Abstract
Background
Transesophageal echocardiography (TEE) has been considered the gold standard for left atrial appendage (LAA) thrombus detection. Nevertheless, TEE may sometimes induce discomfort and cause complications. Cardiac computed tomography has been studied extensively for LAA thrombus detection. We performed this systemic review and meta‐analysis to assess the diagnostic accuracy of cardiac computed tomography for LAA thrombus detection compared with TEE.
Methods and Results
A systemic search was conducted in the PubMed, Embase, and Cochrane Library databases from January 1977 to February 2021. Studies performed for assessment diagnostic accuracy of cardiac computed tomography on LAA thrombus compared with TEE were included. Summary sensitivity, specificity, and posterior probability of LAA thrombus was calculated by using bivariate random‐effects model. The Quality Assessment of Diagnostic Accuracy Studies‐2 tool was used for the quality assessment. A total of 27 studies involving 6960 patients were included in our study. The summary sensitivity of early imaging studies was 0.95 (95% CI, 0.79–0.99), and the specificity was 0.89 (95% CI, 0.85–0.92). The positive posterior probability was 19.11%, and the negative posterior probability was 0.16%. The summary sensitivity of delayed imaging studies was 0.98 (95% CI, 0.92–1.00), and the specificity was 1.00 (95% CI, 0.98–1.00). The positive posterior probability was 95.76%, and the negative posterior probability was 0.12%. The delayed imaging method significantly improved the specificity (1.00 versus 0.89; P<0.05) and positive posterior probability (95.76% versus 19.11%; P<0.05).
Conclusions
Cardiac computed tomography with a delayed imaging is a reliable alternative to TEE. It may save the patient and health care from an excess TEE.
Registration
URL: https://www.crd.york.ac.uk/PROSPERO; Unique identifier: CRD42021236352.
Keywords: cardiac computed tomography, diagnostic accuracy, left atrial appendage thrombus, systemic analysis and meta‐analysis, transesophageal echocardiogram
Subject Categories: Atrial Fibrillation, Computerized Tomography (CT), Diagnostic Testing
Nonstandard Abbreviations and Acronyms
- CCT
cardiac computed tomography
- LAA
left atrial appendage
- PVI
pulmonary vein isolation
Clinical Perspective
What Is New?
This updated meta‐analysis demonstrated that compared with transesophageal echocardiography, cardiac computed tomography showed a high diagnostic accuracy for left atrial appendage thrombus detection when delayed imaging was used.
What Are the Clinical Implications?
Cardiac computed tomography with a delayed imaging method is a reliable alternative tool for left atrial appendage thrombus detection.
Doing a delayed computed tomography scan adds nominal radiation exposure (<1 millisievert) and allows a single test to perform both tasks (pulmonary vein assessment and rule out left atrial thrombus), saving the patient and health care from an excess transesophageal echocardiography before pulmonary vein isolation.
Left atrial appendage (LAA) thrombus, which may present in conditions resulting in left atrial flow stasis, especially in atrial fibrillation, is an important source of cardioembolic stroke. Transesophageal echocardiography (TEE) is currently considered the gold standard for the detection of LAA thrombus, based on 2 large prospective studies. 1 , 2 However, TEE is a semi‐invasive and time‐consuming procedure. Although generally safe when performed by experienced operators, TEE carries physical discomfort for some patients and is associated, although rarely, with potentially life‐threatening complications. 3
In the past 2 decades, cardiac computed tomography (CCT) has been studied extensively for LAA thrombus detection. Almost all of the studies reported that CCT has a high sensitivity for LAA thrombus detection, whereas the specificity has been reported variable. Studies using delayed imaging method reported higher specificity than studies using early imaging method. Moreover, it only takes a few minutes for CCT scan, far less than that of TEE. This will reduce time cost for examiners and patients. Some researchers have assessed the diagnostic accuracy of CCT by conducting meta‐analyses. 4 , 5 , 6 , 7 , 8 The results of these studies have reported that CCT has good diagnostic accuracy for LAA thrombus detection, 4 , 5 , 6 , 7 , 8 especially when the delayed imaging method is used. 4 , 5 However, there are reasons to conduct a new meta‐analysis. First, all studies included in these meta‐analyses were conducted before the year of 2014, and studies using delayed imaging method were relative few. Second, the pooled sensitivity and specificity of 2 meta‐analyses 7 , 8 were relatively low. Third, 2 meta‐analyses 4 , 5 included 1 study 9 that did not meet the criteria because CCT was used for cardiogenic embolus detection, not LAA thrombus detection. In this study, CCT was used for cardiogenic thrombus but not LAA thrombus detection. 9 Moreover, there have been some new studies (at least 10) on LAA thrombus detection using CCT in recent years, some of which reported higher specificity and narrower CIs. 10 , 11 , 12 We therefore conducted this systematic review and meta‐analysis to determine the diagnostic accuracy of CCT versus TEE for LAA thrombus detection.
METHODS
Authors declare that they will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure. The data that support the findings of this study are available from the first author on reasonable request.
This meta‐analysis was performed on the basis of guidelines from the Preferred Reporting Items for a Systematic Review and Meta‐Analysis of Diagnostic Test Accuracy Studies statement (Tables S1 and S2). 13 The literature search, article screening, study selection, quality assessment, and data extraction were performed by 2 authors (S.Y. and H.Z.) independently. Disagreements were resolved by discussion, and a consensus was reached in the selection of the articles for analysis.
Search Strategy
PubMed, Embase, and Cochrane Library databases were searched from January 1977 to February 2021. The search terms are shown in Table S3. In addition, we searched relevant studies from references of the retrieved articles.
Study Selection
Studies fulfilling the following criteria were included: (1) assessment of left atrial thrombus; (2) patients who underwent both CCT and TTE; and (3) sensitivity, specificity, positive predictive value, and negative predictive value data were provided or could be calculated.
Data Extraction and Quality Assessment
Data extraction was performed by 2 authors (S.Y. and H.Z.) independently. We extracted demographics of patients, indications of left atrial thrombus, and CCT method (eg, electrocardiogram (ECG) gated versus non–ECG gated).
