Abstract
Background
Habitual intake of long‐chain omega‐3 fatty acids, especially eicosapentaenoic and docosahexaenoic acid (EPA+DHA) from fish, has been associated with a lower risk of fatal coronary heart disease (CHD) in population‐based studies. Whether that is also the case for patients with CHD is not yet clear. We studied the associations of dietary and circulating EPA+DHA and alpha‐linolenic acid, a plant‐derived omega‐3 fatty acids, with long‐term mortality risk after myocardial infarction.
Methods and Results
We analyzed data from 4067 Dutch patients with prior myocardial infarction aged 60 to 80 years (79% men, 86% on statins) enrolled in the Alpha Omega Cohort from 2002 to 2006 (baseline) and followed through 2018. Baseline intake of fish and omega‐3 fatty acids were assessed through a validated 203‐item food frequency questionnaire and circulating omega‐3 fatty acids were assessed in plasma cholesteryl esters. Hazard ratios (HRs) with 95% CIs were obtained from Cox regression analyses. During a median follow‐up period of 12 years, 1877 deaths occurred, of which 515 were from CHD and 834 from cardiovascular diseases. Dietary intake of EPA+DHA was significantly inversely associated with only CHD mortality (HR, 0.69 [0.52–0.90] for >200 versus ≤50 mg/d; HR, 0.92 [0.86–0.98] per 100 mg/d). Similar results were obtained for fish consumption (HRCHD, 0.74 [0.53–1.03] for >40 versus ≤5 g/d; P trend: 0.031). Circulating EPA+DHA was inversely associated with CHD mortality (HR, 0.71 [0.53–0.94] for >2.52% versus ≤1.29%; 0.85 [0.77–0.95] per 1‐SD) and also with cardiovascular diseases and all‐cause mortality. Dietary and circulating alpha‐linolenic acid were not significantly associated with mortality end points.
Conclusions
In a cohort of Dutch patients with prior myocardial infarction, higher dietary and circulating EPA+DHA and fish intake were consistently associated with a lower CHD mortality risk.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03192410.
Keywords: coronary heart disease, mortality, myocardial infarction, omega‐3 fatty acids, plasma fatty acids, prospective cohort study
Subject Categories: Secondary Prevention, Diet and Nutrition, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- ALA
alpha‐linolenic acid
- DHA
docosahexaenoic acid
- DHD‐15
2015 Dutch Healthy Diet score
- EPA
eicosapentaenoic acid
- FFQ
food frequency questionnaire
Clinical Perspective
What Is New?
Fish intake, eicosapentaenoic and docosahexaenoic acid intake and plasma levels of eicosapentaenoic and docosahexaenoic acid were inversely associated with long‐term mortality risk, especially from coronary heart disease, in Dutch post‐myocardial infarction patients.
What Are the Clinical Implications?
Low intakes of eicosapentaenoic and docosahexaenoic acid, which can be obtained from 1 or 2 weekly servings of fish, may reduce mortality risk in patients who suffered a myocardial infarction.
Increasing the intake of long‐chain omega‐3 fatty acids (n‐3 FAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) is often recommended for the prevention of cardiovascular disease (CVD). 1 , 2 , 3 EPA and DHA can be obtained by consuming oily fish and other seafood. Alpha‐linolenic acid (ALA) is the parent n‐3 FA derived from plant foods, such as flaxseed, nuts, and vegetable oils. EPA (and DHA) may be formed from ALA, but conversion in the human body is probably <10%, as indicated by stable‐isotope studies. 4 , 5 Dietary intake of EPA+DHA in Western populations is generally <500 mg/d, 6 and ALA intake ≈1.5 g/d (<1 energy percent). Concentrations of n‐3 FAs in blood are biomarkers of essential FA intake, especially for EPA+DHA. 1
Most observational studies on n‐3 FAs and mortality have focused on healthy populations. 7 , 8 A recent pooled analysis including 42 466 individuals from 17 prospective cohorts has shown that higher levels of circulating EPA+DHA but not ALA were associated with an ≈20% lower risk for CVD mortality. 8 However, the relationship between n‐3 FAs and mortality is not yet clear in patients with coronary heart disease (CHD). In the Alpha Omega Cohort of Dutch patients who suffered a myocardial infarction (MI) 9 , 10 we examined associations of dietary intake of EPA+DHA, fish, and ALA as well as circulating levels of n‐3 FAs, with fatal CHD (primary outcome), CVD and all‐cause mortality during >12 years follow‐up.
Methods
The data that support the findings of this study are available from the principal investigator (J.M.G.) upon request.
Study Population
The Alpha Omega Cohort comprised 4837 Dutch men and women aged 60 to 80 years with a clinically diagnosed MI up to 10 years before entering the study. Patients were examined by trained research nurses at baseline (2002–2006) and were followed for cause‐specific mortality since study enrollment. During the initial 40 months of follow‐up (Alpha Omega Trial phase), patients received low‐dose supplementation of ALA, EPA+DHA, ALA plus EPA+DHA or placebo, 9 which did not prevent major recurrent CVD events. 10 Medical‐ethical approval was obtained and all patients provided written informed consent. 9 Patients with missing data on circulating n‐3 FAs or >5% unknown FAs were excluded (Figure S1). Patients with missing dietary data or implausible energy intakes (<800 or >8000 kcal for men; <600 or >6000 kcal for women) and extreme unsaturated FA intakes (<2.5th or >97.5th percentile) were further excluded, as described previously, 11 leaving 4067 patients for analysis.
Dietary Assessment
Diet was assessed at baseline using a 203‐item semi‐quantitative Food Frequency Questionnaire (FFQ), an extended version of a biomarker‐validated and reproducible FFQ. 12 , 13 Food intake was assessed over the past month, including preparation methods and brands of foods. Returned FFQs were checked by trained dietitians by telephone to get information on missing or unclear answers from patients, using a predefined protocol. Intakes of total energy and nutrients, including EPA, DHA, and ALA, were calculated through linkage with the Dutch Food Composition Database 2006 (Nederlands Voedingsstoffenbestand [NEVO] 2006). 14 The 2015 Dutch Healthy Diet score was calculated for adherence to dietary guidelines (DHD‐15; scale from zero to maximal adherence [0–150]). 15
Laboratory Measurements
Blood samples (fasted ≥8 hours for 34% of the patients) were collected by trained research nurses to measure serum total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, and plasma glucose using standard laboratory methods. 9 Blood for FAs analysis was collected in EDTA‐treated tubes, and stored in −80 °C until further analysis. Circulating FAs were measured as described previously. 16 Briefly, plasma cholesteryl esters FAs were separated from total lipids, trans‐esterified into FA methyl esters and measured by gas chromatography equipped with a flame‐ionization detector. A total of 38 FAs were quantified and expressed as weight percentage of total FAs (% total FA). For ALA and EPA, within‐ and between‐run coefficients of variation were <5% and for DHA <8%. 16 Correlations between dietary and circulating FAs (in plasma cholesteryl esters) were 0.39 and 0.45 for EPA and DHA, respectively, and −0.02 for ALA. 16
End Points
This study focused on mortality with CHD mortality as the primary outcome. The vital status of the patients was monitored through linkage with municipal registries from baseline through December 31, 2018. From 2002 to 2009 the Alpha Omega Trial period had detailed information on primary and contributing causes of death, 9 , 10 including the national mortality registry of Statistics Netherlands (CBS [Centraal Bureau voor de Statistiek]). Primary and contributing death causes data after the trial up to 2012 were obtained from CBS, however from 2013 to 2018, CBS provided only data on the primary cause of death. Treating physicians were asked to provide additional information on cause of death, which was coded by study physicians not involved in the present analysis. Mortality coding was performed according to International Classification of Diseases, Tenth Revision (ICD‐10). CHD mortality comprised ICD‐10 codes I20‐I25 (ischemic heart disease), I46 (cardiac arrest) and R96 (sudden death, undefined). CVD mortality comprised CHD mortality, I50 (heart failure), and I60‐I69 (stroke). Person‐years were calculated from study enrollment to date of death or December 31, 2018, whichever came first; 1 patient was lost to follow‐up and censored after 2.9 years.
