Abstract
Background
Peripheral artery disease (PAD) and coronary artery disease (CAD) represent atherosclerosis in different vascular beds. We used detailed metabolic biomarker profiling to identify common and discordant biomarkers and clarify pathophysiological differences for these vascular diseases.
Methods and Results
We used 5 prospective cohorts from Finnish population (FINRISK 1997, 2002, 2007, and 2012, and Health 2000; n=31 657; median follow‐up time of 14 years) to estimate associations between >200 metabolic biomarkers and incident PAD and CAD. Metabolic biomarkers were measured with nuclear magnetic resonance, and disease events were obtained from nationwide hospital records. During the follow‐up, 498 incident PAD and 2073 incident CAD events occurred. In age‐ and sex‐adjusted Cox models, apolipoproteins and cholesterol measures were robustly associated with incident CAD (eg, hazard ratio [HR] per SD for higher apolipoprotein B/A‐1 ratio, 1.30; 95% CI, 1.25–1.36), but not with incident PAD (HR per SD for higher apolipoprotein B/A‐1 ratio, 1.04; 95% CI, 0.95–1.14; P heterogeneity<0.001). In contrast, triglyceride levels in low‐density lipoprotein and high‐density lipoprotein were associated with both end points (P heterogeneity>0.05). Lower proportion of polyunsaturated fatty acids relative to total fatty acids, and higher concentrations of monounsaturated fatty acids, glycolysis‐related metabolites, and inflammatory protein markers were strongly associated with incident PAD, and many of these associations were stronger for PAD than for CAD (P heterogeneity<0.001). Most differences in metabolic profiles for PAD and CAD remained when adjusting for traditional risk factors.
Conclusions
The metabolic biomarker profile for future PAD risk is distinct from that of CAD. This may represent pathophysiological differences.
Keywords: biomarker, coronary artery disease, magnetic resonance spectroscopy, metabolomics, peripheral artery disease
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Coronary Artery Disease, Peripheral Vascular Disease
Nonstandard Abbreviations and Acronyms
- GlycA
glycoprotein acetyls
- NMR
nuclear magnetic resonance
Clinical Perspective
What Is New
Blood biomarkers for future risk of peripheral artery disease differ from biomarkers for risk of coronary artery disease in large general population cohorts from Finland.
Low‐density lipoprotein and high‐density lipoprotein cholesterol, as well as apolipoprotein B and A‐1, did not associate with future hospital diagnosis of peripheral artery disease.
Novel biomarkers, measured by nuclear magnetic resonance spectroscopy, including low‐density lipoprotein triglycerides, serum fatty acids levels, and inflammatory protein markers, were strong predictors for future peripheral artery disease.
What Are the Clinical Implications?
The distinct biomarker profiles for risk of peripheral artery disease and coronary artery disease suggest pathophysiology differences for these 2 types of atherosclerotic disease.
Standard cholesterol measures and apolipoproteins are not good indicators for future risk of peripheral artery disease.
Newer means to capture broader panels of blood biomarkers may potentially improve risk assessment for peripheral artery disease and suggest novel targets for therapies.
Peripheral artery disease (PAD) is a common atherosclerotic disease affecting body extremities. It can lead to serious complications, including limb ischemia and amputation, and is predictive of future stroke and myocardial infarction. 1 Atherosclerosis is underpinning the pathogenesis in cardiovascular diseases, but studies suggest that PAD risk factors are partly different from cerebrovascular and coronary artery disease (CAD). 1 , 2 , 3 , 4 For example, smoking and diabetes appear to be stronger risk factors, whereas hypertension and dyslipidemia are more modest for PAD compared with CAD. 1 , 2 , 3 Recent genome‐wide association studies have identified partly distinct genetic signals for PAD compared with CAD. 4 Thus, uncovering precise metabolic pathways underlying PAD is required to better understand the disease pathology and to improve treatment.
Large prospective studies have demonstrated the utility of detailed metabolic profiling (also known as metabolomics) in uncovering biomarkers for cardiovascular event risk 5 , 6 , 7 and elucidating the molecular pathophysiology, 8 which might lead to discovery of new therapeutic targets. The overall pattern of metabolic biomarker associations with a given outcome may also help to dissect similarities and differences between various risk factors 9 , 10 or diseases. 7 This concept was recently used to demonstrate coherent metabolic signatures for future myocardial infarction and ischemic stroke risk, whereas the metabolic signature for intracerebral hemorrhage risk was found to be partly distinct. 7 Only a few studies have examined biomarker associations with future PAD risk because it requires a large sample size to achieve sufficient end points. 11 Recently, Aday et al 12 used advanced lipoprotein testing in the WHS (Women’s Health Study) to identify different association patterns for PAD compared with a composite measure of cardiovascular and cerebrovascular disease, and suggested that primary prevention of PAD should go beyond low‐density lipoprotein (LDL) cholesterol lowering. However, these findings were based on a single cohort of female health professionals and had modest numbers of incident events. Also, as nonlipid pathways seem to play more important roles in PAD pathophysiological features compared with other vascular end points, it is essential to study such biomarkers in greater detail to further characterize these associations. Metabolic profiling studies have shown that several amino acids and fatty acids are associated with future CAD and stroke risk more strongly than routine lipids, 5 , 6 , 7 but the associations with PAD risk have yet to be evaluated.
