Abstract
Background
Little is known about the impact of chronic obstructive pulmonary disease (COPD) in patients with heart failure with preserved ejection fraction (HFpEF).
Methods and Results
We examined outcomes in patients with heart failure with preserved ejection fraction, according to COPD status, in the PARAGON‐HF (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin Receptor Blocker Global Outcomes in Heart Failure With Preserved Ejection Fraction) trial. The primary outcome was a composite of first and recurrent hospitalizations for heart failure and cardiovascular death. Of 4791 patients, 670 (14%) had COPD. Patients with COPD were more likely to be men (58% versus 47%; P<0.001) and had worse New York Heart Association functional class (class III/IV 24% versus 19%), worse Kansas City Cardiomyopathy Questionnaire Clinical Summary Scores (69 versus 76; P<0.001) and more frequent history of heart failure hospitalization (54% versus 47%; P<0.001). The decrement in Kansas City Cardiomyopathy Questionnaire Clinical Summary Scores with COPD was greater than for other common comorbidities. Patients with COPD had echocardiographic right ventricular enlargement, higher serum creatinine (100 μmol/L versus 96 μmol/L) and neutrophil‐to‐lymphocyte ratio (2.7 versus 2.5), than those without COPD. After multivariable adjustment, COPD was associated with worse outcomes: adjusted rate ratio for the primary outcome 1.51 (95% CI, 1.25–1.83), total heart failure hospitalization 1.54 (95% CI, 1.24–1.90), cardiovascular death (adjusted hazard ratio [HR], 1.42; 95% CI, 1.10–1.82), and all‐cause death (adjusted HR, 1.52; 95% CI, 1.25–1.84). COPD was associated with worse outcomes than other comorbidities and Kansas City Cardiomyopathy Questionnaire Clinical Summary Scores declined more in patients with COPD than in those without.
Conclusions
Approximately 1 in 7 patients with heart failure with preserved ejection fraction had concomitant COPD, which was associated with greater functional limitation and a higher risk of heart failure hospitalization and death.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01920711.
Keywords: chronic obstructive pulmonary disease, heart failure with preserved ejection fraction, right ventricle, sacubitril/valsartan
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- I‐Preserve
Irbesartan in Heart Failure With Preserved Ejection Fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- KCCQ‐CSS
Kansas City Cardiomyopathy Questionnaire Clinical Summary Score
- PARAGON‐HF
Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor (ARNI) With Angiotensin Receptor Blocker (ARB) Global Outcomes in Heart Failure With Preserved Ejection Fraction
Clinical Perspective
What Is New?
In patients with heart failure with preserved ejection fraction, chronic obstructive pulmonary disease (COPD) was associated with markedly worse symptoms and quality of life (measured with Kansas City Cardiomyopathy Questionnaire Clinical Summary Score), compared with no COPD, and to other common comorbidities.
COPD was associated with elevation of neutrophils and troponin (but not NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]) and right ventricular enlargement.
COPD was associated with higher rates of nonfatal and fatal outcomes and remained an independent predictor of these, even after adjusting for right ventricular size; COPD did not modify the effect of sacubitril/valsartan compared with valsartan on any prespecified mortality/hospitalization outcome, or on change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Scores.
What Are the Clinical Implications?
Among patients with heart failure with preserved ejection fraction, those with COPD are at high risk, which is not fully explained and merits further investigation.
However, influenza vaccination, smoking cessation and bronchodilators are therapeutic interventions that are beneficial but underused.
In contrast to heart failure with reduced ejection fraction (HFrEF), relatively little is known about chronic obstructive pulmonary disease (COPD) in patients with heart failure with preserved ejection fraction (HFpEF). Indeed, while the 2 conditions can clearly coexist, there has been concern about potential misdiagnosis of HFpEF in patients with COPD, 1 , 2 and sometimes differentiating between the 2 conditions can pose a diagnostic challenge. 3 This concern has led to careful wording of HFpEF trial protocols to exclude individuals with severe COPD, 4 , 5 , 6 , 7 as such patients’ symptoms 3 , 8 and prognosis might be determined as much by their lung disease as their cardiac condition. 9 , 10 This is one reason why the prevalence of COPD in patients with HFpEF in trials (10%–19%) 11 , 12 , 13 is generally much less than in epidemiological and registry studies (prevalence up to 40%). 3 It also why it has been hard to determine the true impact of concurrent COPD in HFpEF, that is, because of the concern that some patient cohorts may have included a significant minority of patients with COPD alone, misdiagnosed as HFpEF. 2 The PARAGON‐HF (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor [ARNI] with angiotensin receptor blocker [ARB] Global Outcomes in Heart Failure With Preserved Ejection Fraction) trial was designed to minimize the possibility of including patients with COPD misdiagnosed as HFpEF. 4 Probable alternative diagnoses that the investigator considered could account for patients’ symptoms (eg, dyspnea, fatigue), such as significant pulmonary disease, were excluded. 4 In addition, eligible patients had to demonstrate structural heart disease consistent with a diagnosis of HFpEF (ie, left ventricular hypertrophy, left atrial enlargement, or both) and an elevated concentration of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide). 4
In this more rigorously defined HFpEF population, we have examined in detail the clinical, biomarker, and echocardiographic characteristics of patients with a concomitant diagnosis of COPD and investigated fatal and nonfatal outcomes in patients with this comorbidity, including cause of death and changes in quality of life.
Methods
PARAGON‐HF was a randomized, double‐blind, parallel‐group, active‐controlled, 2‐arm, event‐driven trial comparing the long‐term efficacy and safety of valsartan and sacubitril/valsartan in patients with chronic symptomatic HFpEF. The study design, baseline characteristics, and primary results are published. 4 Novartis is committed to sharing access to patient‐level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Study Design and Population
Patients in New York Heart Association functional class II, III and IV, with an ejection fraction of 45% or higher, an elevated NT‐proBNP level, evidence of structural heart disease, and taking diuretic therapy were eligible. The NT‐proBNP threshold for inclusion varied on the basis of recent hospitalization for heart failure and the presence of atrial fibrillation (AF) or flutter. The main exclusion criteria included any previous echocardiographic measurement of left ventricular ejection fraction <40%, systolic blood pressure <110 mm Hg, an estimated glomerular filtration rate <30 mL/min per 1.73 m2, and serum potassium level >5.2 mmol/L.
Definition of COPD
The presence of COPD was recorded using a yes/no check box on the case‐report form completed by site investigators at study entry. The protocol specifically excluded patients with “severe COPD,” defined as COPD requiring home oxygen, chronic nebulizer, or chronic oral steroid therapy, or resulting in hospitalization for pulmonary decompensation within the prior 12 months.
Echocardiographic Substudy
Participating investigators sent echocardiographic studies in digital format to a core laboratory at the Brigham and Women’s Hospital, Boston, where quantitative measures were performed in accordance with American Society of Echocardiography guidelines, by dedicated analysts blinded to clinical information and randomized treatment assignment, as described in detail elsewhere. 14 Echocardiographic information was available for 1097 patients (22.8%) in PARAGON‐HF, 162 of the 670 patients (24.2%) with COPD and 934 of the 4121 patients (22.7%) without COPD. One patient had missing information regarding COPD status.
