Abstract
Background
Evidence on the impact of sex on prognoses after myocardial infarction (MI) among older adults is limited. We evaluated sex differences in long‐term cardiovascular outcomes after MI in older adults.
Methods and Results
All patients with MI ≥70 years admitted to 20 Finnish hospitals during a 10‐year period and discharged alive were studied retrospectively using a combination of national registries (n=31 578, 51% men, mean age 79). The primary outcome was combined major adverse cardiovascular event within 10‐year follow‐up. Sex differences in baseline features were equalized using inverse probability weighting adjustment. Women were older, with different comorbidity profiles and rarer ST‐segment–elevation MI and revascularization, compared with men. Adenosine diphosphate inhibitors, anticoagulation, statins, and high‐dose statins were more frequently used by men, and renin‐angiotensin‐aldosterone inhibitors and beta blockers by women. After balancing these differences by inverse probability weighting, the cumulative 10‐year incidence of major adverse cardiovascular events was 67.7% in men, 62.0% in women (hazard ratio [HR], 1.17; CI, 1.13–1.21; P<0.0001). New MI (37.0% in men, 33.1% in women; HR, 1.16; P<0.0001), ischemic stroke (21.1% versus 19.5%; HR, 1.10; P=0.004), and cardiovascular death (56.0% versus 51.1%; HR, 1.18; P<0.0001) were more frequent in men during long‐term follow‐up after MI. Sex differences in major adverse cardiovascular events were similar in subgroups of revascularized and non‐revascularized patients, and in patients 70 to 79 and ≥80 years.
Conclusions
Older men had higher long‐term risk of major adverse cardiovascular events after MI, compared with older women with similar baseline features and evidence‐based medications. Our results highlight the importance of accounting for confounding factors when studying sex differences in cardiovascular outcomes.
Keywords: cohort study, coronary artery disease, gender differences, myocardial infarction, outcomes
Subject Categories: Acute Coronary Syndromes, Epidemiology, Mortality/Survival, Women
Nonstandard Abbreviations and Acronyms
- IPW
inverse probability weighting
- MACE
major adverse cardiovascular event
Clinical Perspective
What Is New?
After adjusting for age, comorbidities, myocardial infarction (MI) type, revascularization, and evidence‐based medications, older men (≥70 years) with MI had a 17% higher 10‐year risk of a major adverse cardiovascular event than women.
These findings persisted in subgroup analyses among patients with MI aged 70 to 79 or ≥80 and among revascularized and nonrevascularized patients.
What Are the Clinical Implications?
Our results highlight the importance of accounting for a wide range of confounding factors, including MI type and treatment, when studying sex differences in cardiovascular outcomes after MI.
Among older adults, female sex is not an independent negative prognostic factor after MI, but long‐term cardiovascular prognosis is worse among men.
Despite decreasing rates of myocardial infarction (MI) during the past 50 years, MI is a major contributor to morbidity in both sexes, and ischemic heart disease remains the leading cause of death globally. 1 Emerging evidence highlights sex differences in the pathophysiology, clinical presentation, and outcomes of MI. 2 , 3 In general, women experience their first MI at an older age and have higher comorbidity burdens than men. Women are traditionally thought to present more often with atypical MI symptoms, although the factuality of this statement has recently been questioned. 4 In addition, women may experience longer delays to reperfusion, lower revascularization rates, and less active pharmacological secondary prevention. 5 , 6 Partly because of these differences, many studies have reported higher mortality after MI among women, compared with men, in unadjusted analyses. 7 , 8 , 9 After adjusting for relevant confounding factors such as age, comorbidities, and treatment of MI, results have been more mixed. 8 , 9 , 10 , 11 Furthermore, worse prognoses after MI in women may be limited to hospitalization periods, not out‐of‐hospital deaths and long‐term outcomes. 9 , 12
Older patients have long been underrepresented in acute coronary syndrome trials, and a paucity of data exists about outcomes and their determinants after MI in older adults. 13 Older patients with acute coronary syndrome are more likely to present atypically, have more comorbidities, and worse outcomes than younger patients. 14 Many studies have demonstrated that, although short‐term mortality after MI may be higher among younger women compared with younger men, this sex difference is attenuated with increasing age. 15 , 16 Analyses of sex differences in MI outcomes focusing on older adults (≥70 years) are few and mostly do not support poorer prognoses among older women. 9 , 16 Although many previous studies exploring sex differences among older adults have focused on short‐ and intermediate‐term mortality after MI, 15 , 17 evidence on long‐term cardiovascular outcomes is limited.
Acknowledging the aforementioned gaps in the current knowledge, we explored sex differences in long‐term cardiovascular outcomes after MI in older adults. We used nationwide longitudinal register‐linkage data and addressed important confounders, including age, comorbidities, and type and treatment of MI by using inverse probability weighting (IPW) adjustments.
Methods
Study Design
From the Care Register for Health Care in Finland, we retrospectively collected data on all consecutive patients with MI aged ≥70 years admitted to participating hospitals in Finland between January 1, 2005 and December 31, 2014 and discharged alive. Participating hospitals comprised all MI‐treating hospitals in Finland equipped with a coronary catherization laboratory (n=20, including 5 hospitals with emergency cardiac surgery). The index MI was defined as the first‐time admission for an out‐of‐hospital MI during the study period, with International Classification of Diseases, Tenth Revision (ICD‐10) code I21 as the primary discharge diagnosis. Exclusion criteria were death within 90 days after MI admission, admission duration >180 days, valvular or aortic surgery, and missing follow‐up data (0.3% of patients) (Figure S1). The IPW method was used to create sex groups with balanced baseline features. Ward and hospital transfers were combined as a single admission.
The primary outcome of interest was composite major adverse cardiovascular event (MACE; new MI, ischemic stroke, or cardiovascular death). Secondary outcomes were cardiovascular death, new MI, and ischemic stroke. Outcomes were followed for 10 years, beginning 90 days after the index MI. In addition, we explored the use of evidence‐based secondary preventive cardiovascular medications within 90 days of discharge from the index MI, including both newly prescribed medications as well as medications that were continued during the 90‐day period after MI. Outcomes, comorbidities, and medications are defined in Data S1, Tables S1 and S2.