In this study, we aimed to characterize the detailed lipoprotein and metabolite profile for PAD in large prospective cohorts of the general population and compare the results with metabolic biomarker pattern for future CAD events.
METHODS
The data are available for purposes of reproducing the results and additional research via application to the Finnish Institute for Health and Welfare Biobank (https://thl.fi/en/web/thl‐biobank).
Study Populations
This observational study examined lipid and metabolite associations with incident PAD and CAD events in 5 population‐based cohorts conducted by the Finnish Institute for Health and Welfare: the FINRISK 1997, 2002, 2007, and 2012 cohorts, and the Health 2000 Study. The cohort studies were approved by the Coordinating Ethical Committee of the Helsinki and Uusimaa Hospital District, Finland. Written informed consent was obtained from all participants. The field surveys for the FINRISK cohort studies have been conducted every 5 years since 1972 in Finland. 13 The cohorts included in the present study were initiated in 1997, 2002, 2007, and 2012. Health 2000 was conducted during 2000 to 2001. Each cohort study is an independent random sample drawn from people aged 25 to 98 years (25–74 years in FINRISK and ≥30 years in Health 2000) in the Finnish population. Participation rates to these cohorts have been between 60% and 70%. The study participants are unique in each cohort. The data were collected by self‐administered questionnaires and basic risk factor assessments (including weight, height, and blood pressure) in each survey. Baseline blood samples were collected for ≈85% of all participants enrolled. Lipid and metabolite biomarker profiling by nuclear magnetic resonance (NMR) metabolomics was conducted from frozen serum samples for all participants in each of the cohorts. All study participants have been followed up using nationwide electronic health registries, including national hospital discharge register, causes‐of‐death register, and drug reimbursement register. Information on use of lipid‐lowering medication and blood pressure treatment was obtained from self‐reports and the drug reimbursement register. Follow‐up has been completed until the end of 2016. All study participants with available lipid and metabolite biomarker data were included in the present study, with the exception of pregnant women and individuals with baseline cardiovascular disease.
Disease Outcome Definitions
Disease outcomes were derived from national electronic health registries, which cover all cardiovascular events that have led to hospitalization or death in Finland. The cardiovascular diagnoses in these registers have been validated. 14 , 15 , 16 PAD was defined as the first occurrence of atherosclerosis of native arteries of the extremities, peripheral vascular operations, unspecified PAD, and diabetes with circulatory complications (International Classification of Diseases, Tenth Revision [ICD‐10] codes: E10.5, E11.5, E12.5, E13.5, E14.5, I70.2, and I73.9). CAD was defined as the first occurrence of CAD event during the follow‐up, comprising fatal or nonfatal myocardial infarction, cardiac revascularization (coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty), or unstable angina (codes: I21–I25, I46, R96, and R98). To focus on biomarkers for incident disease, individuals with CAD, PAD, or stroke at baseline, according to the registry data, were excluded from all analyses. Follow‐up time was censored at the time of first cardiovascular (ie, PAD or CAD) event, and subsequent events were not included in the analysis.
Lipid and Metabolite Biomarker Profiling
Venous blood was drawn from nonfasting samples, but with recommended minimum of 4‐hour fast (median recorded fasting, 5 hours; interquartile range, 4–6 hours). The samples were collected and centrifuged at the field survey sites and then transported to the quality‐controlled central laboratory, where the serum samples have been stored in −70 °C or colder. 13 Lipid and metabolite measures were quantified by high‐throughput NMR metabolomics (Nightingale Health Plc, Helsinki, Finland; biomarker quantification version 2020) using 350 µL aliquots of serum. This NMR platform provides simultaneous quantification of routine lipids, lipoprotein subclass profiling with lipid concentrations within 14 subclasses, fatty acid composition, and various low‐molecular‐weight metabolites, including amino acids, ketone bodies, and gluconeogenesis‐related metabolites, in molar concentration units. Biomarkers were quantified independently for each serum sample without using information from other samples in same well plate or same cohort. The average success rate of metabolite quantification was 99%. Technological details and epidemiological applications of the Nightingale NMR platform have been reviewed previously. 17 , 18 The process of this NMR metabolomics technology has received regulatory approval (CE), and 37 biomarkers in the panel have been certified for diagnostics use. Figure S1 illustrates the consistency of biomarkers measured by both clinical chemistry assays (conducted soon after sample collection) and Nightingale NMR (conducted from samples stored 6–15 years before NMR measurements), with correlation coefficients in line with earlier reports. 7 , 17 , 19 To facilitate visualization, we display results for 57 measures spanning most of the metabolic pathways in the main text; complete results for all the 250 measures quantified by the Nightingale NMR platform are reported in Table S1.