Quality Assessment of Diagnostic Accuracy Studies‐2 was used for the quality assessment of the included studies. 14 Two authors (S.Y. and H.Z.) assessed the risk of bias and applicability concerns independently. The following domains were used to assess bias risk and applicability concerns: patient selection, performance of the index test, performance of the reference standard, and flow and timing (the interval between index test and standard reference, for risk of bias assessment only). 14
Data Synthesis and Statistical Analysis
Metandi and midas commands in Stata 15.0 (StataCorp, College Station, TX) were used for data synthesis and analysis. 15 , 16 The analysis was implemented mainly by midas, and metandi was used to construct hierarchical summary receiver operating characteristic curve. Sensitivity, specificity, and likelihood ratio (LR), along with 95% CIs, were calculated from the contingency 2×2 tables of true‐positive, false‐positive, false‐negative, and true‐negative results using a bivariate random‐effects model estimation. Random effects model was selected because heterogeneity is expected in meta‐analysis of diagnostic accuracy studies. 17
Primarily, midas uses an exact binomial rendition 18 of the bivariate mixed‐effects regression model developed by Van Houwelingen 19 for treatment trial meta‐analysis and modified for synthesis of diagnostic test data. 17 It fits a 2‐level model, with independent binomial distributions for the true positives and true negatives conditional on the sensitivity and specificity in each study and a bivariate normal model for the logit transforms of sensitivity and specificity between studies. The standard output of the bivariate model includes the following: mean logit sensitivity and specificity with their SEs and 95% CIs; and estimates of the between‐study variability in logit sensitivity and specificity and the covariance between them. On the basis of these parameters, we can calculate other measures of interest, such as the likelihood ratio for positive and negative test results, the diagnostic odds ratio (OR), and the correlation between logit sensitivity and specificity. Summary sensitivity, specificity, and the corresponding positive likelihood, negative likelihood, and diagnostic ORs are derived as functions of the estimated model parameters. The derived logit estimates of sensitivity, specificity, and respective variances are used to construct a hierarchical summary ROC curve to display the variation in diagnostic accuracy among studies. 20
Posterior probability of LAA thrombus was also calculated to assess the diagnostic accuracy. The formula is as follows: posterior probability=pretest probability (LAA thrombus incidence)×LR/(pretest probability×LR+1). I2 index was used to assess the heterogeneity. 21 Heterogeneity sources among studies was investigated by using multiple univariable meta‐regression and subgroup analysis. Publication bias was assessed by the Deek method. 22
RESULTS
Search Results
We identified 588 potentially eligible articles. In total, 555 articles were excluded by reviewing the titles and abstracts. The remaining 33 articles were evaluated in detail. Finally, 27 articles that met the inclusion criteria were identified (Figure 1). Six studies were excluded because not all patients underwent TEE, 23 , 24 , 25 no thrombus was found, 26 the sensitivity and specificity could not be calculated because the reference test was surgical finding, 27 and one study was not limited to LAA thrombus detection. 9
Figure 1. Flowchart of selection of studies.
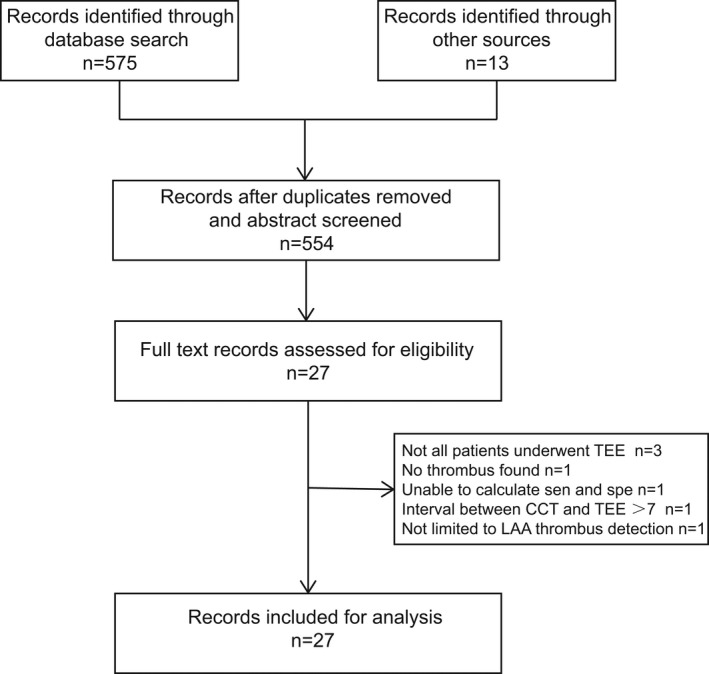
CCT indicates cardiac computed tomography; LAA, left atrial appendage; sen, sensitivity; spe, specificity; and TEE, transesophageal echocardiography.
Baseline Characteristics of the Included Studies
The baseline characteristics of the included studies are shown in Table 1. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 Seventeen studies (63%) had a prospective design, and 10 studies (37%) had a retrospective design. Nineteen studies (70%) were performed with patients scheduled for pulmonary vein isolation (PVI), 4 studies (15%) were performed with patients recently experiencing stroke, 1 study was performed with patients scheduled to direct current cardioversion, and the remaining 3 studies had mixed populations. The ECG‐gated method was used in 16 studies (59%). CCT with delayed imaging was performed in 11 studies (41%). The incidence of LAA thrombus was 3.68% (251/6960).
Table 1.
Baseline Characteristics of Included Studies
| Study | Year | Design | No. of patients | Men, % | Age, y | Indication | CT type | Slice thickness, mm | Diagnostic criteria for TEE |
|---|---|---|---|---|---|---|---|---|---|
| Achenbach 28 | 2004 | Prospective | 52 | 64 | 66±10 | DCCV | ECG‐gated EBCT; early phase | 1.5 | LAT/LAAT |
| Kim 29 | 2007 | Retrospective | 223 | 82 | 57±10 | PVI | ECG‐gated 16‐, 40‐, 64‐slice MDCT; early phase | 1.2, 0.75, 0.6 | LAAT+SEC |
| Shapiro 30 | 2007 | Retrospective | 21 | N/A | N/A | No restrict | ECG‐gated 64 slice; MDCT; early phase | 0.6 | LAAT |
| Feuchtner 31 | 2008 | Prospective | 64 | 68 | 58±13 | PVI/valve surgery | ECG‐gated 64 slice; MDCT | 0.6 | LAT/LAAT |
| Tang 32 | 2008 | Prospective | 170 | 72 | 56±12 | PVI | Non–ECG‐gated 64 slice; MDCT; early phase | N/A | LAT/LAAT |
| Hur 33 | 2008 | Retrospective | 101 | 62 | 67 | Stroke | ECG‐gated 64‐section CCTA; early phase | 0.6 | LAAT |
| Patel 34 | 2008 | Prospective | 72 | 69 | 56±10 | PVI | ECG‐gated 64 slice; MDCT; early phase | 0.625 | LAAT+SEC |
