Abstract
Background
Myocardial strain can identify subclinical left ventricular dysfunction in various cardiac diseases, but its association with clinical outcomes in genetic cardiomyopathies remains unknown. Herein, we assessed myocardial strain in patients with Danon disease (DD), a rare X‐linked autophagic disorder that causes severe cardiac manifestations.
Methods and Results
Echocardiographic images were reviewed and used to calculate myocardial strain from a retrospective, international registry of patients with DD. Regression analyses were performed to evaluate for an association of global longitudinal strain (GLS) and ejection fraction with the composite outcome (death, ventricular assist device, heart transplantation, and implantable cardioverter defibrillator for secondary prevention). A total of 22 patients with DD (male 14 [63.6%], median age 16.5 years) had sufficient echocardiograms for analysis. Absolute GLS was reduced with a mean of 12.2% with an apical‐sparing pattern observed. Univariable regression for GLS and composite outcome showed an odds ratio of 1.32 (95% CI, 1.02–1.71) with P=0.03. For receiver operating characteristic analysis, the areas under the curve for GLS and ejection fraction were 0.810 (P=0.02) and 0.605 (P=0.44), respectively. An absolute GLS cutoff of 10.0% yielded a true positive rate of 85.7% and false positive rate of 13.3%.
Conclusions
In this cohort of patients with DD, GLS may be a useful assessment of myocardial function and may predict clinical outcomes. This study highlights the potential use of myocardial strain phenotyping to monitor disease progression and potentially to predict clinical outcomes in DD and other genetic cardiomyopathies.
Keywords: Danon disease, echocardiography, ejection fraction, genetic cardiomyopathy, hypertrophic cardiomyopathy, left ventricular hypertrophy, strain
Subject Categories: Echocardiography, Genetics, Cardiomyopathy, Heart Failure
Nonstandard Abbreviations and Acronyms
- EF
ejection fraction
- HCM
hypertrophic cardiomyopathy
- LA
left atrial
Clinical Perspective
What Is New?
In Danon disease, a reduction in global longitudinal strain is associated with the composite outcome of death, ventricular assist device, heart transplantation, and implantable cardio‐defibrillator for secondary prevention.
What Are the Clinical Implications?
Global longitudinal strain may be a useful marker to follow longitudinally and predict clinical outcomes in patients with genetic cardiomyopathies.
Left ventricular (LV) ejection fraction (EF) has been the traditional method to assess cardiac function and to prognosticate outcomes in patients with heart disease. Current guidelines recommend EF thresholds for many therapeutic recommendations. 1 , 2 However, EF does not directly measure myocardial contractility and has limited efficacy in predicting outcomes. 1 Reproducible and reliable tools for measuring myocardial function are needed to ensure appropriate patient treatment and improve clinical outcomes. Accordingly, myocardial strain has been increasingly used in cardiovascular disorders because of its ability to detect subclinical myocardial dysfunction independent of motion from translation or tethering. 3
Global longitudinal strain (GLS), measured by speckle‐tracking echocardiography, potentially offers a more accurate and direct measure of myocardial function. GLS can identify cardiac pathology before clinical symptoms and reduction in EF. 4 , 5 GLS has been well validated as a prognostic marker in heart failure, myocardial infarction, chemotherapy cardiotoxicity, and amyloid cardiomyopathy. 6 , 7 , 8 , 9 It has been shown that strain in patients with hypertrophic cardiomyopathy (HCM) is significantly reduced despite normal LV EF. 10 , 11 Furthermore, GLS may be useful in differentiating causes of LV hypertrophy not only of sarcomeric HCM, but also HCM “phenocopies” such as Danon disease (DD), Anderson‐Fabry disease, Pompe disease, and Friedreich ataxia and more common ones such as hypertensive heart disease and aortic stenosis. However, studies evaluating strain in genetic cardiomyopathies remain limited.
The aim of this study was to analyze the association of various echocardiographic parameters with clinical outcomes in a cohort of patients with DD, a rare X‐linked genetic cardiomyopathy that ultimately progresses to end‐stage heart failure. 12
Methods
Study Population
A retrospective, international registry was created to understand the natural history of DD (ClinicalTrials.gov Identifier NCT03766386). Medical records, including past medical and surgical history, medications, laboratory results, genetic testing, ECGs, and Digital Imaging and Communications in Medicine imaging were solicited. Patients gave consent to participate in the registry between January 2018 and December 2019. DD diagnosis was confirmed with genetic testing confirming a LAMP2 mutation and/or with histochemical staining for LAMP2 deficiency on muscle biopsies. All echocardiograms included in the study were completed at an outside facility, but were re‐interpreted at University of California, San Diego. Patients without adequate quality echocardiograms were excluded from the study (Figure 1). Adequate quality was defined by absence of signal dropout or artifacts and <2 myocardial borders obscured. The study was approved by the University of California, San Diego Human Research Protection Program and University of Colorado Multi‐Institutional Review Board. Informed consent was obtained from all patients enrolled in the registry. With the goal of advancing the field of rare genetic diseases, the study data will be available from the corresponding author upon reasonable request.
Figure 1. Study selection and flow diagram.
