Abstract
BAY38-4766 and BAY43-9695 are nonnucleosidic compounds with activities against human cytomegalovirus (HCMV). Two phenotypic assays were used to determine the drug susceptibilities of 36 HCMV clinical isolates to the BAY compounds and ganciclovir. Using either assay, both BAY compounds at a concentration of approximately 1 μM inhibited the replication of all 36 HCMV clinical isolates, including 11 ganciclovir-resistant clinical isolates, by 50%.
Human cytomegalovirus (HCMV) causes considerable morbidity and mortality in immunocompromised hosts (13). Organ transplant patients suffer from hepatitis and pneumonia caused by HCMV infections, whereas AIDS patients suffer from HCMV-induced retinitis and other complications (1). The current Food and Drug Administration-approved therapies for retinitis due to infection with HCMV include ganciclovir, foscarnet, cidofovir, and fomivirsen (2, 11, 12, 14). These antiviral drugs are active against infections caused by HCMV; however, they are not ideal because of their toxicity and poor bioavailability. Furthermore, long-term treatment with these antiviral agents often leads to the selection of viral mutants that are resistant to one or more of these drugs (3–5). Current research has led to the discovery of several novel compounds with in vitro and in vivo activities against HCMV (6, 7, 16). One such compound, BAY38-4766, is a nonnucleosidic inhibitor of HCMV replication (17). Two phenotypic drug susceptibility assays, a flow cytometric fluorescence-activated cell sorter (FACS) assay (8–10) and a plaque reduction assay (PRA) (15), were used to compare the effects of BAY38-4766, its main metabolite, BAY43-9695, and ganciclovir on the in vitro replication of ganciclovir-susceptible and ganciclovir-resistant HCMV clinical isolates. BAY38-4766 and BAY43-9695 inhibited the replication of ganciclovir-sensitive and ganciclovir-resistant HCMV clinical isolates at concentrations less than or equal to 1 μM. These results suggest that these compounds are potentially useful for treating patients infected with ganciclovir-sensitive or ganciclovir-resistant HCMV.
The use of the FACS assay and the PRA for determining 50% inhibitory concentrations (IC50s) for HCMV clinical isolates have been described in detail previously (8–10). These two phenotypic assays were used to determine the susceptibilities of the AD169 laboratory strain of HCMV and 36 HCMV clinical isolates to BAY38-4766, BAY43-9695, and ganciclovir. The FACS assay yielded average IC50s of BAY38-4766, BAY43-9695, and ganciclovir for the AD169 laboratory strain of 0.95 ± 0.17 (mean ± standard deviation), 0.70 ± 0.30, and 3.05 ± 0.21 μM, respectively. The PRA yielded average IC50s of these three drugs for AD169 of 0.64 ± 0.14, 0.55 ± 0.06, and 3.50 ± 0.21 μM, respectively. The average IC50s of the two BAY compounds and ganciclovir for 36 HCMV clinical isolates are presented in Table 1. Both BAY compounds inhibited the replication of all of the HCMV clinical isolates by 50% at essentially the same concentrations. Of the 36 HCMV clinical isolates, 25 were susceptible to ganciclovir (IC50s less than 8 μM) and 11 were partially or completely resistant to ganciclovir (IC50s between 9 and >96 μM). For the ganciclovir-susceptible clinical isolates, the average IC50s of BAY38-4766 and BAY43-9695 were approximately one-third of the average IC50s of ganciclovir. Furthermore, both BAY compounds inhibited the replication of ganciclovir-resistant HCMV clinical isolates by 50% at average concentrations near 1 μM. The PRA yielded very similar ranges for and average IC50s of these three drugs for the HCMV clinical isolates. When the biases and levels of precision of the IC50s of each of the three drugs for these HCMV clinical isolates, determined by the FACS assay and the PRA, were compared, there was a <2-fold difference, suggesting that the more rapid FACS assay is equivalent in efficacy to the PRA. These results show that BAY38-4766 and BAY43-9695 inhibited replication for a large number of HCMV clinical isolates, including some that were partially or completely resistant to ganciclovir.
TABLE 1.