Assessment of Covariables
Information on demographic, lifestyle, medical history, and medication use was collected using questionnaires, as described elsewhere. 9 The highest attained education level was categorized as primary, lower secondary, higher secondary or lower tertiary, and higher tertiary education. Smoking status was categorized as never, former, or current smoker. Physical activity was classified as low (no or only light activity, ≤3 metabolic equivalents [METs]), moderate (>0 to <5 days/week of moderate or vigorous activity, >3 METs) or high (≥5 days/week of moderate or vigorous activity). Medication use was coded according to Anatomical Therapeutic Chemical classification system, 17 with codes C10AA and C10B for statins, B01 for anti‐thrombotic drugs and C02, C03, C07, C08, and C09 for antihypertensive drugs. Physical examination was performed by trained research nurses, in the hospital or the patient’s home. 9 Body weight (kg) and height (m) were assessed, from which body mass index was calculated (kg/m2). Obesity was defined as body mass index ≥30 kg/m2. Systolic and diastolic blood pressures were measured twice in sitting position with an automated device, and values were averaged. Diabetes was considered present if patients reported a diagnosis of diabetes by a physician or the use of antidiabetic drugs, or when they had a fasting plasma glucose ≥7.0 mmol/L or non‐fasting value of ≥11.1 mmol/L. Family history of MI was defined as having any parent with MI before the age of 60 years and family history of diabetes was defined as having any parent with diabetes. Alcohol intake (ethanol in g/d) was calculated from the FFQ and categorized into no (0 g/d), low (>0–10 g/d), moderate (>10–30 g/d for men or >10–20 g/d for women) or high (>30 g/d for men or >20 g/d for women).
Statistical Analysis
Baseline characteristics of patients were presented as mean (±SD) or median and interquartile range for continuous variables or percentages for categorical variables. Intakes of FAs were energy‐adjusted using the residual method by Willett et al 18 or expressed as percentage of total energy intake (en%), where appropriate. Dietary n‐3 FAs and energy‐adjusted fish consumption were categorized in 4 classes. Circulating EPA+DHA and ALA were analyzed in quintiles. For circulating EPA+DHA, additional categories were based on the cut‐offs of erythrocytes from the omega‐3 index, 19 converted to cut‐off values of plasma cholesteryl esters by Stark et al 20 Omega‐3 index cut‐off values of 4% and 8% would correspond to 1.3% and 3.1% in plasma cholesteryl esters, respectively. Continuous associations were estimated per 100 mg/d for dietary EPA+DHA or 1 g/d for dietary ALA, and for circulating EPA+DHA and ALA per 1‐SD increment.
Age‐ and sex‐adjusted hazard ratios (HRs) with 95% CIs for fatal end points were obtained from Cox models, and proportional hazards assumptions were met, based on Schoenfeld residuals. In multivariable Cox models, HRs for dietary intake of n‐3 FAs were adjusted for age, sex, education level (4 categories), physical activity (3 categories), smoking status (3 categories), alcohol intake (4 categories), obesity (no/yes), prevalent diabetes (no/yes), cardiovascular medication use (statins, antihypertensive drugs, antithrombotic drugs, as separate dummies), time since MI (y), and intake of total energy intake (kcal/d), dietary cholesterol (mg/d), fiber (g/d) and trans FAs (g/d).
Multivariable Cox models for circulating n‐3 FAs included age, sex, education level, physical activity, smoking status, alcohol intake, obesity, prevalent diabetes, cardiovascular medication use, time since MI, and serum total cholesterol (mmol/L). In addition, circulating linoleic acid (18:2n‐6; % total FAs) and arachidonic acid (20:4n‐6; % total FAs) were added to the multivariable model. 21 , 22 Covariates with missing values (<5%) were imputed by the sex‐specific median (continuous variables) or mode (categorical variables) to retain patients in multivariable models.
Linear trends were assessed by entering median values for fish intake, dietary n‐3 FAs and circulating n‐3 FAs within the defined categories as continuous variables into the multivariable models. Potential non‐linear associations for dietary and circulating n‐3 FAs were explored using restricted cubic spline analysis with 5 knots located at 5th, 27.5th, 50th, 72.5th, and 95th percentiles. 23 , 24
Stratified analyses were performed for dietary and circulating n‐3 FAs in subgroups of age (<65 versus ≥65 years), sex, prevalent diabetes (no versus yes), obesity (no versus yes), statin use (no versus yes), diet quality (<median versus ≥median) and time since MI (<1 versus ≥1 year). We also stratified for treatment group of the Alpha Omega Trial during the first 40 months of follow‐up (“EPA‐DHA”, “ALA”, “EPA‐DHA plus ALA”, or “any omega‐3 treatment” versus “placebo”). Circulating n‐3 FAs were additionally studied in strata of circulating LA and arachidonic acid (<median versus. ≥median). P values for interaction were obtained by entering product terms for n‐3 FAs and stratification variables into the multivariable models.
Patients with deteriorating health or prescribed diets may have recently changed their dietary intakes and/or lifestyle habits. In sensitivity analyses for dietary and circulating n‐3 FAs, we excluded 146 deaths during the first 2 years of follow‐up to examine reverse causation bias, also subtracting 2 years of follow‐up time for the remainder of the cohort. We also excluded 187 patients who reported the use of fish oil supplements at baseline, because supplemental intake has a large impact on circulating EPA+DHA and we were primarily interested in amounts of EPA+DHA that can be obtained through the diet.
A 2‐sided P‐value <0.05 was considered statistically significant. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
At baseline, patients were on average aged 69 years, 79% were men, 17% were current smokers, and 20% had diabetes (Table 1). Patients had an MI ≈4 year before study enrollment and most patients were treated with cardiovascular medication, such as statins (86%). During a median follow‐up period of 12.4 years (total of 45 229 person‐years), 1877 patients died, including 515 CHD deaths (11.4 per 1000 person‐years) and 834 CVD deaths (18.4 per 1000 person‐years). The median consumption of fish was 14 g/d and that of oily fish 5 g/d. Median EPA+DHA intake was 108 (interquartile range, 46–187) mg/d, with ≈20% consumed >200 mg/d. Mean ALA intake was 1.09 (SD, 0.50) g/d, or 0.5% of energy (en%). EPA+DHA were mainly from fish (89%), while ALA was mainly obtained from cooking oils (26%), grain products (19%), and soft margarine (14%) (Table S1). The median DHD‐15 score for adherence to dietary guidelines was 79 (range, 33–125).
