alcohol at least occasionally were more likely to have lower physical activity levels.
Table 1.
Baseline Characteristics of the Participants in the Study by Categories Based on the 10th, 50th, and 90th Percentiles of Accelerometer‐Measured Total Volume of PA Distribution (n=39 294)
| Variable | Total | <26.28 mg | 26.28–<39.04 mg | 39.04–<56.23 mg | ≥56.23 mg | P value* |
|---|---|---|---|---|---|---|
| Total No. | 39 294 | 3930 | 15 716 | 15 718 | 3930 | |
| Sedentary behavior, min/wk | 4994.02 (676.76) | 5833.86 (542.95) | 5267.30 (496.98) | 4711.65 (504.31) | 4190.70 (558.23) | <0.001 |
| MVPA, min/wk | 716.56 (320.88) | 284.64 (114.26) | 532.20 (132.75) | 862.03 (172.65) | 1303.88 (273.29) | <0.001 |
| Total volume of PA, mg | 40.50 (12.52) | 22.22 (3.14) | 33.21 (3.55) | 46.04 (4.71) | 65.73 (10.66) | <0.001 |
| Sleep patterns, n (%) † | <0.001 | |||||
| Poor | 916 (2.3) | 189 (4.8) | 372 (2.4) | 300 (1.9) | 55 (1.4) | |
| Intermediate | 15 437 (39.3) | 1856 (47.2) | 6569 (41.8) | 5697 (36.2) | 1315 (33.5) | |
| Healthy | 22 941 (58.4) | 1885 (48.0) | 8775 (55.8) | 9721 (61.8) | 2560 (65.1) | |
| Age, y | 58.39 (7.13) | 60.36 (6.75) | 59.16 (6.98) | 57.83 (7.07) | 55.53 (7.25) | <0.001 |
| Male sex, n (%) | 19 857 (50.5) | 2403 (61.1) | 8048 (51.2) | 7453 (47.4) | 1953 (49.7) | <0.001 |
| Obesity=yes, n (%) ‡ | 10 454 (26.6) | 1842 (46.9) | 4997 (31.8) | 3162 (20.1) | 453 (11.5) | <0.001 |
| Education=university/college, n (%) | 23 878 (60.8) | 2528 (64.3) | 9440 (60.1) | 9549 (60.8) | 2361 (60.1) | 0.001 |
| Smoking, n (%) | <0.001 | |||||
| Never | 21 230 (54.0) | 1877 (47.8) | 8451 (53.8) | 8694 (55.3) | 2208 (56.2) | |
| Previous | 15 582 (39.7) | 1611 (41.0) | 6245 (39.7) | 6194 (39.4) | 1532 (39.0) | |
| Current | 2482 (6.3) | 442 (11.2) | 1020 (6.5) | 830 (5.3) | 190 (4.8) | |
| Diet score, n (%) § | <0.001 | |||||
| Poor | 9650 (25.0) | 748 (19.5) | 3716 (24.0) | 4061 (26.2) | 1125 (29.1) | |
| Reasonable | 2142 (5.5) | 329 (8.6) | 897 (5.8) | 740 (4.8) | 176 (4.6) | |
| Good | 26 850 (69.5) | 2759 (71.9) | 10 857 (70.2) | 10 672 (69.0) | 2562 (66.3) | |
| Alcohol use, n (%) || | <0.001 | |||||
| Never | 1104 (2.8) | 153 (3.9) | 463 (2.9) | 402 (2.6) | 86 (2.2) | |
| Previous | 1054 (2.7) | 173 (4.4) | 449 (2.9) | 355 (2.3) | 77 (2.0) | |
| Occasional | 7457 (19.0) | 1045 (26.6) | 3124 (19.9) | 2649 (16.9) | 639 (16.3) | |
| Within guidelines | 13 264 (33.8) | 1075 (27.4) | 5349 (34.0) | 5547 (35.3) | 1293 (32.9) | |
| Double guidelines | 9530 (24.3) | 839 (21.3) | 3627 (23.1) | 4012 (25.5) | 1052 (26.8) | |
| Above double guidelines | 6885 (17.5) | 645 (16.4) | 2704 (17.2) | 2753 (17.5) | 783 (19.9) | |
| HbA1c, mmol/mol | 36.21 (6.23) | 38.20 (8.39) | 36.54 (6.77) | 35.69 (5.22) | 34.97 (4.29) | <0.001 |
| Preexisting CVD=yes, n (%) | 13 686 (34.8) | 1963 (49.9) | 5978 (38.0) | 4785 (30.4) | 960 (24.4) | <0.001 |
| Hypertension medication=yes, n (%) | 12 938 (32.9) | 1877 (47.8) | 5838 (37.1) | 4435 (28.2) | 788 (20.1) | <0.001 |
| Arterial pressure, mm Hg | 109.06 (10.19) | 108.69 (11.16) | 109.04 (10.36) | 109.11 (9.92) | 109.27 (9.50) | 0.071 |
| Diastolic blood pressure, mm Hg | 87.61 (9.79) | 87.33 (10.46) | 87.65 (9.91) | 87.59 (9.61) | 87.76 (9.27) | 0.238 |
| Systolic blood pressure, mm Hg | 151.96 (16.24) | 151.42 (17.65) | 151.82 (16.50) | 152.16 (15.85) | 152.30 (15.24) | 0.028 |
Values represent mean (SD) unless specified otherwise. CVD indicates cardiovascular disease; HbA1c, hemoglobin A1c; mg, milligravities; MVPA, moderate‐to‐vigorous PA; and PA, physical activity.