Study End Points
The primary outcome was a composite of total heart failure hospitalizations and cardiovascular death. Secondary outcomes included the components of the primary outcome and all‐cause mortality. We analyzed change from baseline to 8 months (as prespecified) in the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ‐CSS), Total Symptom Score, and Overall Summary Score, as well as noncardiovascular deaths, in view of the potential impact of COPD on quality of life and deaths from respiratory causes and infection.
Statistical Analysis
Baseline characteristics are presented as means with SD or median with interquartile range for continuous variables, and frequency and percentages for categorical variables. The primary outcome was evaluated with the use of semiparametric proportional rates method of Lin et al stratified according to geographical region. 15 The cumulative recurrent events were displayed using Nelson‐Aalen cumulative hazard curves and cumulative first events were displayed using Kaplan‐Meier curves. Models were adjusted for treatment, age, sex, race, systolic blood pressure, heart rate, body mass index, clinical features of heart failure (left ventricular ejection fraction, NT‐proBNP [log]), New York Heart Association class), hypertension, chronic kidney disease, diabetes, AF, hospitalization for heart failure, myocardial infarction, stroke, and duration of heart failure and stratified by region. We also examined the effect of comorbidities for each outcome in a multivariable model adjusted for treatment, race, sex, and NT‐proBNP(log). Obesity was defined as a body mass index ≥30 kg/m2, and chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min per 1.73 m2. Change in KCCQ‐CSS from baseline to 8 months was analyzed using a multilevel mixed‐effects linear regression model with an interaction term between COPD status and time and adjusted for baseline KCCQ, region, and randomized treatment with a random intercept and slope per patient with an unstructured covariance structure. For patients with and without COPD, the effect of sacubitril‐valsartan was compared with valsartan for the primary outcome, and its components were also examined. All analyses were conducted using STATA version 16. A P value of <0.05 was considered statistically significant.
Results
Among 4796 patients included in the primary efficacy analysis of PARAGON‐HF, 5 did not have information on COPD status. Of the 4791 with information about COPD status, 670 (14%) had a diagnosis of COPD.
Baseline Findings: COPD Versus No COPD
Demographics and Comorbidity
Patients with COPD were older (73.4±7.9 years versus 72.6±8.5 years; P=0.04), less likely to be women (42.7% versus 53.2%; P<0.001), and had a higher heart rate (72±13 versus 70; P=0.001). Current (13.8% versus 5.8%) and prior smoking (48.8% versus 29.2%) were more common in patients with COPD than in those without (both P<0.001). Patients with COPD were more likely than those without to have a history of coronary heart disease and of stroke. However, they did not have a higher prevalence of AF or atrial flutter (Table 1).
Table 1.
Baseline Characteristics: Patients With and Without COPD
|
No COPD n=4121 |
COPD n=670 |
P value | |
|---|---|---|---|
| Age, y | 72.6±8.5 | 73.4±7.9 | 0.04 |
| Female sex, n (%) | 2191 (53.2) | 286 (42.7) | <0.001 |
| Race, n (%) | <0.001 | ||
| White | 3308 (80.3) | 594 (88.7) | |
| Asian | 563 (13.7) | 44 (6.6) | |
| Black or African American | 82 (2.0) | 20 (3.0) | |
| Other | 168 (4.1) | 12 (1.8) | |
| Region, n (%) | <0.001 | ||
| Western Europe | 1171 (28.4) | 216 (32.2) | |
| Central Europe | 1495 (36.3) | 220 (32.8) | |
| North America | 408 (9.9) | 149 (22.2) | |
| Latin America | 350 (8.5) | 20 (3.0) | |
| Asia/Pacific and other | 697 (16.9) | 65 (9.7) | |
| Physical characteristics | |||
| Systolic blood pressure, mm Hg | 131±15.0 | 130±16.0 | 0.72 |
| Heart rate, bpm | 70.0±12.0 | 72.0±13.0 | 0.001 |
| Body mass index, kg/m2 | 30.1±5.0 | 30.9±5.1 | <0.001 |
| Laboratory measures | |||
| Hemoglobin, g/L | 135.0 (125.0–145.0) | 134.0 (125.0–145.0) | 0.93 |
| White blood cells, 109/L | 6.3 (5.3–7.5) | 6.8 (5.7–8.1) | <0.001 |
| Neutrophils, 109/L | 4.0 (3.2–5) | 4.3 (3.6–5.5) | <0.001 |
| Lymphocytes, 109/L | 1.6 (1.3–2.0) | 1.6 (1.3–2.0) | 0.70 |
| Neutrophil/lymphocyte ratio | 2.5 (1.9–3.3) | 2.7 (2.0–3.7) | <0.001 |
| Creatinine, μmol/L | 95.8±27.0 | 100.2±29.0 | <0.001 |
| Smoking history, n (%) | <0.001 | ||
| Never | 2661 (65.0) | 250 (37.4) | |
| Former | 1197 (29.2) | 326 (48.8) | |
| Current | 237 (5.8) | 92 (13.8) | |
| Medical history, n (%) | |||
| Hypertension | 3936 (95.5) | 643 (96.0) | 0.59 |
| Diabetes | 1759 (42.7) | 301 (44.9) | 0.28 |
| AF | 1328 (32.3) | 222 (33.3) | 0.62 |
| ECG AF at randomization | 1200 (29.3) | 195 (29.4) | 0.95 |
| Myocardial infarction | 912 (22.1) | 170 (25.4) | 0.06 |
| Prior CABG | 469 (11.4) | 101 (15.1) | 0.006 |
| Prior PCI | 812 (19.7) | 163 (24.3) | 0.006 |
| Stroke | 420 (10.2) | 87 (13.0) | 0.03 |
AF indicates atrial fibrillation; CABG, coronary artery bypass; COPD, chronic obstructive pulmonary disease; and PCI, percutaneous coronary intervention.
Heart Failure Characteristics
Patients with COPD had worse functional class (New York Heart Association class III/IV, 24.1% versus 19.1%) and a more frequent history of heart failure hospitalization (54.2% versus 47.1%; P<0.001), compared with participants without COPD. There was no difference in NT‐proBNP and left ventricular ejection fraction between patients with and without COPD, even when accounting for electrocardiographic evidence of AF (Table 2). Elevated jugular venous pressure (19.1% versus 12.9%) and rales (10.3% versus 6.7%) were reported more often in patients with COPD (P value for both <0.0001), while peripheral edema was similar in the 2 groups. Right bundle branch block was more prevalent in patients with COPD than in participants without COPD (9.0% versus 6.8%; P=0.04, respectively).
Table 2.
