Abstract
Background
Depressive symptoms are associated with heightened risk of heart failure (HF), but their association with cardiac function and with HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF) in late life is unclear. We aimed to determine the prevalence of depression in HFpEF and in HFrEF in late life, and the association of depressive symptoms with cardiac function and incident HFpEF and HFrEF.
Methods and Results
We studied 6025 participants (age, 75.3±5.1 years; 59% women; 20% Black race) in the ARIC (Atherosclerosis Risk in Communities) study at visit 5 who underwent echocardiography and completed the Center for Epidemiologic Studies Depression Scale questionnaire. Among HF‐free participants (n=5086), associations of Center for Epidemiologic Studies Depression Scale score with echocardiography and incident adjudicated HFpEF and HFrEF were assessed using multivariable linear and Cox proportional hazards regression. Prevalent HFpEF, but not HFrEF, was associated with a higher prevalence of depression compared with HF‐free participants (P<0.001 and P=0.59, respectively). Among HF‐free participants, Center for Epidemiologic Studies Depression Scale score was not associated with cardiac structure and function after adjusting for demographics and comorbidities (all P>0.05). Over 5.5‐year follow‐up, higher Center for Epidemiologic Studies Depression Scale score was associated with heightened risk of incident HFpEF (hazard ratio [HR] [95% CI], 1.06 [1.04–1.12]; P=0.02), but not HFrEF (HR [95% CI], 1.02 [0.96–1.08]; P=0.54), independent of echocardiographic measures, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), troponin, and hs‐CRP (high‐sensitivity C‐reactive protein) (HR [95% CI], 1.06 [1.00–1.12]; P=0.04).
Conclusions
Worse depressive symptoms predict incident HFpEF in late life, independent of common comorbidities, cardiac structure and function, and prognostic biomarkers. Further studies are necessary to understand the mechanisms linking depression to risk of HFpEF.
Keywords: depression, echocardiography, heart failure
Subject Categories: Heart Failure, Mental Health, Epidemiology, Echocardiography
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- CES‐D
Center for Epidemiologic Studies Depression Scale
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
Clinical Perspective
What Is New?
Depressive symptoms are associated with a higher risk of incident heart failure with preserved ejection fraction, but not heart failure with reduced ejection fraction, in late life, independent of cardiovascular risk factors, biomarkers, and echocardiographic measures.
Despite the association with incident heart failure with preserved ejection fraction, depressive symptoms are not associated with cardiac structure and function in late life, independent of demographics and cardiovascular risk factors.
What Are the Clinical Implications?
Further studies are necessary to understand the mechanisms linking depressive symptoms in older adults to development of heart failure with preserved ejection fraction.
Depression is as an important potential contributor to heart failure (HF). The prevalence of depression is higher among people with versus without HF, and comorbid depression is a predictor of mortality and morbidity in HF, including rehospitalization. 1 , 2 , 3 A growing body of evidence suggests that depressive symptoms also predict incident HF, even after accounting for traditional cardiovascular comorbidities. In one large population‐based study, depressive symptoms were associated with incident HF independent of coronary heart disease and cardiovascular risk factors. 4 Although not consistent in all studies, 5 associations between prevalent depression and heightened risk of incident HF have been observed in elderly individuals, 6 , 7 in veterans, 8 and in patients with isolated hypertension, 9 those with coronary artery disease, 10 or those who report poor health at baseline. 11 HF incidence and prevalence are highest in late life, a time of life when depression is also common. Although most of prevalent HF in late life occurs with a preserved ejection fraction (HFpEF), sparse data exist on the association between depressive symptoms and risk of incident HFpEF or HF with reduced ejection fraction (HFrEF). Cardiac structural remodeling and impairments in left ventricular (LV) systolic and diastolic dysfunction underlie the development of clinical HF. However, the extent to which depressive symptoms associate with contemporary measures of cardiac structure and function is unclear, although associations with measures of diastolic function have been reported, 12 , 13 potentially suggesting a higher risk of developing HFpEF. We therefore aimed to determine the prevalence of depressive symptoms in HFpEF and HFrEF, the association of depressive symptoms in late life with incident HFpEF and HFrEF among people free of prevalent HF, and the extent to which the associations of depressive symptoms with cardiac structure and function account for the potential relationships between depressive symptoms and incident HFpEF and HFrEF.
Methods
Data availability and detailed policies for requesting ARIC (Atherosclerosis Risk in Communities) study data can be found at https://sites.cscc.unc.edu/aric/pubs‐policies‐and‐forms‐pg. ARIC study data can also be obtained from the National Heart, Lung, and Blood Institute BioLINCC repository (https://biolincc.nhlbi.nih.gov/studies/aric/).
Study Population
The ARIC study is an ongoing longitudinal cohort study that was initiated in 1987 to 1989, at which time it enrolled 15 792 men and women aged 45 to 64 years from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; suburbs of Minneapolis, MN; and Jackson, MS. A detailed description of the study’s objective and design has been previously published. 14 The cohort participants subsequently underwent 4 study visits between 1987 and 1998. A total of 6538 returned for a fifth study visit in 2011 to 2013, at which time they underwent standardized comprehensive echocardiography and completed the Center for Epidemiologic Studies Depression Scale (CES‐D) questionnaire. Institutional Review Board approved the study at each site, and informed consent was obtained from all participants. The present study included 6025 participants with available CES‐D and LV ejection fraction (LVEF) by echocardiography at visit 5. Participants with prevalent HF at visit 5 were subsequently excluded for analyses of the clinical and echocardiographic correlates of depressive symptoms and the association of depressive symptoms with incident HFpEF and HFrEF after visit 5 (n=5086). Prevalent HF was defined on the basis of an adjudicated HF hospitalization between January 1, 2005, and the visit 5 date, hospitalization with HF‐related International Classification of Diseases, 9th revision, Clinical Modification (ICD‐9‐CM) codes before January 1, 2005, or participant self‐report.
Assessment of Depressive Symptoms
Depressive symptoms were assessed using a short version of the CES‐D. The 11‐item version assesses symptoms of depression within the past week, such as “I felt depressed,” “I felt everything I did was an effort,” and “I felt lonely.” Each item on the scale was scored as 0 (none/rarely), 1 (sometimes), or 2 (often), yielding a total score ranging from 0 to 22, with higher scores indicative of more depressive symptoms. The full 20‐item version was designed to assess symptoms of depression in the general population, 15 and has been validated in a variety of populations, including elderly individuals. 16 The short version has shown a strong correlation with the original version. 17 On the basis of prior recommendations, a score of ≥9 was classified as having significant depressive symptoms, equivalent to ≥16 on the 20‐item questionnaire. 18 CES‐D score was set to missing if there was >1 missing item on the scale.
