Abstract
Background
Whether intravenous thrombolysis before mechanical thrombectomy provides additional benefit for functional outcome in acute ischemic stroke remains uncertain. We performed a meta‐analysis to compare the outcomes of direct mechanical thrombectomy (dMT) to mechanical thrombectomy with bridging using intravenous thrombolysis (bridging therapy [BT]) in patients with acute ischemic stroke.
Methods and Results
We performed a literature search in the PubMed, Excerpta Medica database, and Cochrane Central Register of Controlled Trials from January 1, 2003, to April 26, 2021. We included randomized clinical trials and observational studies that reported the 90‐day functional outcome in patients with acute ischemic stroke undergoing dMT compared with BT. The 12 included studies (3 randomized controlled trials and 9 observational studies) yielded 3924 participants (mean age, 68.0 years [SD, 13.1 years]; women, 44.2%; 1887 participants who received dMT and 2037 participants who received BT). A meta‐analysis of randomized controlled trial and observational data revealed similar 90‐day functional independence (odds ratio [OR], 1.04; 95% CI, 0.90–1.19), mortality (OR, 1.03; 95% CI, 0.78–1.36), and successful recanalization (OR, 0.93; 95% CI, 0.76–1.14) for patients treated with dMT or BT. Compared with those in the BT group, patients in the dMT group were less likely to experience symptomatic intracranial hemorrhage (OR, 0.68; 95% CI, 0.51–0.91; P=0.008) or any intracranial hemorrhage (OR, 0.71; 95% CI, 0.61–0.84; P<0.001).
Conclusions
In this meta‐analysis of patients with acute ischemic stroke, we found no significant differences in 90‐day functional outcome or mortality between dMT and BT, but a lower rate of symptomatic intracranial hemorrhage for dMT. These findings support the use of dMT without intravenous thrombolysis bridging therapy.
Registration
URL: https://www.crd.york.ac.uk/prospero/; Unique identifier: 42021234664.
Keywords: functional independence, ischemic stroke, thrombectomy, thrombolysis
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Cerebrovascular Procedures
Nonstandard Abbreviations and Acronyms
- BT
bridging therapy
- dMT
direct mechanical thrombectomy
- ICH
intracranial hemorrhage
- IVT
intravenous thrombolysis
- MT
mechanical thrombectomy
- sICH
symptomatic intracranial hemorrhage
Clinical Perspective
What Is New?
In this meta‐analysis of patients with acute ischemic stroke eligible for intravenous thrombolysis, there were no significant differences in 90‐day functional outcome or mortality between direct mechanical thrombectomy and bridging therapy, but a lower rate of symptomatic intracranial hemorrhage for direct mechanical thrombectomy.
What Are the Clinical Implications?
Current available evidence suggests that direct mechanical thrombectomy is effective and safe compared with bridging therapy, supporting the use of direct mechanical thrombectomy without intravenous thrombolysis bridging therapy.
Intravenous thrombolysis (IVT) administered within 4.5 hours is the first‐line treatment for acute ischemic stroke. 1 However, only about one third of patients with acute ischemic stroke have improved functional recovery using IVT. 2 , 3 Endovascular intervention using mechanical thrombectomy (MT) has been increasingly used over the past 2 decades based on previous randomized controlled trials (RCTs) and meta‐analyses showing efficacy for acute ischemic stroke caused by proximal occlusion in the intracranial anterior circulation. 4 , 5 The current American Heart Association/American Stroke Association guidelines recommend IVT before MT for eligible patients, evidenced by the fact that all patients in the trials received intravenous alteplase treatment if they did not have contraindications. 6 However, whether IVT provides additional clinical benefits (above “direct” MT [dMT] alone) on functional outcome remains uncertain. Although several recent meta‐analyses suggested potential beneficial effects of IVT pretreatment, 7 , 8 , 9 some observational analyses yielded conflicting results about the additional benefit in terms of 90‐day favorable functional outcome 10 , 11 , 12 , 13 , 14 , 15 or mortality. 10 , 14 IVT pretreatment might facilitate MT by facilitating clot detachment, enhancing collateral circulation, or lysing distal thrombi not accessible to endovascular devices. 16 , 17 , 18 But these hypotheses were not supported by 3 recently published RCTs 19 , 20 , 21 and a prospective cohort study, 22 suggesting that dMT was noninferior but not superior in acute ischemic stroke attributable to large‐vessel occlusion. However, the aforementioned RCTs were heterogeneous in statistical design. We therefore aimed to synthesize all available evidence (from RCTs and observational studies) on the efficacy and safety of IVT before MT in IVT‐eligible patients compared with dMT.
METHODS
The data sets used and analyzed for the current study are available from the corresponding author on reasonable request.
Study Design
We prospectively registered this meta‐analysis in the international prospective register of systematic reviews (PROSPERO CRD: 42021234664) in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analysis guidelines and applying the methods recommended in the Meta‐Analysis of Observational Studies in Epidemiology proposal. 23 , 24 Any modification to this protocol will be updated in PROSPERO.
Data Source and Search Strategy
We performed a literature search up to April 26, 2021, for relevant publications in PubMed, Excerpta Medica database, and Cochrane Central Register of Controlled Trials database. Our search strategy included the following set of terms: (stroke) AND (thrombolysis OR tPA OR plasminogen OR alteplase OR tenectplase) AND (thrombectomy OR endovascular OR bridging treatment). We also manually screened references for additional studies.
Study Selection
Randomized clinical trials and observational studies were eligible if they met the following criteria: (1) original published studies involving human participants regardless of language; (2) patients with acute ischemic stroke eligible for IVT, according to the current US guidelines, 6 aged ≥18 years, regardless of sex, race, and area; and (3) the intervention arm is dMT, and the control arm is MT with bridging using intravenous thrombolysis (bridging therapy [BT]). We applied the following exclusion criteria: (1) patients with IVT contraindications; (2) patients who ultimately did not undergo any endovascular treatment; (3) insufficient data information provided; (4) study with <10 participants in each arm; (5) case reports or case series with <10 eligible patients; (6) review articles, meta‐analyses, literature reviews, and commentaries; and (7) abstracts or posters from conference proceedings before the full‐text article was formally published in a peer‐reviewed journal. Disagreements about inclusion or exclusion criteria were settled by team discussion.
Screening and Data Extraction
Two trained authors (H.L. and S.F.) blindly assessed study inclusion and study quality, and extracted data on study characteristics (ie, authors, date of publication, setting, sample size, and study design), participants' characteristics (ie, mean/median age and sex), inclusion and exclusion criteria, follow‐up time points, and outcome measures using standardized data collection sheets. Articles were imported to a citation manager (Endnote X 8.2; Thompson Reuters, Philadelphia, PA) to automatically exclude most duplicate records at the article importing stage. These 2 reviewers (H.L. and S.F.) then compared studies based on their research teams (eg, authors list), study setting (ie, nation, city, and hospital), and reported study period, to further exclude the potential duplicates from the selected studies. When multiple published literatures were from the same study or center, only data for each outcome from the largest reported sample were extracted to avoid overlap. Data extractions were checked for accuracy by 2 authors (R.H. and H.D.). We extracted the frequency counts and measures of association for main outcomes when reported. When both unadjusted and adjusted odds ratios (ORs) were available, we recorded the adjusted ORs and the variables used in the adjustment. We contacted the corresponding authors to obtain the data needed to quantify the measures of association in case relevant information was not provided in a publication. Disagreements and missing data were settled by team discussion.
Outcomes
The primary outcome was functional independence, defined as a modified Rankin Scale score of 0 to 2 at 3 months. Secondary outcomes included the occurrence of mortality at follow‐up, successful recanalization (defined as Thrombolysis in Cerebral Infarction scores of 2b–3 after the end of MT), and symptomatic or any intracranial hemorrhage (ICH). The differences in onset to artery puncture time between dMT and BT groups were reported in the form of standardized mean differences.