One‐way ANOVA for continuous variables and χ2 test for categorical variables.
Participants were categorized by how many healthy sleep characteristics (morning chronotype, adequate sleep duration [7–8 h/d], never or rare insomnia, never or rare snoring, and infrequent daytime sleepiness) they displayed into 3 groups (healthy, ≥4; intermediate, 2–3; poor, ≤1). 16 , 17
Obesity was ascertained on the basis of body mass index (ie, participants with a body mass index ≥30 kg/m2 were considered obese).
Dietary intake was collected using a touchscreen questionnaire that collected information on food frequency consumption. 18 Dietary pattern was classified on the basis of the UK’s latest‐available National Health Service Eatwell Guide and a previously applied scoring procedure that considered consumption of fruits, vegetables, fish, red meats (unprocessed), and processed meats. One point was awarded for each of the following conditions that were met in each participant’s diet, with possible scores ranging from 0 to 4 points: total fruit and vegetable intake ≥4.5 pieces or servings/d (one serving of vegetables was considered to be 3 tablespoons of vegetables); total fish intake ≥2 times/wk; red meat (unprocessed) intake ≤5 times/wk; and processed meat intake ≤2 times/wk. Diets were categorized as poor (0), reasonable (1), and good (2–4). 19
Guidelines for alcohol use in the United Kingdom recommend no more than 14 units of alcohol per week for both men and women.
Mortality Outcomes
Compared with the lowest category of moderate‐to‐vigorous physical activity, the relative risks (HR and 95% CI) of all‐cause mortality for increasing categories were 0.53 (0.46–0.61), 0.41 (0.34–0.49), and 0.36 (0.26–0.49) (Figure 1). Corresponding values for total physical activity volume were 0.56 (0.48–0.64), 0.41 (0.34–0.50), and 0.30 (0.21–0.41) (Figure 1). We found similar trends for CVD mortality for both moderate‐to‐vigorous physical activity and total physical activity volume (Figure 1). The dose‐response analysis revealed a nonlinear association between moderate‐to‐vigorous physical activity and all‐cause mortality (minimal dose, 676 min/wk; maximal dose, 1147 min/wk; optimal dose, 804 min/wk) and linear dose‐response associations between moderate‐to‐vigorous physical activity and total physical activity volume with CVD mortality (Figure 2). We did not detect an association between moderate‐to‐vigorous physical activity and stroke mortality (Figure 1 and Figure S3). In contrast, we found linear associations of total physical activity volume with stroke and CHD mortality and between moderate‐to‐vigorous physical activity and CHD mortality (Figure 1 and Figure S3).
Figure 1. Adjusted hazard ratios (HRs) for mortality outcomes by categories of average accelerometer‐measured total volume and moderate‐to‐vigorous intensity physical activity in adults with hypertension (n=39 294).