Heart Failure Characteristics in Patients With and Without COPD
|
No COPD n=4121 |
COPD n=670 |
P value | |
|---|---|---|---|
| Prior heart failure hospitalization | 1941 (47.1) | 363 (54.2) | <0.001 |
| NYHA class, n (%) | 0.003 | ||
| I | 125 (3.0) | 12 (1.8) | |
| II | 3206 (77.8) | 496 (74.0) | |
| III | 770 (18.7) | 161 (24.0) | |
| IV | 18 (0.4) | 1 (0.1) | |
| KCCQ‐CSS | 76.0 (60.9–88.5) | 69.3 (55.0–82.8) | <0.001 |
| Signs of congestion, n (%) | |||
| Jugular venous distention | 527 (12.9) | 127 (19.1) | <0.001 |
| Edema | 1552 (37.7) | 271 (40.4) | 0.18 |
| Third heart sound | 91 (2.2) | 20 (3.0) | 0.21 |
| Rales | 276 (6.7) | 69 (10.3) | <0.001 |
| Biomarkers | |||
| NT‐proBNP, pg/mL | 913 (453–1606) | 887 (467–1647) | 0.67 |
| NT‐proBNP, pg/mL with ECG AF | 1563 (1151–2231) | 1586 (1153–2319) | 0.89 |
| NT‐pro BNP, pg/mL without ECG AF | 632 (386–1145) | 639 (404–1156) | 0.56 |
| Troponin, ng/L |
16.0 (11.0–24.0) n=1050 |
18.0 (13.0–26.0) n=209 |
0.004 |
| ST2, ng/mL |
22.1 (18.0–26.8) n=1022 |
23.2 (18.6–27.7) n=205 |
0.14 |
| Procollagen type 1, µg/L |
38.0 (29.0–49.0) n=728 |
38.0 (30.0–49.0) n=135 | 0.77 |
| Procollagen type 3, µg/L |
4.5 (3.5–5.5) n=730 |
4.1 (3.5–5.2) n=133 |
0.25 |
| Collagen type 1, µg/L |
5.9 (4.6–7.8) n=728 |
6.1 (4.9–8.7) n=135 |
0.16 |
| ECG | |||
| LBBB | 219 (5.3) | 43 (6.4) | 0.24 |
| RBBB | 280 (6.8) | 60 (9.0) | 0.04 |
| QRS duration | 103.6±40.2 | 106.2±41.8 | 0.13 |
| Treatments at randomization, n (%) | |||
| Diuretic | 3929 (95.3) | 651 (97.2) | 0.03 |
| Digoxin | 382 (9.3) | 68 (10.1) | 0.47 |
| Beta blocker | 3316 (80.5) | 501 (74.8) | <0.001 |
| Calcium channel blocker | 1406 (34.1) | 223 (33.3) | 0.67 |
| MRA | 1076 (26.1) | 162 (24.2) | 0.29 |
| Oral nitrate | 437 (10.6) | 89 (13.3) | 0.04 |
| Influenza vaccination, n (%) | 1389 (33.9) | 302 (45.1) | <0.001 |
ST2 was measured in 1227 patients, procollagen type 1 and type 3 and collagen type I was measured in 863 patients and high‐sensitivity troponin was measured in 1259 patients. AF indicates atrial fibrillation; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; LBBB, left bundle branch block; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐hormone B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; and RBBB, right bundle branch block.
Heath‐Related Quality‐of‐Life
Patients with COPD had a lower (worse) KCCQ clinical summary score than patients without COPD (69.3 versus 76.0; P<0.001). The decrement in all KCCQ scores examined was greater in patients with COPD compared with other common comorbidities (Figure 1).
Figure 1. Mean baseline Kansas City Cardiomyopathy Questionnaire scores associated with major comorbidities.

CKD indicates chronic kidney disease; and COPD, chronic obstructive pulmonary disease.
Echocardiographic Findings
Patients with COPD had a greater right ventricular end diastolic area (22.4±5.6 cm2 versus 20.8±5.9 cm2; P value=0.022) and right ventricular end systolic area (12.4±4.4 cm2 versus 11.0±4.0 cm2; P=0.002) and a shorter fractional area change (45.0±9.9 versus 47.4±9.1; P=0.024) than those without COPD (Table 3). Peak systolic right ventricular pressure gradient did not differ between patients with and without COPD.
Table 3.
Echocardiography Parameters in Patients With and Without COPD
|
No COPD n=934 |
COPD n=162 |
P value | |
|---|---|---|---|
| Left heart structure | |||
| Interventricular septum, cm |
1.09±0.24 (n=888) |
1.13±0.26 (n=155) |
0.11 |
| LV posterior wall thickness, cm |
0.95±0.20 (n=869) |
0.99±0.22 (n=151) |
0.039 |
| LA area, cm2 |
23.0±5.7 (n=590) |
22.7±4.7 (n=107) |
0.66 |
| LA volume, mL |
75.1±30.6 (n=834) |
72.7±23.4 (n=143) |
0.37 |
| LVOT area, cm |
1.85±0.23 (n=842) |
1.88±0.23 (n=149) |
0.12 |
| LV end‐diastolic volume, mL |
101.7±37.1 (n=763) |
106.9±41.5 (n=133) |
0.14 |
| LV end‐systolic volume, mL |
42.9±22.3 (n=763) |
45.6±24.2 (n=133) |
0.21 |
| LVEF (%) |
58.7±9.7 (n=934) |
58.5±10.3 (n=162) |
0.86 |
| Pulmonary pressure and the right ventricle | |||
| RV end‐diastolic area, cm2 |
20.8±5.9 (n=532) |
22.4±5.6 (n=87) |
0.022 |
| RV end‐systolic area, cm2 |
11.0±4.0 (n=532) |
12.4±4.4 (n=87) |
0.002 |
| RV ejection time, msec |
317±43 (n=560) |
320±39 (n=106) |
0.56 |
| Fractional area change, RV (%) |
47.4±9.1 (n=532) |
45.0±9.9 (n=87) |
0.024 |
| Myocardial RV performance index |
0.27±0.16 (n=323) |
0.26±0.16 (n=64) |
0.94 |
| RV VTI (cm) |
15.0±6.0 (n=559) |
15.1±6.3 (n=105) |
0.90 |
| Tricuspid regurgitation velocity, m/s |
2.67±0.45 (n=422) |
2.67±0.53 (n=66) |
1.00 |
| Peak systolic pressure gradient, RV, mm Hg |
29.2±10.3 (n=422) |
29.6±12.2 (n=66) |
0.83 |
| TAPSE, cm |
1.80±0.43 (n=443) |
1.83±0.41 (n=72) |
0.63 |
| LV diastolic function | |||
| E/A velocity ratio |
1.32±0.73 (n=498) |
1.34±0.75 (n=94) |
0.79 |
| E/e′ septal |
16.9±7.4 (n=633) |
16.5±6.3 (n=114) |
0.55 |
| E/e′ lateral |
12.6±5.8 (n=603) |
12.2±5.1 (n=111) |
0.45 |
| MV deceleration time, msec |
169±40 (n=667) |
178±46 (n=118) |
0.047 |
| IVC maximal diameter, cm |
1.72±0.42 (n=249) |
1.76±0.50 (n=54) |
0.55 |
| IVC minimal diameter, cm |
0.89±0.39 (n=118) |
0.92±0.50 (n=28) |
0.68 |
A indicates peak velocity flow in late diastole caused by atrial contraction; COPD, chronic obstructive pulmonary disease; e′, peak early diastolic mitral annular tissue velocity; E wave, peak early diastolic transmitral flow velocity; IVC, inferior vena cava; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; MV, mitral valve; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; and VTI, velocity time integral.
Laboratory Measures and Cardiac Biomarkers
Patients with COPD had a higher serum creatinine (100.2±29.0 μmol/L versus 95.8±27.0 μmol/L), neutrophil count (4.3 × 109/L versus 4.0 × 109/L) and neutrophil‐to‐lymphocyte ratio (2.7 versus 2.5) than those without COPD (P<0.001 for all comparisons). Patients with COPD had a higher high‐sensitivity troponin when compared with those without (18.0 ng/L versus 16.0 ng/L), but there was no difference in levels of soluble ST2 or the markers of collagen turnover measured (Tables 1 and 2).