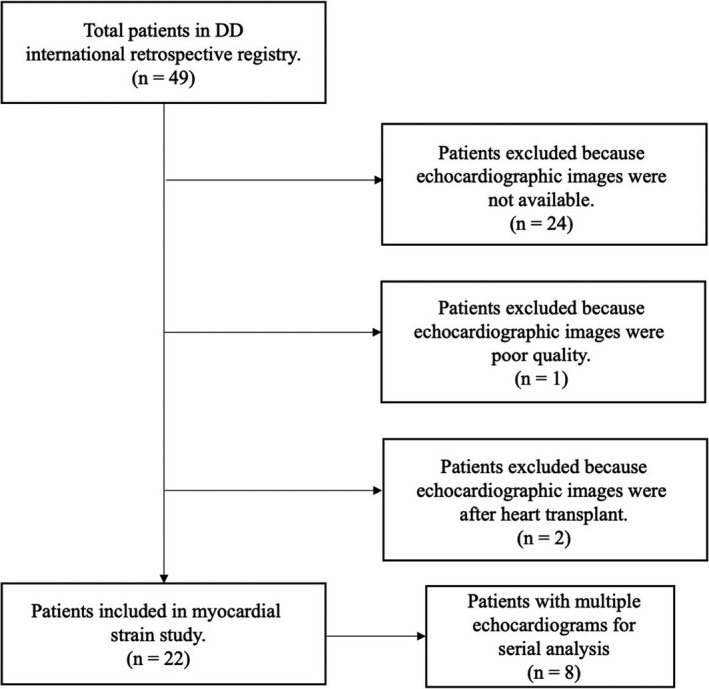
DD indicates Danon disease.
Two‐Dimensional Echocardiographic Evaluation
Echo measurements were obtained according to the adult and pediatric Cardiac Chamber Quantification Guidelines of the American Society of Echocardiography. 13 , 14 EF and left atrial (LA) volume indexed to body surface area were measured using the Simpson biplane method from the apical 4‐ and 2‐chamber views. LV endocardial dimensions, interventricular septal diameter and left ventricular posterior wall diameter (LVPWd) were measured at end‐diastole. LV mass was calculated using the cube formula and relative wall thickness was calculated as 2 times LVPWd divided by LV end‐diastolic diameter. Given a high proportion of pediatric patients (age <18 years), Z‐scores were calculated for interventricular septal diameter, LVPWd, LV end‐diastolic diameter, and LV mass according to a previous imaging assessment. 15 Diastolic parameters, including mitral inflow velocities (early [E] and late [A]), pulsed wave tissue Doppler velocity of the mitral annulus (e’), E/A and E/e’ ratios were assessed using 2016 American Society of Echocardiography diastology guidelines. 16
Two‐Dimensional Strain Evaluation
Strain analysis was completed using EchoInsight software v.3.2.3.5564 (Epsilon Imaging, Ann Arbor, MI, USA). For 2D strain analysis, 2D gray‐scale tracking included the standard 2, 3, and 4‐chamber apical views. Longitudinal strain (LS) was measured globally and regionally (basal, mid, apex) in all 22 patients. To avoid confusion when communicating about strain, values will be reported as an absolute number. Myocardial strain was measured independently (by QB and MK).
Statistical Analysis
For patients with >1 echocardiogram, the most recent study was used for comparison and regression analyses. Echocardiographic parameters and LS were aggregated within the entire DD cohort and then compared by sex. Strain values were compared based on groups that experienced the composite outcome and those that did not. Logistic regression was used to determine associations between GLS and composite outcome. Linear regression was completed to evaluate for relationships between strain and EF with time. Receiver operating characteristic curves were used to propose a GLS cutoff for the composite outcome. Scatter plots displayed GLS and EF across time. Scatter plots were created using data from all available echocardiograms, including patients with multiple studies. Additional scatter plots were provided in the supplemental section that includes data only from the most recent echocardiogram.
Continuous variables were reported either as mean with SD or as median with interquartile range as appropriate based on normality of distribution assessed by Shapiro–Wilk test. Categorical variables were expressed as counts with percentages. Variables were compared using the unpaired Student t test, Mann–Whitney test, and Fisher exact tests, as appropriate. Pearson and Spearman correlations were used based on normality of distribution. The conventional P value of ≤0.05 was used to determine level of statistical significance. All reported P values were 2‐sided. Interobserver reliability was assessed using Cronbach’s alpha, the most widely used objective measure of reliability. Statistical analyses and figures were completed using SPSS v.27.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA).
Results
There were 49 patients included in the registry, but only 22 patients with DD had sufficient quality echocardiograms for both standard echo and myocardial strain evaluation (Figure 1). Patients were predominantly male (14, 63.6%), White (21, 95.5%), and pediatric age <18 years (14, 63.6%) with a median age of 16.5 years (Table 1). Males and females differed significantly in median age (14.3 versus 27.9 years) and body mass index (19.7 versus 24.4 kg/m2), respectively.
Table 1.