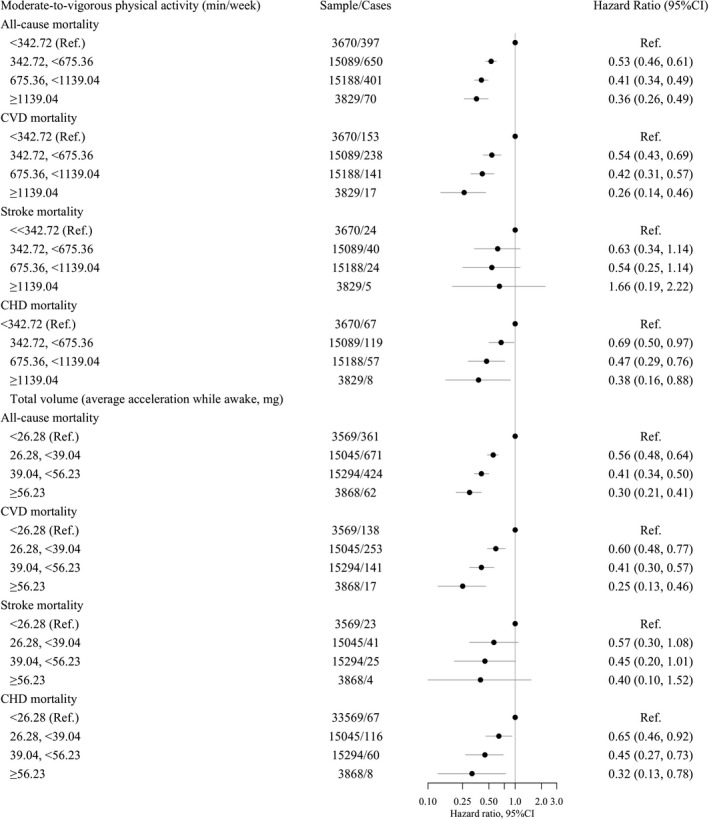
Models adjusted for age, sex, education, sedentary behavior (only models for moderate‐to‐vigorous physical activity), sleep pattern, obesity, smoking, and alcohol use. Lines represent 95% CI and circle is the estimate (hazard ratio). HRs are in logarithmic scale. CHD indicates coronary heart disease; CVD, cardiovascular disease; mg, milligravities; and Ref., reference.
Figure 2. Dose‐response association (adjusted hazard ratios [HRs] and associated 95% CI band) between accelerometer‐measured moderate‐to‐vigorous physical activity (MVPA) and total volume of physical activity with all‐cause (n=39 294; events=1518) and cardiovascular disease (CVD) mortality (n=39 294; events=549).
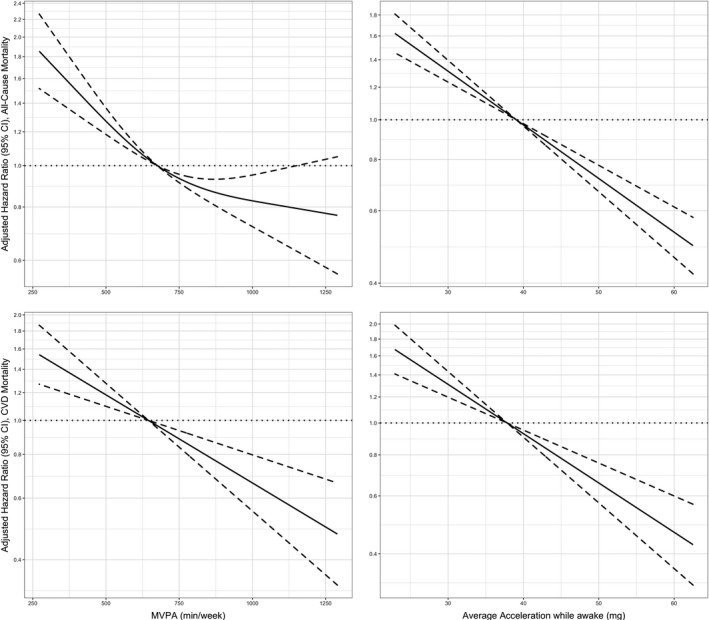
Models adjusted for age, sex, education, sedentary behavior (only models for MVPA), sleep pattern, obesity, smoking, and alcohol use. Dose‐response associations were assessed with restricted cubic splines with knots at 10th, 50th, and 90th centiles of the distribution of the exposure of interest (reference category=675.36 min/wk of MVPA; and 39.04 milligravities [mg] for total volume of physical activity). HRs are in logarithmic scale. Dashed line (the line that goes along y=1) represents the reference value. Solid line is the estimate (hazard ratio) and dotted lines is the 95% CI.