Baseline Cardiovascular Treatment
The greatest difference in cardiovascular therapy between patients with and without COPD was in use of beta blockers, which were prescribed less often to patients with COPD compared with those without (74.8% versus 80.5%; P value <0.001) (Table 1). Conversely, diuretic and nitrate prescriptions were more common in patients with COPD (97.2% versus 95.3; P=0.03; and 13.3% versus 10.6%; P=0.04, respectively). Influenza vaccination rate was low overall, though more frequent in patients with COPD compared with those without COPD (45.1% versus 33.9%; P<0.001).
Baseline Pulmonary Treatment (in Patients With COPD)
The most commonly used treatments were inhaled beta agonists (28.5%), anticholinergics (29.9%), and combination inhalers, including corticosteroids (19.6%). Methylxanthines were used in only 4.0% of cases.
Outcomes: COPD Versus No COPD
Primary Outcome and Mortality
COPD was associated with a significantly higher risk of the primary end point and all secondary outcomes, even after adjustment for conventional prognostic variables (as described in the Methods section): adjusted rate ratio for the primary end point, 1.51 (95% CI, 1.25–1.83; P<0.001); total heart failure hospitalizations, 1.54 (95% CI, 1.24–1.90; P<0.001); cardiovascular death (adjusted hazard ratio [HR], 1.42; 95% CI, 1.10–1.82; P=0.006); and all‐cause death (HR, 1.52; 95% CI, 1.25–1.84; P<0.001) (Table 4 and Figure 2).
Table 4.
Clinical Outcomes According to COPD status
|
Without COPD n=4121 |
With COPD n=670 |
P value | |
|---|---|---|---|
| Primary outcome | |||
| Event number | 1442 | 460 | |
| Event rate per 100 patient‐years | 12.07 (11.47–12.71) | 24.30 (22.18–26.63) | |
| Unadjusted RR | 1.0 (ref) | 1.78 (1.48–2.13) | <0.001 |
| Adjusted RR | 1.0 (ref) | 1.51 (1.25–1.83) | <0.001 |
| Total HFH | |||
| Event number | 1111 | 375 | |
| Event rate per 100 patient‐years | 9.30 (8.77–9.87) | 19.81 (17.90–21.92) | |
| Unadjusted RR | 1.0 (ref) | 1.81 (1.48–2.23) | <0.001 |
| Adjusted RR | 1.0 (ref) | 1.54 (1.24–1.90) | <0.001 |
| Cardiovascular death | |||
| Event number | 331 | 85 | |
| Event rate per 100 patient‐years | 2.77 (2.49–3.08) | 4.49 (3.63–5.55) | |
| Unadjusted HR | 1.0 (ref) | 1.64 (1.29–2.09) | <0.001 |
| Adjusted HR | 1.0 (ref) | 1.42 (1.10–1.82) | 0.006 |
| Noncardiovascular death | |||
| Event number | 214 | 60 | |
| Event rate per 100 patient‐years | 1.79 (1.57–2.05) | 3.17 (2.46–4.08) | |
| Unadjusted HR | 1.0 (ref) | 1.86 (1.39–2.49) | <0.001 |
| Adjusted HR | 1.0 (ref) | 1.67 (1.23–2.27) | 0.001 |
| All‐cause death | |||
| Event number | 545 | 145 | |
| Event rate per 100 patient‐years | 4.56 (4.19–4.96) | 7.65 (6.50–9.01) | |
| Unadjusted HR | 1.0 (ref) | 1.73 (1.43–2.08) | <0.001 |
| Adjusted HR | 1.0 (ref) | 1.52 (1.25–1.84) | <0.001 |
| KCCQ‐CSS | |||
| Mean change (SE) | −1.52 (0.26) | −5.49 (0.66) | |
| Difference | −3.96 (0.72) | <0.001 | |
| Proportion with increase in score ≥5 points at 8 mo (%) | 29.9 | 28.4 | |
| Unadjusted OR | 0.82 (0.67–0.99) | 0.04 | |
| Adjusted OR | 0.95 (0.79–1.15) | 0.62 | |
| Proportion with decrease in score ≥5 points at 8 mo (%) | 29.1 | 37.6 | |
| Unadjusted OR | 1.51 (1.27–1.80) | <0.001 | |
| Adjusted OR | 1.38 (1.16–1.65) | <0.001 | |
Model adjusted for treatment, age, sex, race, systolic blood pressure, heart rate, body mass index, clinical features of heart failure (left ventricular ejection fraction, N‐terminal pro‐B‐type natriuretic peptide [log]), New York Heart Association class, hypertension, chronic kidney disease, diabetes, atrial fibrillation, hospitalization for heart failure, myocardial infarction, stroke, and duration of heart failure and stratified by region. COPD indicates chronic obstructive pulmonary disease; HFH, heart failure hospitalizations; HR, hazard ratio; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; OR, odds ratio; and RR, rate ratio.
Figure 2. Clinical outcomes in heart failure with preserved ejection fraction according to COPD status at baseline.
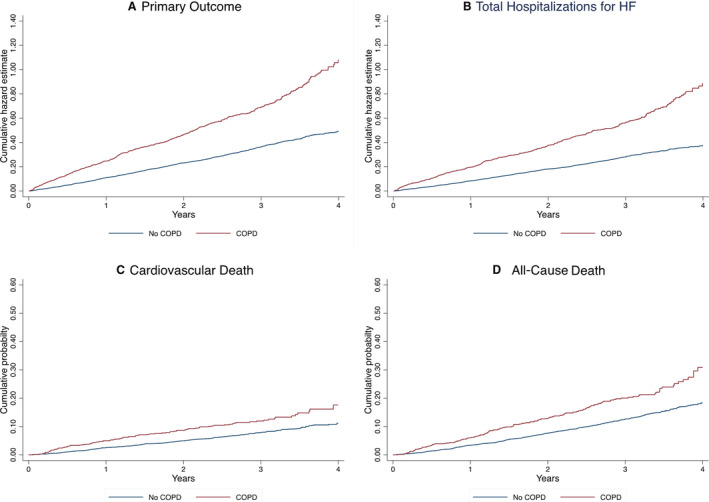
A, Cumulative hazard estimate for the primary composite outcome. B, Cumulative hazard estimate for total hospitalizations for heart failure. C, Cumulative probability of cardiovascular death. D, Cumulative probability of all‐cause death. COPD indicates chronic obstructive pulmonary disease; and HF, heart failure.
Prognostic Importance of Right Ventricular Enlargement
In the subset of patients with a baseline echocardiogram, COPD was associated with a similarly elevated risk for the primary end point (adjusted rate ratio, 1.65; 95% CI, 1.04–2.63; P=0.034) (Table S1). In this subset of patients, right ventricular end systolic area was independently predictive of worse outcomes when added to the multivariable model: the adjusted rate ratio for the primary composite outcome was 1.63 (95% CI, 1.03–2.56; P=0.036).
Kansas City Cardiomyopathy Questionnaire Clinical Summary Scores
On average, KCCQ‐CSS decreased (deteriorated) between baseline and 8 months. The mean decrease was substantially and significantly larger in patients with COPD (−5.49±0.66) than those without COPD (−1.52±0.26). Likewise, a significantly greater proportion (37.6%) of patients with COPD reported a clinically meaningful deterioration (ie, ≥5‐point decrease) in KCCQ‐CSS than among participants without COPD (29.1%; odds ratio [OR], 1.51; 95% CI, 1.27–1.80); COPD patients were also less likely to have a clinically meaningful increase (improvement) in KCCQ‐CSS (28.4% versus 29.9%; unadjusted OR, 0.82; 95% CI, 0.67–0.99) (Table 4).