Study Data
Data of all hospital and emergency room admissions and major interventional procedures in Finland (Care Register for Health Care in Finland registry) and data on malignancies (Finnish Cancer Registry) were obtained from the National Institute for Health and Welfare of Finland (permission no: THL/2245/5.05.00/2019). Data on mortality were obtained from Statistics Finland (permission no: TK‐53‐484‐20). Data on prescription medication purchases and drug reimbursement permissions were obtained from the Social Insurance Institution of Finland (permission no: 91/522/2015). The aforementioned registries are mandatory by law and have full coverage of the Finnish population. 18 As our study used routinely recorded administrative health records, informed consent was not required, nor were the participants contacted. Like all studies utilizing only registry data, the study was exempt by law from institutional review board review. Legal grounds for the data handling are public interest and scientific research (European Union General Data Protection Regulation 2016/679, Article 6(1)(e) and Article 9(2)(j); Data Protection Act, Sections 4 and 6).
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Findata at http://findata.fi/en.
Statistical Analysis
Differences between study groups were analyzed with t test, Jonckheere‐Terpstra test, and chi‐square test. Effect sizes in baseline characteristics between groups were evaluated with standardized mean difference. Propensity scores based on age, alcohol abuse, anemia, atrial fibrillation, cerebrovascular disease, chronic pulmonary disease, coagulopathy, dementia, depression, heart failure, hypertension, hypothyroidism, insulin‐dependent diabetes, liver disease, malignancy, noninsulin dependent diabetes, paralysis, peripheral vascular disease, prior coronary artery bypass grafting, psychotic disorder, rheumatic disease, renal failure, valvular disease, revascularization by percutaneous coronary intervention or coronary artery bypass grafting, type of MI, use of cardiovascular medications after MI (adenosine diphosphate (ADP) receptor inhibitors, anticoagulants, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, aldosterone antagonists, antiarrhythmics, beta blockers, ezetimibe, and statins), treating hospital, and study year for female sex were created with logistic regressions. The inverse probability weight was calculated using a propensity score, and IPW was stabilized (mean 1.00; SD 0.48). Weighting resulted in balanced study groups with 0.003 standardized mean difference of propensity score (Table 1). Separate IPW balancing without post‐MI medications in the propensity score was performed to study medication use. Subgroup analyses with separate propensity scoring and IPW balancing were performed for revascularized and nonrevascularized patients and for patients aged 70 to 79 and ≥80 years. Patient features and used medications were balanced in all subgroups (standardized mean difference ≤0.011 for all variables). The extent of unmeasured confounding was estimated by the E‐value. 19 The Kaplan–Meier method and Cox regression were used to analyze time‐dependent outcomes. Median follow‐up was 4.8 (interquartile range 2.3–7.5) years. Mean follow‐up was 5.1 years in men and 4.9 years in women. Cause‐specific hazard models were applied in outcome analyses. 20 Proportional hazard assumptions were confirmed by Schoenfeld residuals. Logistic regression was used for the analysis of medication use. All analyses of balanced cohorts were weighted with stabilized IPW. In addition, multivariable Cox models adjusted with the same variables that were used for propensity scoring were analyzed. Results are given as the mean, percentage, hazard ratio (HR), or odds ratio with 95% CI or ±SD. Statistical significance was inferred at P value <0.05. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Table 1.
Baseline Features of Inverse Probability Weighted Older Men and Women With Myocardial Infarction
| Variable | Men | Women | P value | |SMD| |
|---|---|---|---|---|
| n=16 083 | n=15 495 | |||
| Age, y (SD) | 79.3 (6.1) | 79.3 (6.1) | 0.858 | 0.002 |
| Comorbidities | ||||
| Alcohol abuse | 1.2% | 1.1% | 0.898 | 0.001 |
| Anemia | 5.0% | 4.9% | 0.849 | 0.002 |
| Atrial fibrillation | 22.1% | 22.4% | 0.619 | 0.006 |
| Cerebrovascular disease | 14.9% | 14.8% | 0.875 | 0.002 |
| Chronic pulmonary disease | 15.5% | 15.7% | 0.655 | 0.005 |
| Coagulopathy | 0.4% | 0.4% | 0.642 | 0.005 |
| Dementia | 8.5% | 8.3% | 0.648 | 0.005 |
| Depression | 10.7% | 10.7% | 0.942 | 0.001 |
| Diabetes | 27.5% | 27.5% | 0.883 | 0.002 |
| Insulin dependent | 9.0% | 9.2% | 0.707 | 0.004 |
| Noninsulin dependent | 18.4% | 18.4% | 0.913 | 0.001 |
| Heart failure | 30.6% | 30.7% | 0.919 | 0.001 |
| Hypertension | 57.6% | 57.8% | 0.715 | 0.004 |
| Hypothyroidism | 5.9% | 5.9% | 0.977 | 0.0003 |
| Liver disease | 0.7% | 0.8% | 0.667 | 0.005 |
| Malignancy | 15.9% | 16.2% | 0.429 | 0.009 |
| Metastatic tumor | 0.3% | 0.3% | 0.887 | 0.002 |
| Paralysis | 0.4% | 0.4% | 0.975 | 0.0003 |
| Peripheral vascular disease | 9.3% | 9.3% | 0.928 | 0.001 |
| Prior CABG | 4.4% | 4.4% | 0.841 | 0.002 |
| Prior MI | 22.9% | 23.0% | 0.893 | 0.002 |
| Psychotic disorder | 2.9% | 2.9% | 0.815 | 0.003 |
| Rheumatic disease | 7.3% | 7.3% | 0.865 | 0.002 |
| Renal failure | 4.2% | 4.1% | 0.624 | 0.006 |
| Valvular disease | 7.4% | 7.4% | 0.906 | 0.001 |
| Revascularization | 42.7% | 42.3% | 0.534 | 0.001 |
| Percutaneous coronary intervention | 36.4% | 36.1% | 0.627 | 0.005 |
| CABG | 6.8% | 6.8% | 0.754 | 0.004 |
| ST‐segment–elevation MI | 29.1% | 29.2% | 0.948 | 0.001 |
| Anterior* | 53.2% | 52.9% | 0.819 | 0.005 |
| Post‐MI medication | ||||
| ADP inhibitor | 53.8% | 53.9% | 0.935 | 0.001 |
| Prasugrel or ticagrelor † | 6.8% | 6.5% | 0.530 | 0.002 |
| Anticoagulant | 17.3% | 17.4% | 0.906 | 0.001 |
| Direct oral anticoagulant ‡ | 0.9% | 0.6% | 0.136 | 0.039 |
| Angiotensin‐converting‐enzyme inhibitor or angiotensin receptor blocker | 63.2% | 63.3% | 0.953 | 0.001 |
| Aldosterone antagonist | 4.5% | 4.4% | 0.770 | 0.003 |
| Antiarrhythmic | 1.3% | 1.4% | 0.640 | 0.005 |
| Beta blocker | 81.9% | 81.9% | 0.874 | 0.002 |
| Ezetimibe | 1.5% | 1.5% | 0.943 | 0.001 |
| Statin | 73.2% | 73.3% | 0.930 | 0.001 |
| High‐dose statin § | 10.6% | 10.6% | 0.976 | 0.0004 |
| Treating hospital (n=20) | 0.234 | 0.003 | ||
| Admission year | 0.816 | 0.003 | ||
ADP indicates adenosine diphosphate; CABG, coronary artery bypass grafting surgery; MI, myocardial infarction; and SMD, standardized mean difference.