Assessment of Echocardiographic Measures
Details of echocardiography at ARIC study visit 5, including acquisition, quantitative analysis, and reproducibility metrics, have been previously described in detail. 19 Briefly, comprehensive echocardiography was performed at visit 5 by certified sonographers at the 4 field centers using a standardized image acquisition protocol and uniform equipment (Philips iE33 Ultrasound systems). All quantitative measures were performed centrally at the Echocardiography Reading Center at Brigham and Women’s Hospital in accordance with American Society of Echocardiography guidelines by analysts blinded to clinical information. 20 , 21 , 22 LV dimensions and wall thickness were obtained from the parasternal long‐axis view. LV mass was indexed to height2.7. Diastolic function was assessed using mitral inflow pulse‐wave Doppler (E‐wave velocities), mitral annular tissue Doppler (eʹ), and pulmonary artery systolic pressure. Left atrial volume was measured in the apical 4‐ and 2‐chamber views by the method of disks and was indexed to the body surface area. Measures of systolic function included LVEF and global longitudinal strain, derived from speckle‐tracking echocardiography (TomTec Imaging system).
Assessment of Demographic Covariates and Socioeconomic Indicators
Demographic covariates and socioeconomic indicators, including age, sex, self‐reported race, and education (≤11, 12–16, and 17–21 years), were assessed at previous visits, whereas information about household income (<$5000, $5000–$7999, $8000–$11 999, $12 000–$15 999, $16 000–$24 999, $25 000–$34 999, $35 000–$49 999, $50 000–$74 999, $75 000–$99 999, or ≥$100 000) was obtained at visit 5, and subsequently categorized into 3 groups: <$15 999, $16 000 to $34 999, and ≥$35 000.
Assessment of Health Behaviors, Prevalent Chronic Conditions, and Cardiovascular Risk Factors
Demographic covariates and socioeconomic indicators, including age, sex, self‐reported race, and education (≤11, 12–16, and 17–21 years), were assessed at previous visits, whereas information about household income (<$5000, $5000–$7999, $8000–$11 999, $12 000–$15 999, $16 000–$24 999, $25 000–$34 999, $35 000–$49 999, $50 000–$74 999, $75 000–$99 999, or ≥$100 000) was obtained at visit 5, and subsequently categorized into 3 groups: <$15 999, $16 000 to $34 999, and ≥$35 000. Physical activity was assessed using an interviewer‐administered modified Baecke Physical Activity questionnaire 23 and categorized according to American Heart Association guidelines as ideal, intermediate, or poor, as previously used in the ARIC study. 24 Smoking and alcohol drinking status (current, ever, or never smokers/alcohol drinkers) were assessed from an interviewer‐administered questionnaire.
Prevalent hypertension and diabetes mellitus were defined on the basis of self‐report, medication use, or if prevalent (blood pressure >140/90 mm Hg and fasting glucose ≥126 mg/dL, respectively) at any study visit. Prevalent atrial fibrillation was defined on the basis of documented atrial fibrillation or atrial flutter on standard 12‐lead ECG rhythm at any study visit, or ICD‐9 code 427.31 or 427.32 in any hospitalization occurring before the visit 5 date. 25 Prevalent coronary heart disease (definite or probable myocardial infarction or coronary revascularization) and stroke were based on ARIC study surveillance of hospital discharge codes and subsequent chart abstraction and committee adjudication, which has been ongoing since study inception, as previously described, or if self‐reported at visit 1. 26 Body mass index (BMI; kg/m2) was calculated from weight and standing height acquired at visit 5. Blood pressure was averaged on the basis of the second 2 of 3 measurements ascertained at visit 5. Estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation. 27
Other Assessments
The cardiac biomarkers hs‐CRP (high‐sensitivity C‐reactive protein), high‐sensitivity troponin T, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) were measured from plasma centrally stored at −80°C. Medical adherence was assessed using the Morisky Medication Adherence Scale, which includes 4 questions with yes/no response options. The Morisky Medication Adherence Scale results in a score ranging from 0 to 4, with 3 levels of medication adherence based on this score: high, medium, and low adherence with 0, 1 to 2, and 3 to 4 points, respectively. Cognitive function was modeled as a categorical variable indicating dementia, mild cognitive impairment, and normal, and was based on expert review of a comprehensive neurocognitive battery, neurological history, informant interviews, and brain magnetic resonance imaging, as previously described in detail elsewhere. 28
Outcome Assessment
Incident HFpEF, HFrEF, and death were ascertained from visit 5 to the end of 2017 (median follow‐up time, 5.5 years; interquartile interval, 5.1–6.0 years). Incident HF was assessed on the basis of ARIC study HF Committee review and adjudication of abstracted hospitalization records of hospitalizations or deaths with HF‐related ICD‐9‐CM, as previously described in detail. 29 Abstraction included results of imaging studies and LVEF. Reviewers determined if evidence of an LVEF <50% at the time of hospitalization was present, and a numerical LVEF was recorded if available. This information from the HF hospitalization was used to determine the incident HF type (HFpEF [LVEF ≥50%] or HFrEF [LVEF <50%]). If no LVEF information was available from that hospitalization, LVEFs recorded from hospitalizations in the prior 6 months were carried forward if no intercurrent myocardial infarction was present. Of the 303 incident HF cases, 136 (45%) were classified as HFpEF, 126 (42%) were classified as HFrEF, and LVEF was not available in 41 (14%). Death was ascertained from the National Death Index.