CVD Incidence Outcomes
During a median follow‐up of 5.9 years (492 person‐years; n=31 968), 4933 participants developed CVD. Compared with participants categorized in the lowest category of moderate‐to‐vigorous physical activity, the relative risks (HR and 95% CI) of CVD incidence for increasing categories were 0.77 (0.71–0.84), 0.65 (0.59–0.72), and 0.57 (0.49–0.67) (Figure 3). Corresponding values for total physical activity volume were 0.77 (0.70–0.84), 0.66 (0.59–0.74), and 0.61 (0.52–0.71) (Figure 3). In both instances, the dose‐response associations were linear (Figure 4). Linear dose‐response associations were also found for moderate‐to‐vigorous physical activity and stroke and CHD incidence (Figure 3 and Figure S4). In contrast, a nonlinear association was detected for total physical activity volume and stroke incidence (minimal dose, 36 milligravities; maximal dose, 47 milligravities; optimal dose, 41 milligravities; Figure 3 and Figure S4).
Figure 3. Adjusted hazard ratios (HRs) for incident cardiovascular disease (CVD) outcomes (total, coronary heart disease [CHD], and stroke) by categories of average accelerometer‐measured total volume and moderate‐to‐vigorous intensity physical activity in adults with hypertension (total CVD, n=31 968; stroke, n=38 978; and CHD, n=36 669).
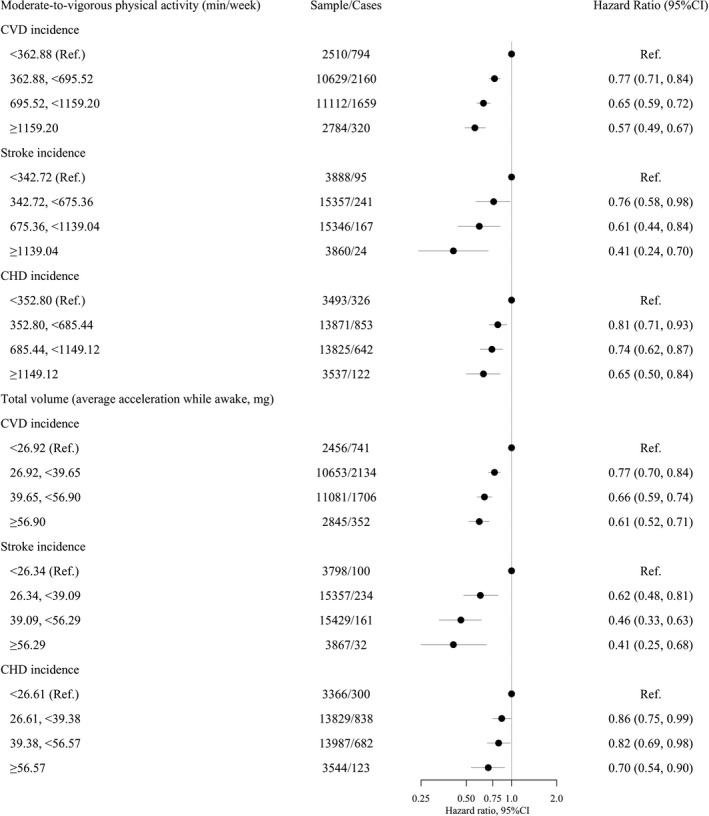
Models adjusted for age, sex, education, sedentary behavior (only models for moderate‐to‐vigorous physical activity), sleep pattern, obesity, smoking, and alcohol use. Lines represent 95% CI and circle is the estimate (hazard ratio). HRs are in logarithmic scale. Mg indicates milligravities; and Ref., reference.
Figure 4. Dose‐response association (adjusted hazard ratios [HRs] and associated 95% CI band) between accelerometer‐measured moderate‐to‐vigorous physical activity (MVPA) and total volume of physical activity with incidence of cardiovascular disease (CVD) (n=31 968; events=4933).
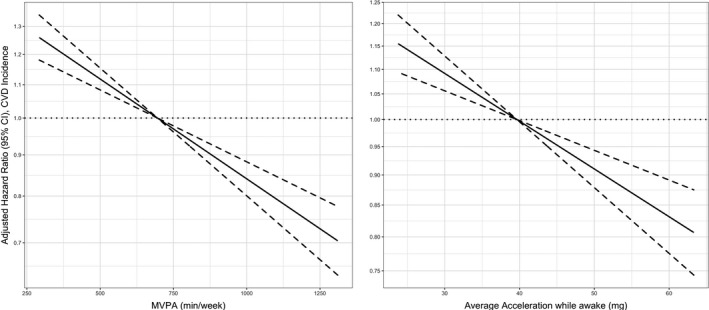
.Models adjusted for age, sex, education, sedentary behavior (only models for MVPA), sleep pattern, obesity, smoking, and alcohol use. Dose‐response associations were assessed with restricted cubic splines with knots at 10th, 50th, and 90th centiles of the distribution of the exposure of interest (reference category=695.52 min/wk of MVPA; and 39.65 milligravities [mg] for total volume of physical activity). HRs are in logarithmic scale. Dashed line (the line that goes along y=1) represents the reference value. Solid line is the estimate (hazard ratio) and dotted lines is the 95% CI.