Of patients with ST‐segment–elevation MI.
Of ADP inhibitor users.
Of anticoagulant users.
Of statin users.
Results
Unadjusted Cohort
In the unadjusted cohort, men were younger and had chronic pulmonary disease, malignancies, peripheral vascular disease, history of coronary artery bypass grafting or MI, and ST‐segment–elevation at presentation more often, and were more frequently revascularized, than women (Table S3). Atrial fibrillation, heart failure, hypertension, hypothyroidism, dementia, psychiatric, and rheumatic diseases were more common among women in the unadjusted cohort. Nonadjusted cumulative incidence of MACE, MI, and cardiovascular death was higher among women (Table S4).
IPW‐Adjusted Cohort
Risk of MACE
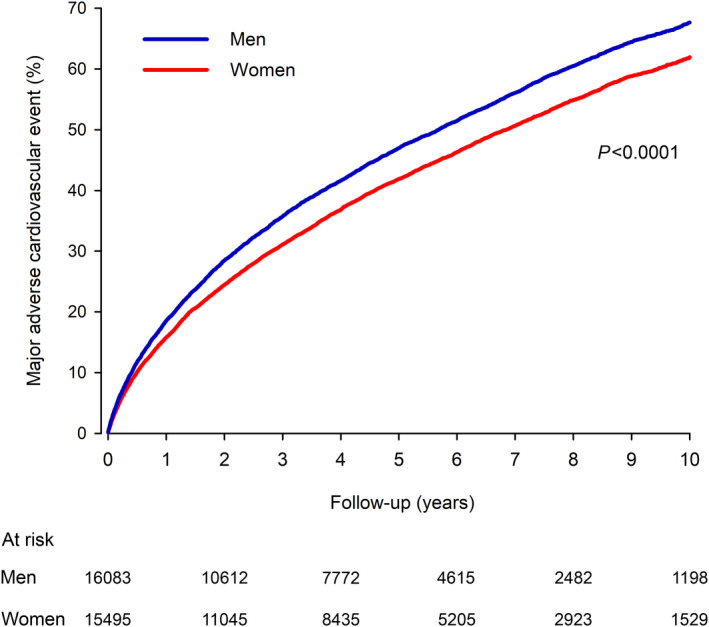
Differences in baseline features and the use of post‐MI medications were equalized with IPW adjustments, resulting in a balanced study population of 16 083 men and 15 495 men with a median age of 79 years (Table 1). MACE occurred in 8508 men and 7620 women in the IPW cohort during the follow‐up period (Figure 1). The cumulative incidence of MACE was 18.6% in men versus 15.8% in women after 1‐year follow‐up (P<0.0001), 47.0% versus 41.9% after 5‐year follow‐up (P<0.0001), and 67.7% versus 62.0% at the end of 10‐year follow‐up (HR, 1.17; CI, 1.13–1.21; P<0.0001). The E‐value was 1.62 (CI, 1.51–1.71). Multivariable adjusted HRs in the unadjusted cohort were comparable to IPW‐adjusted results (Table S4).
Figure 1. Adjusted cumulative incidence of major adverse cardiovascular events in older men and women after myocardial infarction.