Statistical Analysis
The prevalence of severe depressive symptoms (defined as a CES‐D score ≥9) at visit 5 was determined among participants with prevalent HFrEF, prevalent HFpEF, and no HF. Among HF‐free participants at visit 5, participants were classified on the basis of CES‐D score ≥9 (severe depressive symptoms) and quartiles of CES‐D score <9 for descriptive analyses of clinical characteristics and echocardiographic measures. For analyses of associations of depressive symptoms with incident HFpEF and HFrEF, we modeled depressive symptoms continuously. Continuous data are presented as mean and the SD, and categorical variables are presented as frequencies and percentages. All continuous variables were inspected for normality and transformed if necessary to achieve normality. For univariable analyses of the association between baseline characteristics across categories of depressive symptoms, tests for trend were performed. Adjusted analyses were performed using multivariable linear and logistic regression. Analyses comparing clinical characteristics across CES‐D score categories were adjusted for demographics, whereas comparisons of echocardiographic features were adjusted for demographics (age, sex, race, and field center), socioeconomic indicators (education and income), health behaviors (smoking, drinking, and physical activity), and prevalent chronic conditions and cardiovascular risk factors (hypertension, diabetes mellitus, prevalent atrial fibrillation, prevalent coronary heart disease, prevalent stroke, history of myocardial infarction, BMI, and eGFR). Multivariable logistic regressions were used to assess the association of CES‐D score categories with abnormal echocardiographic measures, defined using cutoffs based on normative values from the ARIC study 30 , 31 , 32 and American Society of Echocardiography guidelines. 20 Model covariates were selected on the basis of a priori knowledge of demographic covariates, socioeconomic indicators, health behaviors, and prevalent chronic conditions and cardiovascular risk factors associated with depression and heart failure, as also applied in several similar studies. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11
Multivariable Cox proportional hazards models were used to investigate the association of depressive symptoms (based on CES‐D score modeled continuously) and incident HFpEF or incident HFrEF. For analyses with incident HFpEF as the primary outcome, participants were censored at the time of incident HFrEF or HF with unknown LVEF. Similarly, for the primary outcome of incident HFrEF, participants were censored at the time of incident HFpEF or HF with unknown LVEF. Additional analyses assessed the composite of incident HFpEF or death and the composite of incident HFrEF or death. To develop a parsimonious set of adjustment covariates for Cox models, a linear regression model was generated with CES‐D score as the outcome and the following covariates as predictors: demographics (age, sex, and race), socioeconomic indicators (education and income), health behaviors (smoking, drinking, and physical activity), and prevalent chronic conditions and cardiovascular risk factors (hypertension, diabetes mellitus, stroke, prevalent coronary heart disease, BMI, heart rate, and eGFR). Predictors significantly associated with CES‐D score in this multivariable model were as follows: BMI, diabetes mellitus, heart rate, eGFR, and smoking. Fully adjusted models for all time‐to‐event analyses therefore adjusted for demographics (age, sex, and race), BMI, diabetes mellitus, heart rate, eGFR, and smoking. Additional analyses adjusting for the full set of covariates specified above were performed and are presented in the Supplemental Data. The proportional hazards assumptions for Cox models were tested using Schoenfeld residuals, and no violations were detected. 33 Model covariates were as follows: model 1 included demographics (age, sex, and race), socioeconomic indicators (education and income), health behaviors (smoking, drinking, and physical activity), and prevalent chronic conditions and cardiovascular risk factors (diabetes mellitus, BMI, heart rate, and eGFR); model 2 additionally adjusted for echocardiographic measures (LV mass index, mean LV thickness, septal early diastolic myocardial velocity, and ratio of mitral peak velocity of early filling/early diastolic mitral annular velocity); model 3 additionally adjusted for cardiac biomarkers (log‐transformed NT‐proBNP and log‐transformed high‐sensitivity troponin T). Model 4 additionally adjusted for log‐transformed hs‐CRP. The following sensitivity analyses were also performed: (1) Additional adjustment for cognitive impairment and medical adherence, as late‐life depression may be characterized by greater cognitive impairments 34 and depression is associated with medical nonadherence, which has been shown to affect health outcomes. 35 (2) Exclusion of HF events occurring during the first year of follow‐up to assess for the potential impact of reverse causation. (3) Use of 2 additional models to optimize model fit. First, we included covariates with a P<0.01 using a forward selection model. Second, we determined risk scores for each individual using previously published regression coefficients from the ARIC study HF risk function. 36 (4) Effect measure modification of the observed associations by sex, age, and race was assessed using multiplicative interaction terms. (5) To assess the impact of potential bias caused by visit 5 nonattendance, we performed additional sensitivity analysis using inverse probability of attrition weighting. 37 , 38 Visit 5 nonattendance was modeled among participants alive at the initiation of visit 5 using the following covariates from visit 1: age, sex, race, field center, smoking, drinking, hypertension, diabetes mellitus, atrial fibrillation, stroke, income, education, history of myocardial infarction, prevalent coronary heart disease, physical activity, BMI, heart rate, and eGFR. The resulting calculated weights were incorporated into multivariable models for the association of depressive symptoms with incident HF. All analyses were conducted using STATA software (StataSE 14).
Results
At visit 5, severe depressive symptoms (CES‐D score ≥9) were present in 6% of HF‐free participants, 7% of participants with prevalent HFrEF (odds ratio [OR] [95% CI], 1.29 [0.51–3.28]; P=0.59) versus no HF, and 13% of participants with prevalent HFpEF (OR [95% CI], 2.12 [1.43–3.15]; P<0.001) versus no HF (Figure 1). The prevalence of severe depressive symptoms did not statistically differ between participants with HFrEF and those with HFpEF (OR [95% CI], 0.55 [0.20–1.55]; P=0.26).
Figure 1. Prevalence of depression in heart failure (HF) groups.
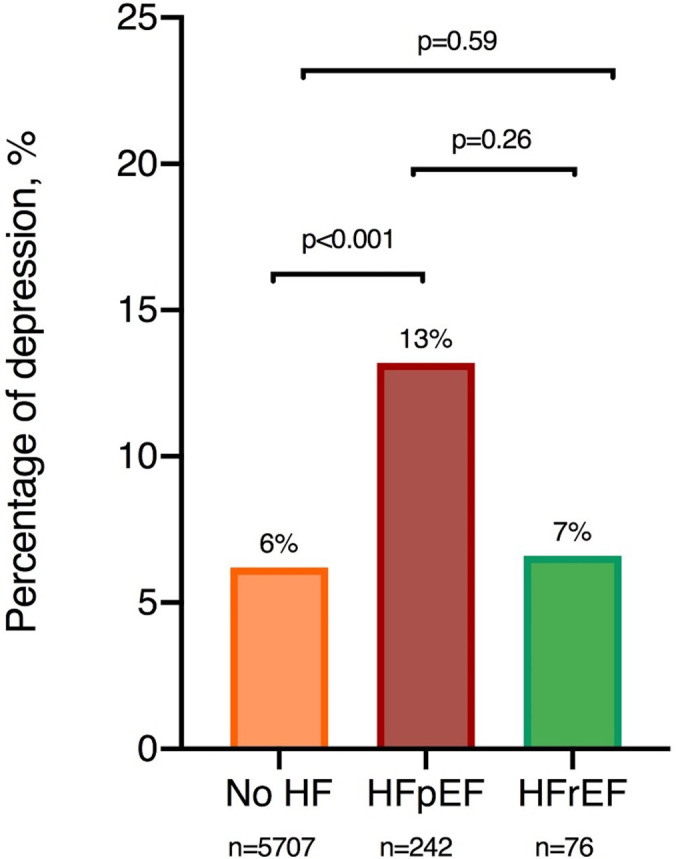
The prevalence of depression by HF subgroups was determined using logistic regression with adjustments for age, sex, race, and field center. The prevalence of depression was higher in those with HF with preserved ejection fraction (HFpEF) compared with those free of HF. No statistically significant difference was found between HF with reduced ejection fraction (HFrEF) and those free of HF, and between those with HFrEF and HFpEF. No detectable difference in the latter comparison could, however, be caused by small sample sizes.
Among the 5086 HF‐free participants in the study sample, the mean age was 75.3±5.1 years (range, 66–90 years), 59% were women, and 41% were men. The median CES‐D score was 2 (interquartile interval [quartile 1–quartile 3], 1–4), and 287 (6%) participants had severe depressive symptoms, defined as a CES‐D score of ≥9. Higher CES‐D score was associated with female sex, White race, higher BMI, a higher prevalence of cardiovascular comorbidities, higher NT‐proBNP and high‐sensitivity troponin T, and higher hs‐CRP (Table 1). Lower CES‐D score was also associated with fewer years of education, lower income, and higher prevalence of poor physical activity.