Statistical Analysis
Studies with data available for the main outcomes in the dMT group and comparator (BT) group were included in the quantitative meta‐analysis. We analyzed data separately for RCTs and observational study designs to calculate summary estimates from the individual studies using a random‐effects (DerSimonian‐Laird) approach 25 and a fixed‐effects model, and displayed the results using forest plots. Dichotomous outcomes of interests were summarized as ORs. We evaluated heterogeneity by inspecting forest plots, and with tests for heterogeneity after calculating the Q statistic and I2 values. We considered the I2 statistic using thresholds of 25%, 50%, and 75% as a low, moderate, and high heterogeneity, respectively. 26 To minimize possible imbalances in baseline characteristics, we statistically combined the adjusted OR resulting from multiple regression or multivariate matching analyses (propensity score matching) when reported. In addition, we compared the pooled ORs from RCTs and observational data using a test of interaction before performing overall analyses. 27 We performed preplanned subgroup analyses stratified by participant region (East Asia and Western countries) of the main outcomes to further understand heterogeneity. We conducted 2 sensitivity analyses by limiting the studies to those on acute ischemic stroke attributable to anterior circulation occlusion, and by including only RCTs and high‐quality observational studies according to the Newcastle‐Ottawa Scale. We additionally combined the RCTs and observational propensity score matching data in another sensitivity analysis. We also performed a separate analysis limited to studies with a full dose of alteplase. All analyses were performed using the STATA 15.0 (StataCorp LP, College Station, TX) and the Cochrane Collaboration’s Review Manager (Rev Man 5.3) Software Package (2014; Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Statistical significance was set at α=0.05 for all analyses.
Assessment of Publication Bias and Study Quality Assessment (Risk of Bias)
Publication bias tests for funnel plot asymmetry and the Egger test were performed for associations described in >10 studies. Two authors (H.L. and S.F.) independently evaluated the quality of the included RCT studies using the Cochrane Collaboration risk of bias tool on the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported result. 26 The Newcastle‐Ottawa Scale was adapted for observational studies, and studies with <5 stars were considered of low quality, studies with 5 to 7 stars were considered of moderate quality, and studies with >7 stars were considered of high quality. 28
RESULTS
Identified Studies
We included 12 eligible studies (3 RCTs 19 , 20 , 21 and 9 observational studies 22 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ) for the final quantitative analysis (Figure 1).
Figure 1. Flowchart of study selection.

BT indicates bridging therapy; dMT, direct mechanical thrombectomy; and IVT, intravenous thrombolysis.
Study Characteristics
Table 1 summarizes the key characteristics of the included studies. The sample size of eligible participants in all included studies ranged from 42 to 1148 (median, 190 [interquartile range, 105–561]). The median baseline National Institutes of Health Stroke Scale score ranged from 14 to 18 points (moderate to severe severity) across studies. The 12 included studies yielded 3924 participants (mean age, 68.0 years [SD, 13.1 years]; women, 44.2%; 1887 participants in the dMT arm and 2037 in the BT arm). Most studies only included patients with acute ischemic stroke involving the anterior circulation, except 2 studies 22 , 29 that included patients involving both anterior and posterior circulation occlusion. Among 9 observational studies, 5 studies 22 , 30 , 31 , 33 , 34 , 35 provided propensity score matching analysis results. One study 29 included patients within a 3‐hour time window. For thrombectomy devices, 1 study 29 only applied the first‐generation devices (Merci retrieval system/Penumbra system). All 12 studies provided the primary outcome (modified Rankin Scale score 0–2 at 90 days), the results of mortality and successful recanalization, and 10 studies reported the outcome for ICH.
Table 1.
Baseline Characteristics
| Study | Study type | Country | Sample size | Age, mean/median, y | Women, n (%) | Baseline NIHSS score, median (IQR) | ASPECTS, median (IQR) | Onset to groin puncture time, median (IQR), min | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dMT | BT | dMT | BT | dMT | BT | dMT | BT | dMT | BT | dMT | BT | |||
| Suzuki et al, 2021 19 | RCT | Japan | 101 | 103 | 74 (67–80) | 76 (67–80) | 45 (45) | 31 (30) | 19 (13–23) | 17 (12–22) | 7 (6–9) | 8 (6–9) | N/A | N/A |
| Yang et al, 2020 20 | RCT | China | 327 | 329 | 69 (61–71) | 69 (61–76) | 138 (42.2) | 148 (45) | 17 (12–21) | 17 (14–22) | 9 (7–10) | 9 (7–10) | N/A | N/A |
| Zi et al, 2021 21 | RCT | China | 116 | 118 | 70 (60–77) | 70 (60–78) | 50 (43.1) | 52 (44.1) | 16 (12–20) | 16 (13–20) | 8 (7–9) | 8 (7–9) | 200 (155–247) | 210 (179–255) |
| Broeg‐Morva et al, 2016 30 | Retrospective | Germany | 40 | 40 | 77 (14) | 78 (12) | 15 (37.5) | 15 (37.5) | 17 (4–38) | 17 (4–36) | N/A | N/A | 228.6 (78.6) | 262.2 (85.2) |
| Casetta et al, 2019 31 | Retrospective | Italy | 513 | 635 | 68.8 (13.1) | 67.6 (14.6) | 262 (51.1) | 322 (50.7) | 18 (14–22) | 18 (14–21) | N/A | N/A | 210 (170–270) | 230 (185–275) |
| Du et al, 2021 32 | Retrospective | China | 57 | 54 | 66.9 (11.9) | 65.2 (12.2) | 25 (43.9) | 26 (48.1) | 18 (13–22) | 18 (16–23) | 9 (8–10) | 9 (7–10) | 198 (156–252) | 218 (175–294) |
| Gong et al, 2019 33 | Retrospective | China | 21 | 21 | 71 (10) | 70 (11) | 10 (48) | 9 (43) | 15 (6–22) | 14 (7–21) | N/A | N/A | 216.5 (57.8) | 172.2 (29.81) |
| Kass‐Hout et al, 2014 29 | Retrospective | United States | 62 | 42 | 69.3 (15.8) | 67.6 (14.9) | 33 (53.2) | 22 (52.4) | 16.0 (5.3) | 14.8 (4.7) | N/A | N/A | 121.9 (36.78) | 227.8 (88) |
| Pienimäki et al, 2021 34 | Retrospective | Finland | 48 | 58 | 72 (11) | 69 (12) | 18 (38) | 21 (36) | 14 (9) | 16.5 (8) | 10 (2) | 9.5 (2) | N/A | N/A |
| Tong et al, 2021 22 | Prospective | China | 394 | 394 | 65 (55–73) | 65 (55–73) | 139 (35.3) | 147 (37.3) | 17 (12–21) | 16 (11–21) | 9 (7–10) | 10 (7–10) | N/A | N/A |
| Wang et al, 2017 35 | Retrospective | China | 138 | 138 | 67 (58.75–75) | 67 (58.75–73) | 62 (44.9) | 60 (43.5) | 16 (13–21) | 17 (13–21.25) | 9 (8–10) | 9 (8–10) | N/A | N/A |