Outcomes Related to COPD Compared With Other Comorbidities
COPD was associated with a higher risk of the primary end point, its components, and all‐cause mortality, after adjustment for treatment, sex, race, region, and NT‐proBNP. The association between other comorbidities and the risk of these outcomes is given for comparison (Figure 3).
Figure 3. Risk of primary outcome and all‐cause mortality associated with major comorbidities.

CKD indicates chronic kidney disease; and COPD; chronic obstructive pulmonary disease. Adjusted for treatment, sex, race, and N‐terminal pro‐B‐type natriuretic peptide (log) and stratified by region.
Effect of Sacubitril/Valsartan Compared With Valsartan
Baseline history of COPD did not modify the effect of sacubitril/valsartan compared with valsartan on any prespecified mortality/hospitalization outcome, or on change in KCCQ‐CSS (Table S2).
Discussion
The present study provides a more detailed description of patients with the combination of HFpEF and COPD than reported previously, including biomarker, and quality‐of‐life data, as well as comprehensive echocardiographic analysis in a core laboratory. In addition, an extensive range of adjudicated fatal and nonfatal outcomes are reported, with adjustment for other important prognostic variables, including NT‐proBNP. We also examined the impact of COPD, compared with other comorbidities, on quality of life and mortality/hospitalization outcomes. Finally, these data provide an interesting comparison with other recent reports in patients with HFrEF who had concomitant COPD.
As anticipated, the prevalence of COPD in patients with HFpEF in PARAGON‐HF (14%) was lower than in most, but not all, epidemiological studies and registries. 16 However, our prevalence findings were consistent with those in I‐Preserve (Irbesartan in Heart Failure With Preserved Ejection Fraction) (10%), 13 TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) (12%), 11 and Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity‐Preserved (COPD was not recorded but 9% patients were prescribed bronchodilator drugs). 17 For comparison, the prevalence of COPD in HFrEF trials is around 11% to 13%. 18 , 19 , 20
In PARAGON‐HF, patients with COPD were older, more commonly men, and had a history of smoking. Patients with COPD were less likely to be treated with a beta blocker and had more severe functional limitation and impairment of quality of life than participants without COPD. While each of these findings are similar to those reported in patients with HFrEF with COPD, 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 there were also differences. Higher levels of NT‐proBNP have been reported in patients with HFrEF with COPD, 19 , 21 , 24 , 28 , 29 compared with patients with HFrEF without COPD. AF was more frequent in patients with HFrEF with COPD, compared with those without. 19 , 21 , 25 , 28 Although not reported in the HFrEF studies, in PARAGON‐HF use of oral nitrates was more common in patients with COPD than without COPD. The reason is not clear but may reflect treatment for angina (given the greater prevalence of coronary heart disease and lower use of beta blockers in patients with COPD) or in the belief that nitrates might be efficacious in heart failure (given the greater symptom severity and functional limitation in patients with COPD). Either way, this is a notable finding given the observation in a recent randomized, placebo‐controlled trial that isosorbide mononitrate did not improve dyspnea and reduced, rather than increased, activity levels in patients with HFpEF. 30
Although used less than in patients without COPD, 75% of patients with COPD were treated with a beta blocker. Yet in a recent randomized, placebo‐controlled trial, the BLOCK COPD (Beta‐Blockers for the Prevention of Acute Exacerbations of Chronic Obstructive Pulmonary Disease) trial, metoprolol exacerbated dyspnea and increased the risk of severe exacerbations in patients with COPD. 31 Although the patients in BLOCK COPD were at high risk of exacerbation, and the risk related to use of beta blockers in patients with less COPD is unknown, it may be prudent to use these drugs in patients with HFpEF with COPD only where there is no other option. While beta blockers are an essential treatment in patients with HFrEF, there is no indication in HFpEF per se, and in PARAGON‐HF only a minority of patients with COPD had a firm alternative indication for beta blockade, such as prior myocardial infarction (25%). One avenue to potentially improve symptoms and outcomes in patients with HFpEF and more severe COPD may be to carefully evaluate whether beta blockade is appropriate, with alternative treatment options available for treatment of hypertension and ventricular rate control in AF.
Interestingly, although NT‐proBNP was not elevated in patients with COPD, markers of systemic inflammation (white blood count, neutrophil count, and neutrophil/lymphocyte ratio) were elevated, compared with patients without COPD (although we did not measure C‐reactive protein). COPD may therefore be a comorbidity driving the systemic inflammatory state that one hypothesis proposes is central to the pathophysiology of HFpEF. 32
The detailed echocardiographic substudy of PARAGON‐HF provided unique information in a trial about the cardiac abnormalities associated with COPD. The only other studies reporting echocardiographic findings in HFpEF patients with COPD were too small to detect differences or did not report right heart measurements, 33 , 34 , 35 , 36 and in the TOPCAT trial the echocardiography findings described were for a group of patients with “pulmonary disease,” including both COPD and asthma. 37 In our study, left ventricular volume and left atrial size were identical in patients with and without COPD, but those with COPD had increased left ventricular posterior wall thickness, right ventricular size, and lower right fractional area change. Although the latter findings are consistent with the view that hypoxia‐induced pulmonary vasoconstriction may lead to right ventricular afterload, enlargement, and failure, we observed no difference in right ventricular systolic pressure in patients with and without COPD. 38 , 39 This may reflect the recognized difficulty in estimating right ventricular systolic pressure in patients with COPD, and we could estimate this in only 41% of COPD patients in PARAGON‐HF. 40 , 41 , 42 Alternatively, right ventricular systolic pressure may not be elevated at rest unless HFpEF or COPD is severe, it may be elevated on exertion in patients with less severe disease.
The effects of COPD on the left side of the heart are more complex. Right ventricular hypertrophy and dilatation may cause a leftward shift of the interventricular septum, reducing left ventricular cavity size, compliance, and stroke volume. 43 Conversely, severe COPD may lead to a reduced pulmonary vein cross‐sectional area, reduced left ventricular filling and volumes, and a reduction in cardiac output. 44 , 45 It has been suggested that COPD may lead to left ventricular hypertrophy as a result of increased residual volume, negative inspiratory pleural pressure increased left ventricular transmural pressure and wall stress, and we found that patients with COPD had a thicker left ventricular posterior wall. 44 , 45 Notably, however, left ventricular ejection fraction was similar in patients with COPD and those without, and there was also a lack of difference in NT‐proBNP between patients with and without COPD. We found that right ventricular size was an independent predictor of the composite of cardiovascular death and heart failure hospitalization, in keeping with other studies in HFpEF. 46 , 47 , 48 , 49 However, those prior studies had not linked right ventricular enlargement and dysfunction to COPD. Moreover, COPD remained an independent predictor of worse outcomes even after including indices of right ventricular size in the multivariable model; that is, the excess risk related to COPD was not explained by right ventricular impairment.