After excluding participants in the study who died within the first 24 months of follow‐up (n=317), results did not materially differ from the main analysis (Figure S5) but the dose‐response association between moderate‐to‐vigorous physical activity and all‐cause mortality became linear. Results including diet (Figure S6), blood pressure (Figure S7), and preexisting CVD/hemoglobin A1c (Figure S8) yielded consistent results. Results were also robust to total physical activity estimated for the time between 7 am to 9 pm and 6 am to 10 pm (Figure S9).
Discussion
This is, to our knowledge, the largest examination of the associations of device‐measured physical activity and CVD incidence and mortality in a population sample of adults living with hypertension. We found a linear inverse dose‐response association between accelerometry‐assessed moderate‐to‐vigorous physical activity and CVD incidence and mortality. A linear inverse association of similar magnitude was also found for total physical activity volume. Our findings address an important evidence gap identified by World Health Organization physical activity and sedentary behavior Guidelines Development Group 12 and the 2018 US Physical Activity Guidelines Advisory Committee, 5 and underscore the importance of physical activity for prevention of CVD among people living in this prevalent population group.
The results of this study expand on the existing limited and methodologically weaker evidence base, which has suggested some beneficial associations of physical activity with all‐cause and CVD among adults with hypertension. 10 , 11 , 25 More important, our study offers unique and novel insights in several ways. First, we used accelerometry to assess physical activity, thus overcoming a major limitation of previous studies that relied on subjective self‐report methods that are prone to recall and social desirability bias. 26 , 27 This may partially explain why our observed associations were markedly stronger than those previously reported in the self‐reported physical activity literature. 7 Second, we report for the first time on the detailed dose‐response associations between physical activity and all‐cause and CVD mortality and incidence among adults with hypertension, a population with an elevated risk for major CVD events.
All‐Cause Mortality
We found a nonlinear dose‐response association between moderate‐to‐vigorous physical activity and total physical activity volume with all‐cause mortality. However, this association became linear after excluding participants who died in the first 2 years of follow‐up. These results contrast with recent findings reported for the general population using accelerometry, 28 where the dose‐response was nonlinear (L‐shape), and highlight the differences between the general population and people with hypertension, for whom no low or high physical activity threshold was revealed (ie, any activity is better than none; and the more the better).
Total CVD Incidence and Mortality
We also uncovered a linear inverse dose‐response association between physical activity and total CVD incidence and mortality. Although not directly comparable, these findings concur with those from a previous study that reported gradually stronger associations between nonfatal CVD events and self‐reported physical activity intensity. 11 More important, we did not detect any evidence for increased risk among participants with hypertension who engaged in large amounts of physical activity. This finding contrasts with the results observed in a large population‐based study of British women with self‐reported measures of physical activity, 29 which found that an increased participation in physical activity was associated with higher risk of CHD, cerebrovascular disease, and venous thromboembolic events. These differences may underscore the importance of using accelerometers and 24‐hour continuous measures of physical activity to derive more precise estimates 28 , 30 , 31 , 32 and may also indicate that hypertension does not seem to add a major risk for engaging in large amounts of physical activity.