| Weber et al, 2017 36 | Retrospective | Switzerland | 70 | 105 | 70.7 (17.1) | 70.2 (12.6) | 32 (45.7) | 53 (50.5) | 15 (10–18) | 15.5 (12–20) | N/A | N/A | 183 (132–225) | 233 (198–295) |
| Study | sICH definition | Mortality definition | SR definition | FI definition | Adjustment method | Rescue therapy | Occlusion vessel | rtPA dose, mg/kg | MT devices |
|---|---|---|---|---|---|---|---|---|---|
| Suzuki et al, 2021 19 | NINDS | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | N/A | Yes | AC | 0.6 | Penumbra/stent retriever |
| Yang et al, 2020 20 | Heidelberg criteria | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | N/A | Yes | AC | 0.9 | Stent retriever/aspiration |
| Zi et al, 2021 21 | ECASS II | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | N/A | Yes | AC | 0.9 | Stent retriever/aspiration |
| Broeg‐Morvayet al, 2016 30 | PROACT II | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | Multivariable PS matching | Yes | AC | 0.9 or 0.6 | Stent retriever/aspiration |
| Casetta et al, 2019 31 | ECASS II | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | PS matching | N/A | AC | 0.9 | Stent retriever/aspiration |
| Du et al, 2021 32 | ECASS II | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | Multivariable | Yes | AC | 0.9 | Stent retriever |
| Gong, et al, 2019 33 | NA | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | PS matching | N/A | AC | N/A | Stent retriever |
| Kass‐Hout et al, 2014 29 | ECASS III | All cause (in hospital) | mTICI score 2B/3 | mRS score 0–2 (discharge) | N/A | Yes | AC/PC | 0.9 or 0.6 | Merci retrieval system/Penumbra system |
| Pienimäki et al, 2021 34 | NA | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | Multivariable | Yes | AC | 0.9 | Stent retriever/aspiration |
| Tong et al, 2021 22 | Heidelberg | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | PS matching | Yes | AC/PC | 0.9 | Stent retriever/aspiration |
| Wang et al, 2017 35 | Heidelberg Bleeding Classification | All cause (90 d) | mTICI score 2B/3 | mRS score 0–2 (90 d) | PS matching | Yes | AC | Not mentioned | Stent retriever |
| Weber et al, 2017 36 | ECASS III | All cause (5.7 mo) | mTICI score 2B/3 | mRS score 0–2 (5.7 mo) | N/A | N/A | AC | Not mentioned | Stent retriever |
Data are generally displayed as mean (SD) or median (IQR) if not otherwise specified. AC indicates anterior circulation; ASPECTS, Alberta Stroke Program Early CT [Computed Tomography] Score; BT, bridging therapy; dMT, direct mechanical thrombectomy; ECASS, Europe cooperative acute stroke study; FI, functional independence; IQR, interquartile range; mRS, modified Rankin Scale; MT, mechanical thrombectomy; mTICI, modified Thrombolysis in Cerebral Ischemia; NA, not available; NIHSS, National Institutes of Health Stroke Scale; NINDS, national institute of neurological disease and stroke; PC, posterior circulation; PROACT, prolyse in acute cerebral thromboembolism; PS, propensity score; RCT, randomized controlled trial; rtPA, recombinant tissue plasminogen activator; sICH, symptomatic intracranial hemorrhage; and SR, successful recanalization.
RCT Evidence
Table 2 summarizes the pooled estimated effect sizes for the RCT and observational evidence using a random‐effects approach. RCT data showed there were no statistically significant differences for functional independence (OR, 1.08; 95% CI, 0.84–1.38; Figure 2A), mortality (OR, 0.93; 95% CI, 0.66–1.31; Figure 2B), successful recanalization (OR, 0.77; 95% CI, 0.54–1.10; Figure 2C), and symptomatic ICH (sICH) (OR, 0.72; 95% CI, 0.43–1.22; Figure 2D). However, patients treated with dMT had significantly lower odds of any ICH (OR, 0.68; 95% CI, 0.50–0.92; Figure 2E). Only 1 study reported that patients in the dMT group had a shorter delay in onset to artery puncture time (−0.21; 95% CI, −0.47 to 0.05). 21 We also performed a separate analysis for RCTs using a fixed‐effects model because of similar treatments and populations. The results were similar to those derived from the random‐effects model (Figure 3A through 3F).
Table 2.
Summary Pooled OR (95% CI) Values for Main Outcomes
| Variable | 90‐d mRS score 0–2 | Mortality | Recanalization | sICH | Any ICH |
|---|---|---|---|---|---|
| RCTs | 1.08 (0.84–1.38), I2=0.0%, P=0.538 | 0.93 (0.66–1.31), I2=0.0%, P=0.690 | 0.77 (0.54–1.10), I2=0.0%, P=0.152 | 0.72 (0.43–1.22), I2=0.0%, P=0.222 | 0.68 (0.50–0.92), I2=23.3%, P=0.014 |
| Observational studies | 1.02 (0.86–1.21), I2=0.0%, P=0.854 | 1.03 (0.70–1.53), I2=57.5%, P=0.866 | 1.03 (0.79–1.35), I2=20.2%, P=0.837 | 0.66 (0.47–0.93), I2=0.0%, P=0.018 | 0.72 (0.57–0.90), I2=21.2%, P=0.004 |
| Overall analysis | 1.04 (0.90–1.19), I2=0.0%, P=0.615 | 1.03 (0.78–1.36), I2=45.8%, P=0.832 | 0.93 (0.76–1.14), I2=10.1%, P=0.486 | 0.68 (0.51–0.91), I2=0.0%, P=0.008 | 0.71 (0.60–0.84), I2=13.9%, P<0.001 |
| East Asia | 1.08 (0.91– 1.29), I2=0.0%, P=0.358 | 1.04 (0.83–1.30), I2=0.0%, P=0.762 | 1.01 (0.70–1.43), I2=40.4%, P=0.977 | 0.70 (0.51–0.95), I2=0.0%, P=0.024 | 0.69 (0.58–0.82), I2=0.0%, P<0.001 |
| Western countries | 0.96 (0.73–1.26), I2=6.0%, P=0.776 | 0.78 (0.35–1.73), I2=76.6%, P=0.534 | 0.88 (0.68–1.15), I2=0.0%, P=0.359 | 0.59 (0.29–1.21), I2=0.0%, P=0.148 | 0.80 (0.50–1.26), I2=33.0%, P=0.335 |
| RCT+PSM | 0.97 (0.84–1.13), I2=0.0%, P=0.723 | 1.07 (0.87–1.32), I2=0.0%, P=0.510 | 0.85 (0.66–1.09), I2=23.0%, P=0.201 | 0.66 (0.51–0.85), I2=0.0%, P=0.001 | 0.82 (0.68–0.98), I2=17.2%, P=0.030 |
| RCT+observational studies with an NOS score >7 | 1.03 (0.89–1.20), I2=0.9%, P=0.665 | 1.04 (0.79–1.36), I2=42.6%, P=0.785 | 0.94 (0.73–1.20), I2=29.8%, P=0.611 | 0.68 (0.50–0.91), I2=0.0%, P=0.010 | 0.72 (0.62–0.85), I2=6.0%, P<0.001 |
| Anterior circulation occlusion | 1.01 (0.85–1.19), I2=0.0%, P=0.945 | 0.96 (0.67–1.37), I2=54.0%, P=0.810 | 1.00 (0.76–1.32), I2=24.8%, P=0.983 | 0.74 (0.52–1.07), I2=0.0%, P=0.110 | 0.68 (0.55–0.84), I2=20.0%, P<0.001 |
ICH indicates intracranial hemorrhage; mRS, modified Rankin Scale; NOS, Newcastle‐Ottawa Scale; OR, odds ratio; PSM, propensity score matching; RCT, randomized controlled trial; and sICH, symptomatic ICH.
Figure 2. Overall pooled estimate effect size by combining randomized controlled trials (RCTs) and observational studies using a random‐effects model.

A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). F, Onset to artery puncture time. BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; and ID, identifier.
