Abstract
Background
The serum uric acid/serum creatinine ratio (SUA/SCr), which represents renal function‐normalized SUA, is associated with diverse adverse outcomes. The aim of this study was to investigate the association between SUA/SCr and cardiovascular disease (CVD), and determine whether and to what extent this association is mediated by cardiometabolic factors.
Methods and Results
This prospective study enrolled 96 378 participants from the Kailuan study without stroke and myocardial infarction at baseline (2006). Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Mediation analyses were conducted to separately explore the mediating effects of cardiometabolic factors on the association between SUA/SCr and CVD. During median follow up of 11.01 years, 6315 (6.55%) individuals developed incident CVD. After adjustment for potential confounders, the highest quartile of SUA/SCr was associated with the highest risk of CVD (HR, 1.15; 95% CI, 1.07–1.23), stroke (HR, 1.16; 95% CI, 1.07–1.26), ischemic stroke (HR, 1.12; 95% CI, 1.02–1.22), and hemorrhagic stroke (HR, 1.36; 95% CI, 1.11–1.65), but not with myocardial infarction (HR, 1.07; 95% CI, 0.92–1.25). The association was consistent across different degrees of kidney function and glucose tolerance statuses. Additionally, the association between high SUA/SCr and CVD was partially mediated by triglycerides (30.74%), body mass index (BMI) (19.52%), total cholesterol (15.06%), hs‐CRP (high‐sensitivity C‐reactive protein) (13.06%), diastolic blood pressure (11.75%), and blood glucose (−16.38%).
Conclusions
SUA/SCr and CVD were positively associated. Furthermore, this association was partially mediated through blood lipids, BMI, blood pressure, hs‐CRP, and blood glucose.
Keywords: cardiometabolic factors, cardiovascular disease, mediation analysis, serum uric acid to serum creatinine ratio
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- FBG
fasting blood glucose
- SBP
systolic blood pressure
- SUA
serum uric acid
- SUA/SCr
serum uric acid to serum creatinine ratio
- TC
total cholesterol
- TG
triglyceride
Clinical Perspective
What Is New?
Higher levels of serum uric acid/serum creatinine ratio (SUA/SCr) were significantly associated with the risk of cardiovascular disease.
The association between SUA/SCr and cardiovascular disease was partially mediated through blood lipids, body mass index, blood pressure, high‐sensitivity C‐reactive protein, and blood glucose.
What Are the Clinical Implications?
SUA/SCr is a practical and effective risk factor for cardiovascular disease in a large‐scale community population in China.
These findings emphasize the important roles of SUA/SCr and cardiometabolic factors as conjunctive intervention targets in the prevention of cardiovascular disease.
Cardiovascular disease (CVD) is the most common cause of death worldwide and a significant contributor to morbidity. In 2017, ≈17.8 million CVD deaths occurred worldwide, corresponding to 330 million years of life lost and another 35.6 million years lived with disability. 1 Reducing the major risk factors for CVD is a major strategy for reducing its prevalence. Therefore, primary prevention of CVD through understanding and reduction of risk factors may have significant implications for public health and clinical practice.
Serum uric acid (SUA), the final oxidation product of purine metabolism, 2 has been shown to be a modifiable risk factor for CVD. However, conflicting data exist. 3 , 4 , 5 , 6 , 7 These inconsistencies may be explained by the fact that the concentration of endogenous SUA depends primarily on renal clearance function. Increased SUA often occurs as a consequence of renal dysfunction, although some previous studies have ignored the effect of renal function on SUA. 3 , 5 , 8 Consequently, renal function‐normalized SUA (the SUA to serum creatinine ratio [SUA/SCr]) has emerged as a new biomarker and is considered a superior indicator of net SUA production. Several studies have suggested that SUA/SCr is significantly associated with several metabolic diseases and mortality. 9 , 10 , 11 , 12 , 13 However, evidence on the effect of SUA/SCr on CVD is limited and, to date, the potential pathway linking SUA/SCr to CVD remains incompletely characterized. SUA/SCr was confirmed to be associated with various cardiometabolic factors, 9 , 14 , 15 , 16 , 17 , 18 which raised the question as to whether SUA/SCr can lead directly to the outcome of CVD, or if cardiometabolic factors, such as indicators of obesity, blood glucose, blood lipids, blood pressure, and indicators of inflammation play potentially mediating roles in the association between SUA/SCr and CVD.
The aim of the present study was to investigate the association between SUA/SCr and CVD and, using mediation analysis, assess whether and to what extent the association is mediated by cardiometabolic factors.
METHODS
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Study Design and Participants
The Kailuan study is a prospective cohort study conducted in the Kailuan community in Tangshan, China, involving employees of the Kailuan company and their family members. The detailed study design and procedures have been described previously. 19 , 20 Beginning from June 2006, a total of 101 510 active and retired employees from the Kailuan Coal Company (81 110 men and 20 400 women, aged 18–98 years; one‐third coal miners) were enrolled during the first survey, and underwent questionnaire assessments, clinical examinations, and laboratory tests every 2 years. All participants were followed up until their death or the end of the study (December 31, 2017). We excluded 3725 participants with history of stroke or myocardial infarction (MI), and 1407 participants with missing data on SUA or SCr at the baseline survey in 2006. Ultimately, 96 387 participants were enrolled in the final analysis (Figure S1). The study was performed in accordance with the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital and Beijing Tiantan Hospital. All participants provided written informed consent.
Measurement of SUA, SCr, and SUA/SCr
Fasting blood samples were collected in the morning following an overnight fast of 8 to 12 hours and transferred to vacuum tubes containing ethylene diamine tetra‐acetic acid. The concentrations of SUA were determined using a commercial kit (Ke Hua Biological Engineering Corporation, Shanghai, China) and an automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan) following the manufacturer’s instructions. SCr was measured using the sarcosine oxidase assay method with a Hitachi 7600 P autoanalyzer (Hitachi 7600, Tokyo, Japan). SUA/SCr was calculated as the concentration of SUA (mmol/L) divided by the concentration of SCr (mmol/L).