Stroke and CHD Incidence and Mortality
We did not detect a statistically significant association between physical activity and stroke mortality. However, the point estimates for both moderate‐to‐vigorous physical activity and total volume were remarkably similar to those obtained for total CVD mortality, suggesting that the lack of significance may be attributable to the compromised statistical power and the wide 95% CIs. In contrast, we found linear inverse dose‐response associations between total volume and moderate‐to‐vigorous physical activity with stroke incidence and CHD incidence and mortality. These unique findings are highly relevant to clinical practice, as they highlight potential benefits of physical activity for preventing 2 of the most common causes of death and disability among people with hypertension. 33 , 34
Another novel and clinically relevant result of our study was the similar in magnitude associations for moderate‐to‐vigorous physical activity and total physical activity volume, a finding similar to what was recently reported in the general population. 35 A possible explanation is that participants who accumulated more total volume of physical activity also engaged in more moderate‐to‐vigorous physical activity in our study. It is unclear if this finding reflects a genuine behavioral pattern in the UK Biobank sample, or it is driven by how moderate to vigorous physical activity is calculated in studies using wrist‐worn accelerometry. Several previous studies have shown cardiometabolic benefits of light intensity activities 36 , 37 and added benefits for vigorous intensity physical activity. 38
Strengths and Limitations
Our study had several strengths. First, this is the largest analysis of device‐based measurement of physical activity in participants with hypertension to date. Accelerometers have several distinct advantages over self‐reports, including minimal recall bias and the ability to capture both structured exercise and incidental physical activity of light intensity. 28 , 36 It is nonetheless important to note the differences between device‐assessed and self‐reported physical activity estimates. For example, we found that our participants spent ≈700 min/wk of moderate‐to‐vigorous physical activity level, which is much higher than the recommended level of physical activity (ie, 150 min/wk). A previous study has shown that 150 min/wk of self‐reported moderate‐to‐vigorous physical activity corresponded to ≈1000 min/wk of device‐assessed physical activity. 39 We envisage that future guidelines will be informed by device‐assessed physical activity studies such as ours to reconcile future recommendations with newer types of evidence. 39 Another key strength is that the UK Biobank relies on registry‐based prospectively collected data, which increases the internal validity of our estimates. Unlike analogous previous studies in people with hypertension, we used methods to account for the presence of competing risk factors in our cause‐specific outcome analyses. 23
Our study has several limitations also. Physical activity may not be stable over time, particularly after developing chronic disease, such as hypertension. 40 Therefore, the lack of repeated physical activity measures in this study limits our ability to make strong causal claims. Accelerometry measurement in this study may be biased because of reproducibility issues (eg, seasonal reproducibility, weekday‐holiday reproducibility, and weather reproducibility) and reactivity issues (ie, participants may have modified their activity levels in response to wearing an accelerometer). An intrinsic limitation to this data is that covariate measurement was not undertaken at the present study baseline (ie, accelerometer wear date). Nonetheless, the responses have been shown to be generally stable over time and therefore the associations between accelerometer‐assessed physical activity and health outcomes are valid. 41 Despite the consistency of our estimates after removing participants who died within the first 2 years of follow‐up, some potential for reverse causation may still exist. Although we were able to account for several factors known to influence the associations between physical activity and mortality and morbidity outcomes, residual (unmeasured) confounding may still be present. The UK Biobank had a low response rate, and participants are not representative of the overall UK population (ie, participants in our study were majority White race, educated, and from less socially disadvantaged areas). 42 Nonetheless, recent studies have demonstrated that the lack of representativeness in the UK Biobank does not materially affect the associations of physical activity and mortality outcomes, including CVD. 43 , 44 Last, the number of events for some narrow CVD outcomes analyses was low, compromising our ability to make meaningful inference.
Clinical Implications
Our results have 2 main implications for clinical and public health practice. First, we found no minimal or upper threshold for the beneficial effect of device‐assessed physical activity on risk of cardiovascular mortality and disease incidence, which echoes the “any physical activity counts, a little is better than nothing campaign of the recent 2020 WHO Guidelines on Physical Activity and Sedentary Behaviour” 8 and highlights the lack of risks associated with high amounts of physical activity in people with hypertension. The second main practical implication was that the beneficial associations for total physical activity volume and for time in moderate‐to‐vigorous activity were comparable. If confirmed in other cohorts, our findings could support the use of physical activity among people with hypertension not only for the management of high blood pressure, 45 but for primary prevention of CVD events.
Conclusions
In this large‐scale study of device‐based physical activity in people with hypertension, we found a linear dose‐response association with all‐cause and cardiovascular mortality and morbidity. The risk reduction associated with total physical activity volume was similar to that observed for moderate‐to‐vigorous physical activity. Future clinical and public health guidelines aimed at people living with hypertension may use our findings to support the prevention of serious CVD consequences associated with the ever‐increasing prevalence of high blood pressure.
Sources of Funding
This work was partly supported by University of Southern Denmark (Dr del Pozo Cruz) and partly by a National Health and Medical Research Council Investigator Grant (APP1194510; Dr Stamatakis).
Disclosures
None.
Supporting information
Tables S1‐S2
Figures S1–S9
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023290
For Sources of Funding and Disclosures, see pages 10.