| Martinez 35 | 2009 | Prospective | 402 | 76 | 56±10 | PVI | 64 Slice; MDCT; early phase | 0.6 | LAAT |
| Hur 36 | 2009 | Prospective | 55 | 65 | 61 | Stroke | ECG‐gated 64‐section CCTA; late phase | 0.6 | LAAT |
| Kim 37 | 2010 | Prospective | 314 | 59 | 65±13 | Stroke | ECG‐gated 64‐slice MDCT; late phase | 0.625 | LAAT |
| Kapa 38 | 2010 | Prospective | 255 | 78 | 59±11 | PVI | ECG‐gated DSCT; early phase | 0.6 | LAAT |
| Maltagliati 39 | 2011 | Prospective | 171 | 83 | 60±11 | PVI | 64‐Slice MDCT; early phase | N/A | LAA/LAAT |
| Hur 40 | 2011 | Prospective | 83 | 67 | 63±10 | Stroke | ECG‐gated DSCT; late phase | 0.6 | LAAT+SEC |
| Swait 41 | 2012 | Retrospective | 70 | N/A | N/A | PVI | ECG‐gated (patient in sinus rhythm) and nongated (patients in AF) 256‐, 128‐, and 64‐slice CCT; late phase | N/A | LAT+LAAT |
| Hur 42 | 2013 | Prospective | 101 | 70 | 62±10 | PVI | ECG‐gated 128‐, 64‐slice CCT; late phase | 0.6 | LAT+LAAT |
| Dorenkamp 43 | 2013 | Prospective | 329 | 65 | 62±10 | PVI | ECG‐gated 64‐slice MDCT; early phase | 0.625 | LAT+LAAT |
| Budoff 44 | 2014 | Retrospective | 86 | 81 | 66 | PVI | 64‐Slice CCTA; late phase | N/A | LAAT |
| Hong 45 | 2014 | Retrospective | 678 | 78 | 57±11 | PVI | ECG‐gated 64‐slice MDCT; early phase | 0.6 | LA/LAAT+SEC |
| Homsi 46 | 2016 | Prospective | 124 | 83 | 58±12 | AF/stroke | 64‐Slice MDCT; early phase | 0.9 | LAAT+SEC |
| Lazoura 11 | 2016 | Retrospective | 122 | 78 | 60 | PVI | ECG‐gated DSCT; late phase | 0.5 | LAAT |
| Wang 47 | 2016 | Retrospective | 831 | 75 | 61±10 | PVI | Non–ECG‐gated 64 slice; MDCT; early phase | 0.625 | LAAT+SEC |
| Zhai 48 | 2017 | Retrospective | 783 | 72 | 55±11 | PVI | ECG‐gated 64 slice; MDCT; late phase | 0.625 | |
| Kottmaier 49 | 2019 | Prospective | 622 | 69 | 60±10 | PVI |
ECG‐gated (patient in sinus rhythm) and nongated (patients in AF) 64‐slice DSCT early phase image |
0.6 | LAT |
| Kuronuma 12 | 2019 | Prospective | 81 | 75 | 68±11 | PVI | ECG‐gated CCT; late phase | N/A | LAAT |
| Li 50 | 2019 | Prospective | 302 | 54 | 64±7 | PVI | 64‐Slice DSCT; late phase | 0.6 | LAAT |
| Guha 51 | 2020 | Retrospective | 480 | 66 | 63 | PVI | 64‐Slice MDCT; early phase | 0.63 | LAAT |
| Spagnolo 10 | 2020 | Prospective | 260 | 77 | 59±11 | PVI | ECG‐gated 64‐slice CCT; late phase | N/A | LAAT |
AF indicates atrial fibrillation; CCT, cardiac CT; CCTA, coronary CT angiography; CT, computed tomography; DCCV, direct current cardioversion; DSCT, dual‐source CT; ECG, electrocardiogram; EBCT, electron‐beam CT; LAAT, left atrial appendage thrombus; LAT, left atrial thrombus; MDCT, multidetector CT; N/A, not available; PVI, pulmonary vein isolation; SEC, spontaneous echo contrast; and TEE, transesophageal echocardiography.
Quality Assessment
The results of the quality assessment are summarized in Table S4. In total, 3.70% (1/27) of the studies showed an unclear risk of bias in the patient selection domain, 7.41% (2/27) of the studies showed an unclear risk of bias in the index test domain, 33.33% (9/27) of the studies showed an unclear risk of bias in the reference standard domain, and 7.41% (2/27) of the studies showed a high or unclear risk of bias in the flow and timing domain.
Main Analysis
Analysis was based on study design (prospective or retrospective), imaging methods (early or delayed imaging; ECG gated or non–ECG gated), indication (PVI or not PVI), and sample size (patient number >100 or ≤100). The results are shown in Table 2. Sensitivity and negative LR (LR−) were not influenced by any factors, but the delayed imaging method had a significant impact on specificity and positive LR (LR+). The pooled sensitivity and specificity of the early and delayed imaging subgroups are also shown in Figures 2A and 2B and 3A and 3B.
Table 2.
Sensitivity and Specificity of Each Subgroup
| Subgroup | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR− (95% CI) | Incidence of thrombus |
|---|---|---|---|---|---|
| Prospective | 0.97 (0.82–1.00) | 0.97 (0.93–0.99) | 29.91 (13.36–66.96) | 0.03 (0.00–0.20) | 169/3467 |
| Retrospective | 0.98 (0.85–1.00) | 0.92 (0.82–0.97) | 12.63 (5.30–30.12) | 0.02 (0.00–0.19) | 82/3493 |
| Early | 0.95 (0.79–0.99) | 0.89 (0.85–0.92)* | 8.99 (6.61–12.21)* | 0.06 (0.01–0.26) | 120/4695 |
| Delayed | 0.99 (0.92–1.00) | 1.00 (0.98–1.00)* | 368.27 (41.94–3233.86)* | 0.01 (0.00–0.08) | 131/2265 |
| ECG gated | 0.98 (0.87–1.00) | 0.97 (0.93–0.99) | 36.30 (12.99–101.46) | 0.02 (0.00–0.14) | 158/3604 |
| Non–ECG gated | 0.97 (0.73–1.00) | 0.91 (0.85–0.95) | 11.32 (6.61–19.39) | 0.03 (0.00–0.36) | 93/3356 |
| PVI | 0.98 (0.84–1.00) | 0.95 (0.91–0.97) | 19.69 (10.13–38.3) | 0.03 (0.00–0.19) | 134/6146 |
| Non‐PVI | 0.99 (0.76–1.00) | 0.96 (0.86–0.99) | 28.11 (6.75–117.02) | 0.01 (0.00–0.30) | 117/814 |
| PVI delayed | 0.99 (0.79–1.00) | 1.00 (0.93–1.00) | 302.20 (14.3–6386.8) | 0.01 (0.00–0.25) | 52/1511 |
| Stroke | 0.99 (0.87–1.00) | 0.99 (0.93–1.00) | 172.40 (13.8–2151.4) | 0.01 (0.00–0.14) | 77/553 |
| Small sample | 0.99 (0.70–1.00) | 0.94 (0.83–0.98) | 17.56 (5.47–56.43) | 0.01 (0.00–0.44) | 84/592 |
| Large sample | 0.98 (0.85–1.00) | 0.96 (0.92–0.98) | 23.98 (11.61–49.54) | 0.03 (0.00–0.17) | 167/6368 |
ECG, electrocardiogram; LR indicates likelihood ratio; and PVI, pulmonary vein isolation.
indicates a statistical difference.
Figure 2. Forest plot of diagnostic accuracy of cardiac computed tomography (CCT) with early imaging method vs transesophageal echocardiography (TEE).