Assessment of Outcomes
The outcome of the present study was the first occurrence of CVD. Subtypes of CVD included stroke (ischemic stroke and hemorrhagic stroke) and MI. We used International Classification of Diseases 10th revision codes to identify cases of CVD (I63 for ischemic stroke, I60–I61 for hemorrhagic stroke, and I21 for MI). The database of CVD diagnoses, which covers all Kailuan study participants, was obtained from the Municipal Social Insurance Institution and Hospital Discharge Register, and was updated annually during the follow‐up period. To further identify potential CVD events, an expert panel collected and reviewed annual discharge records from 11 local hospitals and requested a history of CVD via a questionnaire during the biennial interview. For all suspected CVD events, 3 experienced physician adjudicators blinded to the study design reviewed the medical records. Stroke was diagnosed based on neurological signs, clinical symptoms, and neuroimaging tests, including computed tomography or magnetic resonance imaging, according to the World Health Organization criteria. 21 Given the small proportion (0.12%), we did not include the subarachnoid hemorrhage group in the subgroup analysis. The criteria for MI were based on combinations of chest pain symptoms, electrocardiographic changes, and cardiac enzyme levels. 22
Assessment of Covariates
Demographic and clinical features, including age, sex, smoking habits, alcohol use, educational level, and medical history, were collected via questionnaires. Educational attainment was categorized as illiteracy or primary school, middle school, or high school or above. Income level was categorized as <800 RMB/month or ≥800 RMB/month. Physical activity was classified as inactive activity (<80 minute per week) or active activity (≥80 minute per week). Smoking status and alcohol use were classified as never, former, or current according to self‐reported information. Weight and height were measured, and body mass index (BMI) was calculated as weight (kg)/height (m)2. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times with participants in a seated position using a mercury sphygmomanometer, and the average of three readings was used in analyses. All blood samples were tested using a Hitachi 747 autoanalyzer (Hitachi, Tokyo, Japan) at the central laboratory of Kailuan Hospital. Automated dipstick urinalysis (H12‐MA, DIRUI N‐600) was used to measure urinary protein. Women were analyzed during the nonmenstrual period. Urinary protein was recorded semiquantitatively as “none,” “trace,” “1+,” “2+,” or “≥3+.” The biochemical indicators tested included fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), and hs‐CRP (high‐sensitivity C‐reactive protein) . Estimated glomerular filtration rate (eGFR) was calculated using the creatinine‐based Chronic Kidney Disease Epidemiological Collaboration equation using the formula designated for White race. 23
Hypertension was defined as any self‐reported hypertension or use of antihypertensive drugs, or blood pressure ≥140/90 mm Hg. Prediabetes was defined as FBG of 5.6 to 6.9 mmol/L, and diabetes mellitus was defined as any self‐reported diabetes mellitus or use of glucose‐lowering drugs, or FBG ≥7 mmol/L. 24 Dyslipidemia was defined as any self‐reported history or use of lipid‐lowering drugs, or serum TC ≥5.17 mmol/L or TG ≥1.69 mmol/L or LDL‐C ≥3.62 mmol/L or HDL‐C ≤1.04 mmol/L. Chronic kidney disease was defined as eGFR <60 mL/min per 1.73 m2, mildly decreased kidney function was defined as eGFR from 60 to 89 mL/min per 1.73 m2, and normal kidney function was defined as eGFR ≥90 mL/min per 1.73 m2. 25 , 26
Statistical Analysis
Participants were divided into four categories according to quartiles of SUA/SCr. Continuous variables are presented as mean±SD and were compared using ANOVA. Categorical variables are presented as frequency (percentage) and were compared using the chi‐square test. Incidence rates (per 1000 person‐years) with 95% CI were calculated from the data at baseline to the data at CVD diagnosis, death, or end of follow up (December 31, 2017), whichever occurred first.
The associations between SUA/SCr and CVD and its subtypes were evaluated using Cox proportional hazard models. Validity of the proportionality assumption was verified using scaled Schoenfeld residuals. The multivariable‐adjusted model was adjusted for age, sex, BMI, education, income, current smoking, current drinking, physical activity, history of hypertension, diabetes, dyslipidemia, proteinuria, diuretics, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blocker treatment, SBP, DBP, FBG, TC, and hs‐CRP. Trend tests were performed in the Cox regression models after the median SUA/SCr values of each quartile were entered into the model and treated as continuous variables. When SUA/SCr was treated as a continuous variable, a multivariable Cox proportional hazard model was used to estimate the hazard ratio (HR) and 95% CI of incident CVD per SD increment in SUA/SCr. Additionally, we used restricted cubic splines to examine the shape of the association between SUA/SCr and the outcomes with five knots (at the 5th, 25th, 50th, 75th, and 95th percentiles). The reference point for SUA/SCr was the median (2.10) of the reference group (the first quartile) and the HR was adjusted for all confounding variables.
To validate the robustness of our results, sensitivity analyses were performed. The first sensitivity analysis was conducted using Fine–Gray models by considering non‐CVD deaths as competing risk events. Second, to explore the potential impact of reverse causality, we repeated the primary analysis using a 2‐year lag period by excluding incident CVD cases from the first 2 years of follow up. Subgroup analyses were performed across different ages (<45, 45–65, and ≥65 years), degrees of kidney function according to eGFR levels (chronic kidney disease, mildly decreased, and normal function), and glucose tolerance statuses (normoglycemia, prediabetes, and diabetes). Using the likelihood ratio test, we assessed the interaction effect by including interaction terms between SUA/SCr and each above‐noted indicator in the model. Furthermore, we compared the strength of the association between SUA/SCr, SUA, and SCr and risk of CVD estimated using the multivariable Cox proportional hazard model.