Table 1.
Baseline Characteristics Among Participants With CES‐D Score ≥9 and Quartiles of CES‐D Scores <9
| Characteristic | CES‐D Score 0–1 (n=1990) | CES‐D Score 2 (n=865) | CES‐D Score 3–4 (n=1074) | CES‐D Score 5–8 (n=870) | CES‐D Score ≥9 (n=287) | P Value | Adjusted P Value* |
|---|---|---|---|---|---|---|---|
| Age, y | 74.9±4.9 | 75.3±5.0 | 75.8±5.2 | 75.9±5.3 | 75.3±5.3 | 0.001 | |
| BMI, kg/m2 | 28.1±5.0 | 28.0±5.1 | 28.6±5.6 | 28.7±6.0 | 29.9±6.0 | 0.001 | 0.001 |
| Men, n (%) | 928 (47) | 377 (44) | 401 (37) | 279 (32) | 92 (32) | 0.001 | |
| Black, Asian, and Native American, n (%) | 369 (19) | 146 (17) | 180 (17) | 228 (26) | 84 (29) | 0.001 | |
| Smoking status, n (%) | 0.004 | 0.001 | |||||
| Current smoker | 85 (5) | 46 (6) | 75 (8) | 57 (7) | 31 (12) | ||
| Ever smoker | 943 (51) | 407 (56) | 486 (50) | 398 (50) | 124 (50) | ||
| Never smoker | 816 (44) | 337 (43) | 412 (42) | 335 (42) | 94 (38) | ||
| Drinking status, n (%) | 0.001 | 0.001 | |||||
| Current drinker | 1105 (57) | 475 (56) | 533 (51) | 339 (41) | 96 (36) | ||
| Ever drinker | 469 (24) | 194 (23) | 302 (29) | 289 (35) | 98 (36) | ||
| Never drinker | 366 (19) | 174 (21) | 210 (20) | 209 (25) | 76 (28) | ||
| Atrial fibrillation, n (%) | 115 (6) | 44 (5) | 71 (7) | 54 (6) | 19 (7) | 0.30 | 0.17 |
| Hypertension, n (%) | 1556 (78) | 690 (80) | 882 (82) | 744 (86) | 251 (88) | 0.001 | 0.001 |
| Diabetes mellitus, n (%) | 594 (30) | 295 (34) | 371 (35) | 345 (40) | 139 (48) | 0.001 | 0.001 |
| History of MI, n (%) | 110 (6) | 75 (9) | 83 (8) | 66 (8) | 23 (9) | 0.01 | 0.006 |
| CHD, n (%) | 183 (9) | 106 (13) | 118 (11) | 83 (10) | 25 (9) | 0.81 | 0.11 |
| History of stroke, n (%) | 41 (2) | 22 (3) | 28 (3) | 34 (4) | 11 (4) | 0.004 | 0.005 |
| Heart rate, bpm | 61±10 | 62±10 | 63±11 | 64±10 | 66±12 | 0.001 | 0.001 |
| Systolic BP, mm Hg | 130±18 | 131±17 | 130±18 | 132±18 | 130±18 | 0.008 | 0.41 |
| Diastolic BP, mm Hg | 67±10 | 66±10 | 66±11 | 67±11 | 67±10 | 0.87 | 0.57 |
| eGFR, mL/min /1.73 m2 | 71.8±15.7 | 71.1±15.8 | 70.5±16.8 | 69.4±17.8 | 68.9±17.5 | 0.001 | 0.001 |
| Income, n (%) | 0.001 | 0.001 | |||||
| ≤$15 999 | 149 (8) | 103 (13) | 139 (14) | 162 (20) | 72 (28) | ||
| $16 000–$34 999 | 409 (22) | 200 (25) | 305 (31) | 263 (33) | 96 (37) | ||
| ≥$35 000 | 1269 (70) | 491 (62) | 547 (55) | 368 (46) | 93 (36) | ||
| Education, n (%) | 0.001 | 0.001 | |||||
| Basic or no education (≤11 y) | 150 (8) | 101 (12) | 130 (12) | 132 (15) | 78 (27) | ||
| Intermediate education (12–16 y) | 726 (37) | 374 (43) | 513 (48) | 395 (45) | 138 (48) | ||
| Advanced education (17–21 y) | 1107 (56) | 389 (45) | 431 (40) | 343 (39) | 70 (25) | ||
| Physical activity, n (%) | 0.001 | 0.001 | |||||
| Ideal | 1144 (58) | 457 (54) | 505 (48) | 347 (41) | 80 (29) | ||
| Intermediate | 326 (17) | 166 (20) | 198 (19) | 198 (24) | 56 (21) | ||
| Poor | 491 (25) | 226 (27) | 353 (33) | 299 (35) | 137 (50) | ||
| NT‐proBNP, pg/mL | 108 (58–210) | 125 (63–239) | 131 (67–231) | 137 (72–266) | 138 (59–266) | 0.001 | 0.001 |
| hs‐TnT, ng/L | 1.0 (0.7–1.5) | 1 (0.7–1.5) | 1.0 (0.7–1.5) | 1.0 (0.8–1.6) | 1.1 (0.8–1.5) | 0.001 | 0.001 |
| hs‐CRP, mg/L | 1.8 (0.9–3.6) | 1.9 (0.9–4.1) | 2.0 (1.0–4.2) | 2.2 (1.0–4.3) | 2.5 (1.1–6.1) | 0.001 | 0.001 |
Values are mean±SD, number (percentage), or median (quartile 1–quartile 3). BMI indicates body mass index; BP, blood pressure; bpm, beats per minute; CES‐D, Center for Epidemiologic Studies Depression Scale; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnT, high‐sensitivity troponin T; MI, myocardial infarction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Adjusted for demographics (age, sex, race, and field center).
Association of Depressive Symptoms With Risk of Incident HFpEF and HFrEF
Of the 303 incident HF events occurring over a median follow‐up of 5.5 years (quartile 1–quartile 3, 5.1–6.0 years), 126 were classified as HFrEF (42%), 136 were classified as HFpEF (45%), and LVEF was not available in 41 (14%). Higher CES‐D score was associated with incident HFrEF in models adjusted for demographics, but this association attenuated appreciably after further adjusting for health behaviors, prevalent chronic conditions, and cardiovascular risk factors (Figure 2). In contrast, higher CES‐D score was associated with heightened risk for incident HFpEF, such that each 1‐unit increase in CES‐D was associated with a 6% increase in risk of incident HFpEF. This association persisted after adjustment for cardiovascular comorbidities, associated echocardiographic features, NT‐proBNP, and high‐sensitivity troponin T concentrations without attenuation of the effect estimate (Table 2). Similar findings were observed for the composite of incident HFpEF or death (Figure 2), in models adjusting for the full set of potential covariates (Table S1 and Figure S1), in a sensitivity analysis treating unclassified incident HF events as incident HFpEF events (Table S2), and in models further adjusting for cognitive function and medication adherence (Table S3). Excluding events that occurred during the first year of follow‐up did not appreciably alter the results (Table S4). Similar findings were observed in models using forward selection of variables and HF risk scores (Tables S5 and S6). No effect modification by sex, race, or age was observed on the relationship between CES‐D score and incident HFrEF (interaction P=0.99, P=0.82, and P=0.93, respectively) or incident HFpEF (interaction P=0.11, P=0.12, and P=0.91, respectively). Finally, comparable results were observed in analyses incorporating inverse probability of attrition weighting (Figure S2).