Figure 3. Overall pooled estimate effect size by combining randomized controlled trials (RCTs) and observational studies using a fixed‐effects model.

A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). F, Onset to artery puncture time. BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; and ID, identifier.
Observational Evidence
Compared with BT participants, there were no statistically significant differences for dMT in 90‐day functional independence (OR, 1.02; 95% CI, 0.86–1.21; Figure 2A), mortality (OR, 1.03; 95% CI, 0.70–1.53; Figure 2B), or successful recanalization (OR, 1.03; 95% CI, 0.79–1.35; Figure 2C). However, patients receiving dMT had lower odds of sICH (OR, 0.66; 95% CI, 0.47–0.93; Figure 2D) or any ICH (OR, 0.72; 95% CI, 0.57–0.90; Figure 2E); those receiving dMT had a shorter delay in onset to artery puncture time (−0.50; 95% CI, −0.94 to −0.07; Figure 2F). A separate analysis for observational studies using a fixed‐effects model yielded similar results to those derived from the random‐effects model (Figure 3A through 3F).
Overall Analysis
A test of interaction showed no significant differences between pooled ORs derived from RCTs and observational studies for 90‐day functional independence (Z=0.146; P=0.884), mortality (Z=0.385; P=0.700), successful recanalization (Z=0.227; P=0.820), sICH (Z=0.318; P=0.751), or any ICH (Z=0.194; P=0.846). A combination of RCTs and observational data using a random‐effects approach showed no significant differences for 90‐day functional independence (OR, 1.04; 95% CI, 0.90–1.19; Figure 2A), mortality (OR, 1.03; 95% CI, 0.78–1.36; Figure 2B), or successful recanalization (OR, 0.93; 95% CI, 0.76–1.14; Figure 2C) between dMT and BT. However, dMT was associated with lower odds of sICH (OR, 0.68; 95% CI, 0.51–0.91; P=0.008; Figure 2D), lower odds of any ICH (OR, 0.71; 95% CI, 0.60–0.84; P<0.001; Figure 2E), and a shorter delay in onset to artery puncture time (−0.46; 95% CI, −0.81 to −0.10; Figure 2F). The results derived from the fixed‐effects model were similar to those derived from the random‐effect model (Figure 3A through 3F).
Subgroup Analysis
Our predetermined subgroup analysis, stratified by participant region (East Asia and Western countries), yielded results consistent with the overall analyses for 90‐day functional independence (for the East Asian population: OR, 1.08; 95% CI, 0.91–1.29; for the Western population: OR, 0.96; 95% CI, 0.73–1.26; Figure 4A), mortality (for the East Asian population: pooled OR, 1.04; 95% CI, 0.83–1.30; for the Western population: OR, 0.78; 95% CI, 0.35–1.73; Figure 4B), and successful recanalization (for the East Asian population: OR, 1.01; 95% CI, 0.70–1.43; for the Western population: OR, 0.88; 95% CI, 0.68–1.15; Figure 4C). dMT was associated with significantly lower odds of sICH (OR, 0.70; 95% CI, 0.51–0.95; P=0.024; Figure 4D) and any ICH (OR, 0.69; 95% CI, 0.58–0.82; P<0.001; Figure 4E) in the East Asian patients. Similarly, Western patients with stroke in dMT group were at lower odds of experiencing an sICH (OR, 0.59; 95% CI, 0.29–1.21; Figure 4D) and any ICH (OR, 0.80; 95% CI, 0.50–1.26; Figure 4E), although these results were not statistically different (Table 2).
Figure 4. Pooled odds ratio, stratified by participant region.

A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; ID, identifier; and RCT, randomized controlled trial.
Sensitivity Analyses
A sensitivity analysis in studies on acute ischemic stroke attributable to anterior circulation occlusion yielded similar findings to the overall analyses for most main outcomes except sICH (Figure 5A through 5E). Another sensitivity analysis, by including RCTs and high‐quality observational studies, showed the stability of our overall analyses results for 90‐day functional independence, mortality, successful recanalization, sICH, and any ICH (Figure 6A through 6E). Additional sensitivity analysis, by including RCTs and observational studies with propensity score matching data, confirmed the results derived from the overall analyses (Figure 7A through 7E). A separate analysis limited to studies with a full dose of alteplase (0.9 mg/kg) yielded similar results to the primary analysis (Figure 8A through 8E).
Figure 5. Pooled odds ratio limited to anterior circulation occlusion.
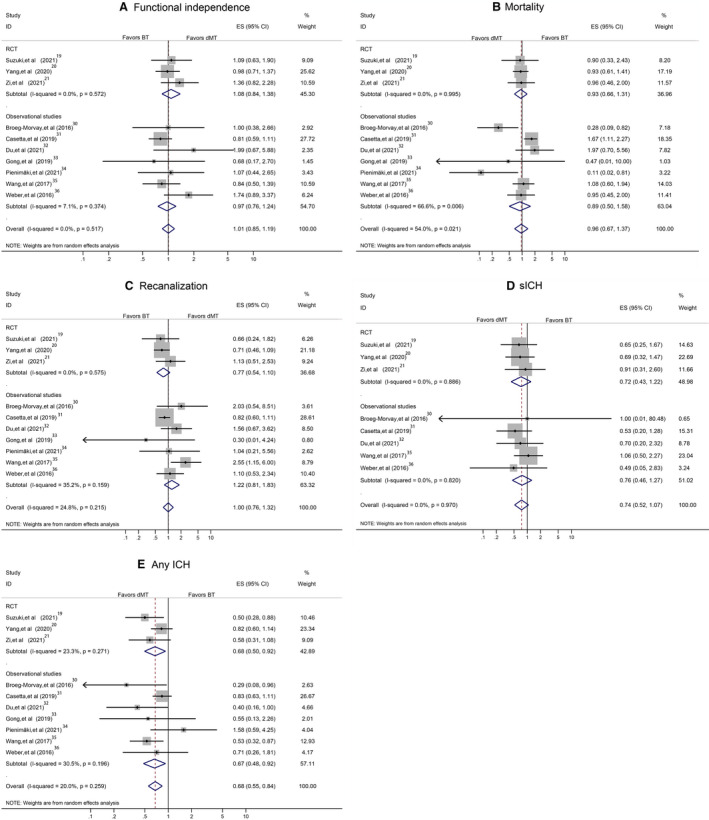
A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; ID, identifier; and RCT, randomized controlled trial.
Figure 6. Pooled odds ratio by including high‐quality studies.
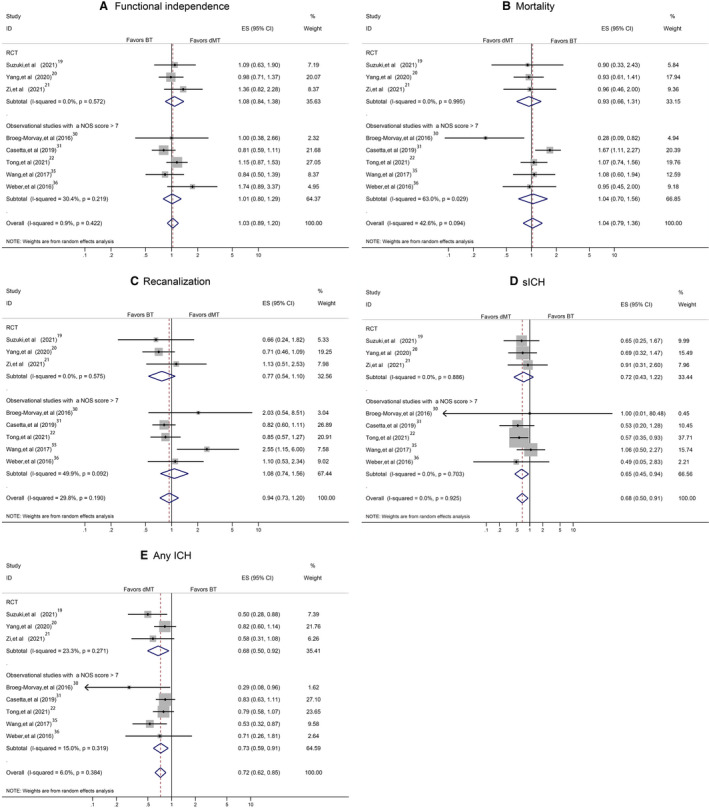
A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; ID, identifier; and RCT, randomized controlled trial.