Upon establishment of the temporal relationship between SUA/SCr and CVD, the mediation method was applied to examine whether the associations were mediated by metabolic factors. SUA/SCr was the predictor variable (X); cardiometabolic factors were mediators (M); and CVD was the outcome variable (Y). Generally, four steps are involved in mediation analyses: (1) demonstrating that the predictor variable is associated with the outcome (Model Y=βTot X) (βTot=total effect); (2) demonstrating that the predictor variable is associated with the mediator (Model M=β1 X) (β1=indirect effect 1); (3) demonstrating which part of the outcome is explained by controlling for the predictor (Model Y=β2 M+βDir X) (β2=indirect effect, βDir=direct effect); and (4) calculating the proportion of mediation: mediation effect (%)=(β1×β2/βTol)×100%. This statistical approach was conducted in accordance with that described by Valeri and VanderWeele and has been applied successfully in previous studies to demonstrate the role of mediators. 27 , 28 , 29 We included age, sex, education, income, smoking status, and drinking status as covariates to adjust for the mediating effect of these biomarkers on the association between SUA/SCr and CVD because when using mediation models, it is necessary that baseline covariates are sufficient to control for exposure‐outcome, mediator‐outcome, and exposure‐mediator confounding. 30
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A two‐sided P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
A total of 96 378 eligible participants were included in this study. Mean age was 51.48±12.57 years, and 76 662 (79.54%) were men. Baseline characteristics according to quartiles of SUA/SCr are presented in Table. Significant differences were observed in age, sex, educational level, family income, smoking status, drinking status, physical activity, hypertension, diabetes, dyslipidemia, antihypertensive agents, hypoglycemic agents, lipid‐lowering agents, BMI, SBP, DBP, TC, TG, HDL‐C, LDL‐C, FBG, and hs‐CRP between the four categories (all P<0.05).
Table 1.
Baseline Characteristics of Participants According to Quartiles of SUA/SCr Ratio
| Characteristics | Total | Quartiles of SUA/SCr ratio | P value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| No. of participants | 96 378 | 24 094 | 24 095 | 24 095 | 24 094 | |
| SUA/SCr ratio | 3.17 (2.54–3.92) | 2.14 (1.83–2.36) | 2.87 (2.71–3.02) | 3.51 (3.33–3.71) | 4.55 (4.20–5.15) | <0.0001 |
| Age, y | 51.48±12.57 | 50.62±12.23 | 51.57±12.53 | 51.64±12.63 | 52.08±12.83 | <0.0001 |
| Men, n (%) | 76 662 (79.54) | 18 424 (76.47) | 18 809 (78.06) | 19 526 (81.04) | 19 903 (82.61) | <0.0001 |
| High school or above, n (%) | 6528 (7.01) | 993 (4.24) | 1536 (6.61) | 1898 (8.18) | 2101 (9.05) | <0.0001 |
| Income ≥800 RMB/mo, n (%) | 13 209 (14.20) | 2382 (10.19) | 3065 (13.20) | 3615 (15.59) | 4147 (17.88) | <0.0001 |
| Current smoker, n (%) | 32 089 (34.21) | 6280 (26.82) | 7766 (33.23) | 8742 (37.21) | 9301 (39.52) | <0.0001 |
| Current alcohol, n (%) | 35 142 (37.45) | 6287 (26.83) | 8241 (35.25) | 9720 (41.37) | 10 894 (46.29) | <0.0001 |
| Active physical activity, n (%) | 13 994 (14.52) | 2895 (12.02) | 3377 (14.02) | 3709 (15.39) | 4013 (16.66) | <0.0001 |
| Hypertension, n (%) | 41 633 (43.20) | 11 199 (46.48) | 9969 (41.37) | 9738 (40.42) | 10 727 (44.52) | <0.0001 |
| Diabetes, n (%) | 8709 (9.04) | 2751 (11.42) | 2156 (8.95) | 1892 (7.85) | 1910 (7.93) | <0.0001 |
| Dyslipidemia, n (%) | 33 137 (34.38) | 7285 (30.24) | 7566 (31.40) | 8339 (34.61) | 9947 (41.28) | <0.0001 |
| Proteinuria ≥1+, n (%) | 3414 (3.72) | 1196 (5.09) | 741 (3.20) | 667 (2.91) | 810 (3.63) | <0.0001 |
| Antihypertensive agents, n (%) | 9436 (9.79) | 1631 (6.77) | 2038 (8.46) | 2444 (10.14) | 3323 (13.79) | <0.0001 |
| Diuretics, n (%) | 962 (1.03) | 123 (0.53) | 152 (0.65) | 228 (0.98) | 459 (1.98) | <0.0001 |
| ACEI/ARB treatment, n (%) | 647 (0.70) | 95 (0.41) | 144 (0.62) | 162 (0.70) | 246 (1.06) | <0.0001 |
| Hypoglycemic agents, n (%) | 2061 (2.14) | 594 (2.46) | 519 (2.15) | 480 (1.99) | 468 (1.94) | 0.0002 |
| Lipid‐lowering agents, n (%) | 709 (0.74) | 116 (0.48) | 161 (0.67) | 176 (0.73) | 256 (1.06) | <0.0001 |
| Body mass index, kg/m2 | 25.02±3.49 | 24.72±3.34 | 24.80±3.48 | 25.07±3.52 | 25.49±3.57 | <0.0001 |
| Systolic blood pressure, mm Hg | 130.64±20.88 | 131.09±20.63 | 130.14±21.15 | 129.79±20.63 | 131.52±21.08 | <0.0001 |
| Diastolic blood pressure, mm Hg | 83.38±11.76 | 83.55±11.66 | 83.05±11.85 | 82.85±11.66 | 84.08±11.82 | <0.0001 |
| Total cholesterol, mg/dL | 4.95±1.15 | 4.78±1.27 | 4.92±1.09 | 5.00±1.05 | 5.08±1.14 | <0.0001 |
| Triglyceride, mg/dL | 1.67±1.37 | 1.53±1.20 | 1.63±1.28 | 1.64±1.38 | 1.89±1.57 | <0.0001 |
| HDL‐C, mg/dL | 1.55±0.4 | 1.59±0.39 | 1.54±0.40 | 1.53±0.41 | 1.54±0.41 | <0.0001 |
| LDL‐C, mg/dL | 2.34±0.91 | 2.30±0.91 | 2.29±0.90 | 2.33±0.93 | 2.46±0.89 | <0.0001 |
| Fasting blood glucose, mmol/L | 5.47±1.67 | 5.62±2.04 | 5.44±1.65 | 5.40±1.49 | 5.42±1.41 | <0.0001 |
| hs‐CRP, mg/L | 2.39±6.48 | 2.13±6.93 | 2.32±6.24 | 2.38±5.72 | 2.72±6.91 | <0.0001 |
ACEI indicates angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; HDL‐C, high density lipoprotein cholesterol; hs‐CRP, high sensitivity C‐reactive protein; LDL‐C, low density lipoprotein cholesterol; SCr, serum creatinine; and SUA, serum uric acid.