Baseline Characteristics
| Baseline characteristics | Patients with DD (n=22) | Male (n=14) | Female (n=8) | P value | |||
|---|---|---|---|---|---|---|---|
| Age, median y (IQR) | 22 | 16.5 (10.4, 24.2) | 14 | 14.3 (9.08, 17.7) | 8 | 27.9 (17.4, 38.9) | 0.001 |
| Pediatric, n (%) | 22 | 14 (63.6%) | 14 | 12 (85.7%) | 8 | 2 (25.0%) | 0.004 |
| White patients, n (%) | 22 | 21 (95.5%) | 14 | 13 (92.9%) | 8 | 8 (100.0%) | 0.439 |
| BMI, median kg/m2 (IQR) | 22 | 22.7 (18.8, 26.5.0) | 14 | 19.7 (18.2, 25.8) | 8 | 24.4 (23.1, 33.4) | 0.020 |
Pediatric, age <18 years; BMI indicates body mass index; DD, Danon disease; and IQR, interquartile range.
Parameters from the most recent echocardiogram available included median (interquartile range [IQR]) EF of 60.0 (12.5) %, median (IQR) interventricular septal diameter 12.8 (9.80) mm, median (IQR) LVPWd 11.6 (12.1) mm, mean (SD) LV end‐diastolic diameter 40.7 (8.54) mm, median (IQR) LV mass index 125.9 (112.5) g/m2, and median (IQR) LA volume index 21.2 (10.3) mL/m2 (Table 2). There were notable sex differences in LV end‐diastolic diameter, LA volume index, and LV mass index (Table 2). Compared with normal values described in the adult and pediatric American Society of Echocardiography cardiac chamber quantification guidelines, LV mass, interventricular septal diameter, and LVPWd were significantly greater in patients with DD. 13 , 14 This observation held true for pediatric patients when looking at associated Z‐scores (Table 2). Interestingly, of the 21 patients who had available LA volume index data, 90.5% were either normal (16–34 mL/m2) or below normal (<6 mL/m2).
Table 2.
Echo Parameters at Last Follow‐up
| Echo parameters | Patients with DD (n=22) | Male (n=14) | Female (n=8) | P value | |||
|---|---|---|---|---|---|---|---|
| Age at last echocardiogram, median y (IQR) | 22 | 17.6 (16.7) | 14 | 14.3 (8.10) | 8 | 29.6 (13.3) | <0.05 |
| EF, median % (IQR) | 22 | 60.0 (13.5) | 14 | 66.0 (13.5) | 8 | 55.0 (11.5) | 0.082 |
| IVSd, median mm (IQR) | 22 | 12.8 (9.80) | 14 | 16.0 (19.7) | 8 | 11.4 (3.55) | 0.188 |
| Z score, median (IQR) | 13 | 6.77 (16.9) | 12 | 7.80 (19.3) | 1 | 5.34* | … |
| LVPWd, median mm (IQR) | 22 | 11.6 (12.1) | 14 | 18.0 (16.9) | 8 | 10.6 (3.65) | 0.212 |
| Z score, median (IQR) | 13 | 7.71 (12.2) | 12 | 10.0 (12.5) | 1 | 3.98* | … |
| LVEDD, mean mm (SD) | 22 | 40.7 (8.54) | 14 | 38.1 (8.59) | 8 | 45.2 (6.77) | 0.049 |
| Z score, mean (SD) | 13 | −2.41 (2.02) | 12 | −2.38 (2.11) | 1 | −2.84* | … |
| LVESD, mean mm (SD) | 22 | 27.5 (10.5) | 14 | 24.3 (9.18) | 8 | 33.0 (11.0) | 0.080 |
| LA volume index, median mL/m2 (IQR) | 21 | 21.2 (10.3) | 13 | 15.5 (10.4) | 8 | 24.2 (20.5) | 0.020 |
| LV mass, median g/m2 (IQR) | 22 | 228.3 (175.6) | 14 | 250.2 (688.7) | 8 | 175.0 (111.4) | 0.402 |
| Z score, median (IQR) | 13 | 8.13 (22.5) | 12 | 9.19 (26.7) | 1 | 3.06 | … |
| LV mass index, median g (IQR) | 22 | 125.9 (112.5) | 14 | 149.4 (303.5) | 8 | 97.2 (49.2) | 0.042 |
| E/A, mean (SD) | 20 | 1.84 (0.39) | 13 | 1.82 (0.38) | 7 | 1.88 (0.42) | 0.779 |
| e' lat, mean cm/s (SD) | 16 | 10.1 (4.86) | 12 | 9.20 (5.19) | 4 | 12.6 (2.69) | 0.117 |
| e' med, mean cm/s (SD) | 15 | 5.80 (2.20) | 12 | 5.69 (2.38) | 3 | 6.25 (1.56) | 0.640 |
| RWT, median (IQR) | 22 | 0.59 (0.80) | 14 | 0.94 (1.06) | 8 | 0.45 (0.17) | 0.082 |
| RVSP, mean mm Hg (SD) | 19 | 22.1 (6.96) | 13 | 21.6 (7.78) | 6 | 23.2 (5.19) | 0.616 |
DD indicates Danon disease; EF, ejection fraction; IQR, interquartile range; IVSd, interventricular septal diameter; LA, left atrium; LV, left ventricular; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; LVPWd, left ventricular posterior wall diameter; RVSP, right ventricular systolic pressure; and RWT, relative wall.