IC50s of compounds for HCMV clinical isolates
| Assay | Drug | No. of clinical isolates | IC50 (μM)
|
|
|---|---|---|---|---|
| Range | Avg ± SD | |||
| FACS | BAY38-4766 | 36 | 0.54–1.99 | 1.06 ± 0.34 |
| BAY43-9695 | 36 | 0.49–1.81 | 0.95 ± 0.34 | |
| Ganciclovira | 11 | 9.20–>96 | 30.07 ± 33.08 | |
| Ganciclovirb | 25 | 1.07–7.59 | 3.07 ± 1.40 | |
| PRA | BAY38-4766 | 36 | 0.25–2.30 | 1.00 ± 0.40 |
| BAY43-9695 | 36 | 0.24–2.49 | 1.11 ± 0.38 | |
| Ganciclovira | 11 | 7.61–>96 | 44.35 ± 36.65 | |
| Ganciclovirb | 25 | 1.00–7.00 | 3.51 ± 1.32 | |
The data are for 11 ganciclovir-resistant isolates.
The data are for 25 ganciclovir-susceptible isolates.
To be therapeutically useful, antiviral compounds must be effective in the presence of serum proteins. To determine the effect of serum proteins on the ability of these BAY compounds to inhibit HCMV replication, human foreskin fibroblasts were infected with the AD169 laboratory strain of HCMV in the presence of various concentrations of these compounds in medium containing 4% human albumin and 1 mg of α-1 acid glycoprotein/ml and the cells were analyzed for the percentage of IE antigen-positive cells by FACS analysis. In the absence of any additives, the IC50s for AD169 were 0.65 μM for BAY38-4766 and 0.53 μM for BAY43-9695. In the presence of serum proteins, the IC50s were 6.2 μM for BAY38-4766 and 8.42 μM for BAY43-9695. In contrast, the IC50s of ganciclovir were 5.36 μM in the absence of serum proteins and 4.53 μM in the presence of serum proteins, confirming that ganciclovir does not bind extensively to serum proteins. These results show that the presence of plasma binding proteins decreases the virological activities of these compounds 10- to 16-fold and that ganciclovir was unaffected by the presence of these binding proteins.
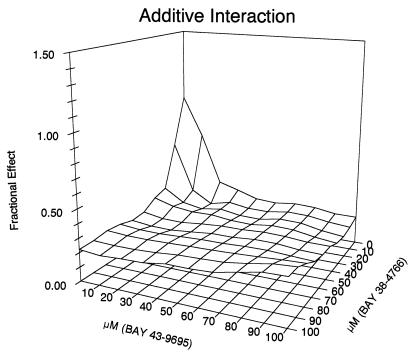
The preceding data show that both compounds have activities against HCMV and that they bind serum proteins. To determine if combinations of the two drugs are additive or synergistic, the AD169 laboratory strain was grown in the presence of serum binding proteins and various concentrations of the two compounds and the percentage of IE antigen-positive cells was determined by FACS analysis. The results are shown in Fig. 1. The value of the α parameter was 0.216, and the 95% confidence interval was −0.074 to 0.358. The value of α ± the 95% confidence interval overlaps zero; therefore, the interaction is additive. These data suggest that BAY43-9695, the main in vivo metabolite of BAY38-4766, may enhance the antiviral effect of the parent compound by establishing longer-lasting inhibitor levels after application.
FIG. 1.
Additive effects of BAY38-4766 and BAY43-9695. Human foreskin fibroblast monolayers were infected with the AD169 laboratory strain of HCMV in the presence of serum binding proteins and various amounts of BAY 38-4766 and BAY 43-9695, and the percentage of IE antigen-positive cells was determined by FACS analysis. The data show that the two drugs are additive.
We have demonstrated that micromolar concentrations of BAY38-4766 and BAY43-9695 inhibited the replication of both ganciclovir-susceptible and ganciclovir-resistant HCMV clinical isolates. The antiviral activities of the two compounds are additive. However, protein binding studies demonstrated that approximately 90% of each of the two BAY compounds is bound to proteins. Both BAY compounds inhibit the replication of ganciclovir-resistant HCMV clinical isolates, suggesting that the BAY compounds have a different mode of action. These BAY compounds join other anti-HCMV compounds that have modes of action different from those of ganciclovir, foscarnet, and cidofovir (6, 7, 16). These novel compounds represent new classes of drugs that are potentially useful for controlling HCMV infections in immunocompromised patients infected with HCMV isolates that are resistant to the currently used antiviral drugs.