Statistical Analysis
All metabolic markers were scaled to SD units to enable comparison of biomarker associations for measures with different units and across wide concentration ranges. Results in absolute concentrations are reported in Table S1. No transformation of the metabolite concentrations was used.
We used Cox proportional hazard modeling to estimate associations between biomarkers and incident PAD and CAD separately in each cohort. Hazard ratios (HRs) from each cohort were meta‐analyzed using inverse‐variance weighted fixed‐effect models. We used 2 different sets of covariates: our primary analysis was conducted adjusting for sex and age, using age as the time scale (model 1). In secondary analyses, we additionally adjusted for systolic blood pressure, blood pressure treatment, body mass index, prevalent diabetes, smoking (current versus nonsmoker), and lipid‐lowering medication (model 2). Differences in association magnitude for incident PAD and CAD were estimated by , and the corresponding P value for heterogeneity derived from the normal distribution. The overall concordance of the biomarker association pattern with incident PAD and CAD was summarized using the linear fit of the HRs. 7 , 20 We also conducted sensitivity analyses, excluding all individuals with prevalent diabetes and those who developed diabetes before PAD and CAD events.
To account for multiple testing, we used a threshold of P<0.05/(50×2)=0.0005 for statistical significance, which is based on 50 independent tests in the NMR metabolomics data (number of principal components explaining 99% of variation) and 2 disease outcomes studied. Statistical analyses were conducted using R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria) and plots made using the ggforestplot package.
RESULTS
The present study included 31 657 participants from 5 general population cohorts in Finland, all free from cardiovascular disease at baseline and with NMR metabolomics measurements available. Baseline characteristics of the study participants from each cohort are shown in the Table. During follow‐up (median, 14 years; interquartile range, 9–15 years; 370 000 person‐years in total), 498 incident PAD events and 2073 CAD events occurred as the first atherosclerotic event. The median age of the first event was 70 years for both CAD and PAD.
Table 1.
Baseline Characteristics and Event Numbers in the 5 Cohorts
| Variable | FINRISK 1997 | FINRISK 2002 | FINRISK 2007 | FINRISK 2012 | Health 2000 |
|---|---|---|---|---|---|
| No. of participants | 7254 | 7575 | 5550 | 5231 | 6047 |
| Women, N (%) | 3678 (51) | 4195 (55) | 2939 (53) | 2765 (53) | 3317 (55) |
| Age, mean (SD), y | 48 (13) | 48 (13) | 50 (14) | 51 (14) | 54 (15) |
| Body mass index, mean (SD), kg/m2 | 26.6 (4.5) | 26.8 (4.7) | 27.1 (4.8) | 27.0 (4.9) | 27.0 (4.6) |
| Systolic blood pressure, mean (SD), mm Hg | 136 (20) | 135 (20) | 136 (20) | 134 (19) | 135 (21) |
| Total cholesterol, mean (SD), mmol/L | 5.5 (1.1) | 5.6 (1.1) | 5.3 (1.0) | 5.3 (1.0) | 6.0 (1.1) |
| HDL cholesterol, mean (SD), mmol/L | 1.4 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 1.5 (0.4) | 1.4 (0.4) |
| Triglycerides, mean (SD), mmol/L | 1.5 (1.0) | 1.4 (0.9) | 1.4 (0.9) | 1.4 (0.9) | 1.6 (1.0) |
| Lipid‐lowering medication, N (%) | 184 (2.5) | 416 (5.5) | 664 (12) | 683 (13) | 342 (5.7) |
| Antihypertensive medication, N (%) | 915 (13) | 1022 (13) | 1064 (19) | 1081 (21) | 962 (16) |
| Current smoking, N (%) | 1719 (24) | 1948 (26) | 1159 (21) | 997 (19) | 1264 (21) |
| Prevalent diabetes, N (%) | 415 (6) | 393 (5) | 439 (8) | 582 (11) | 274 (5) |
| Incident CAD, N (%) | 752 (10) | 435 (6) | 228 (4) | 81 (2) | 577 (10) |
| Incident PAD, N (%) | 190 (3) | 99 (1) | 70 (1) | 16 (0) | 123 (2) |
| Follow‐up time, median, y | 18.8 | 13.8 | 8.9 | 3.9 | 15.3 |
Data are number (percentage) or mean (SD) when appropriate. CAD indicates coronary artery disease; HDL, high‐density lipoprotein; and PAD, peripheral artery disease.