Secondary Outcomes
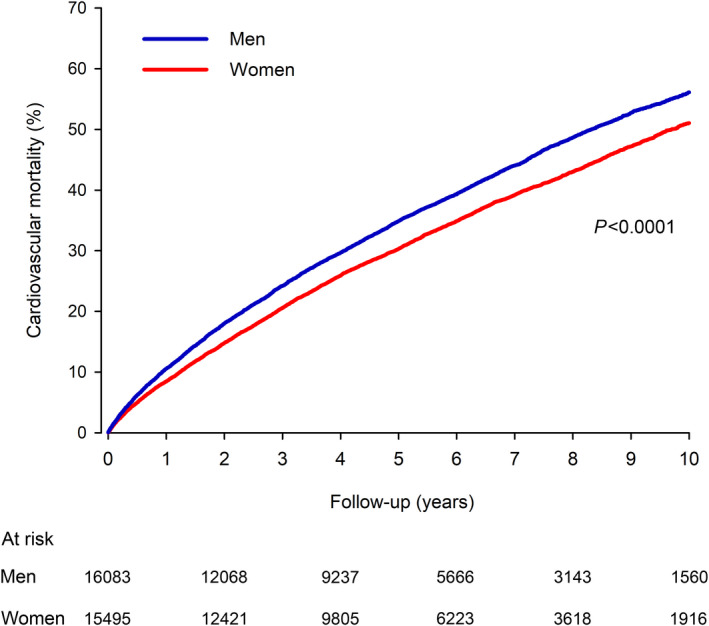
A new MI occurred in 4059 men and 3617 women (Figure 2). The cumulative incidence of new MI was 10.5% in men versus 9.1% in women within the 1‐year follow‐up period (P=0.0003). At 10 years, the cumulative incidence of new MI was 37.0% in men and 33.1% in women (HR, 1.16; CI, 1.11–1.21; P<0.0001). During the follow‐up period, 1874 men and 1783 women had an ischemic stroke, with cumulative incidence of 3.7% in men versus 3.2% in women (P=0.045) at 1 year and 21.1% versus 19.5% at 10 years (HR, 1.10; CI, 1.03–1.18; P=0.004) (Figure 2). Deaths due to cardiovascular causes occurred in 6596 men and 5876 women. The cardiovascular mortality rate was 10.6% in men versus 8.5% in women at 1 year (P<0.0001) and 56.0% versus 51.1% at 10‐year follow‐up (HR, 1.18; CI, 1.13–1.22; P<0.0001) (Figure 3). Also for secondary outcomes, multivariable adjusted HRs in the unadjusted cohort were comparable to IPW‐adjusted results (Table S4).
Figure 2. Adjusted cumulative incidence of new myocardial infarction (A) and ischemic stroke (B) in older men and women after myocardial infarction.
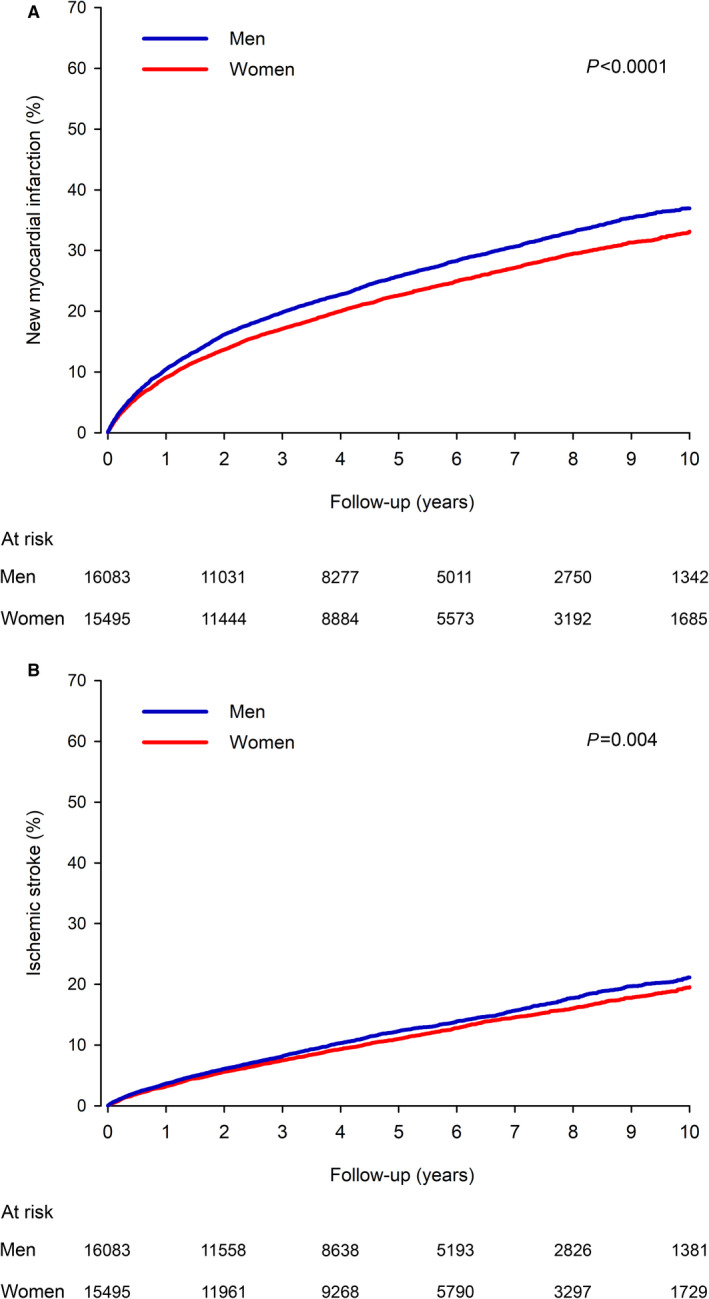
Figure 3. Adjusted cardiovascular mortality in older men and women after myocardial infarction.
Subgroup Analyses
Men had a higher hazard of MACE after MI in the subgroups of revascularized and non‐revascularized patients, and in patients aged 70 to 79 and ≥80 years (Table 2). Differences in adjusted hazards of MACE, ischemic stroke, and cardiovascular death were similar in the revascularized and nonrevascularized patients and in both age groups. A new MI was more frequent in non‐revascularized men compared with nonrevascularized women, but no sex difference was detected in revascularized patients (Table 2).
Table 2.