Association Between SUA/SCr and CVD and its Subtypes
During median follow up of 11.01 years (interquartile range, 10.67–11.20), a total of 6315 (6.55%) individuals developed incident CVD, including 4943 (5.12%) cases of stroke, 4091 (4.24%) cases of ischemic stroke, 816 (0.85%) cases of hemorrhagic stroke, and 1381 (1.43%) cases of MI. The incidence of CVD increased substantially with increasing SUA/SCr quartiles, reaching an incidence of 7.00/1000 person‐years (95% CI, 6.68–7.34) in the highest quartile of SUA/SCr (Figure 1). After adjustment for potential confounding factors, the highest quartile of SUA/SCr remained significantly associated with the risk of incident CVD. Compared with the lowest quartile of SUA/SCr, the HR was 1.15 (95% CI, 1.07–1.23; P for trend<0.0001). The significant association with CVD disappeared when SUA and SCr were treated as the exposure variables (Table S1).
Figure 1. Association between quartiles of SUA/SCr and risk of cardiovascular disease and its subtypes.
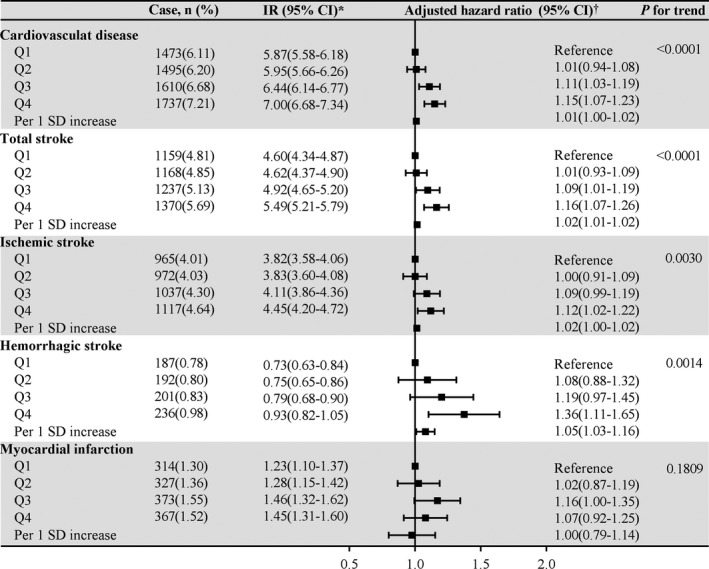
HR indicates hazard ratio; IR, incidence rate; and SUA/SCr, serum uric acid to serum creatinine ratio. *Incidence rate per 1000 person‐years. †Adjusted for age, sex, body mass index, education, income, smoke, drink, physical activity, hypertension, diabetes, dyslipidemia, proteinuria, diuretics, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers treatment, systolic blood pressure, diastolic blood pressure, fasting blood glucose, total cholesterol, and high sensitivity C‐reactive protein.
In the analyses of CVD subtypes, similar results were obtained for total stroke (HR, 1.16; 95% CI, 1.07–1.26; P for trend <0.0001), ischemic stroke (HR, 1.12; 95% CI, 1.02–1.22; P for trend=0.0030), and hemorrhagic stroke (HR, 1.36; 95% CI, 1.11–1.65; P for trend=0.0014). However, most HRs pertaining to MI were not statistically significant (HR, 1.07; 95% CI, 0.92–1.25) (Figure 1).
All sensitivity analyses using Fine–Gray models (Table S2) and excluding outcome events within the first 2 years (N=1500, Table S3) generated similar findings with the primary analysis. The multivariable‐adjusted spline model showed a J‐shaped relationship between SUA/SCr and the risk of CVD, stroke, ischemic stroke, and hemorrhagic stroke, but not risk of MI (Figure 2). In the subgroup analyses, the interactions between SUA/SCr and age, kidney function, and glucose tolerance status were confirmed without effects on the incidence of CVD and its subtypes (all P for interaction>0.05), suggesting that individuals with different ages, degrees of kidney function (chronic kidney disease, mildly decreased, or normal), or glucose tolerance statuses (diabetic or prediabetic) and with higher SUA/SCr have the same relative risk of CVD (Figure 3).
Figure 2. HRs and 95% CIs for SUA/SCr with risk of (A) CVD and (B–E) its subtypes by using restricted cubic spline regression with 5 knots with placed at the 5th, 25th, 50th, 75th, 95th percentiles of SUA/SCr.