Figure 2. Associations of depressive symptoms with incident heart failure with preserved ejection fraction (HFpEF), heart failure with reduced ejection fraction (HFrEF), and the composite outcomes HFpEF/death and HFrEF/death.
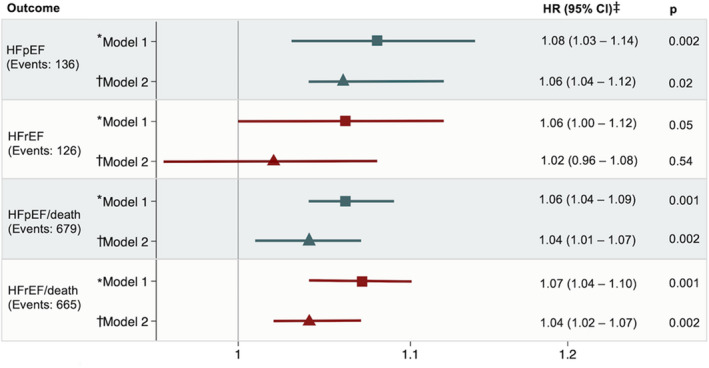
Greater depressive symptoms were associated with a higher risk of developing HFpEF but not HFrEF after adjusting for demographics and cardiovascular risk factors. *Model 1 adjusts for demographics (age, sex, and race). †Model 2 adjusts for model 1, socioeconomic indicators (education and income), health behaviors (smoking, drinking, and physical activity), and prevalent chronic conditions and cardiovascular risk factors (diabetes mellitus, body mass index, heart rate, and estimated glomerular filtration rate). ‡Hazard ratio (HR) is per unit increase in Center for Epidemiologic Studies Depression Scale score.
Table 2.
Association Between Depressive Symptoms and Risk of Incident HFpEF per Unit Increase in CES‐D Score
| Variable | No. | Events | HR (95% CI) | P Value |
|---|---|---|---|---|
| HFpEF | ||||
| Adjusted for demographic covariates, socioeconomic indicators, health behaviors, prevalent chronic conditions, and cardiovascular risk factors* | 4843 | 128 | 1.06 (1.01–1.12) | 0.02 |
| +Echocardiography † | 4795 | 126 | 1.06 (1.00–1.12) | 0.03 |
| +Cardiac biomarkers ‡ | 4495 | 120 | 1.06 (1.00–1.12) | 0.04 |
| +hs‐CRP | 4493 | 120 | 1.06 (1.00–1.12) | 0.04 |
CES‐D indicates Center for Epidemiologic Studies Depression Scale; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; and hs‐CRP, high‐sensitivity C‐reactive protein.
Demographic covariates include age, sex, and race; socioeconomic indicators include income and education; health behaviors include smoking, drinking, and physical activity; prevalent chronic conditions and cardiovascular risk factors include diabetes mellitus, body mass index, heart rate, and estimated glomerular filtration rate.
Echocardiography includes left ventricular mass index, left ventricular mean wall thickness, septal early diastolic myocardial velocity, and ratio of mitral peak velocity of early filling/early diastolic mitral annular velocity.
Cardiac biomarkers include NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) and troponin T.
Association of Depressive Symptoms With Cardiac Structure and Function
Among participants free of HF at visit 5, modest but statistically significant associations were observed between higher CES‐D score and greater LV wall thickness (mean wall thickness), LV cavity size, and LV mass index, and with worse indexes of diastolic function (lower tissue Doppler imaging eʹ, higher E/eʹ, and higher pulmonary artery systolic pressure; Table 3). After adjusting for demographics, higher LV mass index, LV mean wall thickness, eʹ, and E/eʹ remained associated, but after further adjustment for lifestyle factors and cardiovascular risk factors, all associations were attenuated and no longer significant. Analyses modeling echocardiographic measures as categorical variables demonstrate similar results. Adjustment for LV mass index, LV mean wall thickness, eʹ, and E/eʹ did not appreciably attenuate the association of CES‐D score with risk of incident HFpEF (Table 2).
Table 3.
Cardiac Structure and Function Among Participants With CES‐D Score ≥9 and Quartiles of CES‐D Scores <9
| Echocardiography | CES‐D Score 0–1 (n=1990) | CES‐D Score 2 (n=865) | CES‐D Score 3–4 (n=1074) | CES‐D Score 5–8 (n=870) | CES‐D Score ≥9 (n=287) | P Value | Adjusted P Value* | Adjusted P Value † |
|---|---|---|---|---|---|---|---|---|
| LV structure | ||||||||
| MWT, cm | 0.97±0.13 | 0.98±0.14 | 0.98±0.13 | 0.99±0.13 | 1.00±0.12 | 0.001 | 0.001 | 0.07 |
| LVEDD, cm | 4.40±0.48 | 4.39±0.48 | 4.37±0.51 | 4.34±0.49 | 4.34±0.48 | 0.002 | 0.19 | 0.67 |
| LVEDVi, mL/m2 | 43.2±10.1 | 43.1±9.8 | 42.1±10.2 | 41.5±9.3 | 41.1±10.6 | 0.08 | 0.88 | 0.81 |
| Abnormal LVEDVi, % | 13 | 13 | 14 | 13 | 18 | 0.19 | 0.62 | |
| LVMI, g/height2.7 | 36.5±9.7 | 37.0±9.6 | 37.4±9.4 | 37.8±10.1 | 39.5±10.0 | 0.001 | 0.001 | 0.20 |
| Abnormal LVMI, % | 20 | 22 | 25 | 26 | 30 | 0.001 | 0.52 | |
| LV systolic function | ||||||||
| LVEF, % | 65.6±6.0 | 65.7±5.7 | 65.7±6.0 | 66.1±5.6 | 65.6±5.9 | 0.10 | 0.70 | 0.43 |
| Abnormal LVEF, % | 14 | 13 | 15 | 12 | 14 | 0.83 | 0.30 | |
| Longitudinal strain, % | −18.1±2.4 | −18.1±2.4 | −18.1±2.5 | −18.0±2.4 | −17.9±2.4 | 0.17 | 0.03 | 0.33 |
| Abnormal longitudinal strain, % | 18 | 18 | 20 | 19 | 21 | 0.04 | 0.32 | |
| LV diastolic function | ||||||||
| Eʹ, cm/s | 5.8±1.5 | 5.8±1.4 | 5.7±1.5 | 5.7±1.6 | 5.5±1.4 | 0.001 | 0.01 | 0.33 |
| Abnormal Eʹ, % | 18 | 17 | 19 | 20 | 25 | 0.20 | 0.89 | |
| E/Eʹ, cm/s | 12.0±4.2 | 12.0±4.0 | 12.2±4.1 | 12.4±4.0 | 12.8±4.2 | 0.001 | 0.02 | 0.66 |
| Abnormal E/Eʹ, % | 21 | 23 | 23 | 22 | 28 | 0.08 | 0.54 | |
| LAVI, mL/m2 | 25.5±8.3 | 25.3±8.5 | 25.1±7.9 | 25.6±7.9 | 25.9±12.6 | 0.70 | 0.66 | 0.57 |
| Abnormal LAVI, % | 21 | 20 | 21 | 24 | 24 | 0.12 | 0.08 | |
| PASP, mm Hg | 27.7±5.4 | 27.7±5.2 | 28.1±5.9 | 28.6±6.0 | 28.0±5.5 | 0.007 | 0.10 | 0.49 |
| Abnormal PASP, % | 17 | 18 | 20 | 24 | 19 | 0.02 | 0.13 | |
Data are given as mean±SD, unless otherwise indicated. All reference limits were derived in the ARIC (Atherosclerosis Risk in Communities) study, as previously described. The following abnormal sex‐specific limits were used: LVEDVi, <37.5 mL/m2 (men), <30.1 mL/m2 (women); LVMI, >45 g/height2.7 (men), >41.5 g/height2.7 (women); LVEF, <59.6% (men), <60.4% (women); longitudinal strain, >−16.0% (men), >−16.0% (women); Eʹ, <4.6 cm/s (men), <4.5 cm/s (women); E/Eʹ, >13.3 cm/s (men), >15.1 cm/s (women); LAVI, >31 mL/m2 (men), >30 mL/m2 (female); PASP, 31.4 mm Hg (men), 32.5 mm Hg (women). CES‐D indicates Center for Epidemiologic Studies Depression Scale; Eʹ, septal early diastolic myocardial velocity; E/Eʹ, ratio of mitral peak velocity of early filling/early diastolic mitral annular velocity; LAVI, left atrial volume index; longitudinal strain, average peak longitudinal strain; LV, left ventricular; LVEDD, LV end‐diastolic diameter; LVEDVi; LV end‐diastolic volume indexed to body surface area; LVEF, LV ejection fraction; LVMI, LV mass index; MWT, mean wall thickness; and PASP, pulmonary artery systolic pressure.