Table 1.
Baseline Characteristics of Population for Analysis and Across Circulating EPA+DHA Quintiles
|
Total population (n=4067) |
Quintiles of circulating EPA+DHA | P value* | |||
|---|---|---|---|---|---|
| Q1 (n=818) | Q3 (n=813) | Q5 (n=815) | |||
| Age, y | 69.0±5.6 | 69.3±5.6 | 68.9±5.6 | 69.1±5.6 | 0.93 |
| Men | 3221 (79.2) | 687 (84.0) | 612 (75.3) | 627 (76.9) | <0.001 |
| Body mass index, kg/m2 † | 27.7±3.8 | 27.3±3.6 | 27.9±4.0 | 27.9±3.9 | 0.014 |
| Obese (≥30 kg/m2) | 953 (23.4) | 169 (20.7) | 204 (25.1) | 196 (24.1) | 0.21 |
| Time since MI, y † | 3.7 (1.7–6.3) | 4.2 (1.9–6.6) | 3.3 (1.4–5.8) | 3.6 (1.5–6.2) | 0.13 |
| Smoking status † | 0.22 | ||||
| Never | 663 (16.3) | 116 (14.2) | 145 (17.8) | 150 (18.4) | |
| Former | 2730 (67.1) | 559 (68.3) | 539 (66.3) | 534 (65.5) | |
| Current | 673 (16.6) | 143 (17.5) | 129 (15.9) | 131 (16.1) | |
| Physical activity † | <0.001 | ||||
| Low | 1652 (40.8) | 359 (44.2) | 346 (42.7) | 302 (37.3) | |
| Middle | 1528 (37.8) | 272 (33.5) | 295 (36.4) | 335 (41.4) | |
| High | 865 (21.4) | 182 (22.4) | 170 (21.0) | 173 (21.4) | |
| Highest level of education † | 0.002 | ||||
| Primary | 803 (19.8) | 183 (22.5) | 159 (19.7) | 139 (17.1) | |
| Lower secondary | 1462 (36.1) | 310 (38.1) | 297 (36.8) | 296 (36.5) | |
| Higher secondary or lower tertiary | 1275 (31.5) | 242 (29.8) | 257 (31.8) | 262 (32.3) | |
| Higher tertiary | 506 (12.5) | 78 (9.6) | 95 (11.8) | 114 (14.1) | |
| Alcohol intake | <0.001 | ||||
| No | 203 (5.0) | 50 (6.1) | 42 (5.2) | 46 (5.6) | |
| Low | 2155 (53.0) | 489 (59.8) | 417 (51.3) | 385 (47.2) | |
| Moderate | 1067 (26.2) | 195 (23.8) | 242 (29.8) | 216 (26.5) | |
| High | 642 (15.8) | 84 (10.3) | 112 (13.8) | 168 (20.6) | |
| Medication use | |||||
| Statins | 3494 (85.9) | 618 (75.6) | 737 (90.7) | 728 (89.3) | <0.001 |
| Antithrombotic drugs | 3978 (97.8) | 795 (97.2) | 801 (98.5) | 790 (96.9) | 0.08 |
| Antihypertensive drugs | 3650 (89.8) | 719 (87.9) | 729 (89.7) | 727 (89.2) | 0.19 |
| Serum lipids, mmol/L ‡ , § | |||||
| Total cholesterol | 4.71±0.95 | 4.72±0.98 | 4.63±0.87 | 4.75±0.96 | 0.21 |
| LDL cholesterol | 2.57±0.81 | 2.61±0.85 | 2.49±0.74 | 2.62±0.84 | 0.21 |
| HDL cholesterol | 1.29±0.34 | 1.24±0.33 | 1.27±0.34 | 1.35±0.35 | <0.001 |
| Triglycerides | 1.65 (1.21–2.31) | 1.68 (1.19–2.37) | 1.68 (1.21–2.40) | 1.51 (1.18–2.10) | <0.001 |
| Plasma glucose, mmol/L ‡ | 5.61 (5.05–6.59) | 5.57 (4.98–6.48) | 5.72 (5.08–6.85) | 5.62 (5.10–6.45) | 0.64 |
| Blood pressure (mm Hg) † | |||||
| Systolic | 142±22 | 142±22 | 143±21 | 142±22 | 0.67 |
| Diastolic | 80±11 | 81±11 | 80±11 | 80±11 | 0.41 |
| Prevalent diabetes | 813 (20.0) | 154 (18.8) | 186 (22.9) | 155 (19.0) | 0.08 |
| Family history of MI | 467 (11.5) | 95 (11.6) | 84 (10.3) | 104 (12.8) | 0.55 |
| Family history of diabetes | 834 (20.5) | 176 (21.5) | 180 (22.1) | 159 (19.5) | 0.07 |
| Dietary factors | |||||
| Energy, kcal/d | 1921±518 | 1957±517 | 1865±496 | 1761±485 | <0.001 |
| Protein, en% | 15.0±2.8 | 14.6±2.9 | 15.0±2.8 | 16.4±2.9 | <0.001 |
| Total fat, en% | 33.8±6.2 | 34.7±6.1 | 33.9±6.2 | 34.6±6.2 | <0.001 |
| SFAs, en% | 12.5±3.1 | 12.9±3.0 | 12.6±3.1 | 12.6±3.1 | <0.001 |
| cis MUFAs, en% | 9.5±2.2 | 9.6±2.3 | 9.4±2.2 | 10.0±2.3 | 0.20 |
| PUFAs, en% | 7.2±2.2 | 7.5±2.3 | 7.3±2.3 | 7.4±2.3 | <0.001 |
| Total n‐3 FAs, en% | 0.71±0.25 | 0.69±0.26 | 0.71±0.25 | 0.75±0.26 | <0.001 |
| ALA, g/d | 1.09±0.50 | 1.10±0.51 | 1.13±0.52 | 1.06±0.46 | 0.019 |
| EPA+DHA, mg/day | 108 (46–187) | 50 (21–104) | 104 (52–169) | 189 (114–357) | <0.001 |
| Total n‐6 FAs, en% | 5.5±2.1 | 6.0±2.2 | 5.7±2.2 | 5.2±2.0 | <0.001 |
| trans FAs, g/d || | 1.6±0.6 | 1.6±0.6 | 1.6±0.6 | 1.4±0.6 | <0.001 |
| Carbohydrates, en% | 46.7±6.8 | 47.2±6.8 | 46.6±6.7 | 46.3±6.8 | 0.002 |
| Fiber, g/d || | 21.5±6.8 | 22.2±7.1 | 21.2±6.4 | 21.3±6.6 | 0.026 |
| Cholesterol, mg/d || | 184±69 | 178±68 | 183±64 | 187±73 | 0.016 |
| Total fish (g/d) | 14 (5–20) | 7 (1–15) | 13 (6–19) | 18 (13–40) | <0.001 |
| Oily fish (g/d) | 5 (1–11) | 1 (0–5) | 5 (2–10) | 11 (6–22) | <0.001 |
| Diet quality score (DHD‐15) | 79.2±13.6 | 78.0±13.6 | 78.2±13.0 | 81.9±14.1 | <0.001 |
| Circulating FAs, % total FAs | |||||
| SFAs | 13.1±1.1 | 12.7±1.3 | 13.2±1.0 | 13.5±1.0 | <0.001 |
| MUFAs | 22.5±3.2 | 21.2±3.0 | 23.0±3.2 | 23.0±3.2 | <0.001 |
| PUFAs | 63.0±4.0 | 64.8±3.9 | 62.4±4.0 | 62.1±3.9 | <0.001 |
| Total n‐3 PUFAs | 2.35 (1.94–2.98) | 1.65 (1.51–1.82) | 2.32 (2.19–2.46) | 3.80 (3.41–4.45) | <0.001 |
| ALA, 18:3n‐3 | 0.51±0.14 | 0.48±0.15 | 0.51±0.14 | 0.52±0.15 | <0.001 |
| EPA, 20:5n‐3 | 1.06 (0.79–1.52) | 0.61 (0.51–0.70) | 1.05 (0.97–1.16) | 2.21 (1.88–2.73) | <0.001 |
| DHA, 22:6n‐3 | 0.66 (0.53–0.84) | 0.47 (0.40–0.55) | 0.67 (0.58–0.75) | 0.96 (0.87–1.10) | <0.001 |
| Total n‐6 PUFAs | 60.2±4.4 | 63.0±4.0 | 59.9±4.0 | 57.8±4.2 | <0.001 |