Sex‐Based Outcomes in IPW‐adjusted Subgroups of Revascularized and Nonrevascularized Patients and of Patients Aged 70 to 79 and ≥80 Years After Myocardial Infarction
| Ten‐year incidence | HR (95% CI) | P value | ||
|---|---|---|---|---|
| Men | Women | |||
| All patients | ||||
| Major adverse cardiovascular event | 67.7% | 62.0% | 1.17 (1.13–1.21) | <0.0001 |
| New myocardial infarction | 37.0% | 33.1% | 1.16 (1.11–1.21) | <0.0001 |
| Ischemic stroke | 21.1% | 19.5% | 1.10 (1.03–1.18) | 0.004 |
| Cardiovascular death | 56.0% | 51.1% | 1.18 (1.13–1.22) | <0.0001 |
| Revascularized patients | ||||
| Major adverse cardiovascular event | 52.7% | 47.3% | 1.15 (1.08–1.21) | <0.0001 |
| New myocardial infarction | 25.9% | 23.9% | 1.06 (0.97–1.15) | 0.181 |
| Ischemic stroke | 16.8% | 15.0% | 1.13 (1.01–1.26) | 0.027 |
| Cardiovascular death | 37.7% | 33.8% | 1.17 (1.09–1.25) | <0.0001 |
| Nonrevascularized patients | ||||
| Major adverse cardiovascular event | 79.5% | 73.4% | 1.21 (1.16–1.25) | <0.0001 |
| New myocardial infarction | 47.0% | 41.5% | 1.23 (1.16–1.29) | <0.0001 |
| Ischemic stroke | 25.9% | 24.1% | 1.10 (1.01–1.19) | 0.024 |
| Cardiovascular death | 70.8% | 64.5% | 1.21 (1.16–1.26) | <0.0001 |
| Age 70 to 79 y | ||||
| Major adverse cardiovascular event | 55.9% | 49.8% | 1.20 (1.14–1.26) | <0.0001 |
| New myocardial infarction | 29.4% | 26.2% | 1.16 (1.08–1.24) | <0.0001 |
| Ischemic stroke | 18.6% | 17.3% | 1.10 (1.00–1.20) | 0.0499 |
| Cardiovascular death | 40.4% | 35.6% | 1.23 (1.16–1.30) | <0.0001 |
| Age ≥80 y | ||||
| Major adverse cardiovascular event | 85.1% | 80.5% | 1.18 (1.13–1.23) | <0.0001 |
| New myocardial infarction | 49.6% | 45.0% | 1.19 (1.12–1.27) | <0.0001 |
| Ischemic stroke | 26.1% | 24.1% | 1.11 (1.00–1.23) | 0.046 |
| Cardiovascular death | 80.4% | 74.7% | 1.17 (1.12–1.23) | <0.0001 |
HR indicates hazard ratio, IPW indicates inverse probability weight
Use of Secondary Preventive Medications
The use of evidence‐based medication after MI differed between sexes after IPW adjustment for baseline features including age, multiple comorbidities, revascularization, and MI type (Table 3). ADP receptor inhibitors, anticoagulants, and statins were used more frequently by men. Furthermore, when used, statins were more often at high dosages in men (Table 3). Beta blockers, renin‐angiotensin‐aldosterone system inhibiting medications, and ezetimibe were used more frequently by women (Table 3). Sex differences in ADP receptor inhibitor use were present in nonrevascularized patients with MI (35.1% in men versus 31.6% in women) but not in revascularized patients (81.7% versus 81.6%, respectively) (Table S5). Women were more likely to use angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, and ezetimibe, regardless of revascularization status. There was no sex difference in statin use (88.6% in both sexes) and the proportion of high‐dose statin users in the subgroup of revascularized patients.
Table 3.
Adjusted Use of Cardiovascular Prescription Medication After Myocardial Infarction, by Sex, in Older Patients
| Men | Women | OR (95% CI) | P value | |
|---|---|---|---|---|
| n=16 085 | n=15 489 | |||
| ADP inhibitor | 55.0% | 52.7% | 1.10 (1.05–1.15) | <0.0001 |
| Prasugrel or ticagrelor* | 6.7% | 6.7% | 1.00 (0.87–1.13) | 0.998 |
| Anticoagulant | 17.7% | 16.9% | 1.10 (1.05–1.15) | <0.0001 |
| Direct oral anticoagulant † | 0.5% | 0.9% | 0.61 (0.32–1.17) | 0.139 |
| Angiotensin‐converting‐enzyme inhibitor or angiotensin receptor blocker | 61.9% | 64.7% | 0.89 (0.85–0.93) | <0.0001 |
| Aldosterone antagonist | 4.1% | 4.8% | 0.86 (0.77–0.95) | 0.005 |
| Antiarrhythmic | 1.4% | 1.3% | 1.05 (0.86–1.27) | 0.642 |
| Beta‐blocker | 80.7% | 83.1% | 0.85 (0.81–0.90) | <0.0001 |
| Ezetimibe | 1.2% | 1.9% | 0.60 (0.50–0.72) | <0.0001 |
| Statin | 73.8% | 72.7% | 1.06 (1.01–1.11) | 0.029 |
| High‐dose statin ‡ | 11.0% | 10.0% | 1.10 (1.02–1.20) | 0.021 |
ADP indicates adenosine diphosphate; and OR, odds ratio.
Of ADP inhibitor users.
Of anticoagulant users.
Of statin users.
Discussion
Focusing on adults aged ≥70 years, this IPW study investigated sex differences in long‐term outcomes following admission for MI in a nationwide Finnish setting. Older men were found to have 1.2‐fold hazard of 10‐year MACE, compared with older women, after equalizing differences in baseline features, including secondary preventive medications, comorbidities, MI type, and revascularization. Worse long‐term prognoses in men were apparent in subgroups aged 70 to 79 and ≥80 years, as well as in revascularized and nonrevascularized patients. Our findings were consistent across our secondary outcomes, which comprised cardiovascular death, new MI, and ischemic stroke.
Despite the high burden of coronary heart disease in older adults, evidence to guide decision‐making in older patients with MI is sparse. 13 Means for risk stratification applicable to older adults are needed to identify those patients most likely to benefit from interventions and more aggressive secondary prevention. 21 Previous studies have suggested that, while younger women may have impaired short‐term prognoses after MI compared with younger men, this sex difference disappears or even reverses in older adults. 9 , 16 Our finding of male sex as a predictor of adverse long‐term cardiovascular outcomes is well aligned with a few previous studies focusing on adults aged ≥70 years. 6 , 17 A Spanish multicenter study evaluating long‐term outcomes among 680 acute coronary syndrome patients aged ≥90 years showed that, despite more frailty, disability, and lower percutaneous coronary intervention frequency, women had better survival after percutaneous coronary intervention. 17 A Swedish registry study reported lower 1‐year mortality after MI among female 28‐day survivors aged 75 to 84 years, compared with their male counterparts. 9 In our previous study within the same register‐linkage setting, 30‐day and 1‐year case fatality rates after MI were lower in women ≥70, compared with men, after extensive adjustments for comorbidity burdens and guideline‐recommended treatments. 22 Now, we have discovered that this survival disadvantage in older men extends 10 years after an index MI.