Apolipoproteins and Lipids
To best enable comparison of pathophysiological differences in biomarker patterns for PAD and CAD, the primary results are reported with adjustment for age and sex only. As expected, apolipoproteins and cholesterol measures were robustly associated with incident CAD events. However, the effects were weak for incident PAD, with HRs close to unity (Figure 1). For example, higher ratio of apolipoprotein B/apolipoprotein A‐1 was associated with increased risk for CAD (HR, 1.30 per 1 SD; 95% CI, 1.25–1.36), but not with PAD (HR, 1.04 per 1 SD; 95% CI, 0.95–1.14; P heterogeneity<0.001). Similar results were observed for apolipoprotein B and LDL cholesterol measures. However, higher concentrations of triglycerides in very‐low‐density lipoprotein, LDL, and high‐density lipoprotein (HDL) were consistently associated with increased risk for both CAD and PAD (P heterogeneity>0.05).
Figure 1.

Apolipoprotein (Apo) and lipid associations with incident coronary artery disease (CAD) (2073 incident events; red) and peripheral artery disease (PAD) (498 incident events; dark blue).
Hazard ratios (HRs) and 95% CIs are shown per 1‐SD higher biomarker concentration, with numerical results indicated on the right‐hand side. Models are adjusted for sex and age. Results are meta‐analyzed for 31 657 individuals from 5 prospective cohorts. Open circles denote P≥0.0005, and closed circles denote P<0.0005. Asterisks denote P<0.001 for heterogeneity between PAD and CAD associations. Complete numerical results in absolute concentrations and SD‐scaled units are listed in Table S1. Clinical low‐density lipoprotein (LDL) cholesterol (C) and size‐specific LDL‐C refer to different methods for defining LDL. 21 HDL indicates high‐density lipoprotein; IDL, intermediate‐density lipoprotein; P‐het, P value for heterogeneity; TG, triglycerides; and VLDL, very‐low‐density lipoprotein.
Lipoprotein Subclasses and Particle Size
Figure 2 shows the associations of particle concentrations in 14 lipoprotein subclasses and particle size measures with incident CAD and PAD. For particle concentrations, only increased concentrations of very‐low‐density lipoprotein particles were robustly associated with higher risk for PAD, whereas the associations for LDL and HDL subclasses were generally weak for PAD. In contrast, almost all lipoprotein particle concentrations were robustly associated with CAD, with the strongest direct associations observed for medium very‐low‐density lipoprotein (1.24; 95% CI, 1.20–1.29) and medium LDL (1.26; 95% CI, 1.21–1.31; P heterogeneity<0.001 with PAD association). Also, large HDL particles were inversely associated with incident CAD, but not with PAD (P heterogeneity<0.001). Results for specific lipid types within the 14 lipoprotein subclasses broadly followed the same pattern as illustrated for particle concentrations (Figure S2). For the measures of particle size, larger very‐low‐density lipoprotein particle size was associated with higher risk for incident CAD. Larger LDL particle size was associated with lower risk for both end points, whereas larger HDL particle size was robustly associated with lower risk for CAD only (P heterogeneity<0.001).
Figure 2. Lipoprotein subclass associations with incident coronary artery disease (CAD) (2073 incident events; red) and peripheral artery disease (PAD) (498 incident events; dark blue).
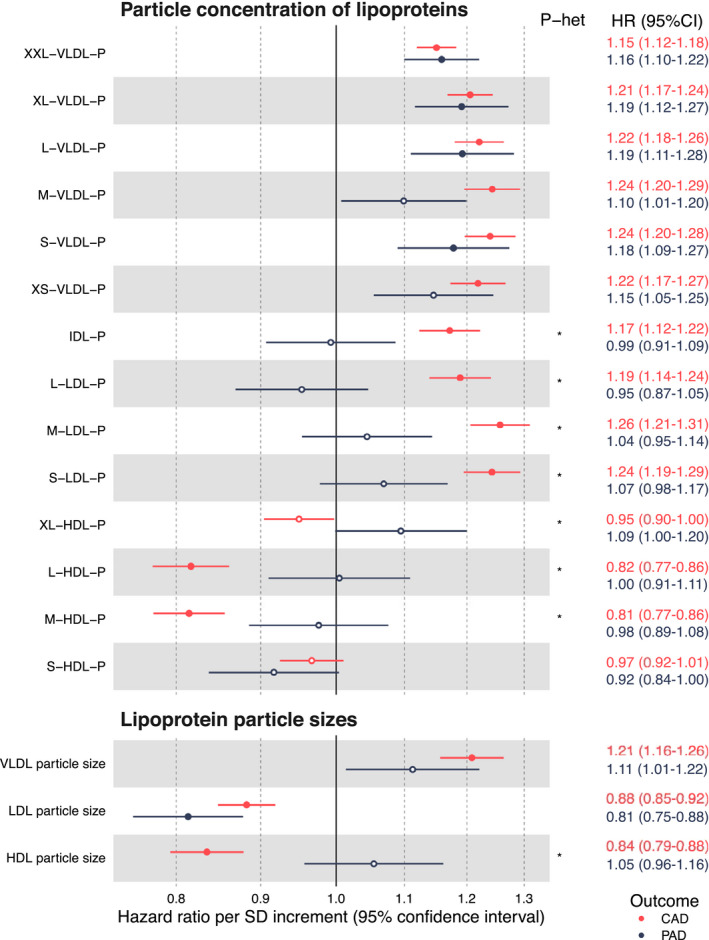
Hazard ratios (HRs) and 95% CIs are shown per 1‐SD higher biomarker concentration, with numerical results indicated on the right‐hand side. Models are adjusted for sex and age. Open circles denote P≥0.0005, and closed circles denote P<0.0005. Asterisks denote P<0.001 for heterogeneity between PAD and CAD associations. Average particle sizes of the 14 lipoprotein subclasses (from XXL VLDL to S HDL) are defined in Reference 18. HDL indicates high‐density lipoprotein; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; P, particle concentration; P‐het, P value for heterogeneity; and VLDL, very‐low‐density lipoprotein.