Figure 7. Pooled odds ratio by including randomized controlled trials (RCTs) and observational propensity score matching data.
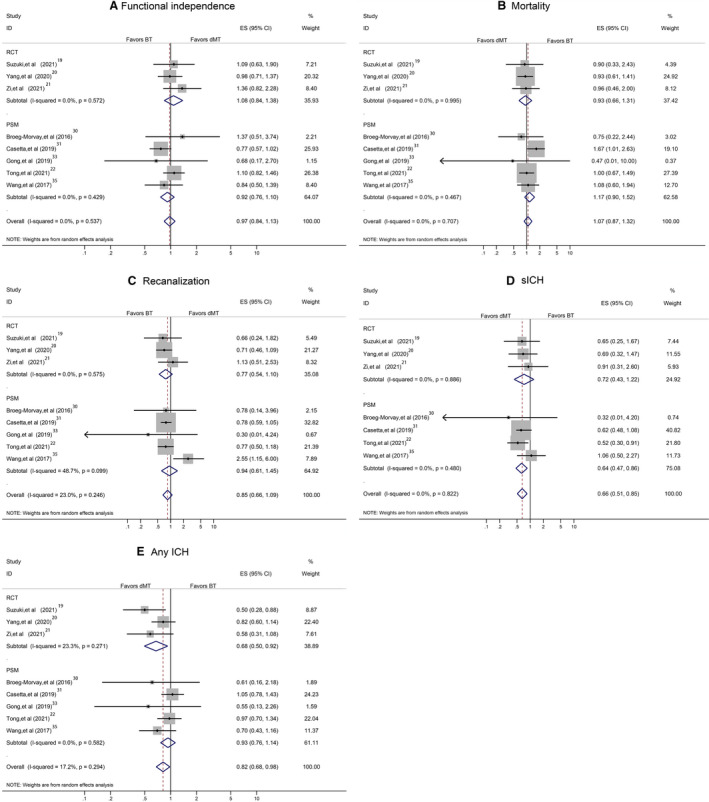
A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; and ID, identifier.
Figure 8. Pooled odds ratio limited to studies with a full dose of alteplase.
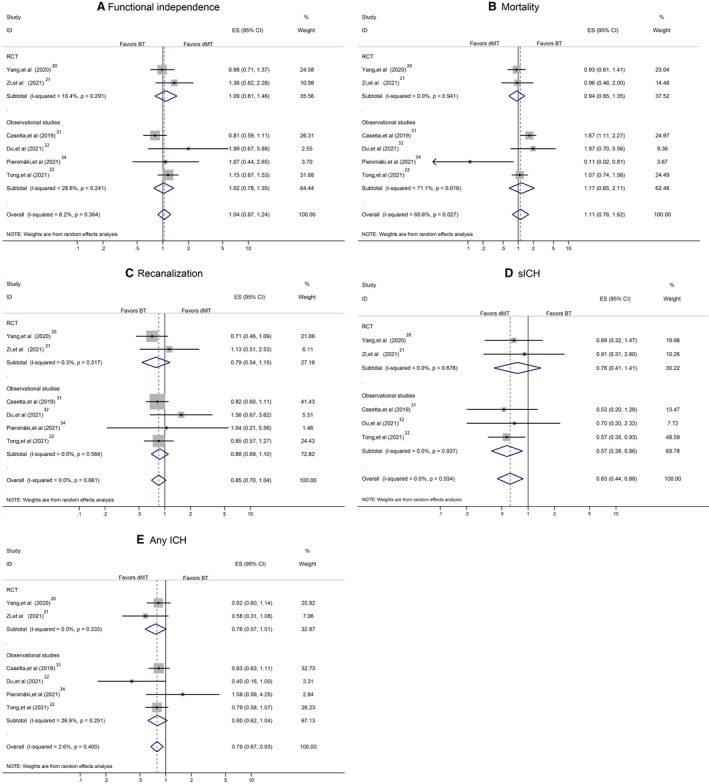
A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracranial hemorrhage (sICH). E, Any intracranial hemorrhage (ICH). BT indicates bridging therapy; dMT, direct mechanical thrombectomy; ES, effect size; ID, identifier; and RCT, randomized controlled trial.
Study Quality Evaluation and Publication Bias Assessment
All 3 RCTs in this meta‐analysis were investigator initiated, using web‐based randomization, and complying with reported open‐label treatment with blinded end point evaluation (Prospective randomized open blinded end‐point [PROBE] design) with low risks of reporting bias assessed by the Cochrane collaboration’s tool (Figure 9). The overall score on the Newcastle–Ottawa scale was 67 of 81 (82.7%), representing overall high quality (Table 3). The reporting bias risk of included observational studies was generally regarded low because of appropriate adjustments for potential confounders in 7 studies (Table 4). There was low evidence of publication bias on the basis of minimal asymmetry in the visual inspection of the funnel plot for 90‐day functional independence, mortality, successful recanalization, sICH, and any ICH (Figure 10A through 10E). The Egger test showed no significant evidence of small study effect (P=0.732 for 90‐day functional independence, P=0.150 for mortality, P=0.537 for recanalization, P=0.350 for sICH, and P=0.592 for any ICH).
Figure 9.
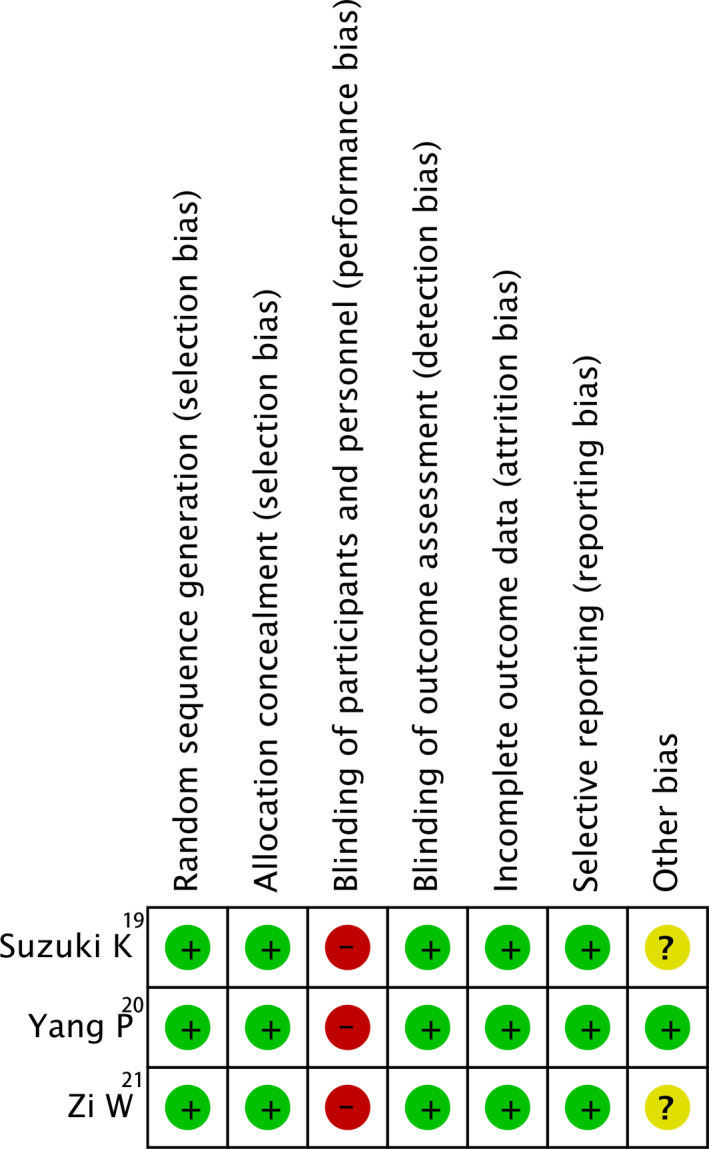
Reporting bias of randomized controlled trials (RCTs), assessing by Cochrane Collaboration's tool. ES, effect size.