Only 1 female patient met pediatric definition and therefore only a single Z score was reported.
GLS was reduced with a mean (SD) of 12.2 (5.18) % (normal reference GLS value <18%) with an observed regional strain gradient: mean (SD) apex (16.7 [7.15] %), mid (11.1 [5.09] %), and basal (9.06 [4.74] %) (Table 3). 17 Comparisons of apex with basal and mid regional strains were significantly different, both with P values <0.0001 (Figure 2). GLS and regional strain did not differ between males and females. Bull’s eye plot in patients with DD reflected an apical‐sparing pattern that has been previously described (Figure 3). 18 In patients with multiple echocardiograms, strain did not change significantly over time, likely because of short follow‐up time. The interobserver reliability for strain measurements for GLS, apex, mid, and base segments was >0.800.
Table 3.
GLS and Regional LS at Last Follow‐Up
| LS | Patients with DD (n=22) | Males (n=14) | Females (n=8) | P value |
|---|---|---|---|---|
| GLS, mean % (SD) | 12.2 (5.18) | 11.1 (4.56) | 14.1 (5.94) | 0.234 |
| Apex LS, mean % (SD) | 16.7 (7.15) | 16.0 (6.91) | 18.1 (7.82) | 0.533 |
| Mid LS, mean % (SD) | 11.1 (5.09) | 9.72 (4.09) | 13.5 (6.02) | 0.141 |
| Basal LS, mean % (SD) | 9.06 (4.74) | 7.79 (4.19) | 11.3 (5.08) | 0.124 |
DD indicates Danon disease; GLS, global longitudinal strain; and LS, longitudinal strain.
Figure 2. Observed differences in regional longitudinal strain.
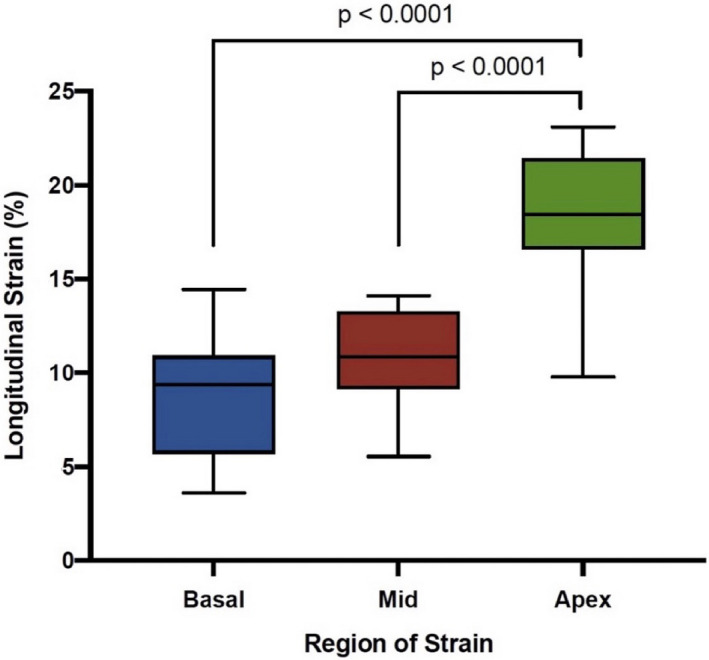
A regional longitudinal strain gradient was observed from base to apex that was significant.
Figure 3. Representative Danon disease longitudinal strain bull’s eye plots.
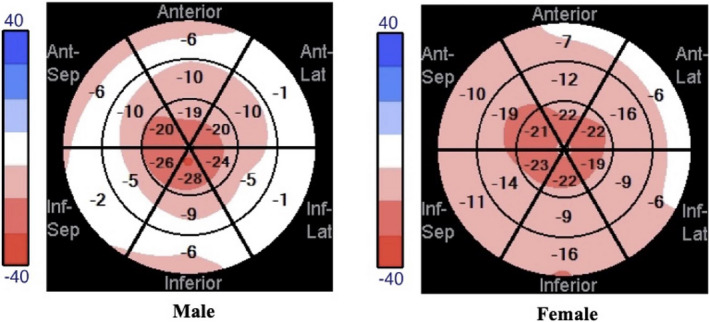
Representative bull’s eye plots from male and female patients with Danon disease demonstrate an apical sparing that is similar to the “cherry on top” pattern seen with cardiac amyloidosis. Lat indicates lateral; and Sep indicates septal.