Fatty Acids, Polar Metabolites, and Inflammatory Proteins
In addition to lipoprotein and lipid measures, the Nightingale NMR platform simultaneously quantifies several fatty acids, polar metabolites, and 2 inflammatory protein measures. Many of these nontraditional biomarkers showed strong associations with both CAD and PAD events (Figure 3). The novel biomarkers tended to be particularly strong for PAD, with 11 of the measures having HRs >1.2 or <0.8 per 1 SD. For comparison, the HR for body mass index is 1.15 (95% CI, 1.05–1.26) per SD in this data set.
Figure 3. Fatty acid, polar metabolite, and inflammatory protein associations with incident coronary artery disease (CAD) (2073 incident events; red) and peripheral artery disease (PAD) (498 incident events; dark blue).

Hazard ratios (HRs) and 95% CIs are shown per 1‐SD higher biomarker concentration, with numerical results indicated on the right‐hand side. Models are adjusted for sex and age. Open circles denote P≥0.0005, and closed circles denote P<0.0005. Asterisks denote P<0.001 for heterogeneity between PAD and CAD associations. BCAA indicates branched‐chain amino acids; DHA, docosahexaenoic acid; LA, linoleic acid; MUFA, monounsaturated fatty acids; P‐het, P value for heterogeneity; PUFA, polyunsaturated fatty acids; and SFA, saturated fatty acids.
For fatty acids, higher proportions of saturated as well as monounsaturated fatty acids, relative to total fatty acids, were associated with increased risk for both PAD and CAD. Higher proportions of polyunsaturated fatty acids were associated with decreased risk for both PAD and CAD events. Glycolysis‐related measures (glucose, lactate, pyruvate, and glycerol) associated with PAD risk, and 2 of these (glucose and lactate) had stronger associations with PAD risk than with CAD risk (P heterogeneity<0.001). For amino acids, the strongest association for higher CAD and PAD risk was for alanine. Also, the aromatic amino acids (phenylalanine and tyrosine) were robustly associated with higher risk for both CAD and PAD. Branched‐chain amino acids had similar HRs as aromatic amino acids in the case of CAD risk, but for PAD the associations were slightly weaker and not statistically significant. Glutamine displayed an inverse association with PAD risk but was not significantly associated with CAD risk. The inflammation‐related protein markers albumin and glycoprotein acetyls (GlycA) were associated with both end points. GlycA displayed the single strongest HR per SD change for both CAD (HR, 1.31; 95% CI, 1.26–1.37) and PAD (HR, 1.53; 95% CI, 1.42–1.65) risk among all the metabolic biomarkers analyzed in this study.
Biomarker Signature for PAD Compared With CAD
The overall consistency and differences in the biomarker associations for PAD and CAD are illustrated in Figure 4. The consistency of the overall pattern of biomarker associations for the 2 end points was moderate when comparing the HRs for the 57 highlighted biomarkers (R 2=0.49). The plot reinforces 2 observations: First, LDL and HDL lipid biomarkers were robustly associated with future CAD but not strongly with PAD. Second, for biomarkers associated with both outcomes, the largest heterogeneity was observed for GlycA and glycolysis‐related metabolites, which all had stronger associations with future PAD.
Figure 4. Consistency of metabolic biomarker associations for coronary artery disease (CAD) (2073 incident events; red) and peripheral artery disease (PAD) (498 incident events; dark blue).