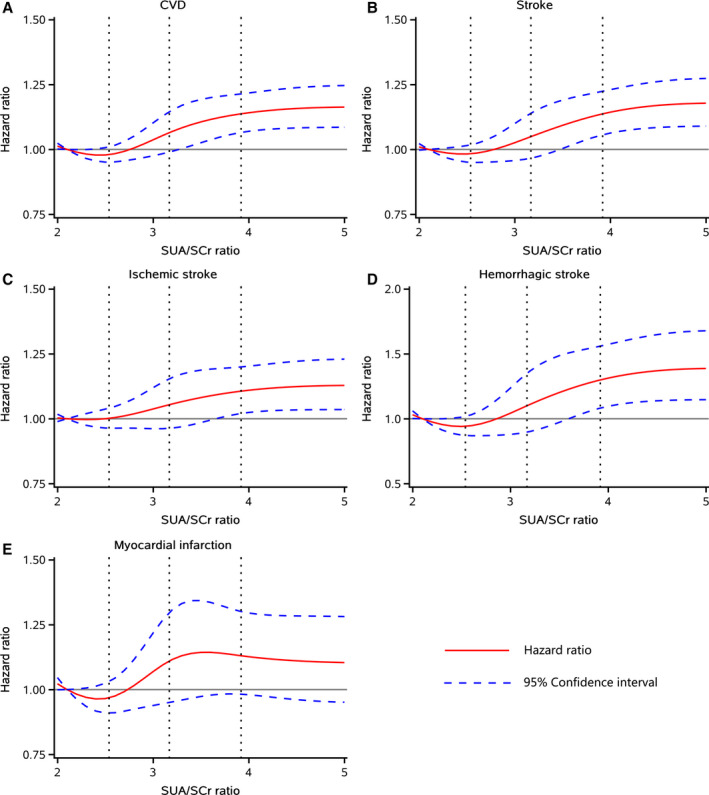
The red line represented HR and blue lines represented 95% CI. Adjusted for age, sex, body mass index, education, income, smoke, drink, physical activity, hypertension, diabetes, dyslipidemia, proteinuria, diuretics, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers treatment, systolic blood pressure, diastolic blood pressure, fasting blood glucose, total cholesterol, and high sensitivity C‐reactive protein. CVD indicates cardiovascular disease; HR, hazard ratio; and SUA/SCr, serum uric acid to serum creatinine ratio.
Figure 3. Adjusted hazard ratio for the association between high SUA/SCr ratio (Q4) and risk of cardiovascular diseases stratified by (A) age, (B) kidney function status, and (C) glucose status subgroups.
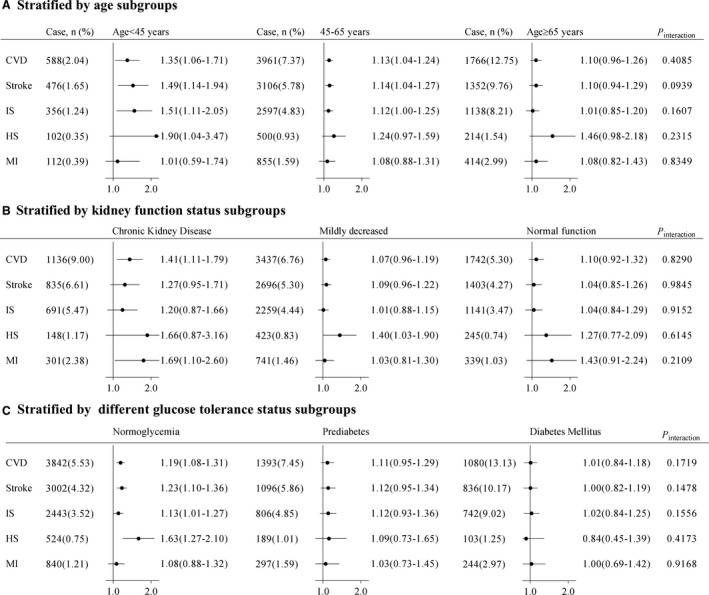
Adjusted for age, sex, body mass index, education, income, smoke, drink, physical activity, hypertension, diabetes, dyslipidemia, proteinuria, diuretics, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers treatment, systolic blood pressure, diastolic blood pressure, fasting blood glucose, total cholesterol, and high sensitivity C‐reactive protein other than variables for stratification. Chronic kidney disease was defined as eGFR<60 mL/min per 1.73 m2, mild decrease was defined as eGFR ranged of 60 to 89 mL/min per 1.73 m2, normal function was defined as eGFR ≥90 mL/min per 1.73 m2. Normglycemia was defined as a fasting plasma glucose <5.6 mmol/L, prediabetes was defined as a fasting plasma glucose of 5.6 to 6.9 mmol/L, and diabetes mellitus was defined as a fasting plasma glucose ≥7.0 mmol/L, or in those taking oral hypoglycemic agents or insulin, or having a history of diabetes mellitus. CVD indicates cardiovascular disease; HS, hemorrhagic stroke; IS, ischemic stroke; MI, myocardial infarction; and SUA/SCr, serum uric acid to serum creatinine ratio.
Mediation Analysis Between SUA/SCr and CVD
We tested BMI, SBP, DBP, FBG, TC, TG, LDL‐C, HDL‐C, and hs‐CRP as potential mediators of the association between SUA/SCr and CVD. The results of mediation analyses are presented in Table S4 and Figure 4. The total effect of SUA/SCr on CVD was 0.0061 (95% CI, 0.0024–0.0096; P=0.0010). The association between SUA/SCr and CVD was partially mediated by TG (βindir=0.0019, mediation proportion [MP]=30.74%), BMI (βindir=0.0012, MP=19.52%), TC (βindir=0.0009, MP=15.06%), hs‐CRP (βindir=0.0008, MP=13.06%), and DBP (βindir=0.0007, MP=11.75%). In contrast, FBG had a negative mediating effect on this association (βindir=−0.0010, MP=−16.38%). The remaining factors—SBP, LDL‐C, and HDL‐C—did not play significant mediating roles in the association between SUA/SCr and CVD.
Figure 4. Mediation analyses of the association between SUA/SCr and CVD.
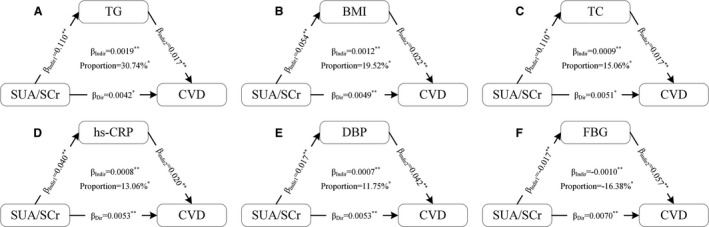
A, Contribution of TG; (B) Contribution of BMI; (C) Contribution of TC; (D) Contribution of hs‐CRP; (E) Contribution of DBP; (F) Contribution of FBG. Adjusted for age, sex, education, income, smoking status, drinking status, and physical activity. BMI indicates body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; hs‐CRP, high sensitivity C‐reactive protein; SUA/SCr, serum uric acid/serum creatinine ratio; TC, total cholesterol; and TG, triglyceride. * P<0.05; ** P<0.01.