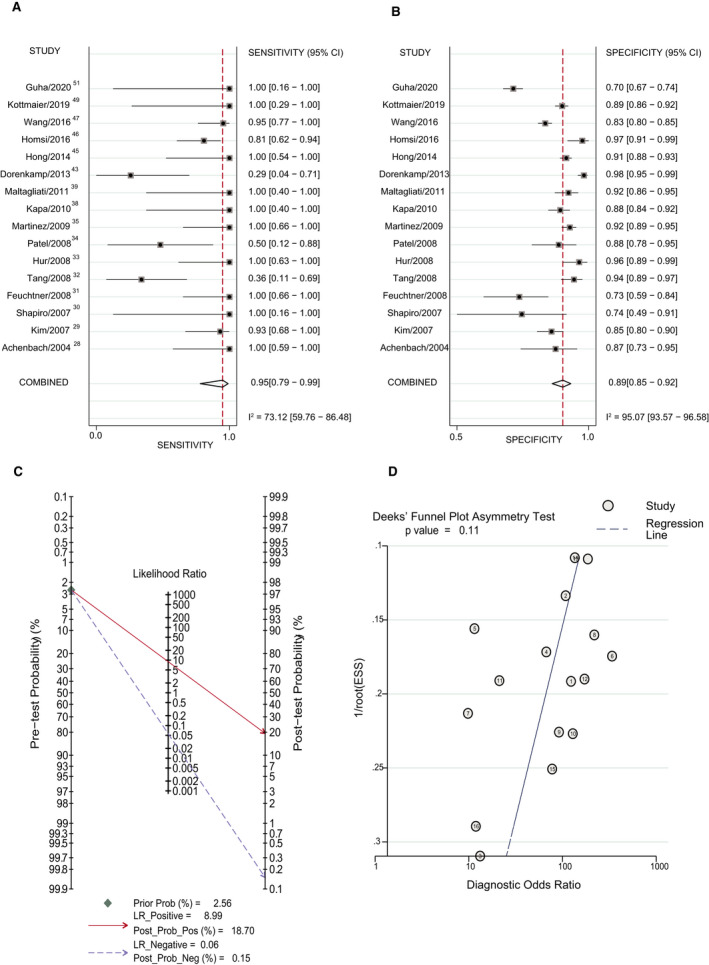
A, Sensitivity of CTT with the early imaging method vs TEE. B, Specificity of CCT with the early imaging method vs TEE. C, Posterior probability of CCT with the early imaging method vs TEE. D, The Deek method for assessment of publication bias. ESS, effective sample size; LR indicates likelihood ratio; Post Prob Neg, negative posterior probability; Post Prob Pos, positive posterior probability; and Prob, probability.
Figure 3. Forest plot of the diagnostic accuracy of cardiac computed tomography (CCT) with the delayed imaging method vs transesophageal echocardiography (TEE).
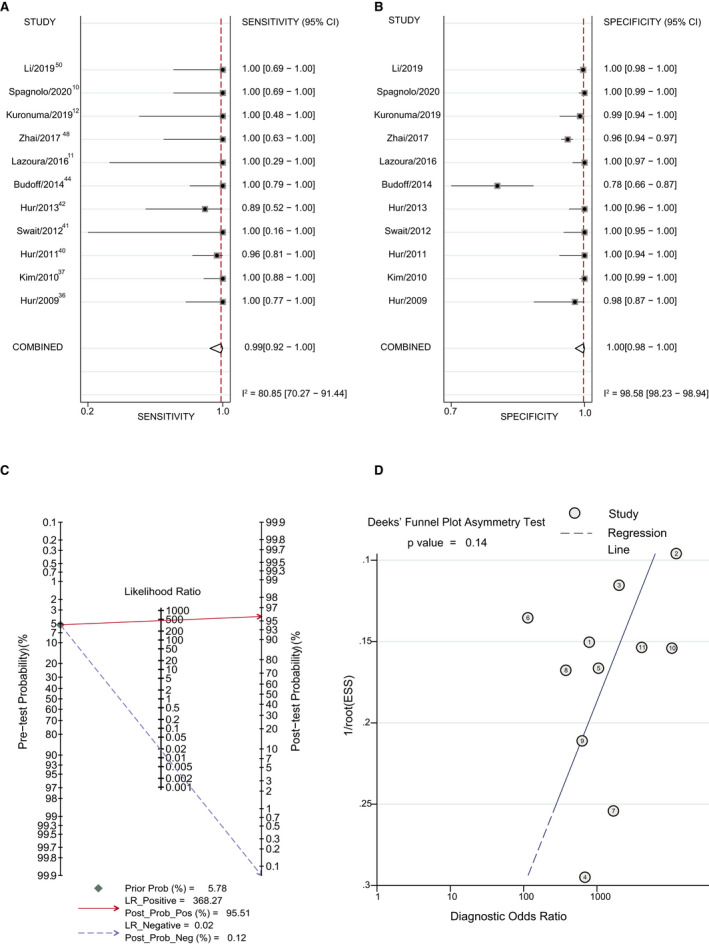
A, Sensitivity of CTT with the delayed imaging method vs TEE. B, Specificity of CCT with the delayed imaging method vs TEE. C, Posterior probability of CCT with the delayed imaging method vs TEE. D, The Deek method for assessment of publication bias. ESS, effective sample size; LR indicates likelihood ratio; Post Prob Neg, negative posterior probability; Post Prob Pos, positive posterior probability; and Prob, probability.
The incidence of LAA thrombus in the early imaging subgroup and delayed imaging group was 2.56% (120/4695) and 5.78% (131/2265), respectively. The positive posterior probability of the early imaging subgroup was 18.70%, and the negative posterior probability of the early imaging subgroup was 0.15% (Figure 2C). P=0.11 suggests no strong evidence of publication bias has been found (Figure 2D). The positive posterior probability of the delayed imaging subgroup was 95.51%, and the negative posterior probability of the delayed imaging subgroup was 0.12% (Figure 3C). P=0.14 suggests no strong evidence of publication bias has been found (Figure 3D).
Compared with the early imaging group, the delayed imaging method had a significantly higher LR+ and similar LR−, meaning that the delayed imaging method significantly improved the diagnostic accuracy. The positive posterior probability of the delayed imaging group was significantly higher than that of the early imaging group.
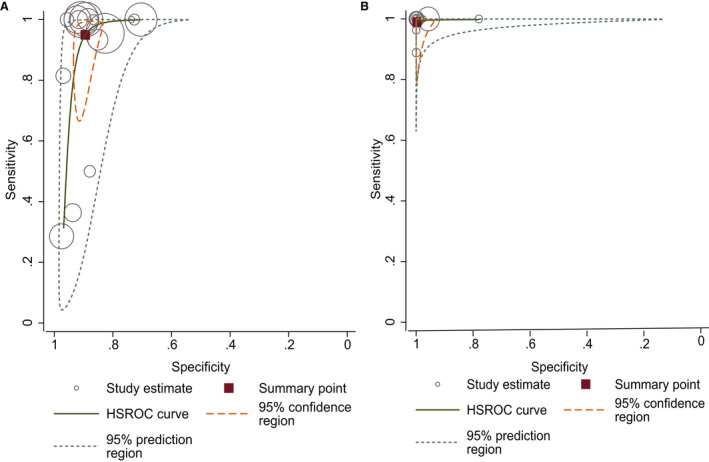
The hierarchical summary receiver operating characteristic curves of the early imaging group and delayed imaging group are shown in Figure 4A and 4B. The 95% prediction region and confidence region of the delayed imaging group (Figure 4A) were smaller than those of the early imaging group (Figure 4B), indicating that the diagnostic accuracy of the delayed imaging group was better than that of the early imaging group.
Figure 4. Hierarchical summary receiver operating characteristic (HSROC) curve of studies using the early imaging method (A) and delayed imaging method (B).
Analysis Based on Indications
Most patients in these studies were patients scheduled for PVI or patients experiencing stroke. Because these 2 indications have different LAA thrombus incidence (pretest probability), the posterior probability may be different. The incidence of LAA thrombus in the PVI with delayed imaging subgroup was 3.44% (52/1511). And the incidence of LAA thrombus in the stroke subgroup was 13.92% (77/553). Because the early imaging method has a low LR+ value, we mainly analyzed data from the delayed imaging group. The results are shown in Table 2 and Figures 5 and 6. The pooled sensitivity and specificity were similar between the 2 subgroups (Table 2 and Figures 5A and 5B and 6A and 6B). Although the estimated LR+ of the stroke subgroup was lower than that of the PVI subgroup, the positive posterior probability of the stroke subgroup was higher (96.00% versus 91.22%) because of the higher LAA thrombus incidence (Figures 5C and 6C). P=0.71 and P=0.09 suggested no strong evidence of publication bias has been found (Figures 5D and 6D).