The hazard ratio of each biomarker is given with 95% CIs in gray vertical and horizontal error bars. The red dashed line denotes the diagonal. Biomarkers with P<0.001 for heterogeneity between associations with PAD and CAD are marked by black color coding. Apo indicates apolipoprotein; C, cholesterol; GlycA, glycoprotein acetyls; HDL, high‐density lipoprotein; IDL, intermediate‐density lipoprotein; L, large; LA, linoleic acid; LDL, low‐density lipoprotein; M, medium; P, particle concentration; S, small; and XL, very large.
Complete Biomarker Associations and Sensitivity Analyses
Results for all 250 metabolic measures quantified by the Nightingale NMR metabolomics platform are shown in Figure S2. All numerical results are listed in Table S1. The findings in Figures 1, 2, 3, 4 are presented as meta‐analysis of the 5 prospective cohorts; the results were consistent within individual cohorts for both CAD and PAD, despite large differences in follow‐up time and fraction of individuals on lipid‐lowering medication at baseline (Figures S3 and S4).
We further examined the influence of traditional cardiovascular risk factors on the biomarker associations. The overall biomarker association profiles did not change substantially when further adjusting for systolic blood pressure, body mass index, diabetes status, smoking, and lipid‐lowering medication (Figure S5). In particular, the pronounced differences in CAD and PAD associations for apolipoprotein and cholesterol were similar. Many of the nontraditional biomarkers, such as omega‐6 fatty acids, glycolysis metabolites, phenylalanine, albumin, and GlycA, remained significantly associated with future PAD events after adjusting for the traditional risk factors, including prevalent diabetes.
To examine if inclusion of diabetes with circulatory complications in the PAD event definition could partly underpin the observed biomarker differences, the performed sensitivity analyses were all diabetes cases that were registered before incidence of PAD and CAD were excluded (Figure S6). Some of the biomarker associations for PAD were attenuated in this sensitivity analysis (eg, glucose, alanine, and glutamine), whereas other biomarker associations with PAD, such as lactate, phenylalanine, and GlycA, remained similar. Furthermore, the differences in apolipoprotein and lipid associations for CAD compared with PAD were similar.
DISCUSSION
Detailed lipoprotein and metabolite profiling in large population‐based cohorts uncovered several novel circulating biomarkers reflecting future risk for PAD events. The overall biomarker association profile showed several molecular differences in comparison to biomarkers associated with future CAD events. We have 2 main findings: First, the most heterogeneous biomarker associations were for standard lipid measures, which were strongly associated with future CAD events but not with the risk for PAD. This difference was particularly marked for apolipoprotein B and LDL cholesterol. Second, a broad range of emerging biomarkers, including triglycerides in LDL and HDL particles, circulating fatty acids, glycolysis metabolites, amino acids, and inflammatory protein markers, showed robust association with both end points; and in many cases, the HRs were stronger for PAD than for CAD. These findings exemplify the possibility for characterization of molecular similarities and differences of related cardiovascular diseases via large‐scale metabolic profiling to enhance causative understanding and potentially help to point toward novel treatment opportunities.
The cause of atherosclerotic diseases in any arterial bed has historically been considered equivalent, but recently, more detailed lipid and metabolic profiling has revealed partly distinct association profiles for different atherosclerosis subtypes. 7 , 12 With the aging of the global population and increasing prevalence of obesity and diabetes, it is likely that especially PAD will become more common in future. 1 We identified several novel biomarkers for PAD, which could help to elucidate pathophysiological differences of PAD in relation to other atherosclerotic diseases. For example, our findings suggest that standard lipid testing does not capture the risk for developing PAD. Similar overall conclusions were recently also suggested by Aday et al, 12 based on standard and advanced lipid testing in the WHS. In contrast to Aday et al, 12 however, we did not observe HDL cholesterol, apolipoprotein A1, or LDL particle concentrations to be associated with future PAD events in our study. These deviating results might be explained by the differences in cohort characteristics and subtle differences in PAD outcome definitions. Although the WHS only included female health care professionals, the participants of the present study are representative of the general Finnish population. Furthermore, our PAD definition was wider compared with theirs, as it also included cases of diabetes with circulatory complications. However, in our sensitivity analyses that excluded all diabetes cases before PAD and CAD events, the prominent differences in lipid associations for CAD compared with PAD were not altered. The overall pattern of biomarker associations was also distinct for the associations of these metabolic biomarkers previously reported for future diabetes risk 20 ; for example, branched‐chain amino acids are strongly associated with incident diabetes, but were not associated with incident PAD in this study. As an additional novelty, we identified several nonlipid biomarkers to be strongly associated with future PAD events. Many of these novel biomarkers for PAD risk, including fatty acid measures and biomarkers related to glycolysis and inflammation, remained associated in the sensitivity analysis excluding all diabetes cases before PAD and CAD events.