| Linoleic acid, 18:2n‐6 | 50.0±5.0 | 53.5±4.5 | 49.1±4.6 | 47.8±4.6 | <0.001 |
| Arachidonic acid, 20:4n‐6 | 8.4±2.0 | 7.8±2.1 | 8.8±2.0 | 8.2±1.9 | 0.07 |
Values are shown as mean±SD, median (interquartile range), or n (%) unless stated otherwise. ALA indicates alpha‐linolenic acid; DHA, docosahexaenoic acid; DHD‐15, 2015 Dutch Healthy Diet score; EPA, eicosapentaenoic acid; FAs, fatty acids; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids; Q1, quintile 1; Q3, quintile 3; Q5, quintile 5; and SFAs, saturated fatty acids.
P value for linear trend, through median values across categories of intake using a linear regression model or obtained from Chi‐square test for categorical variables.
<1% of patients had missing values for body mass index, time since myocardial infarction, smoking status, physical activity, education level, and blood pressure.
Part of the cohort had missing values for total cholesterol, high‐density lipoprotein cholesterol and triglycerides (n=61), low‐density lipoprotein cholesterol (n=252), and plasma glucose (n=33).
To convert to mg/dL, divide by 0.02586 for total, low‐density lipoprotein, high‐density lipoprotein cholesterol and by 0.01129 for triglycerides.
Values for dietary trans‐fatty acid (TFAs), fiber, and cholesterol were non‐energy adjusted.
Dietary n‐3 FAs and Fish Intake
Patients with higher dietary EPA+DHA intake were more likely to be physically active, had higher alcohol intake, higher high‐density lipoprotein cholesterol and slightly lower non‐fasting serum triglyceride levels (Table S2), but this was not seen for ALA intake (Table S3). Patients with higher EPA+DHA and ALA intakes had higher DHD‐15 scores. The risk of CHD mortality was significantly lower for EPA+DHA intakes >200 mg/d (HR, 0.69; 95% CI, 0.52–0.90) as compared with intakes ≤50 mg/d (Table 2). Figure 1 shows the continuous association for dietary EPA+DHA with CHD mortality (HR per 100 mg/d: 0.92, 0.86–0.98; P non‐linearity=0.75). Inverse trends of EPA+DHA intake with CVD and all‐cause mortality were not statistically significant.
Table 2.
Associations of Dietary EPA+DHA and Total Fish Intakes With CHD, CVD, and All‐Cause Mortality in the Alpha Omega Cohort
| Dietary EPA+DHA intake, adjusted for energy | P trend* | ||||
|---|---|---|---|---|---|
| ≤50 mg/d (n=1113) | >50 to 100 mg/d (n=815) | >100 to 200 mg/d (n=1234) | >200 mg/d (n=905) | ||
| Median dietary EPA+DHA, mg/d | 26 | 75 | 141 | 339 | |
| Person‐years | 12 065 | 9069 | 13 826 | 10 269 | |
| CHD mortality | |||||
| Cases, n | 167 | 103 | 154 | 91 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference.) | 0.82 (0.64–1.04) | 0.86 (0.69–1.07) | 0.67 (0.52–0.87) | 0.005 |
| Multivariable HR † | 1.00 (reference) | 0.80 (0.63–1.03) | 0.85 (0.68–1.06) | 0.69 (0.52–0.90) | 0.015 |
| CVD mortality | |||||
| Cases, n | 251 | 161 | 262 | 160 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.85 (0.70–1.03) | 0.98 (0.82–1.16) | 0.79 (0.65–0.96) | 0.043 |
| Multivariable HR | 1.00 (reference) | 0.85 (0.69–1.03) | 0.99 (0.83–1.19) | 0.84 (0.68–1.04) | 0.22 |
| All‐cause mortality | |||||
| Cases, n | 566 | 368 | 570 | 373 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.86 (0.75–0.98) | 0.94 (0.84–1.06) | 0.81 (0.71–0.92) | 0.007 |
| Multivariable HR | 1.00 (reference) | 0.85 (0.74–0.97) | 0.96 (0.85–1.08) | 0.86 (0.75–0.99) | 0.11 |
| Total fish intake, adjusted for energy | P trend* | ||||
|---|---|---|---|---|---|
| ≤5 g/d (n=1002) | >5 to 20 g/d (n=2069) | >20 to 40 g/d (n=523) | >40 g/d (n=473) | ||
| Median total fish, g/d | 1.4 | 13.6 | 27.0 | 44.8 | |
| Median oily fish, g/d | 0.6 | 5.9 | 15.0 | 25.1 | |
| Median dietary EPA+DHA, mg/d | 25 | 112 | 241 | 394 | |
| Person‐years | 10 917 | 23 091 | 5914 | 5307 | |
| CHD mortality | |||||
| Cases, n | 155 | 253 | 59 | 48 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.81 (0.66–0.99) | 0.71 (0.52–0.96) | 0.71 (0.51–0.98) | 0.013 |
| Multivariable HR ‡ | 1.00 (reference) | 0.85 (0.70–1.04) | 0.73 (0.54–0.99) | 0.74 (0.53–1.03) | 0.031 |
| CVD mortality | |||||
| Cases, n | 221 | 431 | 101 | 81 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.97 (0.83–1.14) | 0.86 (0.68–1.09) | 0.84 (0.65–1.09) | 0.11 |
| Multivariable HR | 1.00 (reference) | 1.04 (0.88–1.22) | 0.91 (0.72–1.16) | 0.91 (0.70–1.18) | 0.33 |
| All‐cause mortality | |||||
| Cases, n | 494 | 948 | 241 | 194 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.95 (0.85–1.06) | 0.91 (0.78–1.06) | 0.90 (0.76–1.06) | 0.15 |
| Multivariable HR | 1.00 (reference) | 1.03 (0.92–1.15) | 0.98 (0.84–1.15) | 0.97 (0.82–1.15) | 0.64 |
CHD indicates coronary heart disease; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; and HR, hazard ratio. Values in Table represent hazard ratios (HRs) with 95% CIs, estimated from multivariable Cox models.