In line with our results, previous studies have revealed a pattern of improving prognoses in women, along with increasing adjustments for sex differences in age, risk factors, comorbidities, and treatments. 9 , 12 A meta‐analysis of 35 observational studies exploring sex differences in 1‐year mortality after primary percutaneous coronary intervention‐treated ST‐segment–elevation MI concluded that the seemingly higher mortality rate in women is likely to be confounded by differences in cardiovascular risk factors and clinical profile. 2 A report from the SWEDEHEART register showed that, although women with ST‐segment–elevation MI had a lower probability of reperfusion therapy and higher short‐term mortality than men, long‐term mortality was higher in men in multivariable‐adjusted analyses. 12 The nonadjusted incidence of cardiovascular outcomes was also higher among women in our data. Adequate adjustments are thus crucial in studies aiming to explore the independent effects of sex on cardiovascular outcomes.
Biological, social, environmental, and community factors may contribute to sex/gender differences in health. Why female sex has, in many previous studies, been a predictor of case fatality after MI, especially among younger adults, is a matter of debate. Although adjustments for age and comorbidities have attenuated sex differences, additional explanations include dissimilarities in pathophysiology and inequities in evidence‐based treatment. The mechanisms of MI may differ between sexes, too, with more plaque erosions, coronary spasms, and spontaneous coronary artery dissections, especially in younger women. 3 , 23 , 24 These differences, however, may not characterize older women. A study of 6022 ST‐segment–elevation MI patients showed that worse prognoses among women were apparent only in patients with prehospital delays lasting more than 1 hour, suggesting that women may be more vulnerable to prolonged, untreated ischemia. 25
Both younger and older women may receive less active pharmacological and invasive treatments after MI than their male counterparts. 6 , 26 , 27 , 28 An Austrian study of MI patients ≥80 years revealed a lower intention to coronary intervention among women, compared with men, which was not explained by differences in risk factors for adverse events. 6 Despite this, the benefit of coronary procedures was higher among older women. 6 In line with these findings, we observed a lower revascularization rate in older women, compared with men (35% versus 49% in unadjusted cohorts), but among revascularized and nonrevascularized patients, men had worse long‐term outcomes. The low revascularization rate among women with MI may be related to an increased prevalence of nonobstructive coronary artery disease in coronary angiography, a finding that is linked to better prognoses. 29
Factors possibly contributing to poorer long‐term outcomes among older men are multifold. Men with acute coronary syndrome often present with more severe atherosclerosis, obstructive lesions, necrotic plaques, and plaque ruptures in their coronary arteries than women. 3 , 30 We did not have data on smoking, which may contribute to worse long‐term outcomes among men. These factors may have contributed to the 16% higher risk of reinfarction among men in our cohort, which was apparent in subgroups aged 70 to 79 and ≥80, and in nonrevascularized patients. In general, women have a higher life expectancy and lower risk of sudden cardiac death than men, which is likely to be reflected in the observed sex differences in cardiovascular mortality rates. 31 Hypothetical biological explanations for this survival gap include an asymmetric inheritance of sex chromosomes that exposes males to deleterious recessive mutations in the X chromosome and differing hormonal profiles and responses to oxidative stress and inflammatory stimuli. 32
In older adults, frailty is a strong predictor of mortality and associated with impaired outcomes after MI. 33 , 34 An interesting phenomenon linked to sex, frailty, and survival is the “male–female health‐survival paradox,” which refers to the inverse association of older women experiencing more multimorbidity and frailty but living longer than men. 35 Even though we accounted for a wide range of comorbidities associated with frailty, it may be considered a potential confounder in our analyses.
The sex differences in the secondary preventive medications noted in our study deserve some attention. Although differences were small, older women were dispensed angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, beta blockers, aldosterone antagonists, and ezetimibe slightly more often than older men were. Conversely, men were more often dispensed ADP receptor inhibitors, anticoagulants, and statins. This difference was also apparent in high‐intensity statin treatment. Among older populations, the benefits of statin use are well established in secondary prevention 36 and may be associated with lower mortality rates also in primary prevention. 37 Interestingly, following high‐intensity statin treatment, coronary atheroma regression measured by intravascular ultrasound may be more pronounced among women than men. 38 Overall, although statins are the cornerstone of MI secondary prevention, the proportion of patients with a dispensed statin prescription was rather low in both sexes (72%–74%), warranting special attention to post‐MI pharmacotherapy in older adults. Usage of new ADP receptor inhibitors and direct oral anticoagulants was low during our study period and there were no sex‐dependent differences in their appliance. Potential sex differences in prasugrel or ticagrelor and direct oral anticoagulants usage later in the transition phase to these new more potent therapies remains to be studied. Poor adherence to secondary preventive medications has been associated with female sex in both younger and older patients and especially after non‐ST‐segment–elevation MI. 39 Because we described the frequency of drugs dispensed from pharmacies within the maximum length of 1 reimbursement period (90 days) after discharge, our findings reflect the early usage of secondary preventive medications after MI rather than long‐term adherence. Of note, unlike all other cardiovascular medications, aspirin is available over the counter without prescription in Finland and was therefore not included in our analyses.