DISCUSSION
In this prospective cohort study, we found that relative to the lowest quartile, the highest quartile of SUA/SCr was associated with elevated risk of CVD, as well as stroke, ischemic stroke, and hemorrhagic stroke, even after adjustment for multiple covariates. The associations were consistent across different degrees of kidney function and glucose tolerance statuses. Mediation analyses showed that BMI, TG, TC, DBP, FBG, and hs‐CRP may play important mediating roles in the association between SUA/SCr and CVD.
The role of SUA in the development of CVD has long been debated. Some epidemiological studies, such as the Apolipoprotein MOrtality RISk study and the Rotterdam study, support a significantly positive association. 3 , 4 Other observational studies, such as the Fremantle Diabetes Study and the results of a Mendelian randomization analysis, failed to demonstrate a significant association between SUA and CVD. 5 , 6 This was likely because of the following factors: SUA levels were determined primarily by renal clearance function, those with lower eGFR are more likely to have higher SUA levels, and renal dysfunction may be a major confounder in studies on the association between SUA and CVD. 31 , 32 , 33 Therefore, if SUA is in fact a risk factor for CVD, baseline renal function‐normalized SUA, which may reflect net SUA production, will be a more precise predictor of incident CVD.
Previous studies reported that SUA/SCr is associated with certain adverse health outcomes under specific conditions, such as metabolic syndrome in postmenopausal women or diabetic patients, chronic kidney disease in diabetic individuals, and liver function in healthy subjects. All of these adverse outcomes are proven risk factors for CVD and may contribute to the pathology of CVD. 9 , 10 , 11 , 34 , 35 , 36 To our knowledge, the association between SUA/SCr and CVD has not yet been fully characterized. To address this knowledge gap, we conducted the present study. Our results showed that higher SUA/SCr was associated with elevated risk of CVD, which redefines the role of renal function‐normalized SUA in the management of CVD. The biological mechanisms linking SUA/SCr to CVD are primarily considered to be oxidative stress and promotion of inflammation. Increases in oxidative stress associated with SUA/SCr can result in senescence and apoptosis of human umbilical vein endothelial cells. 37 Furthermore, SUA/SCr can promote proliferative and proinflammatory responses in cultured vascular smooth muscle cells. 38 , 39 Other evidence indicates that SUA may have a pathogenic role through vascular effects. 40 Additionally, SUA/SCr is associated with many cardiometabolic factors 14 , 15 , 16 that may provide a mediating pathway for the association between SUA/SCr and CVD.
To investigate the potential pathways between SUA/SCr and CVD, mediation analyses were performed by considering cardiometabolic factors as mediators. The results showed that TG, TC, BMI, DBP, hs‐CRP, and FBG played fundamental roles in these pathways. Regarding the mediating role of lipids, previous studies reported that higher SUA had an unfavorable impact on lipids, and was associated with dyslipidemia. 41 SUA and lipid metabolic disorder are mutually associated. An increase in SUA can lead to a decline in lipoprotein enzymes that affect lipid metabolism, eventually resulting in alteration of adipose factors that regulate fat synthesis. 42 Therefore, lipids may mediate the association between SUA/SCr and CVD. Regarding obesity and indicators of inflammation, SUA can activate the NLRP3 inflammasome and induce the production of inflammatory factors, and SUA acts on fat cells, which induces an inflammatory response. In the state of obesity, SUA can be converted into an oxidant and participate directly in proliferation and oxidative stress of fat. 30 , 43 Inflammation and oxidative stress are major contributors in the pathology of CVD. Regarding blood pressure, many previous studies confirmed that SUA is associated with the risk of hypertension. Moreover, SUA can activate the renin‐angiotensin system, decrease renal blood flow, induce endothelial cell dysfunction, and further stimulate vascular smooth muscle cell proliferation in systemic circulation, and these mechanisms lead to the occurrence of hypertension and CVD. 44 , 45 However, in the present study, FBG had a negative mediating effect on the association between SUA and CVD, suggesting an inverse link between SUA and FBG. Indeed, the negative association between SUA and FBG is consistent with findings from previous studies. A cross‐sectional survey in Southern China reported a correlation coefficient of −0.26 between SUA and FBG in patients with type 2 diabetes. 46 Data from the Riyadh 2 cohort showed that FBG was decreased with increased SUA/SCr, and subjects in the highest tertile of SUA/SCr had the lowest levels of FBG. 47 Similar results were also observed in a retrospective study, which showed a negative correlation between SUA/SCr and diabetes (r=−0.075) and FBG (−0.059). 9 One study has shown that the hyperfiltration state caused by hyperglycemia can promote excretion of SUA, which partially explains the existence of the inverse association. 46 Our results showed that TG, TC, and DBP played mediating roles in the association between SUA/SCr and CVD. Although LDL‐C, HDL‐C, and SBP were not shown to play mediating roles in this association, this does not necessarily imply that they are not associated with SUA/SCr or CVD. Possible explanations required further investigations.
Our study had some strengths, including a large sample size and long follow‐up period. Additionally, we quantified the contribution of cardiometabolic factors in the pathways between SUA/SCr and CVD. However, the study also had limitations. First, we did not collect information on commonly used urate‐lowering management strategies or medication use such as allopurinol. The potential for these treatment strategies to lower CVD risk is relatively understudied. Second, we separately assessed the mediating effects of an indicator of obesity, blood pressure, blood lipids, blood glucose, and an indicator of inflammation on the association between SUA/SCr and CVD. However, because of the complexity associated with the numerous permutations of mediators, it may not be feasible to mutually consider the combined mediating and interactive effects of these mediators. Third, because all participants were employees of the Kailuan Coal Company and most were men, the sex distribution of participants was unbalanced. Therefore, this cohort is not nationally representative. However, the constitution of the population was complex, consisting of individuals from all levels of society and across various occupations, including coal miners, administrators, secretaries, accountants, and support and service staff, such as physicians, nurses, vendors, and teachers. Study of such a geographically confined and controlled population greatly reduces residual confounding factors because of diverse socioeconomic factors and lifestyle patterns. Finally, because this was an observational study, we could not establish a causal association between SUA/SCr and CVD.