References
- 1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schneider RH, Salerno J, Brook RD. 2020 International Society of Hypertension global hypertension practice guidelines–lifestyle modification. J Hypertens. 2020;38:2340–2341. doi: 10.1097/HJH.0000000000002625 [DOI] [PubMed] [Google Scholar]
- 5. Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, Campbell WW, Dietz S, Dipietro L, George SM, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2019;51:1314–1323. doi: 10.1249/MSS.0000000000001943 [DOI] [PubMed] [Google Scholar]
- 6. Smart NA, Howden R, Cornelissen V, Brook R, McGowan C, Millar PJ, Ritti‐Dias R, Baross A, Carlson DJ, Wiles JD, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2020;52:1001–1002. doi: 10.1249/MSS.0000000000002263 [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Zhang D, Liu YU, Sun X, Han C, Wang B, Ren Y, Zhou J, Zhao Y, Shi Y, et al. Dose‐response association between physical activity and incident hypertension: a systematic review and meta‐analysis of cohort studies. Hypertension. 2017;69:813–820. doi: 10.1161/HYPERTENSIONAHA.116.08994 [DOI] [PubMed] [Google Scholar]
- 8. Bull FC, Al‐Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput J‐P, Chastin S, Chou R, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu G, Jousilahti P, Antikainen R, Tuomilehto J. Occupational, commuting, and leisure‐time physical activity in relation to cardiovascular mortality among Finnish subjects with hypertension. Am J Hypertens. 2007;20:1242–1250. doi: 10.1016/j.amjhyper.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 11. Joseph G, Marott JL, Torp‐Pedersen C, Biering‐Sørensen T, Nielsen G, Christensen A‐E, Johansen MB, Schnohr P, Sogaard P, Mogelvang R. Dose‐response association between level of physical activity and mortality in normal, elevated, and high blood pressure. Hypertension. 2019;74:1307–1315. doi: 10.1161/HYPERTENSIONAHA.119.13786 [DOI] [PubMed] [Google Scholar]
- 12. DiPietro L, Al‐Ansari SS, Biddle SJH, Borodulin K, Bull FC, Buman MP, Cardon G, Carty C, Chaput J‐P, Chastin S, et al. Advancing the global physical activity agenda: recommendations for future research by the 2020 WHO physical activity and sedentary behavior guidelines development group. Int J Behav Nutr Phys Act. 2020;17:143. doi: 10.1186/s12966-020-01042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hildebrand M, Hansen BH, van Hees VT, Ekelund U. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand J Med Sci Sports. 2017;27:1814–1823. doi: 10.1111/sms.12795 [DOI] [PubMed] [Google Scholar]
- 15. Hildebrand M, Van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist‐ and hip‐worn monitors. Med Sci Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 16. Bradbury KE, Young HJ, Guo W, Key TJ. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. doi: 10.1017/jns.2017.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutten‐Jacobs LC, Larsson SC, Malik R, Rannikmäe K, Sudlow CL, Dichgans M, Markus HS, Traylor M. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank participants. BMJ. 2018;363:k4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, Qi L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK Biobank participants. Eur Heart J. 2020;41:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morgan K, Hartescu I. Sleep duration and all‐cause mortality: links to physical activity and prefrailty in a 27‐year follow up of older adults in the UK. Sleep Med. 2019;54:231–237. [DOI] [PubMed] [Google Scholar]
- 20. Obesity . https://www.who.int/health‐topics/obesity. Accessed May 18, 2021.
- 21. NHS website . Alcohol units. https://www.nhs.uk/live‐well/alcohol‐support/calculating‐alcohol‐units/. Accessed May 18, 2021.