Adjusted for demographics (age, sex, race, and field center).
Adjusted for demographics (age, sex, race, and field center), socioeconomic indicators (education and income), health behaviors (smoking, drinking, and physical activity), and prevalent chronic conditions and cardiovascular risk factors (hypertension, diabetes mellitus, prevalent atrial fibrillation, prevalent coronary heart disease, prevalent stroke, history of myocardial infarction, body mass index, and estimated glomerular filtration rate).
Discussion
Depression is increasingly recognized as an important risk factor for incident HF and for adverse outcomes among people with prevalent HF. However, little is known about the association of depressive symptoms with measures of cardiac structure and function predisposing to HF, and the extent to which depressive symptoms differentially relate to HFpEF compared with HFrEF. In this community‐based study of 5086 elderly people followed up for >5 years, we report 3 novel findings. First, when compared with HF‐free people, severe depressive symptoms were more common in people with HFpEF, but not with HFrEF. Second, greater severity of depressive symptoms was associated with incident HFpEF, but not incident HFrEF, independent of clinical cardiovascular risk factors and cardiac biomarkers. Finally, among HF‐free people, greater depression severity was associated with greater LV remodeling and worse diastolic indexes. However, these associations were largely attenuated after accounting for traditional cardiovascular risk factors associated with depression and cardiac structure and function. Associations of depressive symptoms with incident HFpEF persisted after further adjustment of these echocardiographic measures. Together, these findings suggest an important role for depression in the development of HFpEF that appears orthogonal to traditional imaging and biomarker‐based metrics of cardiac dysfunction.
Most studies of the prevalence of depression in HF included patients with HFrEF, 1 although reports also exist of heightened prevalence in HFpEF. 39 , 40 , 41 Among patients with HFpEF from the Americas enrolled in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) (trial, 27% experienced moderate‐to‐severe depressive symptoms, and both depressive symptoms at baseline and worsening in these symptoms over 12 months were associated with greater risk of mortality. 42 The results of prior studies comparing the prevalence of depression between HFrEF and HFpEF have shown conflicting results. 40 , 41 , 43 , 44 In a study of 1404 patients with HF, no significant difference in prevalence was found between HFpEF (36%), heart failure with midrange ejection fraction (32%), and HFrEF (31%), 40 although in another study of 233 patients with HF, a significant trend for higher depression prevalence was observed across HFpEF (20%), heart failure with midrange ejection fraction (33%), and HFrEF (35%) groups. 43 Two smaller studies found no significant differences in prevalence when comparing HFpEF with HFrEF (25% versus 24%, respectively, n=105 in one study; 11% versus 22%, respectively, n=202 in the other study). 41 , 44 However, these prior studies compared depression prevalence between HF phenotypes and not with HF‐free participants. Our results indicate a higher prevalence of depression in HFpEF, but not HFrEF, compared with HF‐free participants. However, the number of participants with severe depressive symptoms in the HFpEF and HFrEF groups was relatively small, which limited the power of our analysis. Therefore, these findings should be evaluated further in future studies with larger sample sizes.
Previous studies have reported a relationship between depressive symptoms and incident HF, 6 , 7 , 8 , 9 although this association has been inconsistent. 5 , 11 To our knowledge, this is the first study to report the association of depressive symptoms with incidence of HFpEF compared with HFrEF. This analysis also accounted for a greater number of potential confounders in multivariable models to mitigate the impact of residual confounding. Greater depressive symptoms among older adults free of HF were associated with the development of HFpEF, but not HFrEF, over a median of 5.5 years of follow‐up. It is possible that observed differences in the association of depression with HF in prior reports may partly relate to study differences in incidence of HFpEF versus HFrEF. 5 , 11
The mechanisms linking depression to incident HFpEF are unclear; however, both depression and HFpEF are highly comorbid. Furthermore, both have been characterized by decreased heart rate variability, 45 endothelial dysfunction, 46 , 47 and increased inflammation. 48 , 49 Notably, however, adjustment for traditional cardiovascular risk factors, echocardiographic measures, cardiac biomarkers, and hs‐CRP did not attenuate the magnitude or statistical significance of the association of depressive symptoms with incident HFpEF in our study. This suggests that depression influences HFpEF risk through pathways orthogonal to impairments in cardiac performance traditionally associated with HFpEF. Our findings are particularly important as HFpEF incidence and prevalence are greatest in late life. Although the prevalence of clinical depression decreases with advancing age, 50 depression screening scales are more likely to identify depression in older adults, and these scales have comparable clinical impact as a clinical depression diagnosis with respect to healthcare use. 51 Our findings therefore provide a rationale for further investigation of potential mechanisms by which depression contributes to HFpEF.