Figure 5. Forest plot of the diagnostic accuracy of cardiac computed tomography (CCT) in patients with pulmonary vein isolation (PVI) using delayed imaging method vs transesophageal echocardiography (TEE).
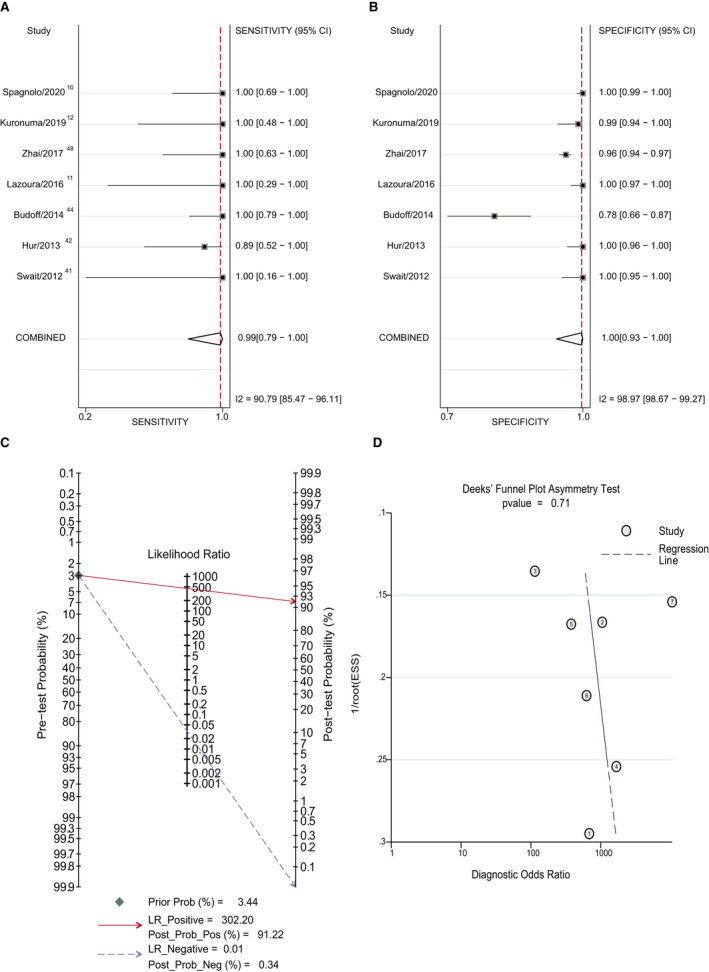
A, Sensitivity of CCT in patients with PVI using the delayed imaging method vs TEE. B, Specificity of CCT in patients with PVI using the delayed imaging method vs TEE. C, Posterior probability of CCT in patients with PVI using the delayed imaging method vs TEE. D, The Deek method for assessment of publication bias. ESS, effective sample size; LR indicates likelihood ratio; Post Prob Neg, negative posterior probability; Post Prob Pos, positive posterior probability; and Prob, probability.
Figure 6. Forest plot of the diagnostic accuracy of cardiac computed tomography (CCT) in patients with stroke using delayed imaging method vs transesophageal echocardiography (TEE).
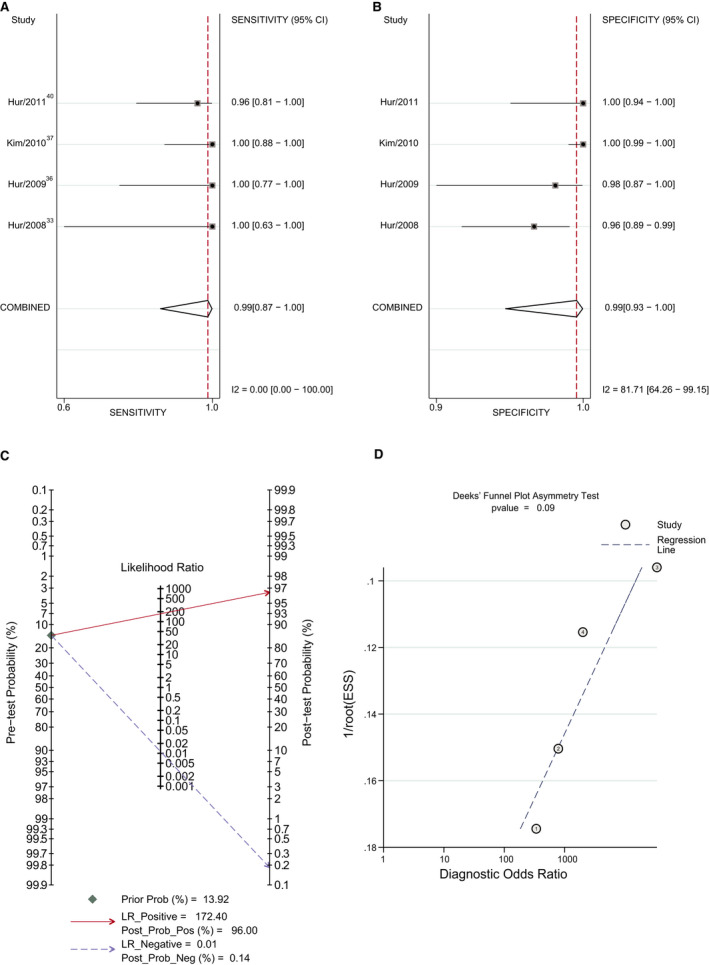
A, Sensitivity of CCT in patients with stroke using the delayed imaging method vs TEE. B, Specificity of CCT in patients with stroke using the delayed imaging method vs TEE. C, Posterior probability of CCT in patients with stroke using the delayed imaging method vs TEE. D, The Deek method for assessment of publication bias. ESS, effective sample size; LR indicates likelihood ratio; Post Prob Neg, negative posterior probability; Post Prob Pos, positive posterior probability; and Prob, probability.
Meta‐regression was performed to explore the source of heterogeneity. The results showed that the delayed imaging method, ECG‐gated method, and PVI may be the source of heterogeneity (Figure S1). When the delayed imaging method was defined as the interval between contrast injection and image capture of >1 minute, the heterogeneity of the delayed imaging subgroup decreased significantly (Figure S2).
DISCUSSION
In this comprehensive meta‐analysis of 27 studies, we assessed the diagnostic accuracy of CCT compared with TEE. The results demonstrated that CCT showed a high diagnostic accuracy for LAA thrombus detection when delayed imaging was used. In the delayed imaging subgroup, the positive posterior probability was 95.76%, and the negative posterior probability was 0.12%. Accurate identification of LAA thrombi is important for patients with atrial fibrillation and suspected cardiogenic stroke. For patients with atrial fibrillation, it can change the subsequent treatment strategy; for patients with suspected cardiogenic stroke, it can clarify a diagnosis. In the subgroup analysis based on these 2 indications, the positive posterior probabilities of PVI with delayed imaging and stroke subgroups were 91.22% and 96%, respectively. The negative posterior probabilities of these 2 subgroups were 0.34% and 0.14%, respectively. In the stroke subgroup, the LR+ value of the study with the early imaging method 33 was 25. This relatively low LR+ value underestimated the positive posterior probability. Therefore, the actual positive posterior probability would be higher. If the LR+ value of PVI with the delayed imaging subgroup was used, the positive posterior probability would be 98%. This means that CCT with delayed imaging method has a better diagnostic accuracy for LAA thrombus detection in patients with stroke.