In addition to the causative insights, there are also potential clinical implications of our results. First, our findings of novel metabolic biomarkers could guide the development of preventative therapies. These results reinforce the importance of focusing beyond LDL cholesterol levels for assessing PAD risk, 12 and we extend this message to suggest a focus also beyond small LDL particle numbers and HDL lipids. Ongoing trials are testing novel treatments for triglyceride‐rich lipoproteins and atherogenic dyslipidemia more widely and may hold potential to improve many of the triglyceride and fatty acid composition biomarkers herein shown to associate robustly with incident PAD. However, we emphasize that our findings are of observational nature, meaning we cannot make deductions on causation. Mendelian randomization analyses may eventually help to provide further information on the causative roles of fatty acids and triglyceride‐related pathways in PAD. 22 Second, although we concluded that standard lipid testing is unlikely to be adequate in PAD risk prediction, we identified novel risk factors for PAD that could potentially improve risk prediction. However, the additive predictive value of these biomarkers was not tested in the present study and needs to be evaluated in large independent data sets.
Our study has strengths and limitations. Strengths include large sample size, spanning 5 prospective cohorts. This enabled analyses of incident symptomatic PAD and CAD with a high number of events, obtained from nationwide registries with close to complete coverage of inpatient diagnoses. 13 , 14 , 15 , 16 We used a widely used high‐throughput metabolomics platform that has received regulatory approvals for diagnostics use. The biomarker associations were consistent across the cohorts, despite differences in follow‐up time and use of lipid‐lowering medication. Furthermore, the correlations between the routine lipids measured by clinical chemistry assays from fresh samples and by NMR 6 to 15 years after sample collection were high. The findings should be interpreted in the context of limitations of this study. First, our PAD diagnosis was based on hospitalized PAD events and, thus, does not cover milder forms of PAD. Second, because of observational study settings, causal conclusions cannot be made. However, these results demonstrate the potential of more accurate lipoprotein and metabolic profiling and call for additional studies to evaluate causality of these biomarkers. Third, although the samples were only semifasting in this study (median, 5 hours), we have previously shown that the analyzed panel of NMR‐based biomarkers has highly similar associations with incident cardiovascular events for nonfasting and fasting samples. 23 Finally, this study was conducted in Finnish population cohorts only. Large prospective studies in other populations are needed to evaluate generalizability and ethnic differences in the biomarker associations.
In conclusion, metabolic profiling of large prospective cohorts highlights prominent differences in the biomarker profile associated with future PAD events compared with CAD events. Although routine cholesterol and apolipoprotein measures were not robustly associated with the risk for PAD, metabolic biomarkers reflecting triglyceride metabolism, fatty acid balance, glycolysis, and chronic inflammation were stronger associated with future PAD events than with CAD event. This may suggest that the drivers for atherosclerosis processes are partly distinct in peripheral compared with coronary arteries; however, further studies are needed to determine whether these findings represent meaningful and clinically actionable pathophysiological differences. Our results highlight the need to look beyond atherogenic dyslipidemia for identification of patients at high risk for PAD while still in asymptomatic stages, and for informing development of preventative options and pharmacological treatments for this increasingly prevalent disease.
Sources of Funding
This work was primarily funded by Nightingale Health Plc. In addition, this research was also supported by Academy of Finland. Dr Salomaa was supported by the Finnish Foundation for Cardiovascular Research. Dr Holmes works in a unit that receives funding from the UK Medical Research Council and is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/23/33512) and the National Institute for Health Research Oxford Biomedical Research Centre. Dr Ala‐Korpela is supported by a research grant from the Sigrid Juselius Foundation, Finland. We acknowledge study participants for their availability and commitment, and THL biobank for providing the data.
Disclosures
Drs Tikkanen, Jägerroos, and Würtz are current or former shareholders and/or employees of Nightingale Health Plc, a company offering nuclear magnetic resonance–based biomarker profiling. Dr Salomaa has consulted for Novo Nordisk and Sanofi and received honoraria from these companies. He also has ongoing research collaboration with Bayer AG, all unrelated to this study. Dr Holmes has collaborated with Boehringer Ingelheim in research, and in accordance with the policy of the Clinical Trial Service Unit and Epidemiological Studies Unit (University of Oxford), did not accept any personal payment. Dr Sattar reports personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from Merck Sharp & Dohme, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Pfizer, and personal fees from Sanofi, outside the submitted work.