- 22. Harrell FE. Regression modeling strategies. Springer Series in Statistics. 2001. doi: 10.1007/978-1-4757-3462-1 [DOI] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24. Singh PN, Wang X. Simulation study of the effect of the early mortality exclusion on confounding of the exposure‐mortality relation by preexisting disease. Am J Epidemiol. 2001;154:963–971. doi: 10.1093/aje/154.10.963 [DOI] [PubMed] [Google Scholar]
- 25. Vatten LJ, Nilsen TIL, Holmen J. Combined effect of blood pressure and physical activity on cardiovascular mortality. J Hypertens. 2006;24:1939–1946. [DOI] [PubMed] [Google Scholar]
- 26. Brenner PS, DeLamater JD. Social desirability bias in self‐reports of physical activity: is an exercise identity the culprit? Soc Indic Res. 2014;117:489–504. doi: 10.1007/s11205-013-0359-y [DOI] [Google Scholar]
- 27. Celis‐Morales CA, Perez‐Bravo F, Ibañez L, Salas C, Bailey MES, Gill JMR. Objective vs. self‐reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7:e36345. doi: 10.1371/journal.pone.0036345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ekelund U, Tarp J, Steene‐Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, et al. Dose‐response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta‐analysis. BMJ. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armstrong MEG, Green J, Reeves GK, Beral V, Cairns BJ. Frequent physical activity may not reduce vascular disease risk as much as moderate activity. Circulation. 2015;131:721–729. [DOI] [PubMed] [Google Scholar]
- 30. Dohrn I‐M, Sjöström M, Kwak L, Oja P, Hagströmer M. Accelerometer‐measured sedentary time and physical activity—a 15 year follow‐up of mortality in a Swedish population‐based cohort. J Sci Med Sport. 2018;21:702–707. doi: 10.1016/j.jsams.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 31. LaMonte MJ, Buchner DM, Rillamas‐Sun E, Di C, Evenson KR, Bellettiere J, Lewis CE, Lee I‐M, Tinker LF, Seguin R, et al. Accelerometer‐measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc. 2018;66:886–894. doi: 10.1111/jgs.15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee I‐M, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE. Accelerometer‐measured physical activity and sedentary behavior in relation to all‐cause mortality: the women’s health study. Circulation. 2018;137:203–205. doi: 10.1161/CIRCULATIONAHA.117.031300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Satoh M, Ohkubo T, Asayama K, Murakami Y, Sugiyama D, Yamada M, Saitoh S, Sakata K, Irie F, Sairenchi T, et al. Lifetime risk of stroke and coronary heart disease deaths according to blood pressure level: EPOCH‐JAPAN (Evidence for Cardiovascular Prevention From Observational Cohorts in Japan). Hypertension. 2019;73:52–59. doi: 10.1161/HYPERTENSIONAHA.118.11635 [DOI] [PubMed] [Google Scholar]
- 34. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 35. Ramakrishnan R, Doherty A, Smith‐Byrne K, Rahimi K, Bennett D, Woodward M, Walmsley R, Dwyer T. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK Biobank cohort study. PLoS Med. 2021;18:e1003487. doi: 10.1371/journal.pmed.1003487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Del Pozo CB, Biddle SJH, Gardiner PA, Ding D. Light‐intensity physical activity and life expectancy: national health and nutrition survey. Am J Prev Med. 2021;61:428–433. doi: 10.1016/j.amepre.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 37. Chastin SFM, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M, Stamatakis E. How does light‐intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta‐analysis of experimental and observational studies. Br J Sports Med. 2019;53:370–376. doi: 10.1136/bjsports-2017-097563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rey Lopez JP, Gebel K, Chia D, Stamatakis E. Associations of vigorous physical activity with all‐cause, cardiovascular and cancer mortality among 64 913 adults. BMJ Open Sport Exerc Med. 2019;5:e000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson D, Batterham AM, Peacock OJ, Western MJ, Booso R. Feedback from physical activity monitors is not compatible with current recommendations: a recalibration study. Prev Med. 2016;91:389–394. doi: 10.1016/j.ypmed.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lei Y, Yeo W, Ho SC, Cheng ACK, Kwok C. Changes in physical activity from pre‐diagnosis to first five years post‐diagnosis: a prospective cohort study in Chinese breast cancer patient. J Clin Oncol. 2020;38:e24121. doi: 10.1200/JCO.2020.38.15_suppl.e24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strain T, Wijndaele K, Dempsey PC, Sharp SJ, Pearce M, Jeon J, Lindsay T, Wareham N, Brage S. Wearable‐device‐measured physical activity and future health risk. Nat Med. 2020;26:1385–1391. doi: 10.1038/s41591-020-1012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health‐related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta‐analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stamatakis E, Owen KB, Shepherd L, Drayton B, Hamer M, Bauman AE. Is cohort representativeness passé? Poststratified associations of lifestyle risk factors with mortality in the UK Biobank. Epidemiology. 2021;32:179–188. doi: 10.1097/EDE.0000000000001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naci H, Salcher‐Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, Ioannidis JPA. How does exercise treatment compare with antihypertensive medications? A network meta‐analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53:859–869. doi: 10.1136/bjsports-2018-099921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2
Figures S1–S9