The strengths of the study deserve brief discussion. We used a combination of previously validated, nationwide registries to avoid selection bias and adjusted the results with a broad coverage of confounders. 40 Propensity scoring and IPW are valuable tools for controlling confounding factors in observational studies. 41 Residual confounding by factors not recognized in the national health registries is nevertheless possible and may influence the results of the study. We lacked more detailed information on laboratory measures, imaging, socioeconomic status, lifestyle, and functional impairment. Based on the E‐value, the observed HR of 1.17 could be explained by an unmeasured confounder that was associated with sex and MACE by a risk ratio of 1.6‐fold each, above and beyond the measured confounders, but weaker confounding could not do so. 19 We did not have data on the race or ethnic backgrounds of study subjects, but because the Finnish population is predominantly White, the generalizability of our results to more diverse populations may be limited. An inherent limitation to administrative registries is that of coding errors. However, it is likely that these errors happen at a similar rate in both sexes, and thus it is unlikely that they would significantly affect our main findings.
Conclusions
In conclusion, after adjustments for a broad spectrum of confounding factors including age, baseline comorbidities, and treatment of MI, older men had worse long‐term cardiovascular prognoses after MI compared with older women. Our results highlight the importance of comprehensive adjustments for confounding factors when studying sex differences in cardiovascular outcomes. Although we recognize that women of all ages may receive fewer evidence‐based treatments after an MI than men, our findings indicate that, when no dissimilarities in risk factors, comorbidities, clinical presentation, and treatment of MI exist, male sex is an independent negative prognostic factor after MI in older adults. Based on our findings, we believe that, in addition to emphasizing intention to coronary procedures and intensive pharmacological secondary prevention on the same grounds in both sexes, older men may deserve special attention after MI because of their worse long‐term cardiovascular prognoses.
Sources of Funding
This study was supported by grant funding for VK from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Paulo Foundation, and the Finnish Governmental VTR‐funding. AK was supported by a research grant from the FOREUM Foundation for Research in Rheumatology.
Disclosures
AK has received speaker fees from Boehringer‐Ingelheim and Sanofi; has attended advisory board meetings of Pfizer, Gilead, and Boehringer‐Ingelheim; and received congress sponsorship from Pfizer, Celgene, UCB Pharma, Mylan, and Roche. AP has received grants from the Finnish Medical Foundation, the Finnish Foundation for Cardiovascular Research, and the Turku University Hospital Research Foundation; a consulting fee from Pfizer; lecture fees from MSD, Pfizer, and Sanofi; and travel expenses from Bristol‐Myers‐Squib and Novartis. VK has received scientific consultancy fees from AstraZeneca; speaker fees from Bayer, Boehringer‐Ingelheim, and Roche; and travel grants and congress sponsorships from AstraZeneca, Boehringer‐Ingelheim, Bayer, and Pfizer. The remaining authors have no disclosures to report.
Supporting information
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022883
For Sources of Funding and Disclosures, see page 10.
References
- 1. Nowbar AN, Gitto M, Howard JP, Francis DP, Al‐Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12:e005375. doi: 10.1161/CIRCOUTCOMES.118.005375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pancholy SB, Shantha GP, Patel T, Cheskin LJ. Sex differences in short‐term and long‐term all‐cause mortality among patients with ST‐segment elevation myocardial infarction treated by primary percutaneous intervention: a meta‐analysis. JAMA Intern Med. 2014;174:1822–1830. doi: 10.1001/jamainternmed.2014.4762 [DOI] [PubMed] [Google Scholar]
- 3. Chandrasekhar J, Mehran R. Sex‐based differences in acute coronary syndromes: insights from invasive and noninvasive coronary technologies. JACC Cardiovasc Imaging. 2016;9:451–464. doi: 10.1016/j.jcmg.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 4. Ferry AV, Anand A, Strachan FE, Mooney L, Stewart SD, Marshall L, Chapman AR, Lee KK, Jones S, Orme K, et al. Presenting symptoms in men and women diagnosed with myocardial infarction using sex‐specific criteria. J Am Heart Assoc. 2019;8:e012307. doi: 10.1161/JAHA.119.012307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 6. Sulzgruber P, Koller L, Pavo N, El‐Hamid F, Rothgerber DJ, Forster S, Maurer G, Goliasch G, Niessner A. Gender‐related differences in elderly patients with myocardial infarction in a European Centre. Eur J Clin Invest. 2016;46:60–69. doi: 10.1111/eci.12567 [DOI] [PubMed] [Google Scholar]
- 7. Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long‐term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–767. doi: 10.1161/CIRCULATIONAHA.114.009480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kvakkestad KM, Fagerland MW, Eritsland J, Halvorsen S. Gender differences in all‐cause, cardiovascular and cancer mortality during long‐term follow‐up after acute myocardial infarction; a prospective Cohort study. BMC Cardiovasc Disord. 2017;17:75–83. doi: 10.1186/s12872-017-0508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berg J, Bjorck L, Nielsen S, Lappas G, Rosengren A. Sex differences in survival after myocardial infarction in Sweden, 1987–2010. Heart. 2017;103:1625–1630. doi: 10.1136/heartjnl-2016-310281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dreyer RP, Dharmarajan K, Kennedy KF, Jones PG, Vaccarino V, Murugiah K, Nuti SV, Smolderen KG, Buchanan DM, Spertus JA, et al. Sex differences in 1‐year all‐cause rehospitalization in patients after acute myocardial infarction: a prospective observational study. Circulation. 2017;135:521–531. doi: 10.1161/CIRCULATIONAHA.116.024993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyto V, Sipila J, Rautava P. Gender and in‐hospital mortality of ST‐segment elevation myocardial infarction (from a multihospital nationwide registry study of 31,689 patients). Am J Cardiol. 2015;115:303–306. doi: 10.1016/j.amjcard.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 12. Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. A gender perspective on short‐ and long‐term mortality in ST‐elevation myocardial infarction–a report from the SWEDEHEART register. Int J Cardiol. 2013;168:1041–1047. doi: 10.1016/j.ijcard.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 13. Tahhan AS, Vaduganathan M, Greene SJ, Alrohaibani A, Raad M, Gafeer M, Mehran R, Fonarow GC, Douglas PS, Bhatt DL, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. 2020;5:714–722. doi: 10.1001/jamacardio.2020.0359 [DOI] [PubMed] [Google Scholar]