Table 3.
Quality Assessment of Observational Studies Using the Newcastle‐Ottawa Scale
| Study name, y | Selection | Comparability | Outcome | Overall score |
|---|---|---|---|---|
| Broeg‐Morvay et al, 2016 30 | 3* | 2* | 3* | 8/9 |
| Casetta et al, 2019 31 | 3* | 2* | 3* | 8/9 |
| Du et al, 2021 32 | 3* | 2* | 2* | 7/9 |
| Gong et al, 2019 33 | 3* | 2* | 2* | 7/9 |
| Kass‐Hout et al, 2014 29 | 3* | 0* | 2* | 5/9 |
| Pienimäki et al, 2021 34 | 3* | 2* | 2* | 7/9 |
| Tong et al, 2021 22 | 3* | 2* | 3* | 8/9 |
| Wang et al, 2017 35 | 4* | 2* | 3* | 9/9 |
| Weber et al, 2017 36 | 3* | 2* | 3* | 8/9 |
Table 4.
Overview of Confounders That Were Used for Adjustment in Eligible Studies
| Study name, y | Confounder adjustment |
|---|---|
| Broeg‐Morvay et al, 2016 30 | Age, NIHSS score, time from symptom onset to diagnosis, hypertension, and thrombus location (internal carotid artery or middle cerebral artery) |
| Casetta et al, 2019 31 | Age, sex, history of diabetes, atrial fibrillation, hypertension, previous stroke, or transient ischemic attack in the previous 3 mo, the presence of carotid stenosis >70%, baseline NIHSS score, baseline ASPECTS, onset to ECC arrival time, onset to groin puncture time, and site of occlusion |
| Du et al, 2021 32 | Age, NIHSS score on admission, ASPECTS on admission and onset to imaging time, clot burden score, successful recanalization, ICH, and collateral status |
| Gong et al, 2019 33 | Age, sex, NIHSS score, vascular risk factors, and laboratory parameters based on a multiple logistic regression model that accounted for additional explanatory variables |
| Pienimäki et al, 2021 34 | Age, onset‐reperfusion time, NIHSS score, atrial fibrillation, mTICI score 2b‐3 |
| Tong et al, 2021 22 | Age, sex, NIHSS score, and the baseline and procedural variables with a significant difference of P<0.05 |
| Wang et al, 2017 35 | Age, sex, previous stroke, premorbid mRS score, time from onset to door, stroke cause, occlusion site, baseline ASPECTS, baseline NIHSS score, and collateral status |
ASPECTS indicates Alberta Stroke Program Early CT [Computed Tomography] Score; ECC, endovascular‐capable center; ICH, intracranial hemorrhage; mRS, modified Rankin Scale; mTICI, modified Treatment in Cerebral Ischemia; and NIHSS, National Institutes of Health Stroke Scale.
Figure 10.

Funnel plot for publication bias. A, The 90‐day functional independence. B, Mortality. C, Successful recanalization. D, Symptomatic intracerebral hemorrhage (sICH). E, Any intracranial hemorrhage (ICH).
DISCUSSION
Our present meta‐analysis of moderate to high quality RCT and observational evidence showed similar 3‐month functional outcome and successful recanalization after dMT compared with dMT and intravenous thrombolysis bridging therapy (BT) in acute ischemic stroke. Our findings also suggest that dMT is associated with a lower odds of symptomatic ICH and a shorter onset to artery puncture time.
Several previous meta‐analyses showed that BT was superior to dMT in achieving a favorable outcome at 90 days, 7 , 8 , 9 , 37 , 38 whereas others showed that outcomes were not significantly different for dMT and BT. 39 , 40 However, most previous meta‐analyses included both IVT‐eligible and IVT‐ineligible patients in the MT group or compared IVT‐eligible patients (undergoing BT) with IVT‐ineligible patients (undergoing dMT). 7 , 8 , 9 , 39 , 40 Only a few meta‐analyses provided pooled effect sizes in IVT‐eligible participants based on observational data. 9 , 37 , 38 Our meta‐analysis adds to previous studies by including the most recently published RCTs and large‐sample prospective cohort data, allowing direct and indirect comparison. In addition, to our knowledge, our study includes the highest number of IVT‐eligible patients, minimizing the risk of selection bias.
Notably, heterogeneity was driven by differences in study method (design and sample) and clinical characteristics across the included studies. We therefore look at the results of the 3 RCTs and observational studies separately. The 3 RCTs are all open‐blinded end point designed, allowing greater similarities with real‐world clinical practice. However, information from the 3 RCTs in the present meta‐analysis is not adequately powered to assess the safety of dMT versus BT. Our observational studies contributed a larger sample of IVT‐eligible participants than RCTs (2830 versus 1094), providing more adequate power to evaluate safety outcomes. Notably, our findings should be interpreted with caution because in observational studies, the decision on whether IVT was initiated before MT was based on arbitrary decisions rather than predefined protocol. However, the consistency between RCTs and observational studies for 90‐day functional outcomes may provide evidence that skipping IVT should be considered in a specific population with acute ischemic stroke, particularly in East Asians with large‐vessel occlusion (class of recommendation=I, level of evidence=A in both US and European stroke guidelines). 6 , 41 Moreover, we assessed the estimated effect sizes that have been adjusted for potential confounders and/or minimized for baseline characteristics with propensity score matching analyses (multivariate matched comparison) in observational studies.
The largest study population in the present meta‐analysis was East Asian. Clinicians therefore need to note the differences in clinical features between the Asian and Western population with ischemic stroke as well as stroke care systems. For example, because East Asian populations with acute ischemic stroke have been shown to be more likely to experience ICH after IVT with alteplase, the bleeding risk of IVT before MT may also be higher than for dMT. 22 , 42 Moreover, there might be differences in the prevalence of intracranial stenosis and atrial fibrillation between the East Asian and Western populations with ischemic stroke. 22 , 43 Asian patients were more likely to harbor intracranial arterial stenosis, which frequently requires stent implantation and additional powerful antiplatelets. 44 , 45 A sensitivity analysis of the ANGEL‐ACT (Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke) study showed that rescue stenting was associated with a higher probability of 90‐day functional independence, but was not associated with an increased risk of sICH, any ICH, or mortality (ANGEL‐ACT study group, unpublished data, 2021), supporting the safety and efficacy of rescue stenting in selected patients after thrombectomy. Moreover, data from a subgroup study of ANGEL‐ACT registry showed no statistically significant differences in safety outcomes, efficacy outcomes on successful recanalization, dramatic clinical improvement, or 3‐month modified Rankin Scale score between the tirofiban and nontirofiban groups. 46 These results need to be validated in future large multicenter studies.