P for linear trend, through median values across categories of circulating eicosapentaenoic acid+docosahexaenoic acid, using a linear regression model.
HRs for eicosapentaenoic acid+docosahexaenoic acid were adjusted for age, sex, education level, physical activity, smoking status, alcohol intake, obesity, prevalent diabetes, cardiovascular drugs, time since myocardial infarction, and intake of total energy, cholesterol, fiber, and trans‐Fas.
HRs for fish were adjusted for age, sex, education level, physical activity, smoking status, alcohol intake, obesity, prevalent diabetes, cardiovascular drugs, time since myocardial infarction, and energy‐adjusted intakes of meat, grains, fruits, and vegetables.
Figure 1. Associations of (A) dietary and (B) circulating eicosapentaenoic acid+docosahexaenoic acid (EPA+DHA) with coronary heart disease mortality in 4067 patients with post‐myocardial infarction.
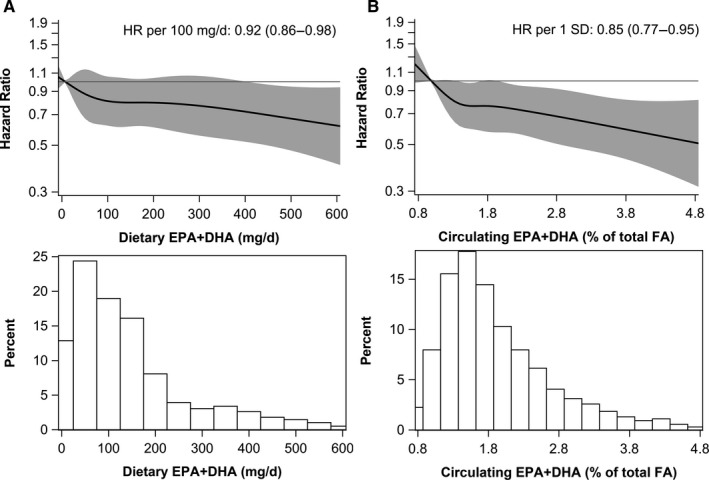
Solid lines are risk estimates evaluated by restricted cubic splines from Cox models showing the shape of the associations on a continuous scale with 5 knots located at 5th, 27.5th, 50th, 72.5th, and 95th percentiles. The y‐axis shows the multivariable‐adjusted hazard ratios for coronary heart disease mortality risk for any dietary or circulating EPA+DHA value, compared with the reference value set at the 5th percentile of dietary (7.8 mg/d) or circulating EPA+DHA (0.99% total fatty acids). Gray areas indicated 95% CIs. One SD of circulating EPA+DHA was 0.95% of total fatty acids. Histograms depict the distributions of dietary or circulating EPA+DHA in the Alpha Omega Cohort. DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; and HR, hazard ratio.
Total fish consumption was significantly inversely associated with CHD mortality (HR, 0.73; 95% CI, 0.54–0.99, for >20 to 40 versus ≤5 g/d; P‐trend=0.031) in multivariable analysis, but not with CVD or all‐cause mortality (Table 2). Findings were similar for oily fish (HR, 0.72; 95% CI, 0.54–0.95, for >11 versus <1 g/d; data not in table). Dietary ALA intake was not associated with mortality (Figure 2 and Figure S2).
Figure 2. Associations of (A) dietary and (B) circulating alpha‐linolenic acid (ALA) with coronary heart disease mortality in 4067 patients with post‐myocardial infarction.
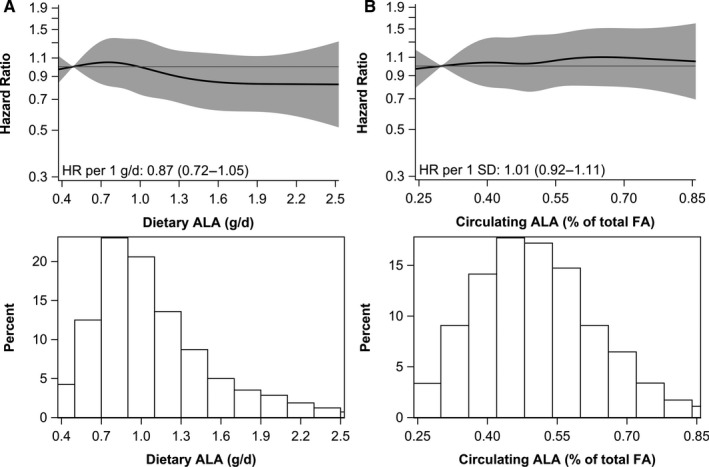
Solid lines are risk estimates evaluated by restricted cubic splines from Cox models showing the shape of the associations on a continuous scale with 5 knots located at 5th, 27.5th, 50th, 72.5th, and 95th percentiles. The y‐axis shows the multivariable‐adjusted hazard ratios for coronary heart disease mortality risk for any dietary or circulating ALA value, compared with the reference values set at fifth percentile of dietary (0.49 g/d) or circulating ALA (0.30% of total fatty acids). Gray areas indicated 95% CIs. One SD of circulating ALA was 0.14% of total fatty acids. Histograms depict the distributions of dietary or circulating ALA in the Alpha Omega Cohort. ALA indicates alpha‐linolenic acid; and FA, fatty acids.
Circulating n‐3 FAs
Patients with higher circulating EPA+DHA were more physically active, had higher alcohol intake and a higher DHD‐15 score (Table 1). Circulating ALA was not associated with DHD‐15 score. Patients in the higher circulating ALA quintiles were less likely to be men and statin users (Table S4). After adjustment for demographic factors, lifestyle factors, and circulating LA and arachidonic acid, patients in higher quintiles of circulating EPA+DHA had a significantly lower CHD mortality risk than those in the lowest quintile (Table 3; HRQ5, 0.71; 95% CI, 0.53–0.94; P for trend: 0.020). Each increment in circulating EPA+DHA (per 1‐SD of 0.95% of total FA) was associated with a 15% lower risk of CHD mortality (Figure 1). Associations were similar for CVD and all‐cause mortality (Table 3). In categorical analyses based on cut‐offs for the omega‐3 index (Table S5), reductions in CHD mortality risk were more pronounced (HR, 0.63 for EPA+DHA >3.1 versus ≤1.3% of total FAs; 95% CI, 0.44–0.90). Circulating ALA was not associated with CHD mortality (Figure 2), and also not with CVD or all‐cause mortality (Figure S2).
Table 3.