Although TEE is currently considered the gold standard for LAA thrombus detection, it is time‐consuming. 52 In the past 2 decades, an increasing number of studies on the diagnostic accuracy of CCT for the detection of LAA thrombi have been performed. Most of these studies reported a high sensitivity and negative predictive value. LAA thrombus detection by CCT relies on filling defects. However, low blood flow velocity may also present as filling defects. It may be difficult to differentiate thrombi from low blood flow for early imaging method because the interval between contrast arrival and LAA image capture is too short. The delayed imaging method helps to differentiate thrombi and low blood flow. Our results showed that the delayed imaging method significantly improved the positive posterior probability compared with the early imaging method. In the subgroup analysis based on indications, our results showed that CCT with delayed imaging method has good diagnostic accuracy for LAA thrombus detection in patients with PVI and stroke. According to our results, we believe that CCT with a delayed imaging method is a reliable alternative tool for LAA thrombus detection. Furthermore, CCT has been recommended to assess left atrial and pulmonary vein anatomical features before PVI. 53 In addition, the cost of CCT is only a few minutes. So, doing a delayed scan at the same time adds nominal radiation exposure (<1 millisievert) and allows a single test to perform both tasks (pulmonary vein assessment and rule out left atrial thrombus), saving the patient and health care from an excess TEE. TEE can be reserved for those with positive CCT to confirm the diagnosis of clot when needed. Given the high diagnostic accuracy and efficiency for LAA thrombus detection, TEE may be prevented in patients before PVI or in patients with stroke.
In previous studies, 4 , 5 , 6 , 7 the diagnostic accuracy of CCT was assessed by sensitivity, specificity, positive predictive value, and negative predictive value. However, the diagnostic accuracy of a test not only depends on sensitivity, specificity, positive predictive value, and negative predictive value but also depends on disease prevalence. The diagnostic accuracy of a test may vary in different populations because of different disease prevalence. The posterior probability calculated on the basis of disease prevalence may be more accurate. In our study, diagnostic accuracy was assessed by calculating the positive posterior probability and negative posterior probability based on the prevalence of LAA thrombi in each group. Our results did show the difference in posterior probability between patients before PVI and patients with stroke. Moreover, all studies included in previous meta‐analyses were conducted before 2014, and studies using delayed imaging method were relatively few. In this study, we included studies published until February 2021, including 11 studies using delayed imaging method.
There are some disadvantages in the use of CCT for the detection of LAA thrombi. First, the contrast agent used during CCT examination may cause contrast‐induced nephropathy and anaphylaxis. The risk of contrast‐induced nephropathy is relatively low in patients with normal renal function. Although the risk may increase in patients with chronic kidney disease, most kidney injuries are reversible. 54 Second, patients are exposed to radiation. Currently, however, as technology has advanced, the level of radiation exposure is relatively low. CCT is most often done in <3 millisieverts, a marked reduction from early reports of ≥15 millisieverts in earlier studies. 55
There are some limitations to our study. First, the heterogeneity was high, and the results of the meta‐regression showed that the heterogeneity was from the delayed imaging method, ECG‐gated method, and PVI. Second, the reference standard was TEE, not surgical validation.
CONCLUSIONS
CCT with a delayed imaging method is superior to CCT with an early imaging method for LAA thrombus detection. It is a reliable alternative to TEE.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (81800292).
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S2
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022505
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Heping Zhang, Email: heping_zhang_bfh@126.com.
Hongwei Li, Email: lhw19656@sina.com.
REFERENCES
- 1. Manning WJ, Weintraub RM, Waksmonski CA, Haering JM, Rooney PS, Maslow AD, Johnson RG, Douglas PS. Accuracy of transesophageal echocardiography for identifying left atrial thrombi: a prospective, intraoperative study. Ann Intern Med. 1995;123:817–822. doi: 10.7326/0003-4819-123-11-199512010-00001 [DOI] [PubMed] [Google Scholar]
- 2. Hwang JJ, Chen JJ, Lin SC, Tseng YZ, Kuan P, Lien WP, Lin FY, Chu SH, Hung CR, How SW. Diagnostic accuracy of transesophageal echocardiography for detecting left atrial thrombi in patients with rheumatic heart disease having undergone mitral valve operations. Am J Cardiol. 1993;72:677–681. doi: 10.1016/0002-9149(93)90884-F [DOI] [PubMed] [Google Scholar]
- 3. Romero J, Cao JJ, Garcia MJ, Taub CC. Cardiac imaging for assessment of left atrial appendage stasis and thrombosis. Nat Rev Cardiol. 2014;11:470–480. doi: 10.1038/nrcardio.2014.77 [DOI] [PubMed] [Google Scholar]
- 4. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta‐analysis. Circ Cardiovasc Imaging. 2013;6:185–194. doi: 10.1161/CIRCIMAGING.112.000153 [DOI] [PubMed] [Google Scholar]
- 5. Vira T, Pechlivanoglou P, Connelly K, Wijeysundera HC, Roifman I. Cardiac computed tomography and magnetic resonance imaging vs. transoesophageal echocardiography for diagnosing left atrial appendage thrombi. Europace. 2019;21:e1–e10. doi: 10.1093/europace/euy142 [DOI] [PubMed] [Google Scholar]
- 6. Aimo A, Kollia E, Ntritsos G, Barison A, Masci PG, Figliozzi S, Klettas D, Stamatelopoulos K, Delialis D, Emdin M, et al. Echocardiography versus computed tomography and cardiac magnetic resonance for the detection of left heart thrombosis: a systematic review and meta‐analysis. Clin Res Cardiol. 2020. Sep 13 [epub ahead of print]. doi: 10.1007/s00392-020-01741-7 [DOI] [PubMed] [Google Scholar]
- 7. Wu X, Wang C, Zhang C, Zhang Y, Ding F, Yan J. Computed tomography for detecting left atrial thrombus: a meta‐analysis. Arch Med Sci. 2012;8:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ning Z, Min ZG, Jiang HX, Hu BL. Diagnostic accuracy of multidetector computed tomography in the detection of left atrial/left atrial appendage thrombus: a meta‐analysis. Intern Med J. 2013;43:573–580. doi: 10.1111/j.1445-5994.2012.02932.x [DOI] [PubMed] [Google Scholar]