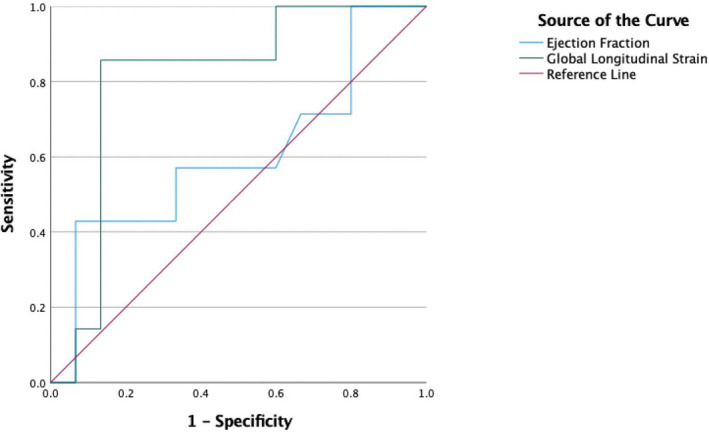
The composite outcome occurred in a total of 7 patients, with 3 of them requiring heart transplantation and 4 requiring implatable cardioverter defibrillator (ICD) for secondary prevention. The indications for ICD were cardiac arrest (n=1), ventricular tachycardia (n=2), and 1 with nonsustained ventricular tachycardia with syncope (n=1). Univariable logistic regression analysis between GLS and composite outcome found a significant odds ratio (OR) for GLS of 1.32 with a P value of 0.03. Univariable analyses for other echocardiographic parameters included LV mass index OR 1.00 (95% CI, 1.00–1.02) with a P value of 0.05, interventricular septal diameter OR 1.10 (95% CI, 0.993–1.22) with P value of 0.07, LVPWd OR 1.12 (95% CI, 0.995–1.27 with P value of 0.06), LV end‐diastolic diameter OR 1.02 (95% CI, 0.913–1.14) with P value of 0.75 and relative wall OR 1.70 (95% CI, 0.491–5.85, with P value of 0.40 [Table S1]). Mean GLS differed by presence of the composite outcome (8.43 versus 13.9%, P=0.016) (Figure 4). For receiver operating characteristic analysis, the area under the curve for GLS and EF was 0.810 (P=0.02) and 0.605 (P=0.44), respectively (Figure 5). An absolute GLS cutoff of 10.0% yielded a true positive rate of 85.7% and false positive rate of 13.3%.
Figure 4. GLS stratified by composite outcome.
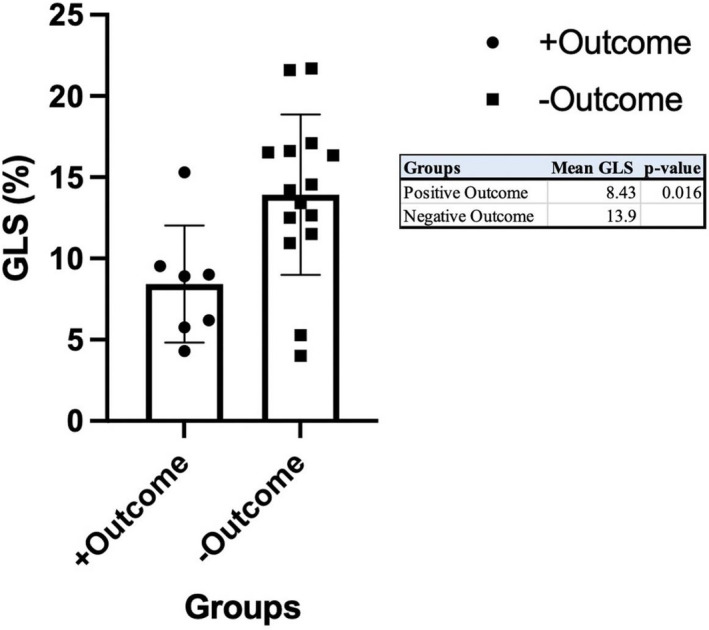
GLS indicates global longitudinal strain.
Figure 5. Receiver operating curve for global longitudinal strain and ejection fraction for the composite outcomes.
Scatter plots and linear regression analyses were completed to show the relationships of GLS and EF from the most recent echocardiogram with time (Figure 6). Both plots show that males have echocardiograms earlier in life compared with females, which correlates with known sex differences in the age‐of‐onset for the natural history of DD. In the GLS scatter plot, there was a linear downward trend with time in the male cohort (R 2=0.488) compared with the female cohort (R 2=0.0590; Figure 6A). Furthermore, all patients who experienced the composite outcome had reduced absolute strain value, defined as a cutoff of GLS <18%. In the EF scatter plot, there was also a downward trend observed with time in males (R 2=0.391) and females (R 2=0.110), but the composite outcome occurred in patients despite a preserved EF (Figure 6B). Additional scatter plots using GLS and EF from all available echocardiograms are provided in the supplemental section (Figure S1).
Figure 6. Scatter plots of (A) GLS and (B) EF with time.
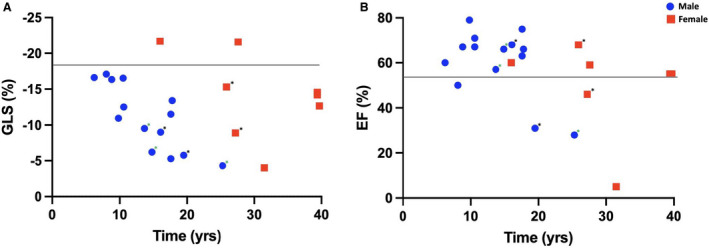
*, Composite outcome; green *, Heart transplant. Scatter plots of global longitudinal strain (GLS) and ejection fraction (EF) with time. All echocardiograms including patients with multiple studies were used to form the plots.