Using the KCCQ, we found patients with COPD to have markedly worse symptoms and health‐related quality of life than participants without COPD. This was also reported in the BIOSTAT‐CHF (Biology Study to Tailored Treatment in Chronic Heart Failure) cohort, and these observations are comparable to the similarly large differences noted in KCCQ scores between patients with HFrEF with and without COPD. 27 Additionally, we found the reduction in health‐related quality of life was greater with COPD than with any other common comorbidity in HFpEF. This suggests that it is not just the combination of 2 conditions per se that explains the worse health‐related quality of life, and that the specific nature of COPD that is important, that is, the combination of cardiac and respiratory conditions that each cause dyspnea and effort intolerance.
During follow‐up, patients with COPD experienced higher rates of the primary composite end point and key secondary end points, and more had a clinically meaningful deterioration (and fewer an improvement) in symptoms and quality of life, compared with those without COPD. These worse hospitalization and mortality outcomes persisted after adjustment for other prognostic variables, including other comorbidities such as diabetes and chronic kidney disease. Indeed, we found that patients with COPD had risks of the primary outcome and all‐cause mortality as high as those associated with other common comorbidities in HFpEF. We think this is underappreciated and that COPD may be a neglected comorbidity in heart failure, relative to its impact on quality of life and hospitalization and mortality outcomes. Our findings suggest that both noncardiovascular causes (such as infection and smoking‐related lung disease and cancer), as well as cardiovascular ones, contribute to the worse outcomes in patients with COPD. However, the small or absent differences in many classical predictors of cardiovascular risk (NT‐proBNP, diabetes, systolic blood pressure) between patients with and without COPD is interesting. Nevertheless, 4 differences do stand out—higher prevalence of coronary disease, higher troponin, elevated markers of inflammation (eg, neutrophil/lymphocyte ratio) and right ventricular dilatation/systolic dysfunction, each of which could increase the risk of death (eg, sudden death) and hospitalization. COPD was an independent predictor of the primary outcome in a multivariable model including classical predictors, likely indicating additional mechanisms and causes that were not measured.
There is currently no evidence‐based therapy for the treatment of HFpEF, and the management is focused on treating fluid overload and comorbidities. Reducing the risk of respiratory infections through vaccination is another important therapeutic approach recommended in both heart failure 50 and COPD guidelines. 51 Although uptake of influenza vaccination was better in patients with COPD (45%) than in those without (34%), it was still significantly underused in both groups. Unfortunately, 14% of patients with COPD continued to smoke, emphasizing the need to intensify smoking cessation efforts in these patients. The relatively low use of bronchodilators is also of concern. Both long‐acting beta‐2 agonists and long‐acting antimuscarinic antagonists improve lung function, dyspnea, and health status and reduce COPD exacerbations. Combination long‐acting beta‐2 agonists/long‐acting antimuscarinic antagonists amplify these benefits and are recommended in most patients with COPD. 51 A recent study of inhaled beta‐adrenergic agonists in patients with HFpEF is particularly relevant to concomitant COPD, especially if there is associated right ventricular dysfunction. In that study, albuterol reduced pulmonary vascular resistance and improved echocardiographic indices of right ventricular systolic function, in keeping with improved right ventricular–pulmonary arterial coupling. 52
Recently, it has been proposed that targeting enzymes that play key roles in systemic and lung inflammation, such as the cyclic nucleotide‐degrading enzymes phosphodiesterases and phosphoinositide‐3 phosphate kinases, might have a specific role in patients with HFpEF and concomitant COPD, and novel approaches of this type are particularly welcome given the impact of COPD on quality and quantity of life in HFpEF. 53
Limitations
Several limitations must be acknowledged, foremost being the investigator‐derived diagnosis of COPD. No prespecified diagnostic criteria were defined in the protocol, and had spirometry been carried out in all patients, the prevalence of COPD would likely have been higher. Moreover, patients with severe pulmonary disease were excluded from PARAGON‐HF. Despite this, the impact of COPD on health status and outcomes in HFpEF was clear and could only have been greater if patients with more severe COPD were included.
Summary and Conclusions
In summary, in PARAGON‐HF, ≈1 in 7 patients with HFpEF had concomitant COPD. Patients with COPD had worse symptoms, functional limitation, and quality of life, compared with those without, and a higher risk of heart failure hospitalization and cardiovascular death, possibly related to right ventricular enlargement.
Sources of Funding
PARAGON‐HF was funded by Novartis. Dr McMurray is supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Disclosures
Drs Desai, Rouleau, Solomon, Zile, Jhund, and McMurray or their institutions were paid by Novartis for their participation in this trial. Dr Lefkowitz is an employee of Novartis. Dr Anand reports receiving fees for serving on a steering committee from AstraZeneca, ARCA biopharma, Amgen, and LivaNova; fees for serving as chair of a data and safety monitoring board from Boston Scientific; fees for serving on an end point committee from Boehringer Ingelheim; and fees for serving on an advisory board from Zensun. Dr Redfield is a nonpaid consultant for Novartis. Dr Maggioni reports receiving fees for serving on a study committee from Bayer and Fresenius. Dr Pfeffer reports grants paid to his institution for serving on the steering committee of PARAGON‐HF and for serving as study chair of Prospective ARNI vs ACE Inhibitor Trial to DetermIne Superiority in Reducing Heart Failure Events After MI from Novartis and personal fees for consulting from AstraZeneca, DalCor, GlaxoSmithKline, Novo Nordisk, Sanofi, Jazz Pharmaceuticals, MyoKardia, Servier, Takeda, and Corvidia. Dr Pfeffer owns stock options of DalCor. Dr Minamisawa has received support from the Japanese Circulation Society, the Japanese Society of Echocardiography, and the Uehara Memorial Foundation Overseas Research Fellowship. Dr Shah reports receiving research support from Novartis, Gilead, and Actelion; and consulting fees from Myocardia. Dr Desai has received consulting fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, DalCor Pharmaceuticals, and Regeneron; grant support (paid to Brigham and Women’s Hospital) and consulting fees from Alnylam Pharmaceuticals and Novartis, and advisory board fees from Corvidia and Relypsa. Dr Zile reports grants and personal fees from Novartis for being a member of the PARAGON‐HF Executive Steering Committee and a local investigator; personal fees from Abbott for serving on the executive committee of the GUIDE‐HF (Hemodynamic‐Guided Management of Heart Failure) trial; personal fees for consulting on product development from Boston Scientific; grants and personal fees for serving on the Executive Steering Committee and being a local investigator for the BeAT HF trial (Baroreflex Activation Therapy for Heart Failure Pivotal Trial) from CVRx; personal fees for serving on the eligibility committee of the SOLVE trial (Stimulation of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in Non‐Responders and Previously Untreatable Patients) from EBR; personal fees for serving on the Clinical Events Committee of the SIRONA trial (A Prospective, Multi‐Center, Open‐Label, Single‐Arm Clinical Trial Evaluating the Safety and Efficacy of the Cordella Heart Failure System in New York Heart Association Class III Heart Failure Patients) from Endotronics; personal fees for serving on the Executive Steering Committee of the CAPACITY HFpEF trial (A Study of the Effect of IW‐1973 on the Exercise Capacity of Patients With Heart Failure With Preserved Ejection Fraction) from Ironwood; personal fees for serving on the data safety monitoring board of the Ertugliflozin trial (MK‐8835/PF‐04971729) from Merck; grants and personal fees for serving on the executive steering committee of the Revamp Trial, Alleviate Trial, Link‐HF trial (Link‐HF, Phase II: Multisensor Non‐invasive Telemonitoring System for Prediction of Heart Failure Exacerbation), and Intervene trial and being a local investigator from Medtronic; personal fees for consulting for product development from MyoKardia; and personal fees for serving on the eligibility committee of the RELIEVE (Reducing Lung Congestion Symptoms in Advanced Heart Failure) trial from V Wave. Dr Rouleau reports personal fees from AstraZeneca. Dr Vaduganathan reports grants from Harvard Catalyst; personal fees from Bayer AG, Baxter Healthcare, AstraZeneca, Amgen, Boehringer Ingelheim, Cytokinetics, and Relypsa, outside the submitted work. Dr Jhund reports receiving grant support from Boehringer Ingelheim and fees for serving on an advisory board from Cytokinetics. Dr Solomon reports grants paid to his institution for chairing PARAGON‐HF from Novartis; grants paid to Brigham and Women’s Hospital from Alnylam, Amgen, AstraZeneca, Bayer, Bellerophon, Bristol‐Myers Squibb, Celladon, Cytokinetics, Gilead, Celladon, Eidos, GlaxoSmithKline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Sanofi Pasteur, and Theracos; and consulting fees from Alnylam, Amgen, AOBiome, AstraZeneca, Bayer, Bristol‐Myers Squibb, Cardiac Dimensions, Corvia, Cytokinetics, Daiichi Sankyo, Gilead, GlaxoSmithKline, Ironwood, Janssen, Merck, MyoKardia, Novartis, Quantum Genomics, Roche, Takeda, Tenaya, and Theracos. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021494
For Sources of Funding and Disclosures, see page 11.