Associations of Circulating EPA+DHA in Quintiles With CHD, CVD, and All‐Cause Mortality in the Alpha Omega Cohort
| Circulating EPA+DHA | P trend* | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| ≤1.29 (n=818) | >1.29 to 1.56 (n=809) | >1.56 to 1.92 (n=813) | >1.92 to 2.52 (n=812) | >2.52 (n=815) | ||
| Median circulating EPA+DHA, % total FAs | 1.12 | 1.43 | 1.73 | 2.17 | 3.14 | |
| Person‐years | 8890 | 8904 | 8966 | 9152 | 9318 | |
| CHD mortality | ||||||
| Cases, n | 123 | 99 | 107 | 89 | 97 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.85 (0.65–1.11) | 0.89 (0.69–1.15) | 0.72 (0.55–0.95) | 0.75 (0.58–0.98) | 0.031 |
| Multivariable HR † | 1.00 (reference) | 0.83 (0.63–1.09) | 0.88 (0.67–1.16) | 0.72 (0.54–0.96) | 0.71 (0.53–0.94) | 0.020 |
| CVD mortality | ||||||
| Cases, n | 183 | 160 | 175 | 159 | 157 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.92 (0.74–1.14) | 0.96 (0.78–1.18) | 0.86 (0.70–1.06) | 0.80 (0.65–0.99) | 0.032 |
| Multivariable HR | 1.00 (reference) | 0.90 (0.72–1.11) | 0.94 (0.75–1.15) | 0.85 (0.68–1.07) | 0.75 (0.60–0.95) | 0.016 |
| All‐cause mortality | ||||||
| Cases, n | 420 | 388 | 370 | 359 | 340 | |
| Age‐ and sex‐adjusted HR | 1.00 (reference) | 0.97 (0.85–1.12) | 0.89 (0.77–1.02) | 0.85 (0.74–0.98) | 0.76 (0.66–0.88) | <0.001 |
| Multivariable HR | 1.00 (reference) | 0.97 (0.84–1.12) | 0.90 (0.77–1.04) | 0.86 (0.74–1.00) | 0.73 (0.63–0.86) | <0.001 |
CHD indicates coronary heart disease; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; HR, hazard ratio; and Q1 to Q5, quintile 1 to quintile 5. Values in Table represent hazard ratios (HRs) with 95% CIs, estimated from multivariable Cox models.
P for linear trend, through median values across categories of circulating eicosapentaenoic acid+ docosahexaenoic acid, using a linear regression model.
HRs were adjusted for age, sex, education level, physical activity, smoking status, alcohol intake, obesity, prevalent diabetes, cardiovascular drugs, serum cholesterol, circulating linoleic acid (18:2 n‐6), and circulating arachidonic acid (20:4 n‐6).
Subgroup and Sensitivity Analyses
HRs for dietary n‐3 FAs (per 100 mg/d) and CHD mortality in subgroups are presented in Figure S3. Dietary EPA+DHA tended to be more strongly inversely associated with fatal CHD in patients who received placebo (HR, 0.82 (95% CI, 0.71–0.95) per 100 mg/d) than in those who received any type of omega‐3 FA treatment during the first 40 months of follow‐up (HR, 0.95 (0.89–1.02) per 100 mg/d; P interaction=0.07). Associations did not essentially differ between subgroups of omega‐3 treatment (HRs between 0.93 and 0.97; data not shown in Figure).
HRs for circulating EPA+DHA and fatal CHD (Figure S4) tended to be lower in patients with circulating arachidonic acid (20:4 n‐6) above the median (HR, 0.74 per 1‐SD; 95% CI, 0.62–0.90; Pinteraction=0.07). Stronger associations were also found in patients who received placebo during the first 40 months follow‐up (HR, 0.77; 95% CI, 0.62–0.94; P interaction=0.25) and in patients with MI <1 year before study enrolment (HR, 0.62; 95% CI, 0.41–0.95; Pinteraction=0.13). Associations for circulating ALA and fatal CHD in subgroups were all non‐significant.
The association between dietary EPA+DHA and fatal CHD was attenuated after excluding the first 2 years of follow‐up (HR, 0.95 per 100 mg/d; 95% CI, 0.89–1.01), whereas HRs for circulating EPA+DHA remained similar (Table S6). Exclusion of fish oil supplement users did not change the HRs for EPA+DHA. For dietary and circulating ALA, results did not materially change in sensitivity analyses.
Discussion
In this analysis of 4067 Dutch patients with prior MI who received state‐of‐the‐art CVD drug treatment, higher habitual intakes of EPA+DHA and fish were associated with ≈30% lower risk of fatal CHD during >12 years of follow‐up. Circulating EPA+DHA (in plasma cholesteryl esters) was similarly associated with fatal CHD and also with CVD and all‐cause mortality. Dietary and circulating ALA were not significantly associated with mortality end points.
Data on habitual dietary intake of EPA+DHA (or fish) and mortality after MI are scarce. In 2412 Norwegian patients with CHD aged ≈62 years, long‐chain n‐3 FAs (from diet and supplements) were not significantly associated with fatal CHD or all‐cause mortality (137 deaths) during 5 years of follow‐up. 25 The Norwegian cohort consumed more fish (64–150 g/d) and marine n‐3 FAs (0.6 to 2.6 g/d). In a 5‐year follow‐up study of 400 Finnish patients with CHD aged ≈61 years, fish intakes >57 g/d (versus no intake) were associated with a markedly lower risk of all‐cause mortality (34 deaths), but not with fatal CHD (16 cases). 26 The Alpha Omega Cohort is characterized by a low fish intake (14 g/d), with 80% of patients consuming <200 mg/d of EPA+DHA.
We observed a 27% lower risk of fatal CHD for 20 to 40 grams of fish per day, which equals 5 to 10 ounces per week. Fish intakes >40 g/d were not associated with additional benefit. Our findings are in line with the recommendation by the American Heart Association of 1 to 2 seafood meals per week (250 mg/d of EPA+DHA). 1 For dietary EPA+DHA, we found a 20% lower risk of fatal CHD for intakes of only 50 to 100 mg/d, which can be achieved with 1 weekly serving of fish. Observational studies of EPA+DHA intake and long‐term mortality risk in patients with CHD are scanty. A meta‐analysis of 9 cohort studies in mainly healthy populations with 6 to 40 years of follow‐up yielded a pooled relative risk of 0.82 (95% CI, 0.69–0.98) for higher versus lower dietary EPA+DHA intakes, showing lower risks for intakes <250 mg/d. 7
Self‐reported fish consumption and EPA+DHA intake over the past month, assessed through our FFQ, may be subject to recall bias and measurement error. EPA+DHA in blood lipid compartments, including plasma cholesteryl esters, is considered an objective biomarker of habitual EPA+DHA and (oily) fish intake, 27 although it may also be influenced by other factors and metabolic processes. 28 Correlations between intake and plasma EPA+DHA were ≈0.4 in the Alpha Omega Cohort, as also reported by others. 27 Consistent with findings for dietary EPA+DHA and fish, we found a 30% lower risk of fatal CHD for higher circulating EPA+DHA. In addition, lower risks were observed for fatal CVD and all‐cause mortality. The concentration of n‐3 FAs in erythrocytes is also used as a biomarker and may be a more suitable biomarker for long‐term intake. 19 , 29 The omega‐3 index is an established metric based on EPA+DHA in erythrocyte cell membranes, 19 but it could not be assessed in the Alpha Omega Cohort because only plasma was available. When converting omega‐3 index cut‐off values to cholesteryl esters, as proposed by Stark et al, 20 we found a 37% lower risk of fatal CHD in the upper (>3.1%) versus lower (<1.3%) category.