- 9. Hur J, Kim YJ, Lee H‐J, Ha J‐W, Heo JH, Choi E‐Y, Shim C‐Y, Kim TH, Nam JE, Choe KO, et al. Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke. 2009;40:2073–2078. doi: 10.1161/STROKEAHA.108.537928 [DOI] [PubMed] [Google Scholar]
- 10. Spagnolo P, Giglio M, Di Marco D, Cannao PM, Agricola E, Della Bella PE, Monti CB, Sardanelli F. Diagnosis of left atrial appendage thrombus in patients with atrial fibrillation: delayed contrast‐enhanced cardiac CT. Eur Radiol. 2021;31:1236–1244. doi: 10.1007/s00330-020-07172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lazoura O, Ismail TF, Pavitt C, Lindsay A, Sriharan M, Rubens M, Padley S, Duncan A, Wong T, Nicol E. A low‐dose, dual‐phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int J Cardiovasc Imaging. 2016;32:347–354. doi: 10.1007/s10554-015-0776-x [DOI] [PubMed] [Google Scholar]
- 12. Kuronuma K, Matsumoto N, Suzuki Y, Makita A, Ashida T, Yokoyama K, Yoda S, Okumura Y. Usefulness of dual‐phase snapshot 320‐detector computed tomography for the detection of a left atrial appendage thrombus. Int Heart J. 2019;60:849–853. [DOI] [PubMed] [Google Scholar]
- 13. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, et al. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies: the PRISMA‐DTA statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 14. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS‐2 Group . QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15. Harbord RM, Whiting P. Metandi: meta‐analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009;9:211–229. doi: 10.1177/1536867X0900900203 [DOI] [Google Scholar]
- 16. Dwamena B. MIDAS: computational and graphical routines for meta‐analytical integration of diagnostic accuracy studies in Stata. Division of Nuclear Medicine, Department of Radiology, University of Michigan Medical School; 2007. [Google Scholar]
- 17. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 18. Chu H, Nie L, Chen Y, Huang Y, Sun W. Bivariate random effects models for meta‐analysis of comparative studies with binary outcomes: methods for the absolute risk difference and relative risk. Stat Methods Med Res. 2012;21:621–633. doi: 10.1177/0962280210393712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Houwelingen HC, Zwinderman KH, Stijnen T. A bivariate approach to meta‐analysis. Stat Med. 1993;12:2273–2284. doi: 10.1002/sim.4780122405 [DOI] [PubMed] [Google Scholar]
- 20. Macaskill P. Empirical Bayes estimates generated in a hierarchical summary ROC analysis agreed closely with those of a full Bayesian analysis. J Clin Epidemiol. 2004;57:925–932. doi: 10.1016/j.jclinepi.2003.12.019 [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 23. Teunissen C, Habets J, Velthuis BK, Cramer MJ, Loh P. Double‐contrast, single‐phase computed tomography angiography for ruling out left atrial appendage thrombus prior to atrial fibrillation ablation. Int J Cardiovasc Imaging. 2017;33:121–128. doi: 10.1007/s10554-016-0973-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer S, De Belder M, Muir D, Williams P. Cardiac computed tomography for assessment of left atrial thrombus in patients undergoing TAVI. Heart. 2016;102:A11. [Google Scholar]
- 25. Kawaji T, Numamoto H, Yamagami S, Mabuchi R, Kitamura T, Enoki N, Koizumi K, Kanao S, Kato M, Yokomatsu T, et al. Real‐time surveillance of left atrial appendage thrombus during contrast computed tomography imaging for catheter ablation: THe Reliability of cOMputed tomography Beyond UltraSound in THROMBUS detection (THROMBUS) study. J Thromb Thrombolysis. 2019;47:42–50. doi: 10.1007/s11239-018-1742-y [DOI] [PubMed] [Google Scholar]
- 26. Munir S, Chang JH, Salahudeen SR, Baranchuk A, Morris C, O'Reilly M, Pal RS. Atrial thrombi detection prior to pulmonary vein isolation: diagnostic accuracy of cardiac computed tomography versus transesophageal echocardiography. Cardiol J. 2015;22:576–582. doi: 10.5603/CJ.a2015.0017 [DOI] [PubMed] [Google Scholar]
- 27. Choi BH, Ko SM, Hwang HK, Song MG, Shin JK, Kang WS, Kim TY. Detection of left atrial thrombus in patients with mitral stenosis and atrial fibrillation: retrospective comparison of two‐phase computed tomography, transoesophageal echocardiography and surgical findings. Eur Radiol. 2013;23:2944–2953. doi: 10.1007/s00330-013-2944-5 [DOI] [PubMed] [Google Scholar]
- 28. Achenbach S, Sacher D, Ropers D, Pohle K, Nixdorff U, Hoffmann U, Muschiol G, Flachskampf FA, Daniel WG. Electron beam computed tomography for the detection of left atrial thrombi in patients with atrial fibrillation. Heart. 2004;90:1477–1478. doi: 10.1136/hrt.2003.027805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim YY, Klein AL, Halliburton SS, Popovic ZB, Kuzmiak SA, Sola S, Garcia MJ, Schoenhagen P, Natale A, Desai MY. Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J. 2007;154:1199–1205. doi: 10.1016/j.ahj.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 30. Shapiro MD, Neilan TG, Jassal DS, Samy B, Nasir K, Hoffmann U, Sarwar A, Butler J, Brady TJ, Cury RC. Multidetector computed tomography for the detection of left atrial appendage thrombus: a comparative study with transesophageal echocardiography. J Comput Assist Tomogr. 2007;31:905–909. doi: 10.1097/rct.0b013e31803c55e3 [DOI] [PubMed] [Google Scholar]
- 31. Feuchtner GM, Dichtl W, Bonatti JO, Jodocy D, Muller S, Hintringer F, Gradl J, Klauser A, Cury RC. Diagnostic accuracy of cardiac 64‐slice computed tomography in detecting atrial thrombi: comparative study with transesophageal echocardiography and cardiac surgery. Invest Radiol. 2008;43:794–801. doi: 10.1097/RLI.0b013e318184cd6c [DOI] [PubMed] [Google Scholar]
- 32. Tang RB, Dong JZ, Zhang ZQ, Li ZA, Liu XP, Kang JP, Yu RH, Long DY, Ma CS. Comparison of contrast enhanced 64‐slice computed tomography and transesophageal echocardiography in detection of left atrial thrombus in patients with atrial fibrillation. J Interv Card Electrophysiol. 2008;22:199–203. doi: 10.1007/s10840-008-9243-0 [DOI] [PubMed] [Google Scholar]
- 33. Hur J, Kim YJ, Nam JE, Choe KO, Choi EY, Shim CY, Choi BW. Thrombus in the left atrial appendage in stroke patients: detection with cardiac CT angiography—a preliminary report. Radiology. 2008;249:81–87. doi: 10.1148/radiol.2491071544 [DOI] [PubMed] [Google Scholar]
- 34. Patel A, Au E, Donegan K, Kim RJ, Lin FY, Stein KM, Markowitz SM, Iwai S, Weinsaft JW, Min JK, et al. Multidetector row computed tomography for identification of left atrial appendage filling defects in patients undergoing pulmonary vein isolation for treatment of atrial fibrillation: comparison with transesophageal echocardiography. Heart Rhythm. 2008;5:253–260. doi: 10.1016/j.hrthm.2007.10.025 [DOI] [PubMed] [Google Scholar]