CONCLUSIONS
In conclusion, we found that higher SUA/SCr was associated with risk of incident CVD. Furthermore, mediation analyses demonstrated that blood lipids, BMI, blood pressure, hs‐CRP, and blood glucose are potential mediators of this association. The identification of a mediating effect between SUA/SCr and development of CVD may improve our understanding of the mechanisms that influence incident CVD, and emphasize the important roles of renal function‐normalized SUA (SUA/SCr) and cardiometabolic factors as conjunctive intervention targets in the prevention of CVD.
Sources of Funding
This work was supported by National Natural Science Foundation of China (81870905, U20A20358, 81773512), Beijing Municipal Science & Technology Commission (D171100003017002), National Key R&D Program of China (2018YFC1312903), Beijing Municipal Administration of Hospitals Incubating Program (PX2020021), Beijing Excellent Talents Training Program (2018000021469G234), Young Elite Scientists Sponsorship Program by CAST (2018QNRC001).
Disclosures
None.
Supporting information
Tables S1–S4
Figure S1
Acknowledgments
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group; and the project development and management teams at the Beijing Tiantan Hospital and the Kailuan Group.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023054
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Yanxia Luo, Email: lyx100@ccmu.edu.cn.
Yongjun Wang, Email: yongjunwang@ncrcnd.org.cn.
REFERENCES
- 1. Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho SK, Chang Y, Kim I, Ryu S. U‐shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol. 2018;70:1122–1132. doi: 10.1002/art.40472 [DOI] [PubMed] [Google Scholar]
- 3. Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the apolipoprotein mortality risk study (AMORIS). J Intern Med. 2009;266:558–570. [DOI] [PubMed] [Google Scholar]
- 4. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4 [DOI] [PubMed] [Google Scholar]
- 5. Ong G, Davis WA, Davis TM. Serum uric acid does not predict cardiovascular or all‐cause mortality in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2010;53:1288–1294. doi: 10.1007/s00125-010-1735-7 [DOI] [PubMed] [Google Scholar]
- 6. Palmer T, Nordestgaard B, Benn M, Tybjærg‐Hansen A, Davey Smith G, Lawlor D, Timpson N. Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. BMJ. 2013;347:f4262. doi: 10.1136/bmj.f4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Muraki I, Imano H, Cui R, Yamagishi K, Umesawa M, Hayama‐Terada M, Ohira T, Kiyama M, Okada T, et al. Serum uric acid and risk of stroke and its types: the Circulatory Risk in Communities Study (CIRCS). Hypertens Res. 2020;43:313–321. doi: 10.1038/s41440-019-0385-5 [DOI] [PubMed] [Google Scholar]
- 8. Hozawa A, Folsom AR, Ibrahim H, Nieto FJ, Rosamond WD, Shahar E. Serum uric acid and risk of ischemic stroke: the ARIC study. Atherosclerosis. 2006;187:401–407. doi: 10.1016/j.atherosclerosis.2005.09.020 [DOI] [PubMed] [Google Scholar]
- 9. Tao J, Shen X, Li J, Cha E, Gu P, Liu J, Zhu W, He L, Li G, Wang Z. Serum uric acid to creatinine ratio and metabolic syndrome in postmenopausal Chinese women. Medicine. 2020;99:e19959. doi: 10.1097/MD.0000000000019959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gu L, Huang L, Wu H, Lou Q, Bian R. Serum uric acid to creatinine ratio: a predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diab Vasc Dis Res. 2017;14:221–225. doi: 10.1177/1479164116680318 [DOI] [PubMed] [Google Scholar]
- 11. Durmus Kocak N, Sasak G, Aka Akturk U, Akgun M, Boga S, Sengul A, Gungor S, Arinc S. Serum uric acid levels and uric acid/creatinine ratios in stable chronic obstructive pulmonary disease (COPD) patients: are these parameters efficient predictors of patients at risk for exacerbation and/or severity of disease? Med Sci Monit. 2016;22:4169–4176. doi: 10.12659/MSM.897759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li M, Gu L, Yang J, Lou Q. Serum uric acid to creatinine ratio correlates with β‐cell function in type 2 diabetes. Diabetes Metab Res Rev. 2018;34:e3001. doi: 10.1002/dmrr.3001 [DOI] [PubMed] [Google Scholar]
- 13. Mazidi M, Katsiki N, Banach M. Α higher ratio of serum uric acid to serum creatinine could predict the risk of total and cause specific mortality—insight from a US national survey. Int J Cardiol. 2020;326:189–193. [DOI] [PubMed] [Google Scholar]
- 14. Moriyama K. The association between the serum uric acid to creatinine ratio and metabolic syndrome, liver function, and alcohol intake in healthy Japanese subjects. Metab Syndr Relat Disord. 2019;17:380–387. doi: 10.1089/met.2019.0024 [DOI] [PubMed] [Google Scholar]
- 15. Kawamoto R, Ninomiya D, Akase T, Kikuchi A, Kasai Y, Kusunoki T, Ohtsuka N, Kumagi T. Serum uric acid to creatinine ratio independently predicts incident metabolic syndrome among community‐dwelling persons. Metab Syndr Relat Disord. 2019;17:81–89. doi: 10.1089/met.2018.0055 [DOI] [PubMed] [Google Scholar]
- 16. Lyubarova R, Robinson JG, Miller M, Simmons DL, Xu P, Abramson BL, Elam MB, Brown TM, McBride R, Fleg JL, et al. Metabolic syndrome cluster does not provide incremental prognostic information in patients with stable cardiovascular disease: a post hoc analysis of the AIM‐HIGH trial. J Clin Lipidol. 2017;11:1201–1211. doi: 10.1016/j.jacl.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jahangiry L, Farhangi M, Rezaei F. Framingham risk score for estimation of 10‐years of cardiovascular diseases risk in patients with metabolic syndrome. J Health Popul Nutr. 2017;36:36. doi: 10.1186/s41043-017-0114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laakso M, Kuusisto J, Stančáková A, Kuulasmaa T, Pajukanta P, Lusis A, Collins F, Mohlke K, Boehnke M. The Metabolic Syndrome in Men study: a resource for studies of metabolic and cardiovascular diseases. J Lipid Res. 2017;58:481–493. doi: 10.1194/jlr.O072629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris‐Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40:1565–1572. doi: 10.2337/dc17-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris‐Etherton PM, Wu Y, Jin C, Huang S, Hu FB, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open. 2019;2:e194758. doi: 10.1001/jamanetworkopen.2019.4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroke—1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 22. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas A, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.CIR.90.1.583 [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2000;23(Suppl 1):S4–S19. [PubMed] [Google Scholar]