References
- 1. Chan MMY, Lam CSP. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. doi: 10.1093/eurjhf/hft062 [DOI] [PubMed] [Google Scholar]
- 2. Caruana L, Petrie MC, Davie AP, McMurray JJV. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Hear Fail. 2017;5:471–482. doi: 10.1016/j.jchf.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 5. Swedberg K, Pfeffer M, Granger C, Held P, McMurray J, Ohlin G, Olofsson B, Ostergren J, Yusuf S. Candesartan in heart failure–assessment of reduction in mortality and morbidity (CHARM): rationale and design. Charm‐Programme Investigators. J Card Fail. 1999;5:276–282. doi: 10.1016/S1071-9164(99)90013-1 [DOI] [PubMed] [Google Scholar]
- 6. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972.e10. doi: 10.1016/j.ahj.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 7. Carson P, Massie BM, Mckelvie R, McMurray J, Komajda M, Zile M, Ptaszynska A, Frangin G. The irbesartan in heart failure with preserved systolic function (I‐PRESERVE) trial: rationale and design. J Card Fail. 2005;11:576–585. doi: 10.1016/j.cardfail.2005.06.432 [DOI] [PubMed] [Google Scholar]
- 8. Caroci ADS, Lareau SC. Descriptors of dyspnea by patients with chronic obstructive pulmonary disease versus congestive heart failure. Heart Lung. 2004;33:102–110. doi: 10.1016/j.hrtlng.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 9. Boudestein LCM, Rutten FH, Cramer MJ, Lammers JWJ, Hoes AW. The impact of concurrent heart failure on prognosis in patients with chronic obstructive pulmonary disease. Eur J Heart Fail. 2009;11:1182–1188. doi: 10.1093/eurjhf/hfp148 [DOI] [PubMed] [Google Scholar]
- 10. Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/S0735-1097(03)00947-1 [DOI] [PubMed] [Google Scholar]
- 11. Shah SJ, Heitner KF, Sweitzer NK, Anand IS, Kim YH, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong JJ, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON‐HF trial. Circ Heart Fail. 2018;11:1–10. doi: 10.1161/CIRCHEARTFAILURE.118.004962 [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJV, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I‐PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 14. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O’Meara E, Ryan JJ, et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2019;74:2858–2873. doi: 10.1016/j.jacc.2019.09.063 [DOI] [PubMed] [Google Scholar]
- 15. Lin DW, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc B. 2000;62:711–730. [Google Scholar]
- 16. Van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co‐morbidities in patients with heart failure: an analysis of the European HEART Failure Pilot Survey. Eur J Heart Fail. 2014;16:103–111. doi: 10.1002/ejhf.30 [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJV, Ostergren K, Pfeffer M, Swedberg K, Granger C, Yusuf S, Held P, Michelson E, Olofsson B, et al. Clinical features and contemporary management of patients with low and preserved ejection fraction heart failure: baseline characteristics of patients in the Candesartan in Heart failure—Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur J Heart Fail. 2003;5:261–270. doi: 10.1016/S1388-9842(03)00052-7 [DOI] [PubMed] [Google Scholar]
- 18. Mogensen UM, Køber L, Kristensen SL, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, et al. The effects of sacubitril/valsartan on coronary outcomes in PARADIGM‐HF. Am Heart J. 2017;188:35–41. doi: 10.1016/j.ahj.2017.02.034 [DOI] [PubMed] [Google Scholar]
- 19. Mentz RJ, Schulte PJ, Fleg JL, Fiuzat M, Kraus WE, Piña IL, Keteyian SJ, Kitzman DW, Whellan DJ, Ellis SJ, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF‐ACTION). Am Heart J. 2013;165:193–199. doi: 10.1016/j.ahj.2012.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Böhm M, Robertson M, Ford I, Borer JS, Komajda M, Kindermann I, Maack C, Lainscak M, Swedberg K, Tavazzi L. Influence of cardiovascular and noncardiovascular co‐morbidities on outcomes and treatment effect of heart rate reduction with ivabradine in stable heart failure (from the SHIFT Trial). Am J Cardiol. 2015;116:1890–1897. doi: 10.1016/j.amjcard.2015.09.029 [DOI] [PubMed] [Google Scholar]
- 21. Ehteshami‐Afshar S, Mooney L, Dewan P, Desai AS, Lang NN, Lefkowitz MP, Petrie MC, Rizkala AR, Rouleau JL, Solomon SD, et al. Clinical characteristics and outcomes of patients with heart failure with reduced ejection fraction and chronic obstructive pulmonary disease: insights from PARADIGM‐HF. J Am Heart Assoc. 2021;10:e019238. doi: 10.1161/JAHA.120.019238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawkins NM, Jhund PS, Simpson CR, Petrie MC, MacDonald MR, Dunn FG, MacIntyre K, McMurray JJV. Primary care burden and treatment of patients with heart failure and chronic obstructive pulmonary disease in Scotland. Eur J Heart Fail. 2010;12:17–24. doi: 10.1093/eurjhf/hfp160 [DOI] [PubMed] [Google Scholar]
- 23. De Blois J, Simard S, Atar D, Agewall S. COPD predicts mortality in HF: the Norwegian heart failure registry. J Card Fail. 2010;16:225–229. doi: 10.1016/j.cardfail.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 24. Mentz RJ, Schmidt PH, Kwasny MJ, Ambrosy AP, O’Connor CM, Konstam MA, Zannad F, Maggioni AP, Swedberg K, Gheorghiade M. The impact of chronic obstructive pulmonary disease in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. J Card Fail. 2012;18:515–523. doi: 10.1016/j.cardfail.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 25. Tavazzi L, Swedberg K, Komajda M, Böhm M, Borer JS, Lainscak M, Robertson M, Ford I. Clinical profiles and outcomes in patients with chronic heart failure and chronic obstructive pulmonary disease: an efficacy and safety analysis of SHIFT study. Int J Cardiol. 2013;170:182–188. doi: 10.1016/j.ijcard.2013.10.068 [DOI] [PubMed] [Google Scholar]