Supporting information
Figures S1–S6
Table S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021995
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849 [DOI] [PubMed] [Google Scholar]
- 2. Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–353. [DOI] [PubMed] [Google Scholar]
- 3. Joshi PH, Martin SS. Unraveling the risk of peripheral artery disease. Circulation. 2018;138:2342–2344. doi: 10.1161/CIRCULATIONAHA.118.036347 [DOI] [PubMed] [Google Scholar]
- 4. Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, Lee KM, Shao Q, Huffman JE, Natarajan P, et al. Genome‐wide association study of peripheral artery disease in the Million Veteran Program. Nat Med. 2019;25:1274–1279. doi: 10.1038/s41591-019-0492-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto‐Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population‐based cohorts. Circulation. 2015;131:774–785. doi: 10.1161/CIRCULATIONAHA.114.013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzoulaki I, Castagné R, Boulangé CL, Karaman I, Chekmeneva E, Evangelou E, Ebbels TMD, Kaluarachchi MR, Chadeau‐Hyam M, Mosen D, et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur Heart J. 2019;40:2883–2896. doi: 10.1093/eurheartj/ehz235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, Guo YU, Xu X, Bian Z, Hu R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71:620–632. doi: 10.1016/j.jacc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng S, Shah SH, Corwin EJ, Fiehn O, Fitzgerald RL, Gerszten RE, Illig T, Rhee EP, Srinivas PR, Wang TJ, et al. Potential impact and study considerations of metabolomics in cardiovascular health and disease: a scientific statement from the American Heart Association. Circ Cardiovasc Genet. 2017;10:e000032. doi: 10.1161/HCG.0000000000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Würtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, Tynkkynen T, Soininen P, Havulinna AS, Kaakinen M, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11:e1001765. doi: 10.1371/journal.pmed.1001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Würtz P, Wang Q, Soininen P, Kangas AJ, Fatemifar G, Tynkkynen T, Tiainen M, Perola M, Tillin T, Hughes AD, et al. Metabolomic profiling of statin use and genetic inhibition of HMG‐CoA reductase. J Am Coll Cardiol. 2016;67:1200–1210. doi: 10.1016/j.jacc.2015.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hazarika S, Annex BH. Biomarkers and genetics in peripheral artery disease. Clin Chem. 2017;63:236–244. doi: 10.1373/clinchem.2016.263798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation. 2018;138:2330–2341. doi: 10.1161/CIRCULATIONAHA.118.035432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borodulin K, Tolonen H, Jousilahti P, Jula A, Juolevi A, Koskinen S, Kuulasmaa K, Laatikainen T, Männistö S, Peltonen M, et al. Cohort profile: the National FINRISK study. Int J Epidemiol. 2017;47:696–696i. doi: 10.1093/ije/dyx239 [DOI] [PubMed] [Google Scholar]
- 14. Tolonen H, Salomaa V, Torppa J, Sivenius J, Immonen‐Räihä P, Lehtonen A, Finstroke R. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil. 2007;14:380–385. doi: 10.1097/01.hjr.0000239466.26132.f2 [DOI] [PubMed] [Google Scholar]
- 15. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. doi: 10.1177/1403494812456637 [DOI] [PubMed] [Google Scholar]
- 16. Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen‐Räihä P, Kärjä‐Koskenkari P, Mähönen M, Niemelä M, Kuulasmaa K, Palomäki P, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–137. [DOI] [PubMed] [Google Scholar]
- 17. Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala‐Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large‐scale epidemiology: a primer on ‐omic technologies. Am J Epidemiol. 2017;186:1084–1096. doi: 10.1093/aje/kwx016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soininen P, Kangas AJ, Würtz P, Suna T, Ala‐Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8:192–206. doi: 10.1161/CIRCGENETICS.114.000216 [DOI] [PubMed] [Google Scholar]
- 19. Tikkanen E, Minicocci I, Hällfors J, Di Costanzo A, D'Erasmo L, Poggiogalle E, Donini LM, Würtz P, Jauhiainen M, Olkkonen VM, et al. Metabolomic signature of angiopoietin‐like protein 3 deficiency in fasting and postprandial state. Arterioscler Thromb Vasc Biol. 2019;39:665–674. doi: 10.1161/ATVBAHA.118.312021 [DOI] [PubMed] [Google Scholar]
- 20. Ahola‐Olli AV, Mustelin L, Kalimeri M, Kettunen J, Jokelainen J, Auvinen J, Puukka K, Havulinna AS, Lehtimäki T, Kähönen M, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62:2298–2309. doi: 10.1007/s00125-019-05001-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holmes MV, Ala‐Korpela M. What is “LDL cholesterol”? Nat Rev Cardiol. 2019;16:197–198. doi: 10.1038/s41569-019-0157-6 [DOI] [PubMed] [Google Scholar]
- 22. Holmes MV, Ala‐Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14:577–590. doi: 10.1038/nrcardio.2017.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tikkanen E, Kanerva N, Aittomaki V, Männistö S, Salomaa VV, Würtz P. Abstract 10212: fasting samples are not required for NMR metabolic profiling studies of cardiovascular disease risk: prospective data for 4,400 individuals profiled few weeks apart. Circulation. 2019;140:A1021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S6
Table S1