Previous studies have demonstrated an association of depressive symptoms with echocardiographic measures of diastolic dysfunction (eʹ and E/eʹ) 12 , 13 and LV structural remodeling (LV mass index). 13 In our study, worse depressive symptoms were also associated with worse diastolic indexes (eʹ, E/eʹ, and pulmonary artery systolic pressure) and LV structural remodeling (LV mass index and mean wall thickness), although these associations were largely accounted for by participant demographics and common cardiovascular comorbidities that were also more frequent with greater depressive symptoms. Given the cross‐sectional nature of this analysis, future studies assessing the association between depressive symptoms and longitudinal changes in cardiac structure and diastolic function will provide additional insights into the temporal relationship of cardiac dysfunction relative to depressive symptoms. However, the present analysis is most notable for the robust association of depressive symptoms with risk of incident HFpEF, despite only modest independent relationships with measures of cardiac structure and function, cardiac biomarkers, and inflammation.
Important strengths of this analysis included the comprehensive longitudinal phenotyping of participants in the ARIC study, which, together with the relatively large sample size, allowed for robust covariate adjustment, including cardiovascular risk factors, socioeconomic status, lifestyle factors, cognition, and markers of systemic inflammation. Furthermore, use of adjudicated incident HF events increased confidence in the validity of the HFpEF diagnosis. Nevertheless, this analysis has important limitations. Similar to previous studies, the measurement of depressive symptoms relied solely on self‐report and not psychiatric documentation, which potentially could lead to misclassification. Furthermore, reliance of self‐report for certain covariates, such as smoking status, may have resulted misclassification.
The absence of repeated measures of depression or information on history of depression precluded assessment of the role of depression duration in the assessed associations. Given the relatively short follow‐up time for incident HF events, reverse causality cannot entirely be excluded. However, excluding events that occurred during the first year of follow‐up did not appreciably alter the results of the primary analysis. In addition, 5‐year follow‐up is particularly clinically relevant when assessing HF risk in late life. A relatively small number of incident HFpEF and HFrEF events may have resulted in overfitting of fully adjusted Cox proportional hazard models for time‐to‐event analyses. However, supplemental analyses using more parsimonious models demonstrated similar results (Tables S5 and S6). Finally, as our primary interest was the prevalence, echocardiographic correlates, and prognostic relevance of depressive symptoms in late life, we do not view death before visit 5 as a limitation of this analysis. However, only a subset of ARIC study participants alive at visit 5 chose to attend. Therefore, attendance bias may limit the generalizability of our findings to older individuals in the community more broadly, although results of analyses incorporating inverse probability of attrition weighting demonstrated similar findings to our primary analysis (Figure S2).
Conclusions
Greater depressive symptoms in late life are associated with heightened risk of incident HFpEF. This association is independent of cardiovascular comorbidities, echocardiographic measures of cardiac structure and function, and circulating cardiac biomarkers. Future prospective studies are necessary to better define the mechanisms linking depressive symptoms in older adults to development of HFpEF.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, Department of Health and Human Services, under contract Nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. The work for this article was also supported by NHLBI grants R01HL135008, R01HL143224, R01HL150342, R01HL14818, and K24HL152008 (Dr Shah).
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S2
Acknowledgments
The authors wish to thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contributions.
Supplementary materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020094
For Sources of Funding and Disclosures, see page 10.
References
- 1. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. DOI: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 2. Jiang W, Kuchibhatla M, Cuffe MS, Christopher EJ, Alexander JD, Clary GL, Blazing MA, Gaulden LH, Califf RM, Krishnan RR, et al. Prognostic value of anxiety and depression in patients with chronic heart failure. J Am Coll Cardiol. 2004;110:3452–3456. DOI: 10.1161/01.CIR.0000148138.25157.F9. [DOI] [PubMed] [Google Scholar]
- 3. Mentz RJ, Babyak MA, Bittner V, Fleg JL, Keteyian SJ, Swank AM, Piña IL, Kraus WE, Whellan DJ, O'Connor CM, et al. Prognostic significance of depression in African Americans with heart failure: insights from HF‐ACTION. Circ Heart Fail. 2015;8:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gustad LT, Laugsand LE, Janszky I, Dalen H, Bjerkeset O. Symptoms of anxiety and depression and risk of heart failure: the HUNT Study. Eur J Heart Fail. 2014;16:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Den Broek KC, Defilippi CR, Christenson RH, Seliger SL, Gottdiener JS, Kop WJ. Predictive value of depressive symptoms and B‐type natriuretic peptide for new‐onset heart failure and mortality. Am J Cardiol. 2011;107:723–729. DOI: 10.1016/j.amjcard.2010.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community‐based study. Psychosom Med. 2002;64:6–12. DOI: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 7. Kamphuis MH, Kalmijn S, Tijhuis MAR, Geerlings MI, Giampaoli S, Nissinen A, Grobbee DE, Kromhout D. Depressive symptoms as risk factor of cardiovascular mortality in older European men: the Finland, Italy and Netherlands Elderly (FINE) study. Eur J Cardiovasc Prev Rehabil. 2006;13:199–206. DOI: 10.1097/01.hjr.0000188242.64590.92. [DOI] [PubMed] [Google Scholar]
- 8. Garfield LD, Scherrer JF, Hauptman PJ, Freedland KE, Chrusciel T, Balasubramanian S, Carney RM, Newcomer JW, Owen R, Bucholz KK, et al. Association of anxiety disorders and depression with incident heart failure. Psychosom Med. 2014;76:128–136. DOI: 10.1097/PSY.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161:1725–1730. DOI: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 10. May HT, Horne BD, Carlquist JF, Sheng X, Joy E, Catinella AP. Depression after coronary artery disease is associated with heart failure. J Am Coll Cardiol. 2009;53:1440–1447. DOI: 10.1016/j.jacc.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 11. Ogilvie RP, Everson‐Rose SA, Longstreth WT, Rodriguez CJ, Diez‐Roux AV, Lutsey PL. Psychosocial factors and risk of incident heart failure: the Multi‐Ethnic study of atherosclerosis. Circ Heart Fail. 2016;9:e002243. DOI: 10.1161/CIRCHEARTFAILURE.115.002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gustad LT, Bjerkeset O, Strand LB, Janszky I, Salvesen Ø, Dalen H. Cardiac function associated with previous, current and repeated depression and anxiety symptoms in a healthy population: the HUNT study. Open Heart. 2016;3:e000363. DOI: 10.1136/openhrt-2015-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YH, Kim SH, Lim SY, Cho GY, Baik IK, Lim HE, Na JO, Han SW, Ko YH, Shin C. Relationship between depression and subclinical left ventricular changes in the general population. Heart. 2012;98:1378–1383. DOI: 10.1136/heartjnl-2012-302180. [DOI] [PubMed] [Google Scholar]