Acknowledgments
A 5 mM stock of ganciclovir was provided by the Virology Quality Assurance Program, Division of AIDS, NIAID. BAY38-4766 and its main metabolite, BAY43-9695, were provided by the Bayer Corporation, West Haven, Conn.
This work was supported in part by a grant from the Bayer Corporation and by grants AI45350 and AI45257 from the National Institutes of Health.
REFERENCES
- 1.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2524. [Google Scholar]
- 2.Crumpacker C. Ganciclovir. N Engl J Med. 1996;335:721–729. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- 3.Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev. 1999;12:286–297. doi: 10.1128/cmr.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabs D A, Enger C, Forman M, Dunn J P for The Cytomegalovirus Retinitis and Viral Resistance Study Group. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. Antimicrob Agents Chemother. 1998;42:2240–2244. doi: 10.1128/aac.42.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabs D, Enger C, Dunn J P, Forman M. Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. J Infect Dis. 1998;177:770–773. doi: 10.1086/514249. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson J G, Renau T E, Nassiri M R, Sweier D G, Breittenbach J M, Townsend L B, Drach J C. Nonnucleoside pyrrolopyrimidines with a unique mechanism of action against human cytomegalovirus. Antimicrob Agents Chemother. 1999;43:1888–1894. doi: 10.1128/aac.43.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krosky P M, Underwood M R, Turk S R, Feng K W-H, Jain R K, Ptak R G, Westerman A C, Biron K K, Townsend L B, Drach J C. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McSharry J J. Antiviral drug susceptibility assays: going with the flow. Antivir Res. 1999;43:1–21. doi: 10.1016/s0166-3542(99)00039-x. [DOI] [PubMed] [Google Scholar]
- 9.McSharry J J, Lurain N S, Drusano G L, Landay A L, Notka M, O'Gorman M R G, Weinberg A, Shapiro H M, Reichelderfer P S, Crumpacker C S. Rapid ganciclovir susceptibility assay using flow cytometry for human cytomegalovirus clinical isolates. Antimicrob Agents Chemother. 1998;42:2326–2331. doi: 10.1128/aac.42.9.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McSharry J M, Lurain N S, Drusano G L, Landay A, Manischewitz J, Nokta M, O'Gorman M, Shapiro H M, Weinberg A, Reichelderfer P, Crumpacker C. Flow cytometric determination of ganciclovir susceptibilities of human cytomegalovirus clinical isolates. J Clin Microbiol. 1998;36:958–964. doi: 10.1128/jcm.36.4.958-964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols W G, Boeckh M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000;16:25–40. doi: 10.1016/s1386-6532(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 12.Palestine A G, Polis M A, DeSmet M D, Baird B F, Falloon J, Kovacs J A, Davey R T, Zurlo J J, Zunich K M, Davis M, Hubbard L, Brothers R, Ferris F L, Chew E, Davis J L, Rubin B I, Mellow S D, Metcalf J A, Maneschewitz J, Minor J R, Nussenblatt R B, Masur H, Lane H C. A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med. 1991;115:665–673. doi: 10.7326/0003-4819-115-9-665. [DOI] [PubMed] [Google Scholar]
- 13.Patel R, Paya C V. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polis M A, Spooner K M, Baird B F, Manischewitz J F, Jaffe H S, Fisher P E, Falloon J, Davey R T, Jr, Kovacs J A, Walker R E, Whitcup S M, Nussenblatt R B, Lane H C, Masur H. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995;39:882–886. doi: 10.1128/aac.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanat S C, Reardon J E, Erice A, Jordan M C, Drew W L, Biron K K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991;35:2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Underwood M R, Harvey R J, Stanat S C, Hemphill M L, Miller T, Drach J C, Townsend L B, Biron K K. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72:717–725. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber O, Bender W, Eckenberg P, Goldman S, Haerter M, Hallenberger S, Henninger K, Reefschlager J, Trappe J, Witt-Laido A, Ruebsamen-Waigmann H. Inhibition of murine cytomegalovirus and human cytomegalovirus by a novel non-nucleosidic compound in vivo. Antivir Res. 2001;49:179–189. doi: 10.1016/s0166-3542(01)00127-9. [DOI] [PubMed] [Google Scholar]