- 35. Martinez MW, Kirsch J, Williamson EE, Syed IS, Feng D, Ommen S, Packer DL, Brady PA. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging. 2009;2:69–76. doi: 10.1016/j.jcmg.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 36. Hur J, Kim YJ, Lee H‐J, Ha J‐W, Heo JH, Choi E‐Y, Shim C‐Y, Kim TH, Nam JE, Choe KO, et al. Left atrial appendage thrombi in stroke patients: detection with two‐phase cardiac CT angiography versus transesophageal echocardiography. Radiology. 2009;251:683–690. doi: 10.1148/radiol.2513090794 [DOI] [PubMed] [Google Scholar]
- 37. Kim SC, Chun EJ, Choi SI, Lee SJ, Chang HJ, Han MK, Bae HJ, Park JH. Differentiation between spontaneous echocardiographic contrast and left atrial appendage thrombus in patients with suspected embolic stroke using two‐phase multidetector computed tomography. Am J Cardiol. 2010;106:1174–1181. doi: 10.1016/j.amjcard.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 38. Kapa S, Martinez MW, Williamson EE, Ommen SR, Syed IS, Feng D, Packer DL, Brady PA. ECG‐gated dual‐source CT for detection of left atrial appendage thrombus in patients undergoing catheter ablation for atrial fibrillation. J Interv Card Electrophysiol. 2010;29:75–81. doi: 10.1007/s10840-010-9505-5 [DOI] [PubMed] [Google Scholar]
- 39. Maltagliati A, Pontone G, Annoni A, Formenti A, Galli CA, Tamborini G, Alimento M, Andreini D, Tondo C, Pepi M. Multidetector computed tomography vs multiplane transesophageal echocardiography in detecting atrial thrombi in patients candidate to radiofrequency ablation of atrial fibrillation. Int J Cardiol. 2011;152:251–254. doi: 10.1016/j.ijcard.2011.07.086 [DOI] [PubMed] [Google Scholar]
- 40. Hur J, Kim YJ, Lee H‐J, Nam JE, Ha J‐W, Heo JH, Chang H‐J, Kim HS, Hong YJ, Kim HY, et al. Dual‐enhanced cardiac CT for detection of left atrial appendage thrombus in patients with stroke: a prospective comparison study with transesophageal echocardiography. Stroke. 2011;42:2471–2477. doi: 10.1161/STROKEAHA.110.611293 [DOI] [PubMed] [Google Scholar]
- 41. Sawit ST, Garcia‐Alvarez A, Suri B, Gaztanaga J, Fernandez‐Friera L, Mirelis JG, D'Anca M, Fuster V, Sanz J, Garcia MJ. Usefulness of cardiac computed tomographic delayed contrast enhancement of the left atrial appendage before pulmonary vein ablation. Am J Cardiol. 2012;109:677–684. doi: 10.1016/j.amjcard.2011.10.028 [DOI] [PubMed] [Google Scholar]
- 42. Hur J, Pak HN, Kim YJ, Lee HJ, Chang HJ, Hong YJ, Choi BW. Dual‐enhancement cardiac computed tomography for assessing left atrial thrombus and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2013;112:238–244. doi: 10.1016/j.amjcard.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 43. Dorenkamp M, Sohns C, Vollmann D, Luthje L, Seegers J, Wachter R, Puls M, Staab W, Lotz J, Zabel M. Detection of left atrial thrombus during routine diagnostic work‐up prior to pulmonary vein isolation for atrial fibrillation: role of transesophageal echocardiography and multidetector computed tomography. Int J Cardiol. 2013;163:26–33. doi: 10.1016/j.ijcard.2011.06.124 [DOI] [PubMed] [Google Scholar]
- 44. Budoff MJ, Shittu A, Hacioglu Y, Gang E, Li D, Bhatia H, Alvergue J, Karlsberg RP. Comparison of transesophageal echocardiography versus computed tomography for detection of left atrial appendage filling defect (thrombus). Am J Cardiol. 2014;113:173–177. doi: 10.1016/j.amjcard.2013.09.037 [DOI] [PubMed] [Google Scholar]
- 45. Hong S‐J, Kim J‐Y, Kim J‐B, Sung J‐H, Wook Kim D, Uhm J‐S, Lee H‐J, Jin Kim Y, Pak H‐N, Lee M‐H, et al. Multidetector computed tomography may be an adequate screening test to reduce periprocedural stroke in atrial fibrillation ablation: a multicenter propensity‐matched analysis. Heart Rhythm. 2014;11:763–770. doi: 10.1016/j.hrthm.2014.01.026 [DOI] [PubMed] [Google Scholar]
- 46. Homsi R, Nath B, Luetkens JA, Schwab JO, Schild HH, Naehle CP. Can contrast‐enhanced multi‐detector computed tomography replace transesophageal echocardiography for the detection of thrombogenic milieu and thrombi in the left atrial appendage: a prospective study with 124 patients. RoFo. 2016;188:45–52. [DOI] [PubMed] [Google Scholar]
- 47. Wang L, Kadiyala M, Koss E, Yarramaneni A, Rapelje K, Kampfer S, Reichek N, Hoch D, Jayam V, Levine J, et al. CTA detection of left atrial stasis and thrombus in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2016;39:1388–1393. doi: 10.1111/pace.12959 [DOI] [PubMed] [Google Scholar]
- 48. Zhai Z, Tang M, Zhang S, Fang P, Jia Y, Feng T, Wang J. Transoesophageal echocardiography prior to catheter ablation could be avoided in atrial fibrillation patients with a low risk of stroke and without filling defects in the late‐phase MDCT scan: a retrospective analysis of 783 patients. Clin Cardiol. 2018;28:1835–1843. doi: 10.1007/s00330-017-5172-6 [DOI] [PubMed] [Google Scholar]
- 49. Kottmaier M, Jilek C, Berglar S, Reents T, Bourier F, Semmler V, Telishevska M, Koch‐Büttner K, Lengauer S, Kornmayer M, et al. Exclusion of left atrial thrombus by dual‐source cardiac computed tomography prior to catheter ablation for atrial fibrillation. Clin Res Cardiol. 2019;108:150–156. doi: 10.1007/s00392-018-1333-0 [DOI] [PubMed] [Google Scholar]
- 50. Li W, Yu F, Zhu W, Zhang W, Jiang T. Detection of left atrial appendage thrombi by third‐generation dual‐source dual‐energy CT: iodine concentration versus conventional enhancement measurements. Int J Cardiol. 2019;292:265–270. doi: 10.1016/j.ijcard.2019.04.079 [DOI] [PubMed] [Google Scholar]
- 51. Guha A, Dunleavy MP, Hayes S, Afzal MR, Daoud EG, Raman SV, Harfi TT. Accuracy of contrast‐enhanced computed tomography for thrombus detection prior to atrial fibrillation ablation and role of novel Left Atrial Appendage Enhancement Index in appendage flow assessment. Int J Cardiol. 2020;318:147–152. doi: 10.1016/j.ijcard.2020.06.035 [DOI] [PubMed] [Google Scholar]
- 52. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey J‐Y, Lowe JE, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d [DOI] [PubMed] [Google Scholar]
- 53. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation: developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS): endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e21. [DOI] [PubMed] [Google Scholar]
- 54. Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, McDonald RJ, Rodby RA, Wang CL, Weinreb JC. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020;2:85–93. doi: 10.1016/j.xkme.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stocker TJ, Deseive S, Leipsic J, Hadamitzky M, Chen MY, Rubinshtein R, Heckner M, Bax JJ, Fang X‐M, Grove EL, et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J. 2018;39:3715–3723. doi: 10.1093/eurheartj/ehy546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S2