All 5 studies performed in Western countries in this meta‐analysis were retrospectively designed. 29 , 30 , 31 , 34 , 36 We therefore could not provide clear evidence which therapy approach (dMT versus BT) might be more beneficial for Western patients with ischemic stroke. The results driven from the ongoing studies (MR CLEAN‐NO IV [Multicenter Randomised Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands], ISRCTN80619088; SWIFT‐DIRECT [Solitaire With the Intention for Thrombectomy Plus Intravenous t‐PA Versus Direct Solitaire Stent‐Retriever Thrombectomy in Acute Anterior Circulation Stroke], NCT03192332; and DIRECT‐SAFE [Direct Endovascular Clot Retrieval Versus Standard Bridging Thrombolysis With Endovascular Clot Retrieval], NCT03494920) permitting direct comparison of dMT to BT will provide more data on this issue.
Previous studies showed that the response to IVT might be partly determined by the site of occlusion (whether internal carotid artery, middle cerebral artery, or basilar artery). 22 , 47 One study showed that the median 90‐day modified Rankin Scale score in anterior circulation occlusion was lower than in posterior circulation occlusion (3 versus 4; P=0.06). 22 Our subgroup analysis, including only patients with anterior circulation occlusion, showed that patients receiving dMT had a similar likelihood of achieving functional independence, mortality, and successful recanalization, but lower rates of sICH and any ICH compared with those in the BT arm. The evidence in the present meta‐analysis supports dMT as a treatment of choice in health systems with rapid access to comprehensive stroke centers. Comparable efficacy for dMT and BT might also raise questions about cost‐effectiveness.
We acknowledge limitations. First, there is selection bias between the dMT and BT groups. Even RCTs and observational studies with propensity score matching data unavoidably introduced selection bias; further studies need to address the factors that might account for the inconsistencies, such as microbleed burden, the sites of occlusion, admission mode (drip‐and‐ship versus mother ship), and procedure parameters (time to start endovascular treatment, anesthetic factors, and thrombectomy devices). Second, our findings were not generalized to the Western population because most included studies were performed in East Asia. Third, because all participants with BT used alteplase in the present meta‐analysis, our findings are not generalized to those who underwent IVT with tenecteplase. Some recent published studies indicated that patients with acute ischemic stroke treated with tenecteplase were superior to those treated with alteplase, 48 , 49 raising concerns about the efficacy of BT using tenecteplase. Fourth, because of limited available information, we could not draw a conclusion in acute ischemic stroke with posterior circulation occlusion. Last, our study only evaluated the 90‐day outcome, so future research with longer follow‐up times is required to better understand longer‐term functional outcome.
CONCLUSIONS
Current available evidence suggests that dMT is effective and safe in comparison to BT. The risk of ICH appears to be lower for patients treated with dMT than BT; sensitivity analyses suggest that the lower ICH risk is more pronounced in the East Asian populations.
Sources of Funding
This study was supported by Fujian Provincial Natural and Science Innovation Project (2016B014). The funders had no role in the study design and the collection, analysis, and interpretation of data or drafting of the article and the decision to submit it for publication.
Disclosures
None.
Acknowledgments
Author Contributions: Concept and design: Drs Du, Werring, and Liu. Acquisition, analysis, or interpretation of data: Drs Du, Lei, Fang, Ambler, Liu, and Werring. Drafting of the manuscript: Drs Du, Lei, and Fang. Critical revision of the manuscript for important intellectual content: Drs He, Yuan, Ambler, Liu, and Werring. Statistical analysis: Drs Du, Lei, and Ambler. Drs Du and Liu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
For Sources of Funding and Disclosures, see page 18.
Contributor Information
David J. Werring, Email: d.werring@ucl.ac.uk.
Nan Liu, Email: xieheliunan1984@fjmu.edu.cn.
References
- 1. Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta‐analysis. Stroke. 2016;47:2409–2412. doi: 10.1161/STROKEAHA.116.014181 [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7:CD000213.; 10.1002/14651858.CD000213.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CBLM, van der Lugt A, de Miquel MA, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5. Rodrigues FB, Neves JB, Caldeira D, Ferro JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta‐analysis. BMJ. 2016;353:i1754. doi: 10.1136/bmj.l1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 7. Kaesmacher J, Mordasini P, Arnold M, López‐Cancio E, Cerdá N, Boeckh‐Behrens T, Kleine JF, Goyal M, Hill MD, Pereira VM, et al. Direct mechanical thrombectomy in tPA‐ineligible and ‐eligible patients versus the bridging approach: a meta‐analysis. J Neurointerv Surg. 2019;11:20–27. doi: 10.1136/neurintsurg-2018-013834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mistry EA, Mistry AM, Nakawah MO, Chitale RV, James RF, Volpi JJ, Fusco MR. Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients: a meta‐analysis. Stroke. 2017;48:2450–2456. doi: 10.1161/STROKEAHA.117.017320 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Wu X, Zhu C, Mossa‐Basha M, Malhotra A. Bridging thrombolysis achieved better outcomes than direct thrombectomy after large vessel occlusion: an updated meta‐analysis. Stroke. 2021;52:356–365. doi: 10.1161/STROKEAHA.120.031477 [DOI] [PubMed] [Google Scholar]
- 10. Goyal N, Tsivgoulis G, Frei D, Turk A, Baxter B, Froehler MT, Mocco J, Pandhi A, Zand R, Malhotra K, et al. Comparative safety and efficacy of combined IVT and MT with direct MT in large vessel occlusion. Neurology. 2018;90:e1274–e1282. doi: 10.1212/WNL.0000000000005299 [DOI] [PubMed] [Google Scholar]
- 11. Minnerup J, Wersching H, Teuber A, Wellmann J, Eyding J, Weber R, Reimann G, Weber W, Krause LU, Kurth T, et al. Outcome after thrombectomy and intravenous thrombolysis in patients with acute ischemic stroke: a prospective observational study. Stroke. 2016;47:1584–1592. doi: 10.1161/STROKEAHA.116.012619 [DOI] [PubMed] [Google Scholar]
- 12. Cappellari M, Saia V, Pracucci G, Sallustio F, Gandini R, Nappini S, Nencini P, Vallone S, Zini A, Bigliardi G, et al. Functional and radiological outcomes after bridging therapy versus direct thrombectomy in stroke patients with unknown onset: bridging therapy versus direct thrombectomy in unknown onset stroke patients with 10‐point ASPECTS. Eur J Neurol. 2021;28:209–219. doi: 10.1111/ene.14529 [DOI] [PubMed] [Google Scholar]
- 13. Coutinho JM, Liebeskind DS, Slater L‐A, Nogueira RG, Clark W, Dávalos A, Bonafé A, Jahan R, Fischer U, Gralla J, et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: a pooled analysis of the SWIFT and STAR Studies. JAMA Neurol. 2017;74:268–274. doi: 10.1001/jamaneurol.2016.5374 [DOI] [PubMed] [Google Scholar]
- 14. Guimarães Rocha M, Carvalho A, Rodrigues M, Cunha A, Figueiredo S, Martins de Campos A, Gregório T, Paredes L, Veloso M, Barros P, et al. Primary thrombectomy versus combined mechanical thrombectomy and intravenous thrombolysis in large vessel occlusion acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:627–631. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 15. Sallustio F, Koch G, Alemseged F, Konda D, Fabiano S, Pampana E, Morosetti D, Gandini R, Diomedi M. Effect of mechanical thrombectomy alone or in combination with intravenous thrombolysis for acute ischemic stroke. J Neurol. 2018;265:2875–2880. doi: 10.1007/s00415-018-9073-7 [DOI] [PubMed] [Google Scholar]
- 16. Tsivgoulis G, Katsanos AH, Mavridis D, Alexandrov AW, Magoufis G, Arthur A, Caso V, Schellinger PD, Alexandrov AV. Endovascular thrombectomy with or without systemic thrombolysis? Ther Adv Neurol Disord. 2017;10:151–160. doi: 10.1177/1756285616680549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barreto AD. Intravenous thrombolytics for ischemic stroke. Neurotherapeutics. 2011;8:388–399. doi: 10.1007/s13311-011-0049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grotta JC, Hacke W. Stroke neurologist's perspective on the new endovascular trials. Stroke. 2015;46:1447–1452. doi: 10.1161/STROKEAHA.115.008384 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, Kamiya Y, Shigeta K, Okubo S, Hayakawa M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325:244–253. doi: 10.1001/jama.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, Peng YA, Han H, Wang J, Wang S, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382:1981–1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 21. Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, Liu S, Yuan J, Song J, Shi Z, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325:234–243. doi: 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tong XU, Wang Y, Fiehler J, Bauer CT, Jia B, Zhang X, Huo X, Luo G, Wang A, Pan Y, et al. Thrombectomy versus combined thrombolysis and thrombectomy in patients with acute stroke: a matched‐control study. Stroke. 2021;52:1589–1600. doi: 10.1161/STROKEAHA.120.031599 [DOI] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 24. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB; Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) Group . Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells G, Shea B, O’Connor D. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analysis. Published 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 19, 2020.