Circulating n‐3 FAs were examined in relationship to CHD mortality in a pooling study of population‐based cohorts (>45 000 individuals, aged ≈59 years) with a median follow‐up of 10 years. 22 Each 1‐SD increase in circulating EPA and/or DHA was associated with a 9% to 10% lower risk of fatal CHD. For circulating EPA+DHA, a 30% lower risk for fatal CHD was estimated when comparing those with an omega‐3 index of 8% versus 4%. 30 ALA was also related to a 9% lower risk of fatal CHD (per 1‐SD) in these cohorts. 22 Data on circulating EPA+DHA and mortality risk in patients with CHD are limited. In a study on Finnish patients with CHD, RRs for CHD and all‐cause mortality were 0.3 to 0.5 for high versus low EPA and DHA in serum cholesteryl esters. 26 In 956 patients with CHD of the Heart and Soul study, those with erythrocyte EPA+DHA above the median had a 27% lower all‐cause mortality risk during 6 years of follow‐up. 31 In 3259 German patients referred for coronary angiography Ludwigshafen Risk and Cardiovascular Health (LURIC study), 32 higher circulating EPA+DHA was associated with a 22% lower risk of both fatal CVD and all‐cause mortality (975 deaths) during 10 years of follow‐up. A more recent case‐cohort study in 2407 hospitalized patients with an acute coronary syndrome (MERLIN‐TIMI 36 study) showed that 1‐SD higher plasma levels of long‐chain n‐3 FAs (EPA, DHA, and docosapentaenoic acid) were associated with a 27% lower odds of sudden cardiac death, which amounted to 63% when comparing extreme quartiles. 33 Associations with CVD mortality were weaker (18% lower odds per 1‐SD). 33 The German patients, MERLIN‐TIMI 36 study and those of the Alpha Omega Cohort showed that the relation of circulating ALA with mortality was neutral.
EPA+DHA may reduce blood pressure, plasma triglycerides and blood coagulation, 34 particularly at higher intakes of >750 mg/d. 3 For lower intakes of EPA+DHA in fish, as in our cohort, protection against fatal CHD has been attributed to the prevention of cardiac arrhythmias. 3 , 34 , 35 , 36 Inverse associations of EPA+DHA with fatal CHD in our patients tended to be stronger in those with an MI <1 year before study enrolment. In patients of MERLIN‐TIMI 36 study where blood samples were collected 24 hours from the start of symptoms, even stronger associations for plasma marine n‐3 FAs were reported. 33 It has been hypothesized that EPA+DHA may have a beneficial effect in attenuating reperfusion ischemia injury in cardiac tissue, although the exact mechanism is not yet understood. 37 , 38
In the Alpha Omega Trial, from which the current cohort emerged, low‐dose EPA+DHA and/or ALA supplementation (through margarine spreads) did not affect CVD or CHD mortality during 40 months of follow‐up. 10 The present observational analysis of the Alpha Omega Cohort, however, shows strong cardioprotective associations for baseline dietary and circulating EPA+DHA. These associations were most pronounced in patients who had not received n‐3 FAs during the trial. Low‐dose supplementation with EPA+DHA and/or ALA significantly increased plasma EPA levels in our cohort, as reported previously. 10 Patients on placebo, with lower plasma EPA levels, could have benefited more from EPA+DHA intake through their habitual diet in relationship to long‐term mortality risk. The discrepancy with trial results may also be related to the amount of EPA+DHA consumed (dietary intake versus supplemental intake of 400 mg/d on top of diet), food matrix (fish versus margarine spreads), and duration of follow‐up (>12 years versus 40 months).
We cannot exclude the possibility of residual confounding in the present analysis because the intake of fish (and EPA+DHA) was higher in patients who were more educated, and also in alcohol users. We did, however, carefully adjust for these and a large number of other potential confounders, including dietary intakes. Mortality coding was done by independent physicians who were not involved in the present analysis. Misclassification of causes of death may have occurred, but we have no reason to believe that it would be differential. If present, this could have led to wider CIs for the hazard ratios. A strength of the present study is completeness of follow‐up, with only 1 patient who had to be censored.
In conclusion, higher levels of dietary and circulating EPA and DHA (mainly from fish) were associated with a lower risk of mortality, in particular from CHD, in Dutch patients with prior MI with on average a low level of fish consumption. In this cohort, circulating EPA+DHA were also associated with lower risks of fatal CVD‐ and all‐cause mortality. These findings could have major implications for dietary advice after MI and warrants confirmation in other cohorts of patients with CHD with long‐term follow‐up.
Sources of Funding
The Alpha Omega Cohort (ClinicalTrials.gov Identifier NCT03192410) was funded by the Dutch Heart Foundation (grant 200T401) and NIH/National Heart, Lung, and Blood Institute and Office of Dietary Supplements, grant R01HL076200. Financial support for laboratory analysis of plasma fatty acids was obtained from Unilever and Upfield, The Netherlands. This research was supported by the Dutch Research Council Nederlandse Organisatie voor Wetenschappelijk Onderzoek through its Graduate Programme on Food Structure, Digestion, and Health.
Disclosures
J.M.G. received funding from Unilever for epidemiological studies of FAs and cardiovascular disease in the Alpha Omega Cohort. P.L.Z. was employed at Unilever until July 2019. Other authors have no disclosures to report.
Supporting information
Tables S1–S6
Figures S1–S4
Acknowledgments
The authors thank Paul Hulshof, Robert Hovenier, and Marlies Diepeveen‐de Bruin (Division of Human Nutrition and Health, Wageningen University) for analysis of plasma fatty acids, and Jeanne de Vries and Els Siebelink (Division of Human Nutrition and Health, Wageningen University) for their expertise on dietary assessment.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022617
For Sources of Funding and Disclosures, see page 11.