- 26. Lipworth B, Skinner D, Devereux G, Thomas V, Ling Zhi Jie J, Martin J, Carter V, Price DB. Underuse of β‐blockers in heart failure and chronic obstructive pulmonary disease. Heart. 2016;102:1909–1914. doi: 10.1136/heartjnl-2016-309458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Ng LL, et al. Non‐cardiac comorbidities in heart failure with reduced, mid‐range and preserved ejection fraction. Int J Cardiol. 2018;271:132–139. doi: 10.1016/j.ijcard.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 28. Dewan P, Docherty KF, Bengtsson O, de Boer RA, Desai AS, Drozdz J, Hawkins NM, Inzucchi SE, Kitakaze M, Køber L, et al. Effects of dapagliflozin in heart failure with reduced ejection fraction, and chronic obstructive pulmonary disease: an analysis of DAPA‐HF. Eur J Heart Fail. 2021;23:632–643. doi: 10.1002/ejhf.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Staszewsky L, Wong M, Masson S, Barlera S, Carretta E, Maggioni AP, Anand IS, Cohn JN, Tognoni G, Latini R. Clinical, neurohormonal, and inflammatory markers and overall prognostic role of chronic obstructive pulmonary disease in patients with heart failure: data from the Val‐HeFT heart failure trial. J Card Fail. 2007;13:797–804. doi: 10.1016/j.cardfail.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 30. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dransfield MT, Voelker H, Bhatt SP, Brenner K, Casaburi R, Come CE, Cooper JAD, Criner GJ, Curtis JL, Han MK, et al. Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med. 2019;381:2304–2314. doi: 10.1056/NEJMoa1908142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 33. Sato Y, Yoshihisa A, Oikawa M, Nagai T, Yoshikawa T, Saito Y, Yamamoto K, Takeishi Y, Anzai T. Prognostic impact of chronic obstructive pulmonary disease on adverse prognosis in hospitalized heart failure patients with preserved ejection fraction – A report from the JASPER registry. J Cardiol. 2019;73:459–465. doi: 10.1016/j.jjcc.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 34. Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Abe S, Sato T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64:256–264. doi: 10.1016/j.jjcc.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 35. Marcun R, Stankovic I, Vidakovic R, Farkas J, Kadivec S, Putnikovic B, Ilic I, Neskovic AN, Lainscak M. Prognostic implications of heart failure with preserved ejection fraction in patients with an exacerbation of chronic obstructive pulmonary disease. Intern Emerg Med. 2016;11:519–527. doi: 10.1007/s11739-015-1319-0 [DOI] [PubMed] [Google Scholar]
- 36. Zhyvotovska A, Yusupov D, Kamran H, Al‐Bermani T, Abdul R, Kumar S, Mogar N, Hartt A, Salcicciolo L, McFarland SI. Diastolic dysfunction in patients with chronic obstructive pulmonary disease: a meta‐analysis of case controlled studies. Int J Clin Res Trials. 2019;4:137. doi: 10.15344/2456-8007/2019/137 31650092 [DOI] [Google Scholar]
- 37. Ramalho SHR, Claggett BL, Sweitzer NK, Fang JC, Shah SJ, Anand IS, Pitt B, Lewis EF, Pfeffer MA, Solomon SD, et al. Impact of pulmonary disease on the prognosis in heart failure with preserved ejection fraction: the TOPCAT trial. Eur J Heart Fail. 2020;22:557–559. doi: 10.1002/ejhf.1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wauthy P, Pagnamenta A, Vassalli F, Naeije R, Brimioulle S. Right ventricular adaptation to pulmonary hypertension: an interspecies comparison. Am J Physiol–Hear Circ Physiol. 2004;286:H1441–H1447. doi: 10.1152/ajpheart.00640.2003 [DOI] [PubMed] [Google Scholar]
- 39. Stenmark KR, Fagan KA, Frid MG. Hypoxia‐induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f [DOI] [PubMed] [Google Scholar]
- 40. Arcasoy SM, Christie JD, Ferrari VA, Sutton MSJ, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC [DOI] [PubMed] [Google Scholar]
- 41. Laaban JP, Diebold B, Zelinski R, Lafay M, Raffoul H, Rochemaure J. Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest. 1989;96:1258–1262. doi: 10.1378/chest.96.6.1258 [DOI] [PubMed] [Google Scholar]
- 42. Tramarin R, Torbicki A, Marchandise B, Laaban JP, Morpurgo M. Doppler echocardiographic evaluation of pulmonary artery pressure in chronic obstructive pulmonary disease. A European multicentre study. Eur Heart J. 1991;12:103–111. doi: 10.1093/oxfordjournals.eurheartj.a059855 [DOI] [PubMed] [Google Scholar]
- 43. Foschi M, Di Mauro M, Tancredi F, Capparuccia C, Petroni R, Leonzio L, Romano S, Gallina S, Penco M, Cibelli M, et al. The dark side of the moon: the right ventricle. J Cardiovasc Dev Dis. 2017;4:18. doi: 10.3390/jcdd4040018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith BM, Kawut SM, Bluemke DA, Basner RC, Gomes AS, Hoffman E, Kalhan R, Lima JAC, Liu C‐Y, Michos ED, et al. Pulmonary hyperinflation and left ventricular mass the multi‐ethnic study of atherosclerosis COPD study. Circulation. 2013;127:1503–1511. doi: 10.1161/CIRCULATIONAHA.113.001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alter P, Jörres RA, Watz H, Welte T, Gläser S, Schulz H, Bals R, Karch A, Wouters EFM, Vestbo J, et al. Left ventricular volume and wall stress are linked to lung function impairment in COPD. Int J Cardiol. 2018;261:172–178. doi: 10.1016/j.ijcard.2018.02.074 [DOI] [PubMed] [Google Scholar]
- 46. Lejeune S, Roy C, Ciocea V, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde J‐L, et al. Right ventricular global longitudinal strain and outcomes in heart failure with preserved ejection fraction. J Am Soc Echocardiogr. 2020;33:973–984. doi: 10.1016/j.echo.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 47. Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, et al. Right ventricular dysfunction in left‐sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. doi: 10.1002/ejhf.873 [DOI] [PubMed] [Google Scholar]
- 48. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;1:873–879. doi: 10.1002/ejhf.664 [DOI] [PubMed] [Google Scholar]
- 49. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CSP, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail. 2016;18:1472–1487. doi: 10.1002/ejhf.630 [DOI] [PubMed] [Google Scholar]
- 50. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 51. GOLD . Global Initiative for Chronic Obstructive. GOLD. 2017;1–44. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdf. Date published April 2015. Date accessed 27/01/2021.
- 52. Reddy Y, Obokata M, Koepp K, Egbe A, Wiley B, Borlaug B. The β‐Adrenergic agonist albuterol improves pulmonary vascular reserve in heart failure with preserved ejection fraction: a randomized controlled trial Yogesh. Circ Res. 2019;124:306–314. doi: 10.1161/CIRCRESAHA.118.313832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sala V, Margaria JP, Murabito A, Morello F, Ghigo A, Hirsch E. Therapeutic targeting of PDEs and PI3K in heart failure with preserved ejection fraction (HFpEF). Curr Heart Fail Rep. 2017;1:187–196. doi: 10.1007/s11897-017-0331-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