- 25. Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. 2020;75:302–308. doi: 10.1161/HYPERTENSIONAHA.119.13643 [DOI] [PubMed] [Google Scholar]
- 26. Stevens P, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 27. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure‐mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowlin S, Cleland C, Parekh N, Hagan H, Melkus G. Racial and ethnic disparities in predictors of glycemia: a moderated mediation analysis of inflammation‐related predictors of diabetes in the NHANES 2007–2010. Nutr Diabetes. 2018;8:56. doi: 10.1038/s41387-018-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y, Zhang T, Han T, Li S, Bazzano L, He J, Chen W. Impact of cigarette smoking on the relationship between body mass index and insulin: longitudinal observation from the Bogalusa Heart Study. Diabetes Obes Metab. 2018;20:1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao Z, Cheng Y, Li S, Yang H, Sun L, Gao Y, Yu P, Li W, Wang Y. Mediation of the effect of serum uric acid on the risk of developing hypertension: a population‐based cohort study. J Transl Med. 2019;17:202. doi: 10.1186/s12967-019-1953-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo Y, Cui L, Ye P, Li J, Wu S, Luo Y. Change of kidney function is associated with all‐cause mortality and cardiovascular diseases: results from the Kailuan study. J Am Heart Assoc. 2018;7:e010596. doi: 10.1161/JAHA.118.010596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al‐Shamsi S, Oulhaj A, Regmi D, Govender R. Use of estimated glomerular filtration rate to predict incident chronic kidney disease in patients at risk of cardiovascular disease: a retrospective study. BMC Nephrol. 2019;20:325. doi: 10.1186/s12882-019-1494-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71:851–865. doi: 10.1053/j.ajkd.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guembe M, Fernandez‐Lazaro C, Sayon‐Orea C, Toledo E, Moreno‐Iribas C. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13‐year prospective study in the RIVANA cohort. Cardiovasc Diabetol. 2020;19:195. doi: 10.1186/s12933-020-01166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP, Robinson BM, Townsend RR, Chen J, Fogelfeld L, et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: findings from the chronic renal insufficiency cohort study. BMC Nephrol. 2019;20:60. doi: 10.1186/s12882-019-1220-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voiosu A, Wiese S, Voiosu T, Bendtsen F, Møller S. Bile acids and cardiovascular function in cirrhosis. Liver Int. 2017;37:1420–1430. doi: 10.1111/liv.13394 [DOI] [PubMed] [Google Scholar]
- 37. Yu MA, Sánchez‐Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin‐angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid‐induced endothelial dysfunction. J Hypertens. 2010;28:1234–1242. doi: 10.1097/HJH.0b013e328337da1d [DOI] [PubMed] [Google Scholar]
- 38. Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh‐Hamad D, Lan HY, Feng L, et al. Uric acid stimulates monocyte chemoattractant protein‐1 production in vascular smooth muscle cells via mitogen‐activated protein kinase and cyclooxygenase‐2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B [DOI] [PubMed] [Google Scholar]
- 39. Kang D‐H, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. doi: 10.1159/000087713 [DOI] [PubMed] [Google Scholar]
- 40. Neogi T, Ellison RC, Hunt S, Terkeltaub R, Felson DT, Zhang Y. Serum uric acid is associated with carotid plaques: the National Heart, Lung, and Blood Institute Family Heart Study. J Rheumatol. 2009;36:378–384. doi: 10.3899/jrheum.080646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Son M, Seo J, Yang S. Association between dyslipidemia and serum uric acid levels in Korean adults: Korea National Health and Nutrition Examination Survey 2016–2017. PLoS One. 2020;15:e0228684. doi: 10.1371/journal.pone.0228684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Sun Y, Wang S, Qian H, Jia P, Chen Y, Li Z, Zhang L. Body adiposity index, lipid accumulation product, and cardiometabolic index reveal the contribution of adiposity phenotypes in the risk of hyperuricemia among Chinese rural population. Clin Rheumatol. 2018;37:2221–2231. doi: 10.1007/s10067-018-4143-x [DOI] [PubMed] [Google Scholar]
- 43. Kim T, Lee S, Yoo J, Kim S, Yoo S, Song H, Kim Y, Choi E, Kim Y. The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:3. doi: 10.1186/1758-5996-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choi Y‐J, Yoon Y, Lee K‐Y, Hien TT, Kang KW, Kim K‐C, Lee J, Lee M‐Y, Lee SM, Kang D‐H, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28:3197–3204. doi: 10.1096/fj.13-247148 [DOI] [PubMed] [Google Scholar]
- 45. Ma H, Wang X, Guo X, Li X, Qi L, Li Y. Distinct uric acid trajectories are associated with different risks of incident hypertension in middle‐aged adults. Mayo Clin Proc. 2019;94:611–619. doi: 10.1016/j.mayocp.2018.08.042 [DOI] [PubMed] [Google Scholar]
- 46. Li Q, Yang Z, Lu B, Wen J, Ye ZI, Chen L, He M, Tao X, Zhang W, Huang Y, et al. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:72. doi: 10.1186/1475-2840-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al‐Daghri NM, Al‐Attas OS, Wani K, Sabico S, Alokail MS. Serum uric acid to creatinine ratio and risk of metabolic syndrome in Saudi type 2 diabetic patients. Sci Rep. 2017;7:12104. doi: 10.1038/s41598-017-12085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figure S1