- 14. Investigators . The Atherosclerosis Risk in Communities study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. DOI: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 16. Lyness J, Noel TK, Caine ED, Cox C, Deborah A. Screening for depression in elderly primary care patients. Arch Intern Med. 1997;157:449–454. DOI: 10.1001/archinte.1997.00440250107012. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter J, Andrykowski M, Wilson J, Hall L, Kay M, Sachs B, Cunningham L. Psychometrics for two short forms of the center for epidemiologic studies. Issues Ment Health Nurs. 1998;19:481–494. [DOI] [PubMed] [Google Scholar]
- 18. Kohout FJ, Berkman LF, Evans DA, Cornoni‐Huntley J. Two shorter forms of the CES‐D depression symptoms index. J Aging Health. 1993;5:179–193. DOI: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 19. Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community‐dwelling elderly persons. Circ Cardiovasc Imaging. 2014;7:173–181. DOI: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 21. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pelikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. DOI: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 22. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. DOI: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23. Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle‐aged women and men. Med Sci Sport Exerc. 1997;29:901–909. DOI: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 24. Hegde SM, Gonc A, Claggett B, Evenson KR, Cheng S, Shah AM, Folsom AR, Solomon SD. Cardiac structure and function and leisure‐time physical activity in the elderly: the Atherosclerosis Risk in Communities Study. Eur Heart J. 2016;37:2544–2551. DOI: 10.1093/eurheartj/ehw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Misialek JR, Rose KM, Everson‐Rose SA, Soliman EZ, Clark CJ, Lopez FL, Alonso A. Socioeconomic status and the incidence of atrial fibrillation in whites and blacks: the Atherosclerosis Risk in Communities (ARIC) Study. J Am Heart Assoc. 2014;3:e001159. DOI: 10.1161/JAHA.114.001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA; The ARIC Investigators . Community surveillance of coronary heart disease in the atherosclerosis risk in communities (ARIC) study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. DOI: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2012;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider ALC, Hengrui S, Alonso A, Coresh J, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement. 2016;2:1–11. DOI: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. DOI: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teramoto K, Santos M, Claggett B, John JE, Solomon SD, Kitzman D, Folsom AR, Cushman M, Matsushita K, Skali H, et al. Pulmonary vascular dysfunction among people aged over 65 years in the community in the Atherosclerosis Risk in Communities (ARIC) study: a cross‐sectional analysis. PLoS Med. 2020;17:e1003361. DOI: 10.1371/journal.pmed.1003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reimer Jensen AM, Zierath R, Claggett B, Skali H, Solomon SD, Matsushita K, Konety S, Butler K, Kitzman DW, Biering‐Sørensen T, et al. The association of left ventricular systolic function with incident heart failure in late life: the Atherosclerosis Risk in Communities (ARIC) study. JAMA Cardiol. 2021;6:509–520. DOI: 10.1001/jamacardio.2021.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, et al. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135:224–240. DOI: 10.1161/CIRCULATIONAHA.116.023361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. DOI: 10.1093/biomet/69.1.239. [DOI] [Google Scholar]
- 34. Haigh EAP, Bogucki OE, Sigmon ST, Blazer DG. Depression among older adults: a 20‐year update on five common myths and misconceptions. Am J Geriatr Psychiatry. 2018;26:107–122. DOI: 10.1016/j.jagp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 35. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease. Arch Intern Med. 2005;165:2508–2513. DOI: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) study. Circ Heart Fail. 2012;5:422–429. DOI: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weuve J, Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, Leon CFM. Accounting for bias due to selective attrition. Epidemiology. 2012;23:119–128. DOI: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen‐Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow‐up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. DOI: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamo CE, Heitner JF, Pfeffer MA, Kim H‐Y, Kenwood CT, Assmann SF, Solomon SD, Boineau R, Fleg JL, Spertus JA, et al. Baseline distribution of participants with depression and impaired quality of life in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Hear Fail. 2015;8:268–277. DOI: 10.1161/CIRCHEARTFAILURE.114.001838. [DOI] [PubMed] [Google Scholar]
- 40. Gastelurrutia P, Lupón J, Moliner P, Yang X, Cediel G, de Antonio M, Domingo M, Altimir S, González B, Rodríguez M, et al. Comorbidities, fragility, and quality of life in heart failure patients with midrange ejection fraction. Mayo Clin Proc Innov Qual Outcomes. 2018;2:176–185. DOI: 10.1016/j.mayocpiqo.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kato N, Kinugawa K, Shiga T, Hatano M, Takeda N, Imai Y, Watanabe M, Yao A, Hirata Y, Kazuma K, et al. Depressive symptoms are common and associated with adverse clinical outcomes in heart failure with reduced and preserved ejection fraction. J Cardiol. 2012;60:23–30. DOI: 10.1016/j.jjcc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 42. Chandra A, Alcala MAD, Claggett B, Desai AS, Fang JC, Heitner JF, Liu J, Pitt B, Solomon SD, Pfeffer MA, et al. Association between depressive symptoms and HFpEF‐related outcomes. J Am Coll Cardiol Heart Fail. 2020;8:1009–1020. [DOI] [PubMed] [Google Scholar]
- 43. Xu SD, Su GH, Lu YX, Shuai XX, Tao XF, Meng YD, Luo P. Elevated soluble ST2 and depression increased the risk of all‐cause mortality and hospitalization in patients with heart failure. Int Heart J. 2014;55:445–450. DOI: 10.1536/ihj.13-371. [DOI] [PubMed] [Google Scholar]
- 44. Warraich HJ, Kitzman DW, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Reeves GR. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail. 2018;11:e005254. DOI: 10.1161/CIRCHEARTFAILURE.118.005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim CK, McGorray SP, Bartholomew BA, Marsh M, Dicken T, Wassertheil‐Smoller S, Curb JD, Oberman A, Hsia J, Gardin J, et al. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005;165:1239–1244. DOI: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 46. Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. DOI: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–659. DOI: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 48. Tromp J, Westenbrink BD, Ouwerkerk W, Veldhuisen DJV, Samani NJ, Ponikowski P, Metra M, Anker SD, Clelang JG, Dickstein K. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2018;72:1081–1090. [DOI] [PubMed] [Google Scholar]
- 49. Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. DOI: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 50. Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Physchol. 2009;5:363–389. DOI: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fulop G, Strain JJ, Stettin G. Congestive heart failure and depression in older adults: clinical course and health services use 6 months after hospitalization. Psychosomatics. 2003;44:367–373. DOI: 10.1176/appi.psy.44.5.367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S2