- 14. Soiza RL, Leslie SJ, Harrild K, Peden NR, Hargreaves AD. Age‐dependent differences in presentation, risk factor profile, and outcome of suspected acute coronary syndrome. J Am Geriatr Soc. 2005;53:1961–1965. doi: 10.1111/j.1532-5415.2005.53573.x [DOI] [PubMed] [Google Scholar]
- 15. Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, et al. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med. 2018;178:632–639. doi: 10.1001/jamainternmed.2018.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex‐based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401 [DOI] [PubMed] [Google Scholar]
- 17. Cepas‐Guillen PL, Echarte‐Morales J, Flores‐Umanzor E, Fernandez‐Valledor A, Caldentey G, Viana‐Tejedor A, Martinez‐Gomez E, Tundidor‐Sanz E, Borrego‐Rodriguez J, Vidal P, et al. Sex‐gender disparities in nonagenarians with acute coronary syndrome. Clin Cardiol. 2021;44:371–378. doi: 10.1002/clc.23545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sipila JO, Ruuskanen JO, Kauko T, Rautava P, Kyto V. Seasonality of stroke in Finland. Ann Med. 2017;49:310–318. doi: 10.1080/07853890.2016.1254350 [DOI] [PubMed] [Google Scholar]
- 19. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 20. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, et al. Knowledge gaps in cardiovascular care of older adults: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: executive summary. Circulation. 2016;133:2103–2122. doi: 10.1161/CIR.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 22. Kytö V, Nuotio M, Rautava P. Sex difference in the case fatality of older myocardial infarction patients. J Gerontol A Biol Sci Med Sci. 2021. May 28 [epub ahead of print]. doi: 10.1093/gerona/glab152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.CIR.97.21.2110 [DOI] [PubMed] [Google Scholar]
- 24. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJM, Rihal CS, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. doi: 10.1161/CIRCULATIONAHA.112.105718 [DOI] [PubMed] [Google Scholar]
- 25. Bugiardini R, Ricci B, Cenko E, Vasiljevic Z, Kedev S, Davidovic G, Zdravkovic M, Miličić D, Dilic M, Manfrini O, et al. Delayed care and mortality among women and men with myocardial infarction. J Am Heart Assoc. 2017;6:e005968. doi: 10.1161/JAHA.117.005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shehab A, Bhagavathula AS, Alhabib KF, Ullah A, Suwaidi JA, Almahmeed W, AlFaleh H, Zubaid M. Age‐related sex differences in clinical presentation, management, and outcomes in ST‐segment‐elevation myocardial infarction: pooled analysis of 15 532 patients from 7 Arabian Gulf Registries. J Am Heart Assoc. 2020;9:e013880. doi: 10.1161/JAHA.119.013880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters SAE, Colantonio LD, Zhao H, Bittner V, Dai Y, Farkouh ME, Monda KL, Safford MM, Muntner P, Woodward M. Sex differences in high‐intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol. 2018;71:1729–1737. doi: 10.1016/j.jacc.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 28. Du X, Spatz ES, Dreyer RP, Hu S, Wu C, Li XI, Li J, Wang S, Masoudi FA, Spertus JA, et al. Sex differences in clinical profiles and quality of care among patients with ST‐segment elevation myocardial infarction from 2001 to 2011: insights from the China Patient‐Centered Evaluative Assessment of Cardiac Events (PEACE)‐Retrospective Study. J Am Heart Assoc. 2016;5:e002157. doi: 10.1161/JAHA.115.002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, Reynolds HR. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry‐GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry‐Get With the Guidelines). Circ Cardiovasc Qual Outcomes. 2017;10:e003443. doi: 10.1161/CIRCOUTCOMES.116.003443 [DOI] [PubMed] [Google Scholar]
- 30. Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, De Bruyne B, Farhat N, Niess G, Jankovic I, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:62. doi: 10.1016/j.jcmg.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 31. Haukilahti MAE, Holmström L, Vähätalo J, Kenttä T, Tikkanen J, Pakanen L, Kortelainen M‐L, Perkiömäki J, Huikuri H, Myerburg RJ, et al. Sudden cardiac death in women. Circulation. 2019;139:1012–1021. doi: 10.1161/CIRCULATIONAHA.118.037702 [DOI] [PubMed] [Google Scholar]
- 32. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kulmala J, Nykanen I, Hartikainen S. Frailty as a predictor of all‐cause mortality in older men and women. Geriatr Gerontol Int. 2014;14:899–905. doi: 10.1111/ggi.12190 [DOI] [PubMed] [Google Scholar]
- 34. Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. Association of frailty with 30‐day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 2019;4:1084–1091. doi: 10.1001/jamacardio.2019.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta‐analysis. Exp Gerontol. 2017;89:30–40. doi: 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 36. Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of statin therapy in older people: a meta‐analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orkaby AR, Driver JA, Ho Y‐L, Lu B, Costa L, Honerlaw J, Galloway A, Vassy JL, Forman DE, Gaziano JM, et al. Association of statin use with all‐cause and cardiovascular mortality in US veterans 75 years and older. JAMA. 2020;324:68–78. doi: 10.1001/jama.2020.7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Puri R, Nissen SE, Shao M, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Raichlen JS, Uno K, et al. Sex‐related differences of coronary atherosclerosis regression following maximally intensive statin therapy: insights from SATURN. JACC Cardiovasc Imaging. 2014;7:1013–1022. doi: 10.1016/j.jcmg.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 39. Eindhoven DC, Hilt AD, Zwaan TC, Schalij MJ, Borleffs CJW. Age and gender differences in medical adherence after myocardial infarction: women do not receive optimal treatment ‐ the Netherlands claims database. Eur J Prev Cardiol. 2018;25:181–189. doi: 10.1177/2047487317744363 [DOI] [PubMed] [Google Scholar]
- 40. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. doi: 10.1177/1403494812456637 [DOI] [PubMed] [Google Scholar]
- 41. Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, Fremes SE. A review of propensity‐score methods and their use in cardiovascular research. Can J Cardiol. 2016;32:259–265. doi: 10.1016/j.cjca.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 42. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