Discussion
To our knowledge, this is the most comprehensive analysis of the standard echo and strain measurements of patients with DD. In this cohort, we observed (1) a reduction in GLS with an apical‐sparing pattern; (2) a significant decrease in GLS in patients who experienced the composite outcome of death, advanced therapies, and ICD for secondary prevention compared with those who did not; and (3) a sizeable and significant area under the curve for GLS with a proposed cutoff of GLS <10% to predict the composite outcome. These results suggest a potential role for using myocardial strain in DD to monitor disease progression, predict clinical outcomes, and evaluate efficacy of therapeutic interventions.
In this relatively small study, GLS appeared to outperform EF with regard to association with clinical outcomes and reliability as a marker to trend over time. Using speckle‐tracking of echocardiograms to quantify GLS has advantages over using EF to measure myocardial function. While EF and GLS are correlated, it is important to note that EF predominantly measures radial myocardial function while GLS measures longitudinal myocardial function. 5 The myocardial fibers that are at greatest risk for disarray are located in the innermost area of the myocardium, the subendocardial region, and are responsible for longitudinal deformation. 19 Therefore, GLS predominantly measures subendocardial function, which is more sensitive to ischemia and changes in wall stress, and can predict subclinical disease before reduction in EF. 4 , 5 , 20 It has been hypothesized that strain may be most useful in settings of significant LV hypertrophy where EF often inadequately assesses systolic function. 7 , 20 Patients with HCM represent a cohort that has preserved or hyperdynamic EF despite overall systolic function being considerably reduced. 10 , 20 The hypertrophied ventricles lead to reductions in cavity volumes, which affect the inputs of calculated EF. 20 On a more mechanistic level, the contractility of individual cardiac myocytes may be impaired because of various reasons including cardiomyocyte hypertrophy, fiber disarray, and interstitial fibrosis, which may not be captured by traditional echocardiographic parameters. 19 Furthermore, impaired cardiac performance may be related to the extent of hypertrophy based on observations that LV mass and regional wall thickness affect global and regional myocardial mechanics. 19 Although the pathophysiology driving hypertrophy in DD is very different compared with sarcomeric HCM, interstitial fibrosis is a common end‐stage finding in both diseases. It is possible that strain is a more sensitive mechanical marker for interstitial fibrosis than EF and thus can be used to predict outcomes earlier.
While the apical‐sparing strain pattern in DD may resemble that in cardiac amyloidosis, differentiating between the 2 cardiac disorders is relatively straightforward since the former predominantly affects a pediatric population and the latter rarely affects people before the age of 40. 21 , 22 Thus, the intriguing question lies in whether strain can discern DD from other pediatric HCM phenocopies, such as Friedrich ataxia, Pompe disease, and Anderson‐Fabry disease. In a cohort of 25 patients with Friedrich ataxia, LS was obtained in apex (−21%±4%), mid (−15%±3%), and basal (−16%±2%) segments from the septum. 23 The authors concluded that a longitudinal base‐to‐apex gradient was rarely detected in patients with Friedrich ataxia. 24 There are limited strain studies in Pompe disease, but 1 study of 12 patients reported a minor reduction in GLS of 20.7±1.9%. 25 In a study that included 21 patients with Anderson‐Fabry disease with LVH, LS was reported for apex (−19.0±4.7%), mid (−18.0±5.5%), and basal regions (−10.1%±2.0%). 26 More recently, Esposito et al 27 described LS in 23 patients with Anderson‐Fabry disease at time of diagnosis and found that LS in the basal segments was always more compromised than in mid and apical segments, but less prominent than the “apical sparing” seen in cardiac amyloidosis. In summary, sarcomeric HCM and associated pediatric phenocopies usually had significant reductions in GLS, but DD appears to have a more pronounced apical‐sparing strain pattern. LA volume index may also help distinguish DD from other pediatric HCM phenocopies. Typically, LA volume is increased in HCM and is associated with obstruction, mitral regurgitation, and diastolic dysfunction. 21 However, in this study, most patients with DD had a normal or below normal LA volume. While this may be related to the young age of this cohort, further work will be needed to validate and understand this mechanism.
As promising, personalized therapies continue to develop for DD, HCM, and other genetic and acquired cardiomyopathies, it is critical to identify potential end points of efficacy as well as biomarkers that may precede onset of severe cardiac dysfunction. 28 , 29 This is particularly true given the size and duration of these trials, which will unlikely be powered to detect mortality end points. Cardiac magnetic resonance imaging has gained popularity in risk stratification through quantification of fibrosis and may be a good end point of efficacy, but has significant limitations of cost, prohibitive use with implantable devices, and required sedation for pediatric patients. Our study raises the intriguing possibility that strain may be a useful marker to follow as GLS deceased linearly with time. In fact, strain has been shown to improve in studies of therapeutics for cardiac amyloidosis. 30 We propose that GLS be reported together with EF for all patients with genetic cardiomyopathies in order to facilitate needed studies to validate whether strain accurately monitors progression of disease and predicts clinical outcomes. Furthermore, assessment of GLS may be of particular utility in syndromes associated with preserved EF, such as heart failure with preserved EF and restrictive cardiomyopathies, both of which are associated with high morbidity and mortality despite having EFs in the normal range.