- 29. Kass‐Hout T, Kass‐Hout O, Mokin M, Thesier DM, Yashar P, Orion D, Jahshan S, Hopkins LN, Siddiqui AH, Snyder KV, et al. Is bridging with intravenous thrombolysis of any benefit in endovascular therapy for acute ischemic stroke? World Neurosurg. 2014;82:e453–e458. doi: 10.1016/j.wneu.2013.01.097 [DOI] [PubMed] [Google Scholar]
- 30. Broeg‐Morvay A, Mordasini P, Bernasconi C, Bühlmann M, Pult F, Arnold M, Schroth G, Jung S, Mattle HP, Gralla J, et al. Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched‐pairs analysis. Stroke. 2016;47:1037. doi: 10.1161/STROKEAHA.115.011134 [DOI] [PubMed] [Google Scholar]
- 31. Casetta I, Pracucci G, Saletti A, Saia V, Padroni M, De Vito A, Inzitari D, Zini A, Vallone S, Bergui M, et al. Combined intravenous and endovascular treatment versus primary mechanical thrombectomy: the Italian Registry of Endovascular Treatment in Acute Stroke. Int J Stroke. 2019;14:898–907. doi: 10.1177/1747493019851279 [DOI] [PubMed] [Google Scholar]
- 32. Du M, Li S, Huang X, Zhang S, Bai Y, Yan B, Guo H, Xu G, Liu X. Intravenous thrombolysis before thrombectomy may increase the incidence of intracranial hemorrhage in treating carotid T occlusion. J Stroke Cerebrovasc Dis. 2021;30:105473. doi: 10.1016/j.jstrokecerebrovasdis.2020.105473 [DOI] [PubMed] [Google Scholar]
- 33. Gong LI, Zheng X, Feng L, Zhang X, Dong Q, Zhou X, Wang H, Zhang X, Shu Z, Zhao Y, et al. Bridging therapy versus direct mechanical thrombectomy in patients with acute ischemic stroke due to middle cerebral artery occlusion: a clinical‐histological analysis of retrieved thrombi. Cell Transplant. 2019;28:684–690. doi: 10.1177/0963689718823206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pienimäki JP, Ollikainen J, Sillanpää N, Protto S. In‐hospital intravenous thrombolysis offers no benefit in mechanical thrombectomy in optimized tertiary stroke center setting. Cardiovasc Intervent Radiol. 2021;44:580–586. doi: 10.1007/s00270-020-02727-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H, Zi W, Hao Y, Yang D, Shi Z, Lin M, Wang S, Liu W, Wang Z, Liu X, et al. Direct endovascular treatment: an alternative for bridging therapy in anterior circulation large‐vessel occlusion stroke. Eur J Neurol. 2017;24:935–943. doi: 10.1111/ene.13311 [DOI] [PubMed] [Google Scholar]
- 36. Weber R, Nordmeyer H, Hadisurya J, Heddier M, Stauder M, Stracke P, Berger K, Chapot R. Comparison of outcome and interventional complication rate in patients with acute stroke treated with mechanical thrombectomy with and without bridging thrombolysis. J Neurointerv Surg. 2017;9:229–233. doi: 10.1136/neurintsurg-2015-012236 [DOI] [PubMed] [Google Scholar]
- 37. Vidale S, Romoli M, Consoli D, Agostoni EC. Bridging versus direct mechanical thrombectomy in acute ischemic stroke: a subgroup pooled meta‐analysis for time of intervention, eligibility, and study design. Cerebrovasc Dis. 2020;49:223–232. doi: 10.1159/000507844 [DOI] [PubMed] [Google Scholar]
- 38. Katsanos AH, Malhotra K, Goyal N, Arthur A, Schellinger PD, Köhrmann M, Krogias C, Turc G, Magoufis G, Leys D, et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol. 2019;86:395–406. doi: 10.1002/ana.25544 [DOI] [PubMed] [Google Scholar]
- 39. Phan K, Dmytriw AA, Maingard J, Asadi H, Griessenauer CJ, Ng W, Kewagamang K, Mobbs RJ, Moore JM, Ogilvy CS, et al. Endovascular thrombectomy alone versus combined with intravenous thrombolysis. World Neurosurg. 2017;108:850–858.e2. doi: 10.1016/j.wneu.2017.08.040 [DOI] [PubMed] [Google Scholar]
- 40. Liu M, Li G. Is direct endovascular treatment as an alternative of bridging therapy in acute stroke patients with large vessel occlusion? J Stroke Cerebrovasc Dis. 2019;28:531–541. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 41. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European Stroke Organisation (ESO)‐European Society of Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke. Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menon BK, Saver JL, Prabhakaran S, Reeves M, Liang LI, Olson DM, Peterson ED, Hernandez AF, Fonarow GC, Schwamm LH, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue‐type plasminogen activator. Stroke. 2012;43:2293–2299. doi: 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- 43. Jia B, Ren Z, Mokin M, Burgin WS, Bauer CT, Fiehler J, Mo D, Ma N, Gao F, Huo X, et al. Current status of endovascular treatment for acute large vessel occlusion in China: a real‐world nationwide registry. Stroke. 2021;52:1203–1212. doi: 10.1161/STROKEAHA.120.031869 [DOI] [PubMed] [Google Scholar]
- 44. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Zhao X, Liu L, Soo YOY, Pu Y, Pan Y, Wang Y, Zou X, Leung TWH, Cai Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–669. doi: 10.1161/STROKEAHA.113.003508 [DOI] [PubMed] [Google Scholar]
- 46. Ma G, Li S, Jia B, Mo D, Ma N, Gao F, Huo X, Luo G, Wang A, Pan Y, et al. Safety and efficacy of low‐dose tirofiban combined intravenous thrombolysis and mechanical thrombectomy in acute ischemic stroke: a matched‐control analysis from a nationwide registry. Front Neurol. 2021;12:666919. doi: 10.3389/fneur.2021.666919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaesmacher J, Kleine JF. Bridging therapy with i. v. rtPA in MCA occlusion prior to endovascular thrombectomy: a double‐edged sword? Clin Neuroradiol. 2018;28:81–89. doi: 10.1007/s00062-016-0533-0 [DOI] [PubMed] [Google Scholar]
- 48. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 49. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Thijs V, Scroop R, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND‐IA TNK part 2 randomized clinical trial. JAMA. 2020;323:1257–1265. doi: 10.1001/jama.2020.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]