References
- 1. Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris‐Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH. Seafood long‐chain n‐3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138:e35–e47. doi: 10.1161/CIR.0000000000000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885 [DOI] [PubMed] [Google Scholar]
- 4. Goyens PLL, Spilker ME, Zock PL, Katan MB, Mensink RP. Compartmental modeling to quantify α‐linolenic acid conversion after longer term intake of multiple tracer boluses. J Lipid Res. 2005;46:1474–1483. doi: 10.1194/jlr.M400514-JLR200 [DOI] [PubMed] [Google Scholar]
- 5. Goyens PLL, Spilker ME, Zock PL, Katan MB, Mensink RP. Conversion of α‐linolenic acid in humans is influenced by the absolute amounts of α‐linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr. 2006;84:44–53. doi: 10.1093/ajcn/84.1.44 [DOI] [PubMed] [Google Scholar]
- 6. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country‐specific nutrition surveys. BMJ. 2014;348:g2272. doi: 10.1136/bmj.g2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta‐analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long‐chain omega‐3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15–29. doi: 10.1016/j.mayocp.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 8. Harris WS, Tintle NL, Imamura F, Qian F, Korat AVA, Marklund M, Djoussé L, Bassett JK, Carmichael P‐H, Chen Y‐Y, et al. Blood n‐3 fatty acid levels and total and cause‐specific mortality from 17 prospective studies. Nat Commun. 2021;12:2329. doi: 10.1038/s41467-021-22370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Oude Griep LM, Teitsma‐Jansen AM, Katan MB, Kromhout D. Effect of low doses of n‐3 fatty acids on cardiovascular diseases in 4,837 post‐myocardial infarction patients: design and baseline characteristics of the Alpha Omega Trial. Am Heart J. 2010;159:539–546.e2. doi: 10.1016/j.ahj.2009.12.033 [DOI] [PubMed] [Google Scholar]
- 10. Kromhout D, Giltay EJ, Geleijnse JM. n–3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603 [DOI] [PubMed] [Google Scholar]
- 11. Mölenberg FJ, de Goede J, Wanders AJ, Zock PL, Kromhout D, Geleijnse JM. Dietary fatty acid intake after myocardial infarction: a theoretical substitution analysis of the Alpha Omega Cohort. Am J Clin Nutr. 2017;106:895–901. doi: 10.3945/ajcn.117.157826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker‐based validity of a food‐frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58:489–496. doi: 10.1093/ajcn/58.4.489 [DOI] [PubMed] [Google Scholar]
- 13. Feunekes IJ, Van Staveren WA, Graveland F, Vos JD, Burema J. Reproducibility of a semiquantitative food frequency questionnaire to assess the intake of fats and cholesterol in The Netherlands. Int J Food Sci Nutr. 1995;46:117–123. doi: 10.3109/09637489509012539 [DOI] [PubMed] [Google Scholar]
- 14. National Institute for Public Health and the Environment (RIVM) . Nederlands Voedingsstoffenbestand (NEVO) (Dutch Food Composition Database). National Institute for Public Health and the Environment (RIVM); 2006.
- 15. Looman M, Feskens EJM, de Rijk M, Meijboom S, Biesbroek S, Temme EHM, de Vries J, Geelen A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20:2289–2299. doi: 10.1017/s136898001700091x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pertiwi K, Kok DE, Wanders AJ, de Goede J, Zock PL, Geleijnse JM. Circulating n‐3 fatty acids and linoleic acid as indicators of dietary fatty acid intake in post‐myocardial infarction patients. Nutr Metab Cardiovasc Dis. 2019;29:343–350. doi: 10.1016/j.numecd.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO Collaborating Centre for Drug Statistics Methodology . Anatomical Therapeutic Chemical Classification System (ATC). World Health Organization; 2009. [Google Scholar]
- 18. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 19. Harris WS. The omega‐3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S [DOI] [PubMed] [Google Scholar]
- 20. Stark KD, Aristizabal Henao JJ, Metherel AH, Pilote L. Translating plasma and whole blood fatty acid compositional data into the sum of eicosapentaenoic and docosahexaenoic acid in erythrocytes. Prostaglandins Leukot Essent Fat Acids. 2016;104:1–10. doi: 10.1016/j.plefa.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 21. Marklund M, Leander K, Vikström M, Laguzzi F, Gigante B, Sjögren P, Cederholm T, de Faire U, Hellénius ML, Risérus U. Polyunsaturated fat intake estimated by circulating biomarkers and risk of cardiovascular disease and all‐cause mortality in a population‐based cohort of 60‐year‐old men and women. Circulation. 2015;132:586–594. doi: 10.1161/circulationaha.115.015607 [DOI] [PubMed] [Google Scholar]
- 22. Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, dela Cruz L, Frazier‐Wood AC, et al. Ω‐3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176:1155–1166. doi: 10.1001/jamainternmed.2016.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 24. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 25. Manger MS, Strand E, Ebbing M, Seifert R, Refsum H, Nordrehaug JE, Nilsen DW, Drevon CA, Tell GS, Bleie Ø, et al. Dietary intake of n–3 long‐chain polyunsaturated fatty acids and coronary events in Norwegian patients with coronary artery disease. Am J Clin Nutr. 2010;92:244–251. doi: 10.3945/ajcn.2010.29175 [DOI] [PubMed] [Google Scholar]
- 26. Erkkilä AT, Lehto S, Pyörälä K, Uusitupa MI. n‐3 fatty acids and 5‐y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. 2003;78:65–71. doi: 10.1093/ajcn/78.1.65 [DOI] [PubMed] [Google Scholar]
- 27. Serra‐Majem L, Nissensohn M, Øverby NC, Fekete K. Dietary methods and biomarkers of omega 3 fatty acids: a systematic review. Br J Nutr. 2012;107:S64–S76. doi: 10.1017/S000711451200147X [DOI] [PubMed] [Google Scholar]
- 28. Lankinen M, Uusitupa M, Schwab U. Genes and dietary fatty acids in regulation of fatty acid composition of plasma and erythrocyte membranes. Nutrients. 2018;10:1785. doi: 10.3390/nu10111785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74 [DOI] [PubMed] [Google Scholar]
- 30. Harris WS, Del Gobbo L, Tintle NL. The Omega‐3 Index and relative risk for coronary heart disease mortality: estimation from 10 cohort studies. Atherosclerosis. 2017;262:51–54. doi: 10.1016/j.atherosclerosis.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 31. Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all‐cause mortality in patients with stable coronary heart disease. Circ Cardiovasc Qual Outcomes. 2010;3:406–412. doi: 10.1161/CIRCOUTCOMES.109.896159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleber ME, Delgado GE, Lorkowski S, März W, von Schacky C. Omega‐3 fatty acids and mortality in patients referred for coronary angiography. the Ludwigshafen risk and cardiovascular health study. Atherosclerosis. 2016;252:175–181. doi: 10.1016/j.atherosclerosis.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 33. Zelniker TA, Morrow DA, Scirica BM, Furtado JD, Guo J, Mozaffarian D, Sabatine MS, O’Donoghue ML. Plasma omega‐3 fatty acids and the risk of cardiovascular events in patients after an acute coronary syndrome in MERLIN‐TIMI 36. J Am Heart Assoc. 2021;10:e017401. doi: 10.1161/JAHA.120.017401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kromhout D, Yasuda S, Geleijnse JM, Shimokawa H. Fish oil and omega‐3 fatty acids in cardiovascular disease: do they really work? Eur Heart J. 2012;33:436–443. doi: 10.1093/eurheartj/ehr362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n‐3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n‐3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33 [DOI] [PubMed] [Google Scholar]
- 36. London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf A, et al. Omega‐3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and office of dietary supplements omega‐3 fatty acids and their role in cardiac arrhythmogenesis workshop. Circulation. 2007;116:e320–e335. doi: 10.1161/CIRCULATIONAHA.107.712984 [DOI] [PubMed] [Google Scholar]
- 37. Arakawa K, Himeno H, Kirigaya J, Otomo F, Matsushita K, Nakahashi H, Shimizu S, Nitta M, Yano H, Endo M, et al. Impact of n‐3 polyunsaturated fatty acids in predicting ischemia/reperfusion injury and progression of myocardial damage after reperfusion in patients with ST‐segment elevation acute myocardial infarction. J Cardiol. 2015;66:101–107. doi: 10.1016/j.jjcc.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 38. Sullivan EM, Pennington ER, Green WD, Beck MA, Brown DA, Shaikh SR. Mechanisms by which dietary fatty acids regulate mitochondrial structure‐function in health and disease. Adv Nutr. 2018;9:247–262. doi: 10.1093/advances/nmy007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S4