Limitations
The main study limitation is the relatively small sample size and events, but DD is a rare disease, and this is the largest study of DD echocardiographic and strain parameters to our knowledge. The recruitment strategy for patients into the registry may have introduced a selection bias given both international and national representation. There were significant differences in baseline characteristics between patients included and excluded from the study, which was determined by echocardiogram availability (Table 4). Patients excluded from the study (“No Echo”) were more likely to be female and older. However, there were no differences in outcomes between those included versus excluded from the study (Table 4). Echocardiograms were obtained by different sonographers on different machines, which could yield variable quality. However, echocardiographic images were rigorously scrutinized to ensure quality by 2 physicians and 1 additional sonographer. It should also be acknowledged that there is a learning curve when assessing echocardiographic parameters in young pediatric patients. Age‐ and sex‐matched controls, including normal and other types of hypertrophic conditions, were not directly compared because of sufficient literature on corresponding strain patterns. Only longitudinal global strain was measured and not radial or circumferential strain. Furthermore, strain analysis was completed using software from a single vendor and hence not generalizable to all methods of LS assessment.
Table 4.
Logistic Regression Analysis for Composite Outcome (Death, HTx, LVAD, ICD for Secondary Prevention)
| Echo (N=22) | No echo (N=27) | P value | |||
|---|---|---|---|---|---|
| Age, median y (IQR) | 22 | 16.5 (13.8) | 27 | 30.0 (22.0) | 0.001 |
| Pediatric, no. (%) | 22 | 14 (63.6%) | 27 | 7 (25.9%) | 0.001 |
| Male, no. (%) | 22 | 14 (63.6%) | 27 | 9 (18.4%) | 0.047 |
| White patients, no. (%) | 22 | 21 (95.5%) | 27 | 23 (85.2%) | 0.362 |
| BMI, median kg/m2 (IQR) | 22 | 22.7 (7.6) | 26 | 25.4 (6.9) | 0.139 |
| Cumulative outcome, no. (%) | 22 | 7 (31.8%) | 27 | 10 (37.0%) | 0.769 |
| HTx, no. (%) | 22 | 3 (13.6%) | 27 | 8 (29.6%) | 0.303 |
| Device implant, no. (%) | 22 | 12 (54.5%) | 27 | 16 (59.3%) | 0.779 |
| Death, no. (%) | 22 | 0 (0%) | 27 | 2 (7.4%) | 0.495 |
BMI indicates body mass index; HTx, heart transplant; ICD, implantable cardioverter defibrillator; IQR, interquartile range; and LVAD, left ventricular assist device.
Conclusions
In this cohort of patients with DD, we found that GLS may be associated with clinical outcomes of mortality, device placement for secondary prevention, and need for advanced therapies. GLS compared with EF may offer further risk stratification of myocardial function in patients with genetic and acquired cardiomyopathies. Future studies should validate the association of strain with clinical outcomes and evaluate whether strain may help assess patient appropriateness for treatments and enrollment in clinical trials for a range of therapeutics in development.
Sources of Funding
Michela Brambatti, Andrew Kahn, Matthew Taylor, and Eric Adler received funding from Rocket Pharmaceuticals (New York, NY). Eric Adler receives funding from the California Institute of Regenerative Medicine (Oakland, CA).
Disclosures
Eric Adler has equity interest and has a licensed patent for methods in treating Danon Disease that is issued to Rocket Pharmaceuticals. Michela Brambatti, Andrew Kahn, and Matthew Taylor received funding/research support from Rocket Pharmaceuticals. Eric Adler serves as a consultant for AstraZeneca and Ionis. Quan Bui serves as a consultant for EcoR1. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1
Acknowledgments
We thank the patients with DD and their families for participating in the International DD registry. We acknowledge Rocket Pharmaceuticals for sponsorship of the DD registry.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. doi: 10.1016/j.jacc.2018.02.064 [DOI] [PubMed] [Google Scholar]
- 2. Pellikka PA, She L, Holly TA, Lin G, Varadarajan P, Pai RG, Bonow RO, Pohost GM, Panza JA, Berman DS, et al. Variability in ejection fraction measured by echocardiography, gated single‐photon emission computed tomography, and cardiac magnetic resonance in patients with coronary artery disease and left ventricular dysfunction. JAMA Netw Open. 2018;1:e181456. doi: 10.1001/jamanetworkopen.2018.1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Negishi K, Negishi T, Kurosawa K, Hristova K, Popescu BA, Vinereanu D, Yuda S, Marwick TH. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc Imaging. 2015;8:489–492. doi: 10.1016/j.jcmg.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 4. Hamada S, Schroeder J, Hoffmann R, Altiok E, Keszei A, Almalla M, Napp A, Marx N, Becker M. Prediction of outcomes in patients with chronic ischemic cardiomyopathy by layer‐specific strain echocardiography: a proof of concept. J Am Soc Echocardiogr. 2016;29:412–420. doi: 10.1016/j.echo.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 5. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334 [DOI] [PubMed] [Google Scholar]
- 6. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035 [DOI] [PubMed] [Google Scholar]
- 7. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538 [DOI] [PubMed] [Google Scholar]
- 8. Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Velazquez EJ, Hassager C, Sogaard P, Kober L. Prediction of all‐cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61:2365–2373. doi: 10.1016/j.jacc.2013.02.061 [DOI] [PubMed] [Google Scholar]
- 9. DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, Lin G, Redfield MM, Lewis G, Semigran MJ, et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail. 2017;19:893–900. doi: 10.1002/ejhf.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, Lafitte S. Global and regional myocardial function quantification by two‐dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1175–1181. doi: 10.1016/j.jacc.2005.10.061 [DOI] [PubMed] [Google Scholar]
- 11. Authors/Task Force members , Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, et al. ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 12. D'Souza RS, Levandowski C, Slavov D, Graw SL, Allen LA, Adler E, Mestroni L, Taylor MR. Danon disease: clinical features, evaluation, and management. Circ Heart Fail. 2014;7:843–849. doi: 10.1161/CIRCHEARTFAILURE.114.001105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 14. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American Society of Echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr. 2010;23:465–495; quiz 576–467. doi: 10.1016/j.echo.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 15. Lopez L, Colan S, Stylianou M, Granger S, Trachtenberg F, Frommelt P, Pearson G, Camarda J, Cnota J, Cohen M, et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the pediatric heart network normal echocardiogram database. Circ Cardiovasc Imaging. 2017;10:e006979. doi: 10.1161/CIRCIMAGING.117.006979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 17. Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2‐dimensional speckle‐tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–84. doi: 10.1016/j.jcmg.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 18. Bui QM, Hong KN, Kraushaar M, Ma GS, Brambatti M, Kahn AM, Bougault C, Boynton K, Mestroni L, Taylor MRG, et al. Apical sparing strain pattern in Danon disease. JACC Cardiovasc Imaging. 2020;13:2689–2691. doi: 10.1016/j.jcmg.2020.05.027 [DOI] [PubMed] [Google Scholar]
- 19. Urbano‐Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG. Investigation of global and regional myocardial mechanics with 3‐dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:11–19. doi: 10.1161/CIRCIMAGING.113.000842 [DOI] [PubMed] [Google Scholar]
- 20. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Losi MA, Nistri S, Galderisi M, Betocchi S, Cecchi F, Olivotto I, Angricola E, Ballo P, Buralli S, D'Andrea A, et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8:1–19. doi: 10.1186/1476-7120-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brambatti M, Caspi O, Maolo A, Koshi E, Greenberg B, Taylor MRG, Adler ED. Danon disease: gender differences in presentation and outcomes. Int J Cardiol. 2019;286:92–98. doi: 10.1016/j.ijcard.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 23. Liu D, Hu K, Niemann M, Herrmann S, Cikes M, Störk S, Gaudron PD, Knop S, Ertl G, Bijnens B, et al. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:1066–1072. doi: 10.1161/CIRCIMAGING.113.000683 [DOI] [PubMed] [Google Scholar]
- 24. Liu D, Hu K, Nordbeck P, Ertl G, Stork S, Weidemann F. Longitudinal strain bull's eye plot patterns in patients with cardiomyopathy and concentric left ventricular hypertrophy. Eur J Med Res. 2016;21:21. doi: 10.1186/s40001-016-0216-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris DA, Blaschke D, Krebs A, Canaan‐Kühl S, Plöckinger U, Knobloch G, Walter TC, Kühnle Y, Boldt L‐H, Kraigher‐Krainer E, et al. Structural and functional cardiac analyses using modern and sensitive myocardial techniques in adult Pompe disease. Int J Cardiovasc Imaging. 2015;31:947–956. doi: 10.1007/s10554-015-0629-7 [DOI] [PubMed] [Google Scholar]
- 26. Labombarda F, Saloux E, Milesi G, Bienvenu B. Loss of base‐to‐apex circumferential strain gradient: a specific pattern of Fabry cardiomyopathy? Echocardiography. 2017;34:504–510. doi: 10.1111/echo.13496 [DOI] [PubMed] [Google Scholar]
- 27. Esposito R, Galderisi M, Santoro C, Imbriaco M, Riccio E, Maria Pellegrino A, Sorrentino R, Lembo M, Citro R, Angela Losi M, et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment‐naive Anderson‐Fabry disease patients. Eur Heart J Cardiovasc Imaging. 2019;20:438–445. [DOI] [PubMed] [Google Scholar]
- 28. (US). CgIBMNLoM . Clinicaltrials.Gov identifier: Nct03882437 gene therapy for male patients with danon disease using rp‐a501; aav9.Lamp2b. 2020.
- 29. Olivotto I, Oreziak A, Barriales‐Villa R, Abraham TP, Masri A, Garcia‐Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 30. Solomon SD, Adams D, Kristen A, Grogan M, González‐Duarte A, Maurer MS, Merlini G, Damy T, Slama MS, Brannagan TH, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin‐mediated amyloidosis. Circulation. 2019;139:431–443. doi: 10.1161/CIRCULATIONAHA.118.035831 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


